-
PDF
- Split View
-
Views
-
Cite
Cite
Yu Kato, Nathaniel I Bloom, Peifang Sun, Corey A Balinsky, Qi Qiu, Ying Cheng, Vihasi Jani, Megan A Schilling, Carl W Goforth, Dawn L Weir, Irene Ramos, Stuart C Sealfon, Andrew G Letizia, Shane Crotty, Memory B-Cell Development After Asymptomatic or Mild Symptomatic SARS-CoV-2 Infection, The Journal of Infectious Diseases, Volume 227, Issue 1, 1 January 2023, Pages 18–22, https://doi.org/10.1093/infdis/jiac319
Close - Share Icon Share
Abstract
The development of memory B cells after asymptomatic severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is not well understood.
We compared spike antibody titers, pseudovirus neutralizing antibody titers, and memory B-cell responses among SARS-CoV-2 PCR-positive Marine recruits who either reported asymptomatic or symptomatic infection.
Thirty-six asymptomatic participants exhibited similar spike IgG titers, spike IgA titers, and pseudovirus neutralization titers compared to 30 symptomatic participants. Pseudovirus neutralization and spike IgG titers showed significant positive correlations with frequency of memory B cells.
Among young adults, asymptomatic SARS-CoV-2 infection induced antibody and memory B-cell responses comparable to mild symptomatic infection.
Clinical outcomes due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of coronavirus disease 2019 (COVID-19), can range from asymptomatic to fatal respiratory failure [1]. Approximately 40%–50% of SARS-CoV-2 infections are asymptomatic in unvaccinated populations, with younger individuals being more likely to have an asymptomatic infection [2]. Immune memory and protective immunity for individuals who experienced asymptomatic SARS-CoV-2 infection is not well understood compared to symptomatic infection. From a public health perspective, it is important to evaluate immune memory after asymptomatic cases [3].
An asymptomatic infection may reflect one of several possible scenarios. The individual may have been infected with a low dose of virus that was cleared more rapidly, resulting in no symptomatic disease [4]. The individual’s innate immune response may have controlled viral replication earlier and to a lower peak titer. The individual may have had a more rapid T-cell response, resulting in improved control of the viral infection [5]. Thus, it is plausible that an individual with an asymptomatic SARS-CoV-2 infection could develop either more or less immune memory to SARS-CoV-2 than an individual who experienced a symptomatic infection. While asymptomatic COVID-19 patients do produce binding and neutralizing antibodies, they tend to be of lower magnitude and may have different decay kinetics than symptomatic cases [6]. Additionally, less is known regarding memory B cells in asymptomatic cases and adaptive immune memory in young adults.
The longitudinal, prospective COVID-19 Health Action Response for Marines (CHARM) study was established to characterize viral transmission dynamics to mitigate the spread of SARS-CoV-2 among new United States Marine Corps (USMC) recruits [7]. After a large number of recruits were infected with SARS-CoV-2, all of whom had asymptomatic or mildly symptomatic disease and none required inpatient treatment, studies were initiated to understand the immune responses of participants in the cohort. Humoral responses were similar between asymptomatic and symptomatic cases [8]. Risk of reinfection within the CHARM cohort was found to be higher for individuals with lower IgG titers after primary infection [9]. To improve our knowledge of immune memory in asymptomatic compared to symptomatic cases of SARS-CoV-2 infection among young adults, we evaluated antibody and memory B-cell responses among CHARM participants, with particular interest in filling knowledge gaps regarding memory B cells in asymptomatic cases.
METHODS
Human Subjects
All participants attended USMC recruit training as recruits between May and November 2020. Only participants that were seronegative for receptor-binding domain (RBD) at the time of enrollment in the study were included in the analyses regardless of spike immunoglobulin G (IgG) status. Unexposed donors were obtained from the La Jolla Institute of Immunology Normal Blood Donor Program. Peripheral blood mononuclear cells (PBMCs) for measuring antigen-specific memory B were isolated from heparin tubes on Ficoll-Paque (GE Healthcare BioSciences) within 24 hours, and stored in liquid nitrogen until used. The study protocol was approved by the Naval Medical Research Center Institutional Review board (protocol number NMRC.2020.0006) in compliance with all applicable Federal regulations governing the protection of human subjects. All participants provided written informed consent for participation.
Quantitative IgA and IgG ELISAs
SARS-CoV-2 spike and RBD-specific IgG and IgA serum levels were determined using an enzyme-linked immunosorbent assay (ELISA), as previously described with modifications [8].
Polymerase Chain Reaction
Polymerase chain reaction (PCR) was performed using the TaqPath COVID-19 Combo Kit (Thermo Fisher Scientific), which is authorized by the Food and Drug Administration.
PSV Neutralization Assay
To determine antibody-mediated neutralization, pseudovirus (PSV)-based neutralization assays were performed. PSV was produced using Lentivirus-derived ZsGreen reporter and packaging plasmids (see supplementary material) generously made available through BEI resources (National Institute of Allergy and Infectious Diseases, National Institutes of Health). PSV neutralization assays were performed as previously described, with modifications [8].
Flow Cytometry
To detect SARS-CoV-2–specific B cells, biotinylated protein antigens were individually multimerized with fluorescently labeled streptavidin. Full-length SARS-CoV-2 spike (2P-stabilized, double Strep-tagged) and RBD were generated in house. Biotinylation was performed using biotin-protein ligase standard reaction kit (Avidity, catalog No. Bir500A) following the manufacturer’s protocol and dialyzed overnight against phosphate-buffered saline. Antigen probes were prepared and PBMCs were stained as previously described [10]. Stained PBMC samples were acquired on Cytek Aurora and analyzed using FlowJo 10.7.1 (BD Bioscience). Additional details for laboratory procedures can be found in the Supplementary Material.
Quantification and Statistical Analysis
Statistical analyses were performed in GraphPad Prism 9.0 (GraphPad software). Nonparametric tests were used for all comparisons. Unpaired Mann-Whitney tests were used for comparison between symptomatic and asymptomatic participant groups. Antibody and neutralization data was reported as median values and all other data was reported as geometric means. Correlations were determined by nonparametric Spearman correlations.
RESULTS
USMC recruits who participated in the CHARM study from May through November 2020 underwent PCR testing from nares swabs and completed a symptom questionnaire (Supplementary Material) upon enrollment, weekly for the first 2 weeks while in quarantine, and then biweekly for an additional 6 weeks while training [9]. The questionnaire qualitatively assessed 14 specific symptoms and contained an opportunity for the participant to write in any other symptoms. This study was conducted prior to SARS-CoV-2 variants of concern emerging or COVID-19 vaccine availability. Participants who were PCR positive for SARS-CoV-2 were subsequently followed every 3–4 days for the first 2 weeks after infection and then biweekly for the rest of their basic training, up to approximately 8 weeks postinfection. PBMCs were obtained upon enrollment and at all encounters after infection. Sixty-six of the participants who tested positive for SARS-CoV-2 were selected to characterize antibody and memory B-cell responses, 36 of whom experienced asymptomatic infection and 30 mild symptomatic infection. Supplementary Table 1 describes the participants in the study, who were predominantly young males (93.9% male, median age 18 years (interquartile range, 18-19)). Among the 30 symptomatic participants, 4 had 1–2 symptoms reported on a single time point while the rest had at least 3 symptoms or reported symptoms on 2 or more different time points postinfection, with the range of symptoms reported at each encounter ranging from 1 to 11. All symptomatic participants were treated as outpatients and none received any type of medication beyond symptomatic treatment including nonsteroidal anti-inflammatory drugs or acetaminophen.
Humoral Responses Among COVID-19 Symptomatic and Asymptomatic Participants
We assessed serum spike IgG and IgA responses of the volunteers at 3 time points: baseline (enrollment), at the time of their first positive PCR tests for SARS-CoV-2, and approximately 8 weeks after their first positive SARS-CoV-2 test. Spike IgG and IgA responses were initially undetectable in the majority of the participants (Figure 1A and 1B). Most volunteers mounted detectable antibody responses by approximately 8 weeks, and spike IgG and IgA titers were comparable between symptomatic and asymptomatic participants (Figure 1A and 1B). SARS-CoV-2 PSV neutralizing antibody titers were also comparable in asymptomatic and symptomatic participants at approximately 8 weeks (Figure 1C). Thus, asymptomatic and mild symptomatic infections with SARS-CoV-2 induced similar short-term IgG and IgA responses capable of neutralizing the virus.
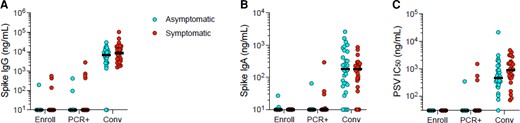
Antibody responses in asymptomatic and mild symptomatic SARS-CoV-2 infections. Data are shown for 3 time points: enrollment (Enroll), date of PCR-positivity (PCR+), and the convalescent period (Conv). A, Serum spike IgG titers; unpaired Mann-Whitney test (P = .4294). B, Serum spike IgA titers; unpaired Mann-Whitney test (P = .4616). C, SARS-CoV-2 PSV neutralization titers; unpaired Mann-Whitney test (P = .0819). Horizontal lines represent the median. Abbreviations: IC50, 50% inhibitory concentration; IgA, immunoglobulin A; IgG, immunoglobulin G; PSV, pseudovirus; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2,
Generation of Memory B Cells Among Symptomatic and Asymptomatic Participants
Whether asymptomatic SARS-CoV-2 infection led to effective formation of B-cell memory was not clear. Fluorescently labeled multimerized protein probes were used to detect memory B cells specific to SARS-CoV-2 spike and RBD, and B-cell Ig isotypes were determined (Figure 2A and Supplementary Figure 1). In unexposed participants, spike-binding memory B cells constituted less than 20 cells per 106 PBMCs (Figure 2B). RBD-binding memory B cells were undetectable (Figure 2C). In convalescent participants after asymptomatic or symptomatic SARS-CoV-2 infection, spike- and RBD-binding memory B-cell frequencies were elevated in the majority of cases (Figure 2B and 2C). The median frequency of spike-binding memory B cells did not differ significantly in symptomatic compared to asymptomatic participants, and spike-binding memory B cells were detectable in 77% of participants in both groups, above that of unexposed participants (Figure 2B). Similarly, RBD-binding memory B-cell responses did not differ significantly in symptomatic compared to asymptomatic participants (Figure 2B and 2C). While substantial variation in memory B-cell responses was observed between individuals (Figure 2B and 2C), the variation was not associated with symptomatic compared to asymptomatic infection. RBD-binding memory B cells constituted approximately 10%–30% of the spike-binding memory B cells in both convalescent symptomatic and asymptomatic cases, with no evident skewing (Figure 2D). IgG was the dominant isotype of spike-binding memory B cells, while IgM and IgA isotypes represented minor fractions in both asymptomatic and symptomatic participants in the early convalescent phase (Figure 2E). Thus, asymptomatic infection with SARS-CoV-2 induced similar spike- and RBD-binding memory B-cell frequencies compared to mild symptomatic infection among young adults in this cohort.
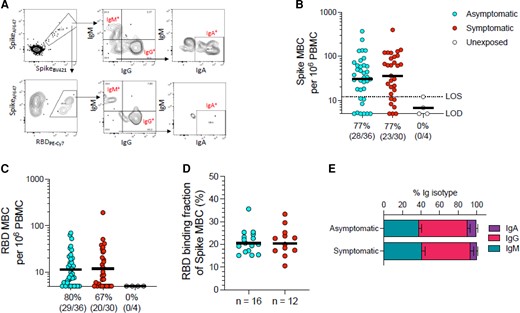
SARS-CoV-2 memory B-cell responses in asymptomatic and mild symptomatic SARS-CoV-2 infections. A, Example flow cytometry plots showing staining patterns of SARS-CoV-2 antigen probes on MBCs (see Supplementary Figure 1 for gating). Spike- and RBD-binding memory B-cell populations are gated. Numbers indicate percentages. Gating strategies to define IgM+, IgG+, or IgA+ SARS-CoV-2 spike-binding or RBD-binding MBCs. B, Frequency of SARS-CoV-2 spike-binding total (IgG+, IgM+, or IgA+) MBCs per 106 PBMCs; unpaired Mann-Whitney test (P = .5365). Axis indicates LOD; dotted line indicates LOS. C, Frequency of SARS-CoV-2 RBD-binding total (IgG+, IgM+, or IgA+) MBCs per 106 PBMCs; unpaired Mann-Whitney test (P = .8790). B and C, Numbers below the graphs show the number of positive participants and total participants, with % responders. D, Fraction of spike-binding MBCs that bound RBD; unpaired Mann-Whitney test (P = .9182). E, Distribution of isotypes among spike-binding MBCs, as gated in (A), for participants who had asymptomatic or symptomatic infections. Horizonal lines in B, C, and D represent the geometric means and the error bars in E represent the standard error of the mean. Abbreviations: IgA, immunoglobulin A; IgG, immunoglobulin G; IgM, immunoglobulin M; LOD, limit of detection; LOS, limit of sensitivity; MBC, memory B cell; RBD, receptor binding domain; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Relationships of Humoral and B-Cell Memory
We next assessed interrelationships between antibody and B-cell memory responses. Spike IgG and spike IgA titers correlated positively with SARS-CoV-2 PSV neutralization titers, as expected (Supplementary Figure 2A and 2B). Spike IgG titers showed significant positive correlations with frequency of spike-binding memory B cells and RBD-binding memory B cells (P = .0014 and P < .0001, respectively; Supplementary Figure 2C and 2D). Of note, participants with undetectable levels of RBD-binding memory B cells at approximately 8 weeks postinfection had significantly lower spike IgG titers compared to participants with detectable RBD-binding memory B cells, irrespective of whether the participants experienced asymptomatic or symptomatic infection (P = .0074; Supplementary Figure 2E). Collectively, the data indicate that among young, healthy adults similar magnitude of antibody and memory B-cell responses are generated irrespective of whether asymptomatic or mild symptomatic infection was reported. This suggests the variability of both antibody and memory B-cell responses in young adults cannot be predicted based upon reported COVID-19 symptomology.
DISCUSSION
Investigating immune responses to symptomatic and asymptomatic COVID-19 infections, including among various demographic groups, improves our understanding of protective immunity and generation of immune memory to SARS-CoV-2. Here, we found that asymptomatic SARS-CoV-2 infection induced comparable memory B-cell responses to mild symptomatic infection in young adults. Additionally, antibody responses correlated with antigen-specific memory B-cell responses.
Published studies of SARS-CoV-2 infected individuals requiring hospitalization have observed positive correlations between COVID-19 disease severity and convalescent antibody titers and [11] memory B-cell frequencies [10]. The association with larger antibody or memory B-cell responses likely reflected higher viral burden or longer infection. However, here no correlation was observed, which may indicate that antigen load may not be an important factor distinguishing most asymptomatic cases and mild symptomatic cases for young adults. Indeed, we found no virological differences between asymptomatic cases and mild symptomatic cases in terms of measured SARS-CoV-2 PCR swab cycle threshold values or days of duration of PCR positivity (Supplementary Figure 3).
Different immune memory components have distinct response kinetics [10]. Memory B-cell frequencies peak between 4 and 5 months after symptomatic SARS-CoV-2 infection, whereas antibody and T-cell responses usually peak within a month of infection [10]. Differential kinetics in distinct immune components add a layer of complexity, and likely dictate how different immune components contribute during reexposure to the virus. Memory B cells are maintained for at least 8 months [10], and likely longer [12], which is consistent with the substantial amount of protective immunity observed in previously infected individuals against infection with Alpha or Delta or hospitalization with Omicron variants of concern [13–15].
This study has limitations. The results are based on samples generated from a relatively homogenous cohort predominantly made up of young, healthy males. A strength of this approach is that it has enabled immunological studies while limiting confounding factors such as age. Notably, the study lacked people of advanced age as well as those with severe symptoms. It is plausible that in older populations, B-cell or antibody responses generated in asymptomatic subjects may differ from mild symptomatic subjects. The data also focus on memory in the first 2 months postinfection, and was not statistically powered to detect small differences between groups. Further studies are warranted on durability of memory after asymptomatic SARS-CoV-2 infections. This cohort of young adults will be followed to assess the long-term antibody and B-cell responses over time before and after SARS-CoV-2 vaccination, which is now mandated in the US military.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgment. We thank the many US Navy corpsmen who assisted in the logistics and sample acquisition and the Marine Corps recruits who volunteered for this study.
Author contributions. C. W. G., D. L. W., and A. G. L. contributed to patient study design, enrollment, and clinical characterization. P. S., C. A. B., Q. Q., Y. C., V. J., and M. A. S. performed PMBC isolation, cryopreservation, and PCR testing. I. R. performed serology assays. Y. K. and N. I. B. designed, executed, and performed data analysis for memory B-cell experiments and wrote the initial manuscript draft. A. G. L., S. C. S., and S. C. obtained funding. S. C. S. provided administrative support and technical coordination. S. C. oversaw memory B-cell experiments and performed data analysis and interpretation. All authors contributed to manuscript drafting and critical review. A. G. L. and S. C. contributed equally. The order of cosenior authors was decided based on the timeline of their contributions.
Disclaimer. The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, nor the US Government. P. S., C. W. G., D. L. W., and A. G. L. are military service member or employees of the US Government. This work was prepared as part of official duties. Title 17, U.S.C., §105 provides that copyright protection under this title is not available for any work of the US Government. Title 17, U.S.C., §101 defines a US Government work as a work prepared by a military service member or employee of the US Government as part of that person’s official duties
Financial support. This work was supported by the Defense Health Agency through the Naval Medical Research Center (grant number 9700130 to A. G. L.); the Defense Advanced Research Projects Agency (contract number N6600119C4022 to S. C. S.); and La Jolla Institute for Immunology institutional funds (to S. C.).
References
Author notes
A. G. L and S. C. contributed equally.
Potential conflicts of interest. S. C. has consulted for GSK, JP Morgan, Citi, Morgan Stanley, Avalia NZ, Nutcracker Therapeutics, University of California, California State Universities, United Airlines, Adagio, and Roche. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.




