-
PDF
- Split View
-
Views
-
Cite
Cite
Neha Dubey, Mehak Zahoor Khan, Suresh Kumar, Aditya Sharma, Lahari Das, Asani Bhaduri, Yogendra Singh, Vinay Kumar Nandicoori, Mycobacterium tuberculosis Peptidyl Prolyl Isomerase A Interacts With Host Integrin Receptor to Exacerbate Disease Progression, The Journal of Infectious Diseases, Volume 224, Issue 8, 15 October 2021, Pages 1383–1393, https://doi.org/10.1093/infdis/jiab081
Close - Share Icon Share
Abstract
Attenuated intracellular survival of Mycobacterium tuberculosis (Mtb) secretory gene mutants exemplifies their role as virulence factors. Mtb peptidyl prolyl isomerase A (PPiA) assists in protein folding through cis/trans isomerization of prolyl bonds. Here, we show that PPiA abets Mtb survival and aids in disease progression by exploiting host-associated factors. While the deletion of PPiA has no discernable effect on bacillary survival in a murine infection model, it compromises the formation of granuloma-like lesions and promotes host cell death through ferroptosis. Overexpression of PPiA enhances the bacillary load and exacerbates pathology in mice lungs. Importantly, PPiA interacts with the integrin α5β1 receptor through a conserved surface-exposed RGD motif. The secretion of PPiA as well as interaction with integrin contributes to disease progression by upregulating multiple host matrix metalloproteinases. Collectively, we identified a novel nonchaperone role of PPiA that is critical in facilitating host–pathogen interaction and ensuing disease progression.
Tuberculosis, caused by Mycobacterium tuberculosis (Mtb), is among the major global health concerns. The persistent nature of the disease is due to the ability of the pathogen to manipulate the host machinery through a large repertoire of secretory virulence factors that Mtb produces. Apart from the classically known virulence proteins such as ESAT-6, Ag85A, SapM, and PPE family of proteins, several highly conserved housekeeping proteins, which are otherwise involved in basal metabolic regulation and stress responses, have emerged as key players in eliciting virulence [1]. Peptidyl prolyl isomerase (PPIase), a well-conserved protein-folding enzyme, has recently gained immense interest owing to its role in virulence of several pathogens [2, 3].
PPIases typically assist in protein folding by catalyzing the conformational cis/trans isomerization of prolyl bonds. Mtb encodes 2 cyclophilin type PPIases: PPiA and PPiB, of which PPiA has garnered particular attention due to its secretory nature [4, 5]. PPiA stimulates the expression of host proinflammatory cytokines, suggesting a possible role in immunomodulation and disease progression [6, 7]. While these studies allude to an important function for PPiA, the studies were performed in either in vitro model systems or in nonpathogenic Mycobacterium smegmatis.
To understand the contribution of PPiA in mycobacterial virulence and host–pathogen dynamics, we set out to investigate its in vivo functions. We show that PPiA provides a survival advantage to Mtb by facilitating bacterial survival and the establishment of granuloma-like lesions in mice. Secreted PPiA interacts with host integrin through a conserved RGD motif, upregulating matrix metalloproteinases and resulting in enhanced bacillary survival.
METHODS
Generation of Constructs and Purification of the Recombinant Proteins
ppiA was amplified from Rv genomic DNA using gene-specific primers containing NdeI and HindIII sites (Supplementary Table 1) and cloned in pVV16 vector. PPiA-G24A and PPiA-R73A point mutants were generated using overlapping extension polymerase chain reaction (PCR) mutagenesis (Supplementary Table 1). To generate the PPiA-∆12 secretion mutant, we designed an alternate forward primer. Amplicons were cloned in pET28a and pNiT-3XFLAG vectors using NdeI-HindIII sites and transformed into Escherichia coli BL21 and RvΔppiA, respectively. Recombinant proteins were purified from E. coli through Ni2+-NTA affinity chromatography and filtered. The details of the constructs are provided in Supplementary Tables 2 and 3.
Animal Infection Studies
The animal infection protocol was followed as described earlier [8]. In brief, different strains were cultured in 7H9-ADC to A600 approximately 0.6–0.8. Cells were harvested, washed twice with Phosphate buffered saline with 0.05% Tween 80 (PBST), and resuspended in neutral buffered saline. Six- to 8-week-old Balb/c mice of either sex were infected (n = 6) with 2 × 108 bacilli through the aerosol route to deposit approximately 200 colony-forming units (CFUs) in the lungs, and bacillary loads were enumerated at 4 and 8 weeks. Infected organs were fixed in 10% formalin for hematoxylin and eosin staining for histopathological evaluation and granuloma scoring was performed as described previously [8].
Bioinformatic Analysis
Integrin (PDB ID: 3vi4) and PPiA (PDB ID: 1w74) PDB files were retrieved from Protein Data Bank and uploaded to the protein–protein interaction server PatchDock [9]. In the input, we specified the potential binding sites for the interaction, that is, residue 23–25 of PPiA (RGD motif) and the fibronectin (cognate ligand) binding cavity on integrin structure, which is 218–230 residues on α-subunit 133–135 residues on β subunit. The software provided different models based on the preset algorithms, which were refined based on their corresponding binding energies and stability parameters by FireDock, and analyzed through the Discovery studio visualizer [10, 11].
Ethical Considerations
Animal experiment protocols were reviewed and approved by the Institutional Animal Ethics Committee of the National Institute of Immunology, New Delhi, India (approval number 389/15). The experiments were carried out as per the guidelines issued by the Committee for Control and Supervision of Experiments on Animals, Government of India.
Statistical Analysis
Data are representative of 2 biologically independent experiments. Error bar represents standard deviation, unless mentioned otherwise. Statistical significance was calculated using a nonparametric Student t test. *P < .01, **P < .001, and ***P < .0001 unless specified otherwise. For mice infections, CFU values were plotted as mean with standard error of the mean, and significance is calculated using 2-way analysis of variance test. *P ≤ .05, **P ≤ .01. GraphPad Prism version 5.0 was used for the plotting and analyzing the results and modified using adobe illustrator CS5.1.
RESULTS
PPiA Assists in Bacterial Survival Under Host-like Stress Conditions
PPiA consists of an N-terminal signal peptide and a C-terminal conserved cyclophilin like domain [5, 12]. Two-dimensional separation, followed by matrix-assisted laser desorption/ionization–time of flight analysis of the Mtb culture filtrate protein (CFP) fraction, predicted PPiA to be a secretory protein [4]. We purified and analyzed different subcellular fractions. While SigA could be detected in the cytosolic fraction, PPiA and well-known secretory antigen Ag85A were detected in both cytosolic and CFP fractions (Figure 1A), indicating that PPiA is indeed a secretory prolyl isomerase. To examine the significance of PPiA for Mtb growth and virulence, we generated the gene replacement mutant strain in H37Rv (Rv) (Supplementary Figure 1A and 1B). Gene replacement in RvΔppiA (mutant) was confirmed by PCR (Figure 1B). For complementation, ppiA was subcloned into mycobacterial integrative pST-CirT [13] and electroporated in RvΔppiA to generate RvΔppiA::ppiA. Western blot analysis confirmed the absence of PPiA in the mutant strain and expression of PPiA in RvΔppiA::ppiA (Figure 1C). We evaluated the in vitro growth profiles of Rv, RvΔppiA, and RvΔppiA::ppiA strains in both nutrient-rich and minimal media (Supplementary Figure 1C–E) and found them to be comparable. However, when cholesterol was used as the sole carbon source, RvΔppiA showed significantly compromised growth compared with Rv and RvΔppiA::ppiA (Figure 1C). These results are in agreement with a high-throughput study wherein ppiA transposon mutant showed compromised growth in cholesterol-rich media [14]. Growth assessment of strains under different in vitro stress conditions revealed that the survival of these strains was equivalent under pH, starvation, and nitrosative stress (Supplementary Figure 2A–C). However, RvΔppiA was markedly attenuated compared with Rv and RvΔppiA::ppiA under oxidative and hypoxic stress (Supplementary Figure 1D andSupplementary Figure 1F). These results suggest a plausible role of PPiA in response to the metabolic adaptations and under host-derived redox insults.
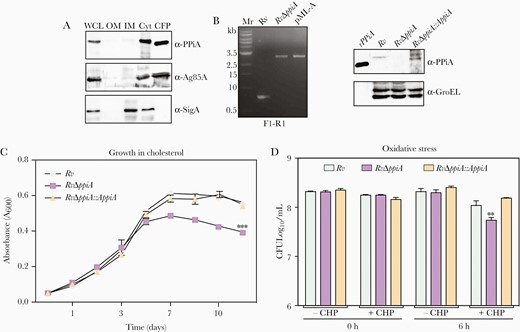
Peptidyl prolyl isomerase A (PPiA) assists in bacterial survival under host-like stress conditions. A, Subcellular localization of PPiA in whole-cell lysate, outer membrane, inner membrane, cytosol, and culture filtrate protein fractions. B, Gel showing amplicons obtained from genomic DNA isolated from Rv and RvΔppiA using external flank primer pairs (Supplementary Figure 1). pML-A DNA was used as positive control. Western blot analysis to probe expression of PPiA. rPPiA-purified recombinant PPiA protein. C, Growth profile in Sauton media supplemented with 0.02% cholesterol and 0.05% tyloxapol. D, Survival under oxidative stress. Abbreviations: CFP, culture filtrate protein; CFU, colony-forming unit; CHP, cumene hydroperoxide; ***p<0.0005; Cyt, cytosol; IM, inner membrane; OM, outer membrane; PPiA, peptidyl prolyl isomerase A; WCL, whole-cell lysate.
PPiA Promotes Ferroptosis-Induced Host Cell Death
Subsequently, we set out to evaluate the role of PPiA in the intracellular bacillary survival. Toward this, we infected THP1 cells with Rv, RvΔppiA, and RvΔppiA::ppiA and examined bacillary survival and host parameters. The intracellular survival of RvΔppiA was found to be attenuated compared with Rv and RvΔppiA::ppiA (Supplementary Figure 3A). The addition of purified PPiA to THP1 cells has been reported to induce tumor necrosis factor alpha (TNF-α) and interleukin (IL) 6 secretion in the host [7]. Congruently, we observed decreased expression of TNF-α, IL-6, IL-12, and IL-4 in RvΔppiA-infected cells, compared with Rv-infected cells (Supplementary Figure 3B). To comprehend the implications, we examined the viability of infected THP1 cells and observed reduced cell death of those infected with RvΔppiA (58%), compared with either Rv (38%) or RvΔppiA::ppiA (28%) (Supplementary Figure 3C). While apoptosis is more favorable host defenses to prompt bacterial clearance, Mtb facilitates necrosis to promote bacterial spread [15]. PPiA is regulated by IdeR (an iron-dependent transcription regulator) [16], an essential gene that is upregulated 24 hours postinfection in THP1 cells [17]. Thus, we sought to examine the role of PPiA in inducing iron-regulated necrotic cell death, that is, ferroptosis. Toward this, we treated THP1 cells with ferrostatin-1 (Fer-1), a potent inhibitor of ferroptosis. Treatment with Fer-1 reduced the cell death in Rv- and RvΔppiA::ppiA-infected cells to the death observed in RvΔppiA-infected cells, suggesting that PPiA induces host cell death through ferroptosis (Figure 2A). Ferroptosis is associated with reduced glutathione levels and glutathione peroxidase-4 expression, which typically function to prevent the accumulation of toxic lipid peroxidases [18, 19]. Compared with Rv and RvΔppiA::ppiA, we observed higher levels of gpx4 transcripts in THP1 cells infected with RvΔppiA (Figure 2B), corroborating the role of PPiA in inducing ferroptosis.
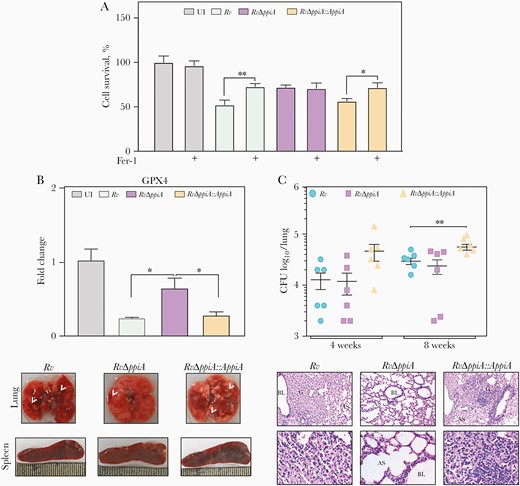
Peptidyl prolyl isomerase A (PPiA) promotes ferroptosis-induced host cell death. A, THP1 cells were infected with indicated strains and treated with 10 μM Fer-1, and survival was quantified 24 hours postinfection by Thiazolyl Blue Tetrazolium Bromide assay. B, THP1 cells were infected with indicated strains at a multiplicity of infection of 1:10 for 24 hours followed by the isolation of host RNA to quantify gpx4. C, Bacterial loads in lungs of Balb/c mice infected with indicated strains. D, Gross morphology of lung and spleen (left panel) harvested from infected mice 8 weeks postinfection. Arrows indicates granulomatous lesions. Histopathological analysis (right panel). Fixed infected lung tissues (8 weeks) were stained with hematoxylin and eosin; photomicrograph indicates granulomatous lesions. *P<.05; **P<.005. Abbreviations: AS, alveolar space; BL, bronchoalveolar lavage; CFU, colony-forming unit; E, epithelioid cells; G, granuloma; L, lymphocytes; UI, uninfected.
Next, we examined the significance of PPiA in Mtb virulence in the murine model of infection (Figure 2C). While we did not observe differences in the lung and spleen bacillary load between Rv and RvΔppiA (Figure 2C and Supplementary Figure 4A), the bacillary loads in the mice infected with RvΔppiA::ppiA were significantly higher, suggesting that higher expression of PPiA in the complemented strain (Figure 1B) augments Mtb survival within the host. Importantly, gross pathology evaluation indicated that the absence of PPiA results in a healthier tissue with fewer lesions on the surface (Figure 2D). Consonantly, histopathological analysis revealed reduced granulomatous lesions in RvΔppiA-infected lungs compared with Rv and RvΔppiA::ppiA (Figure 2D and Supplementary Figure 4B).
Overexpression of PPiA Promotes Mtb Survival in a Murine Model of Infection
To address this, we generated the overexpression strain, wherein the ppiA was cloned into the episomal pVV16 vector and electroporated in Rv to generate Rv::ppiA. Western blot analysis established overexpression of PPiA (Supplementary Figure 4C). Next, we infected mice with Rv::pVV16 (control) and Rv::PPiA. Compared with Rv::pVV16-infected mice lungs, Rv::PPiA-infected mice lungs exhibited an increased number of granulomatous lesions (Figure 3B) and higher granuloma score (Supplementary Figure 4D). Importantly, Rv::PPiA survived approximately 8-fold higher in lungs and spleen of mice compared with control (Figure 3C and 3D). While the absence of PPiA compromises the establishment of granulomatous lesions, which could affect the long-term survival and dissemination of bacteria, its overexpression led to higher bacillary survival. Thus, our results show a positive correlation between disease severity and PPiA expression levels.
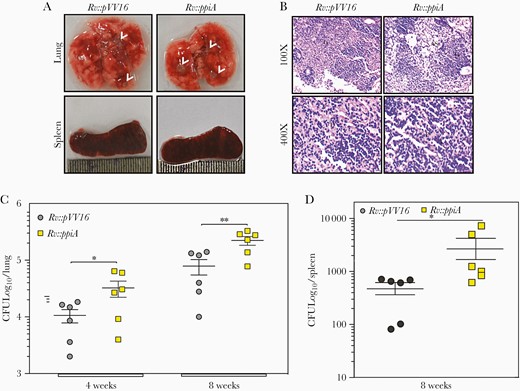
Overexpression of peptidyl prolyl isomerase A (PPiA) promotes Mycobacterium tuberculosis (Mtb) survival in the murine model of infection. A, Gross morphology of lung and spleen of mice infected with indicated strains at 8 weeks postinfection. Arrowheads represent granulomatous lesions. B, Eight weeks postinfection lung tissues from infected animals were harvested, fixed, and stained with hematoxylin and eosin for histopathological analysis. Photomicrograph depicts the granulomatous lesion. C and D, Bacterial loads in the lungs and spleen of Balb/c mice infected with indicated strains. *P<.05; **P<.005. Abbreviations: AS, alveolar space; CFU, colony-forming units; E, epithelioid cells; G, granuloma; L, lymphocytes.
PPiA Can Act as a Ligand to Cell Surface Integrin Receptor
We sought to determine how PPiA, a secretory protein, modulates the disease severity and its possible role in host–pathogen interaction. Analysis of primary sequence and crystal structure of PPiA revealed the presence of surface-exposed RGD motif (Figure 4A and 4B), a characteristic motif present on the ligands of host cell surface integrin receptor. We first examined the interaction between host integrin and PPiA through in silico docking analysis (Figure 4B and 4C). The input specified the ligand-binding region of integrin, that is, α-chain: 218–230 residues and β–chain: 133–135 residues [20]. The curated analysis revealed a stable binding of PPiA in the heterodimeric receptor cavity of the integrin with the global binding energy of –7.78 kcal/mol (Figure 4C). The docking results suggested the presence of multiple intermolecular bonds (Figure 4C). We validated in silico predictions through cell adhesion assay, wherein A549 alveolar epithelial cells were preincubated with different ligands and compared their ability to inhibit adhesion to fibronectin-coated wells (Figure 4D). The preincubation of the cells with collagen, a known ligand of integrin, inhibited the adhesion to fibronectin up to approximately 50% (Figure 4D). While the control RAD peptide did not inhibit adhesion, the extent of inhibition by PPiA and RGD peptide was found to be comparable (35% and 30%, respectively), suggesting that PPiA can establish contact with the host integrin receptor through its surface-exposed RGD motif.
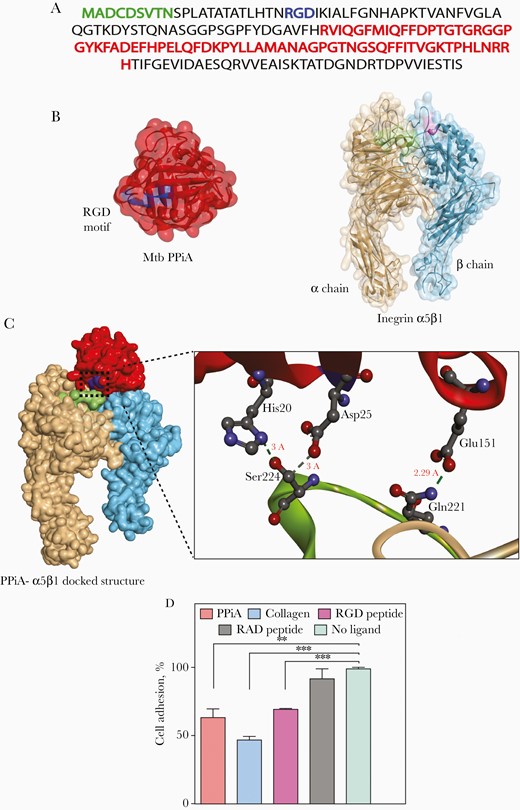
Peptidyl prolyl isomerase A (PPiA) can act as a ligand to cell surface integrin receptor. A, Primary amino acid Mycobacterium tuberculosis (Mtb) PPiA sequence illustrating signal sequence (green), integrin-binding arginine-glycine-aspartic acid (RGD) motif (blue), and active site (red). B, Protein data bank (PDB) structures of Mtb PPiA (PDB ID:1w74) and integrin α5β1(PDB ID:3vi4). PPiA represented as a red surface ribbon with an RGD motif (residue 23–25) highlighted in blue. Integrin α5β1 binding cavity highlighted in green (218–230 residues on α chain) and pink (133–135 on β chain). C, Docked structure of Mtb PPiA with integrin α5β1 as predicted by Patchdock server and refined through Firedock. Inset represents the enlarged docking interface showing intermolecular hydrogen bonding between Ser224(Integrin)-Asp25(PPiA), Ser224(Integrin)-His20(PPiA), and Gln221(Integrin)-Glu151(PPiA) residues. D, A549 cells were pretreated with indicated ligands and seeded on fibronectin-coated plates posttreatment. Cell adhesion was analyzed by staining the adhered cells with 0.1% crystal violet stain, followed by solubilization. A570nm was measured and converted to percentage adhesion inhibition by normalizing the values with no-ligand control. **P<.005; ***P<.0005.
PPiA Modulates the Expression of Host Matrix Metalloproteinases
Integrin signaling is crucial for maintaining tissue integrity through interactions with several extracellular matrix (ECM) components, and maintaining the critical balance of matrix metalloproteinases (MMPs) and tissue inhibitor of MMPs (TIMPs). Mtb skews this critical MMP/TIMP balance to gain access to the host tissue [21–23]. Thus, we sought to determine if PPiA modulates MMP levels. A549 cells were incubated with purified PPiA for different time-points and transcription levels of mmps and integrin α5β1 were quantified. Addition of PPiA resulted in time-dependent upregulation of mmp7, mmp9, mmp13, and integrin transcript levels (Figure 5A and 5B and Supplementary Figure 5A and 5B). Western blot analysis after PPiA treatment confirmed induced MMP9 expression (Supplementary Figure 5C). To corroborate these results, we examined the mmp levels in THP1 cells infected with Rv and RvΔppiA (Figure 5C and 5D and Supplementary Figure 5D and 5E). Infection with Rv resulted in increased mmp transcripts. Importantly, infection with RvΔppiA resulted in lower extent of induction compared with Rv, suggesting that PPiA upregulates transcription of host MMPs.
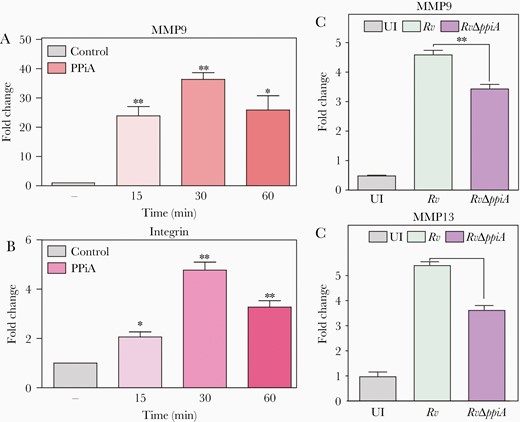
Peptidyl prolyl isomerase A (PPiA) modulates the expression of host matrix metalloproteinases. A and B, A549 cells were treated with 5 µg/mL rPPiA for indicated duration followed by host RNA isolation to examine mmp9 and integrin α5β1. Control: no protein. C and D, THP1 cells were infected at a multiplicity of infection of 1:10. Host cell RNA was extracted 24 hours postinfection to assess mmp9 and mmp13 transcript levels. *P<.05; **P<.005; ***P<.0005. Abbreviations: PPiA, peptidyl prolyl isomerase A; UI, uninfected.
PPiA Secretion and Catalytic Activity, as Well as Interaction With Integrin, Are Necessary for Its Functionality
R73 residue in the active site of PPiA is necessary for its prolyl isomerase activity [12], and the N-terminal 12 amino acids constitute the secretion signal [5]. Intrigued by the ability of PPiA to modulate MMP expression, we sought to investigate the role of (1) prolyl isomerase activity, (2) secretion of PPiA, and (3) its interaction with integrins, in the mmp transcript levels and PPiA-mediated intracellular bacillary survival. While the incubation of A549 cells with recombinant PPiA (rPPiA) could induce the expression of mmp7, mmp9, mmp13, and integrin, incubation with PPiA-G24A (RGD motif mutant), PPiA-R73A (active site mutant), and PPiAΔ12 (secretion mutant) showed markedly reduced induction of these genes (Figure 6A and Supplementary Figure 5F and 5G). Transfection of THP1 cells with integrin α5 endoribonuclease-prepared siRNA (esiRNA) (83.9% reduction) abrogated rPPiA-mediated induction of mmp9, suggesting that PPiA induces MMP expression via integrin (Figure 6B). Subsequently, we sought to validate the above findings in the THP1 model of infection. Toward this, wild-type and mutant ppiA were cloned into pNit-3X-FLAG vector and electroporated into the RvΔppiA strain. Whereas Rv and RvΔppiA::pN-ppiA significantly induced mmp9 transcript expression, neither RvΔppiA mutant nor RvΔppiA complemented with PPiA mutants failed to do so (Figure 6C). The consequence of these results was reflected in bacillary survival (Figure 6D), indicating that activity, secretion, and its interaction with the integrin is necessary for PPiA-mediated modulation of mmp expression and bacillary survival.
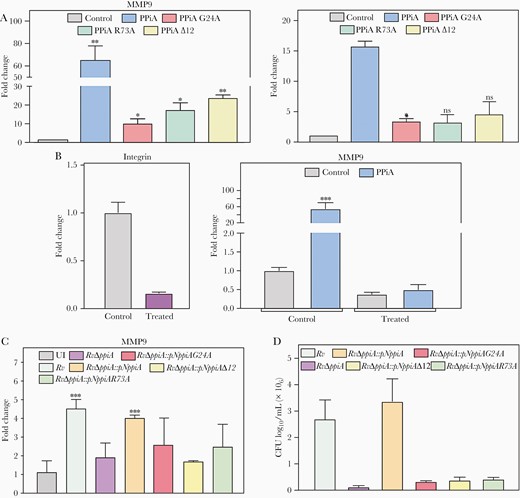
Peptidyl prolyl isomerase A (PPiA) secretion and catalytic activity, as well as interaction with integrin, are necessary for its functionality. A, A549 cells were treated with 5 µg/mL of indicated proteins for 1 hour, followed by isolation of the host RNA to assess mmp9 and integrin α5β1 levels. Control: no protein. B, THP1 cells were either left untreated (control) or transfected with integrin α5 endoribonuclease-prepared siRNA (esiRNA), and the transfection efficiency was assessed by quantifying integrin α5 levels. Transfected cells were treated with 10 µg PPiA for 1 hour and mmp9 levels were quantified. C, THP1 cells were infected with indicated strains at 1:10 multiplicity of infection (MOI) for 24 hours, followed by isolation of host RNA to quantify mmp9 levels. D, THP1 cells were infected with indicated strains at 1:10 MOI, and intracellular bacillary survival was enumerated at 24 hours postinfection. *P<.05; **P<.005; ***P<.0005. Abbreviations: CFU, colony-forming units; ns, not significant; PPiA, peptidyl prolyl isomerase A; UI, uninfected.
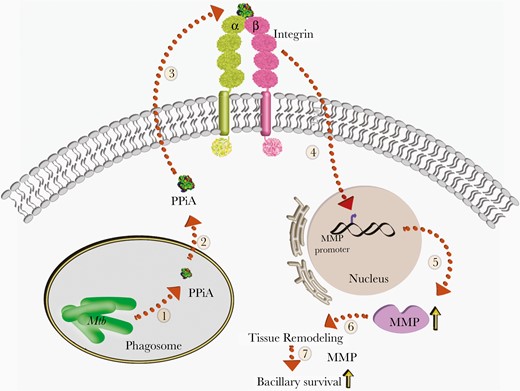
Model depicting the role of peptidyl prolyl isomerase A (PPiA) in host-pathogen interaction. Mycobacterium tuberculosis (Mtb) encodes for PPiA, a propyl isomerase (1) that possesses an N-terminus secretion signal. Upon secretion (2), it interacts with the host integrin receptor (3), with ensuing upregulation of matrix metalloproteinases (MMPs) (4). PPiA-mediated upregulation of MMP transcription and translation (5) is known to remodel host tissues (6), thereby abetting bacillary survival within the host (7).
DISCUSSION
The severity of tuberculosis infection relies on the ability of the pathogen to successfully manipulate the multifaceted host responses. Several secretory proteins such as ESAT-6, PknG, and PtpA play distinct roles during a bacterial infection including host cell lysis, modulation of key regulatory events, phagosomal maturation arrest, etc, creating an accommodating environment for Mtb persistence [24, 25]. In agreement with previous reports, results show that PPiA is indeed a secretory protein.
The evident dispensability of PPiA for in vitro growth in both nutrient-rich and minimal media agrees with the transposon mutagenesis studies [26]. PPiA expression was demonstrated to be unique to the intraphagosomal environment, which explains dispensability for the in vitro growth [27]. We show the importance of PPiA in supporting bacterial growth on cholesterol-rich media (Figure 1), the major carbon source within a nutrient-limited host environment. PPIases promote bacterial survival under multiple stress conditions by facilitating the protein folding process and preventing the accumulation of misfolded proteins [28]. In Mtb, several chaperones are upregulated to prevent proteome destabilization [29]. Consonantly, PPiA deletion compromises Mtb survival under hypoxic and oxidative stress. We speculate that substrate proteins of PPiA include those that are involved in antioxidant defense, hence making the deletion mutant sensitive to oxidative stress. This also explains previous findings wherein PPiA overexpression in M. smegmatis promoted bacterial survival under oxidative stress [30].
Owing to its secretion, PPiA is proposed to participate in host–pathogen interaction [5]. Mtb infection ensues iron accumulation in macrophages to promote host cell death through ferroptosis [18]. PPiA induces ferroptosis, resulting in enhanced intracellular bacillary survival. However, in a much more complex murine model of infection, we did not observe impairment upon PPiA deletion. The ability of PPiA mutant to modulate survival at the early phase of infection cannot be gauged with 4- and 8-week time points. These observed differences between ex vivo and in vivo models could also be due to the complexity of the animal model wherein the network of cellular and immune responses deliver cumulative impact. Interestingly, even though the mutant showed comparable bacillary load, histopathological analysis revealed an important role of PPiA in the formation of the granuloma-like lesions. Similar phenotypes were reported for other Mtb proteins that contribute to disease progression and pathology, despite evident dispensability for the bacterial survival [31–33]. Importantly, we found that PPiA overexpression results in enhanced pathogen survival in the infected mice.
Degradation of the host ECM is an important pathological event that favors disease progression by facilitating the development of tuberculous granuloma and subsequent disease dissemination [21, 22, 34]. The host integrin network coordinates several such physiological responses in the host and is exploited by pathogens for their benefit [35, 36]. In the case of tuberculosis, integrins have been primarily implicated in eliciting protective immune responses by specifically priming the T-cell population to generate effective pulmonary inflammatory responses and through the coordinated expansion of humoral responses [37, 38]. Mtb in turn utilizes the integrin signaling network to its benefit by facilitating multiple stages of infection such as bacterial adhesion and uptake, leukocyte migration, T-cell stimulation, and host tissue and ECM remodeling [39–42]. Mtb cell wall glycolipid phosphatidylinositol mannosides has been shown to bind to α5β1 and promotes adhesion to fibronectin [40]. In silico docking analysis revealed a stable H-bonding network between PPiA (RGD motif) and ligand-binding cavity of integrin α5β1, and mutations in this motif abrogate the interaction. The phenotype of RvΔppiA mirrors the one observed upon infection in α1β1 integrin–deficient mice that forms impaired granulomas despite similar bacillary CFU [44].
Mtb infection results in the induction of mmp through TNF-α and IL-18 in host cells. The mycobacteria-induced mmp9 is negatively regulated by the T-cell–associated cytokines interferon-γ and IL-10 [45]. Hence, MMP-mediated bacterial dissemination [46] is regulated by critical MMP-TIMP balance and associated cytokines. PPiA acts as a key regulator of TNF-α levels, which in turn are important for the induction of mmp9 [45]. Mtb has been reported to upregulate several host MMPs to promote host ECM disintegration and tuberculosis immunopathology [21, 22, 43, 46]. PPiA modulates the expression of host MMPs upon infection, which is dependent on its catalytic activity, secretion, and interactions with integrin. While the role of secretion and RGD domain is comprehensible, the role of catalysis in the process is not apparent. We speculate that PPiA catalytic activity is likely to play a role in modulating the function of proteins in parallel pathways that ultimately effect similar phenotypes. Taken together, we show that PPiA is an important secretory protein that assists host–pathogen interaction, thus promoting the establishment of granuloma-like lesions.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. Conceptualization: N. D., V. K. N., and Y. S. Investigation: N. D., M. Z. K., S. K., A. K. S., L. D. Formal analysis: N. D., M. Z. K., A. K. S., L. D., A. B. Writing, review, and editing: N. D., M. Z. K., V. K. N., Y. S. Resources, supervision, and funding acquisition: V. K. N., Y. S.
Financial support. This work is funded by a JC Bose fellowship to Y. S. (SB/S2/JCB-012/2015) and V. K. N. (JCB/2019/000015). The mycobacterial suicide vector pML523 was a kind gift from Dr Amit Singh’s Lab (IISc, Bengaluru). N. D. was supported through Indian Council of Medical Research, Senior Research Fellowship. L. D. and M. Z. K. are supported through a D. S. Kothari postdoctoral fellowship and Science and Engineering Research Board, respectively. We acknowledge the support of the Tuberculosis Aerosol Challenge Facility at International Centre for Genetic Engineering and Biotechnology and their staff for the animal experiments.
Potential conflicts of interest. All authors: No reported conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
Author notes
N. D. and M. Z. K. contributed equally to this work.




