-
PDF
- Split View
-
Views
-
Cite
Cite
Ali Faisal Saleem, Ondrej Mach, Mohammad Tahir Yousafzai, Zaubina Kazi, Attaullah Baig, Muhammad Sajid, Vishali Jeyaseelan, Roland W Sutter, Anita K M Zaidi, One-Year Decline of Poliovirus Antibodies Following Fractional-Dose Inactivated Poliovirus Vaccine, The Journal of Infectious Diseases, Volume 223, Issue 7, 1 April 2021, Pages 1214–1221, https://doi.org/10.1093/infdis/jiaa504
Close - Share Icon Share
Abstract
Fractional dose (one-fifth of full intramuscular dose) of inactivated poliovirus vaccine (fIPV) administered intradermally is used as IPV dose-sparing strategy. We compared the rate of decline of poliovirus antibodies (PVA) in recipients of 2 doses of fIPV or IPV.
A community-based randomized controlled trial was conducted in Karachi, Pakistan. Children aged 14 weeks were randomized into fIPV or full IPV (study arms A, B) and received 1 vaccine dose at age 14 weeks and 1 at age 9 months. PVAs were measured at age 14, 18 weeks and 10, 21 months.
Seroprevalence of poliovirus type 2 antibodies in 170/250 (68%) children after 2 IPV or fIPV doses at age 10 months in A and B reached 100% vs 99% (P = .339), and at 21 months, 86% vs 67% (P = .004). Between age 10 and 21 months antibody log2 titers dropped from ≥ 10.5 to 6.8 in A and from 9.2 to 3.7 in B.
There was a significant decline in antibody titers 12 months following the second IPV dose. The slope of decline was similar for full IPV and fIPV recipients. The results provide further evidence that fIPV is a viable option for IPV dose-sparing.
NCT03286803.
The Global Polio Eradication Initiative continues to make progress towards poliovirus eradication despite some setbacks in 2019: there has been an increase in paralytic cases caused by wild poliovirus in Pakistan and Afghanistan (the last 2 polio-endemic countries), as well as a dramatic increase of outbreaks caused by circulating vaccine-derived poliovirus type 2. On the other hand, wild poliovirus types 2 and 3 have been certified by the Global Certification Commission as eradicated in 2015 and 2019, respectively. The African continent has been free of wild poliovirus since 2016 [1–3].
A single dose of inactivated poliovirus vaccine (IPV) was recommended to be included into routine immunization schedules in all countries using oral poliovirus vaccine (OPV) prior to the withdrawal of the type 2 component of the OPV in April 2016. The role of IPV is to provide an immunity base for type 2 poliovirus as a risk mitigation measure that could be boosted rapidly when type 2 poliovirus is detected and to close the immunity gaps against poliovirus type 2 and 3 [4]. IPV alone, however, does not provide protection against infection with poliovirus. IPV supply constraints affected almost 50 countries and caused either delays in IPV introduction or stock-outs in countries that had already introduced IPV in their routine immunization programs [5]. This situation was rectified in 2018 and 2019. However, since 2016, the only vaccine in routine immunization providing type 2 poliovirus immunity has been IPV, and because IPV vaccination coverage is low in some countries, considerable immunity gaps persist.
One strategy to alleviate the IPV supply shortage has been the introduction of intradermal administration of one-fifth full dose IPV (0.1 mL instead of 0.5 mL), referred to as fractional IPV (fIPV). fIPV has demonstrated good safety, and while 1 dose of fIPV provides lower immunogenicity than 1 full IPV dose, 2 fIPV doses are superior to 1 full IPV dose [6–10]. Based on the clinical trial evidence, several countries have adopted a 2-dose fIPV schedule in their routine immunization programs (India, Sri Lanka, Nepal, Bangladesh, Cuba, and Ecuador). These countries reported good programmatic experience with the vaccine [11, 12]. Also, several countries have used fIPV to respond to poliovirus outbreaks (eg, India, Pakistan, Nigeria, Syria, Somalia, and others) [13, 14]. While immunogenicity induced by fIPV has been documented well, the decline of antibody titers with time has not and the question remained whether antibodies induced by fIPV are shorter lived than those induced by full IPV.
This article summarizes data obtained from a randomized controlled trial conducted in Pakistan to compare the slope of decline of neutralizing poliovirus antibodies following full or fIPV administration.
METHODS
The study was conducted between July 2017 and June 2019 in 4 low-income areas in and around Karachi (4 periurban, contiguous coastal villages: Rehri Goth, Bhains Colony, Ali Akber Shah, and Ibrahim Hydri) where the Aga Khan University’s Department of Pediatrics and Child Health has a well-established demographic surveillance system (DSS), which captures vital events, including population size, pregnancies, and births. The population of the study area according to the last census from 2015 was 294 171. Each area has a primary health center operated by the Department of Pediatrics and Child Health Research Program of the Aga Khan University, which also provides Expanded Program on Immunizations services. Study participants were selected from the DSS data. All expectant mothers and parents of children younger than 9 months living in the DSS area were approached and asked to participate in the study.
Study subjects were enrolled from 2 distinct age groups: for study arms A and B, children were enrolled at 14 weeks of age, and for study arms C and D, they were enrolled at 9 months of age. Eligibility criteria for the younger children included no prior history of IPV vaccination; in the older age group, only children with documented receipt of 1 full dose of IPV at the age of 14 weeks were enrolled. The primary outcomes are demonstrated on data from arms A and B.
After enrollment, children in each age group were randomized to receive either full IPV or fIPV. In arm A, they received 1 full IPV at 14 weeks of age and another full IPV at 9 months of age; in arm B they received 2 fIPV doses at 14 weeks and 9 months; in arm C children received 1 full IPV at 9 months; and in arm D they received 1 fIPV dose at 9 months of age. Therefore, children in arm C received 2 full IPV doses (1 as part of the routine immunization and the second as part of this study); and children in arm D received 1 full IPV dose as part of the routine immunization and an fIPV dose as part of this study.
For arms A and B, 1 mL of peripheral blood was collected at 14 and 18 weeks, and 10 and 21 months of age; in arms C and D, blood was collected at 9, 10, and 21 months of age.
There was a campaign with IPV in the study area during the period of this study. This campaign was conducted in response to a poliovirus outbreak and targeted all children younger than 5 years, including the children enrolled in this study. While the study staff tried to protect the study children from receiving an additional dose of IPV, a proportion of the enrolled children did receive 1 additional full dose of IPV. These children were subsequently removed from the analysis.
Blood specimens collected at the clinical sites were allowed to clot, centrifuged to separate serum, and transported to the Infectious Disease Research Laboratory at the Aga Khan University where they were stored at −20°C until shipment to the Centers for Disease Control and Prevention (CDC), Atlanta, GA, where the sera were tested for the presence of poliovirus neutralizing antibodies using standard neutralization assays. The maximum dilution tested was 1:1024 (and the highest detectable titer reported was 1:≥1448); the minimum (nondetectable) titer reported was < 1:8 [15].
For each serotype, seropositivity was defined as the reciprocal titer of poliovirus neutralizing antibodies ≥ 8; seroconversion in children with no maternal antibodies (at baseline blood sample at 14 weeks of age) was defined as the change from seronegative to seropositive (from reciprocal titer of < 8 to ≥ 8); in those with maternal antibodies it was defined as a 4-fold rise in reciprocal titers over an expected decline in maternal antibodies with estimated half-life of 28 days. The analysis of seroconversion was restricted to infants with initial titer of antibodies < 362 to be able to demonstrate a 4-fold rise over expected decline given the maximum reported titer of 1:1448. The primary outcome was seroprevalence and seroconversion of antibodies to poliovirus type 2; however, we also report seroprevalence of serotypes 1 and 3.
Adverse events following vaccination were identified by site investigators and reviewed by the principal investigator (A.F.S. and Z.K.). Children were observed for 30 minutes following the administration of the vaccine for immediate adverse events; parents were instructed to immediately report back to the health centers if adverse events occurred. Adverse events were defined as any medical condition in the study participants during the study period. Serious adverse events were defined as any medical condition resulting in either hospitalization or death. Serious adverse events were reported for review to the Data and Safety Monitoring Board (DSMB) and the Ethical Review Committee.
IPV was produced by Bilthoven Biologicals BV, and presented in 1-dose vials (0.5 mL). Bivalent OPV (types 1 and 3) was administered outside the study by the national Expanded Program of Immunization, and was procured from Sanofi Pasteur and BioFarma.
The required minimum sample size was calculated by assuming 90% of immune response at 21 months of age after administration of 2 full doses of IPV, 80% power to detect the difference of 15% between arms, 5% level of significance, and 10% of drop outs; thus the required sample size was 500 (125 in each arm).
Data were analyzed using STATA version 11 (StataCorp LLC). The proportion of seroconversion in different study arms was compared by χ2 test for quantitative variables. P value was calculated to assess differences in immune response between study arms. K sample equality of median test was performed to compare the median titers across the study arms and 95% confidence intervals for median titers were calculated.
The study was approved by the Ethical Review Committee of the Aga Khan University, the National Bioethics Committee of Pakistan, and the Ethical Review Committee of the World Health Organization, Geneva. All activities followed the guidelines of Good Clinical Practice. The trial protocol was registered at ClinicalTrials.gov (NCT03286803). The World Health Organization assisted in study design, trial implementation, and monitoring, and contributed to the writing of the report. The Aga Khan University conducted the trial.
RESULTS
A total of 3897 children were approached and 1424 assessed for eligibility; 500 children were enrolled and randomized (125 in each study arm). All study visits were completed by 104, 111, 117, and 112 children in arms A, B, C, and D, respectively (444/500, 89%; Figure 1). After removing children who received an extra dose of IPV and after considering high baseline antibody titers, there were 81, 89, 96, and 83 (349/500, 70%) children included in the final analysis of serotype 2 immunogenicity.
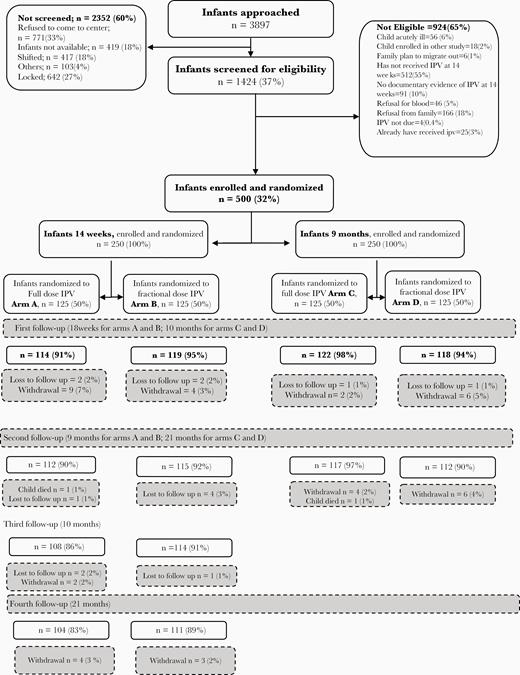
Baseline demographic indicators are shown in Table 1. Except for a significantly higher proportion of male sex in arm B compared with arm A, there were no significant differences between study arms.
| Characteristic . | Arm A, 2 Doses IPV (n = 125) . | Arm B, 2 Doses fIPV (n = 125) . | P Value . | Arm C, 2 Doses IPV (n = 125) . | Arm D, 1 × IPV 1 × fIPV (n = 125) . | P Value . |
|---|---|---|---|---|---|---|
| Sex male, n/N (%) | 56/125 (45) | 76/125 (61) | .011 | 60/125 (48) | 74/125 (59) | .076 |
| Age at enrollment, wk, median (IQR) | 15.7 (14.6–16.9) | 15.1 (14.4–16.4) | .110 | 40 (39.4–41.6) | 40 (39.6–41.7) | .310 |
| Chronic malnutrition moderate and severe, n/N (%) | 30/109 (28) | 30/107 (28) | .930 | 34/92 (37) | 31/90 (34.4) | .720 |
| Monthly income >PKR 10 000, n/N (%)a | 81/125 (65) | 72/125 (58) | .370 | 86/125 (69) | 79/125 (63) | .380 |
| No. of persons sharing household, mean ± SD | 8.9 ± 4.7 | 8.4 ± 3.9 | .370 | 8.6 ± 4.4 | 8.9 ± 4.6 | .590 |
| Ever breastfeed, n/N (%) | 110/114 (97) | 117/119 (98) | .380 | 118/122 (97) | 112/118 (95) | .480 |
| Characteristic . | Arm A, 2 Doses IPV (n = 125) . | Arm B, 2 Doses fIPV (n = 125) . | P Value . | Arm C, 2 Doses IPV (n = 125) . | Arm D, 1 × IPV 1 × fIPV (n = 125) . | P Value . |
|---|---|---|---|---|---|---|
| Sex male, n/N (%) | 56/125 (45) | 76/125 (61) | .011 | 60/125 (48) | 74/125 (59) | .076 |
| Age at enrollment, wk, median (IQR) | 15.7 (14.6–16.9) | 15.1 (14.4–16.4) | .110 | 40 (39.4–41.6) | 40 (39.6–41.7) | .310 |
| Chronic malnutrition moderate and severe, n/N (%) | 30/109 (28) | 30/107 (28) | .930 | 34/92 (37) | 31/90 (34.4) | .720 |
| Monthly income >PKR 10 000, n/N (%)a | 81/125 (65) | 72/125 (58) | .370 | 86/125 (69) | 79/125 (63) | .380 |
| No. of persons sharing household, mean ± SD | 8.9 ± 4.7 | 8.4 ± 3.9 | .370 | 8.6 ± 4.4 | 8.9 ± 4.6 | .590 |
| Ever breastfeed, n/N (%) | 110/114 (97) | 117/119 (98) | .380 | 118/122 (97) | 112/118 (95) | .480 |
Abbreviation: fIPV, fractional inactivated poliovirus vaccine.
aUS$ 1 = PKR 150.
| Characteristic . | Arm A, 2 Doses IPV (n = 125) . | Arm B, 2 Doses fIPV (n = 125) . | P Value . | Arm C, 2 Doses IPV (n = 125) . | Arm D, 1 × IPV 1 × fIPV (n = 125) . | P Value . |
|---|---|---|---|---|---|---|
| Sex male, n/N (%) | 56/125 (45) | 76/125 (61) | .011 | 60/125 (48) | 74/125 (59) | .076 |
| Age at enrollment, wk, median (IQR) | 15.7 (14.6–16.9) | 15.1 (14.4–16.4) | .110 | 40 (39.4–41.6) | 40 (39.6–41.7) | .310 |
| Chronic malnutrition moderate and severe, n/N (%) | 30/109 (28) | 30/107 (28) | .930 | 34/92 (37) | 31/90 (34.4) | .720 |
| Monthly income >PKR 10 000, n/N (%)a | 81/125 (65) | 72/125 (58) | .370 | 86/125 (69) | 79/125 (63) | .380 |
| No. of persons sharing household, mean ± SD | 8.9 ± 4.7 | 8.4 ± 3.9 | .370 | 8.6 ± 4.4 | 8.9 ± 4.6 | .590 |
| Ever breastfeed, n/N (%) | 110/114 (97) | 117/119 (98) | .380 | 118/122 (97) | 112/118 (95) | .480 |
| Characteristic . | Arm A, 2 Doses IPV (n = 125) . | Arm B, 2 Doses fIPV (n = 125) . | P Value . | Arm C, 2 Doses IPV (n = 125) . | Arm D, 1 × IPV 1 × fIPV (n = 125) . | P Value . |
|---|---|---|---|---|---|---|
| Sex male, n/N (%) | 56/125 (45) | 76/125 (61) | .011 | 60/125 (48) | 74/125 (59) | .076 |
| Age at enrollment, wk, median (IQR) | 15.7 (14.6–16.9) | 15.1 (14.4–16.4) | .110 | 40 (39.4–41.6) | 40 (39.6–41.7) | .310 |
| Chronic malnutrition moderate and severe, n/N (%) | 30/109 (28) | 30/107 (28) | .930 | 34/92 (37) | 31/90 (34.4) | .720 |
| Monthly income >PKR 10 000, n/N (%)a | 81/125 (65) | 72/125 (58) | .370 | 86/125 (69) | 79/125 (63) | .380 |
| No. of persons sharing household, mean ± SD | 8.9 ± 4.7 | 8.4 ± 3.9 | .370 | 8.6 ± 4.4 | 8.9 ± 4.6 | .590 |
| Ever breastfeed, n/N (%) | 110/114 (97) | 117/119 (98) | .380 | 118/122 (97) | 112/118 (95) | .480 |
Abbreviation: fIPV, fractional inactivated poliovirus vaccine.
aUS$ 1 = PKR 150.
Seroprevalence of poliovirus type 2 antibodies is shown in Figure 2. At 18 weeks of age, after 1 dose of either full or fIPV, seroprevalence was significantly higher in the full IPV arm than in the fIPV arm (72% vs 46%, P < .001); at 10 months, after 2 doses of either full IPV or fIPV, it was 100% vs 99% (P = .339); and at 21 months it was 86% vs 67% (P = .004). In arms C and D at 9 months, seroprevalence was 46% vs 41%, respectively (P = .67), 96% vs 90% at 10 months (P = .206), and 77% vs 68% at 21 months (P = .148). Between 10 and 21 months of age, type 2 seroprevalence dropped by 14% vs 32% in arms A and B (P = .027), and by 19% vs 23% in arms C and D (P = .276).
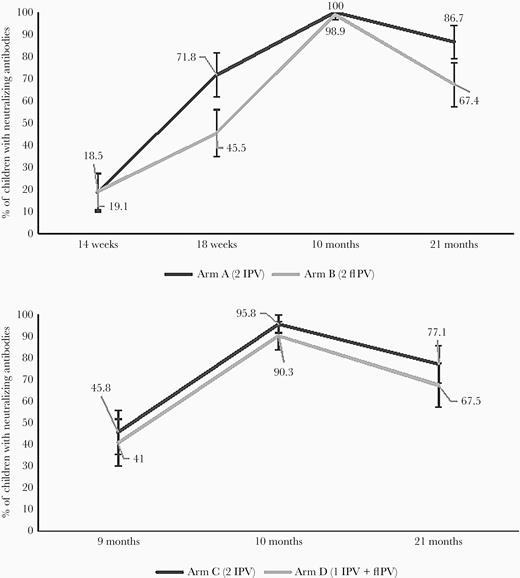
Seroprevalence of poliovirus type 2 antibodies in study arms A, B, C, and D. Abbreviation: fIPV, fractional inactivated poliovirus vaccine.
We evaluated type 2 seroconversion in arms A and B between weeks 14 and 18; it was 68% for arm A and 41% for arm B (P < .001).
Median reciprocal titers of type 2 antipolio antibodies are shown in log2 scale in Figure 3. The median titers dropped from ≥ 10.5 to 6.8, from 9.2 to 3.7, from 9.8 to 5.2, and from 8.5 to 3.8 for study arms A, B, C, and D, respectively, between 10 and 21 months of age. Reverse cumulative titer curves are shown in Figure 4.
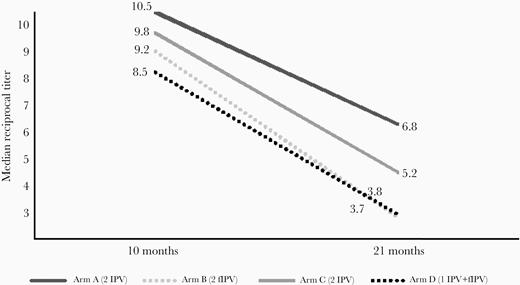
Median reciprocal titer of poliovirus type 2 antibodies at 10 and 21 months in study arms A, B, C, and D. Abbreviation: fIPV, fractional inactivated poliovirus vaccine.
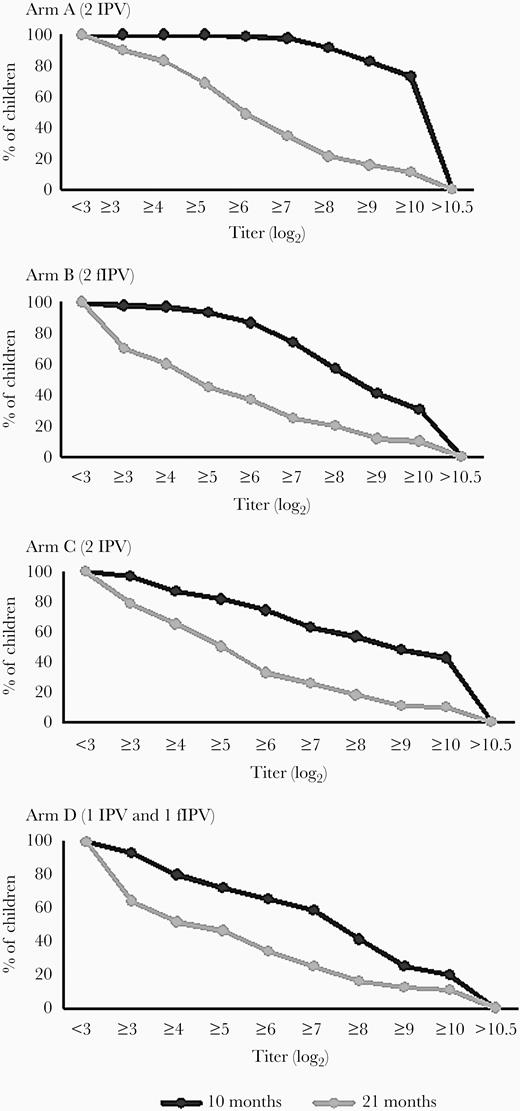
Reverse cumulative curves of poliovirus type 2 antibody titers. Abbreviation: fIPV, fractional inactivated poliovirus vaccine.
Among those who received an additional full IPV dose as part of the outbreak response campaign, 92/96 (96%) remained seropositive for poliovirus serotype 2 at 21 months of age.
Seroprevalence of poliovirus antibodies type 1 and 3 is shown in Table 2. It was close to 100% for serotype 1 at all study visits and for all study arms, and between 85% and 100% for serotype 3.
| Poliovirus Vaccine . | Seroprevalence, % (No. Seropositive/Total No.) . | . | . | . | . |
|---|---|---|---|---|---|
| . | 14 weeks (n = 250) . | 18 weeks (n = 224) . | 9 months (n = 250) . | 10 months (n = 462) . | 21 months (n = 445) . |
| Poliovirus type 1 | |||||
| Arm A (2 IPV) | 94 (117/125) | 98 (106/108) | … | 100 (108/108) | 100 (104/104) |
| Arm B (2 fIPV) | 96 (120/125) | 97 (113/116) | … | 100 (114/114) | 100 (111/111) |
| Arm C (2 IPV) | … | … | 100 (125/125) | 100 (122/122) | 100 (117/117) |
| Arm D (1 IPV + 1fIPV) | … | … | 99 (124/125) | 100 (118/118) | 100 (113/113) |
| Poliovirus type 3 | … | … | … | … | … |
| Arm A (2 IPV) | 88 (110/125) | 95 (103/108) | … | 100 (108/108) | 100 (104/104) |
| Arm B (2 fIPV) | 86 (108/125) | 94 (109/116) | … | 98 (112/114) | 99 (110/111) |
| Arm C (2 IPV) | … | … | 95 (119/125) | 100 (122/122) | 100 (117/117) |
| Arm D (1 IPV + 1fIPV) | … | … | 99 (124/125) | 100 (118/118) | 100 (113/113) |
| Poliovirus Vaccine . | Seroprevalence, % (No. Seropositive/Total No.) . | . | . | . | . |
|---|---|---|---|---|---|
| . | 14 weeks (n = 250) . | 18 weeks (n = 224) . | 9 months (n = 250) . | 10 months (n = 462) . | 21 months (n = 445) . |
| Poliovirus type 1 | |||||
| Arm A (2 IPV) | 94 (117/125) | 98 (106/108) | … | 100 (108/108) | 100 (104/104) |
| Arm B (2 fIPV) | 96 (120/125) | 97 (113/116) | … | 100 (114/114) | 100 (111/111) |
| Arm C (2 IPV) | … | … | 100 (125/125) | 100 (122/122) | 100 (117/117) |
| Arm D (1 IPV + 1fIPV) | … | … | 99 (124/125) | 100 (118/118) | 100 (113/113) |
| Poliovirus type 3 | … | … | … | … | … |
| Arm A (2 IPV) | 88 (110/125) | 95 (103/108) | … | 100 (108/108) | 100 (104/104) |
| Arm B (2 fIPV) | 86 (108/125) | 94 (109/116) | … | 98 (112/114) | 99 (110/111) |
| Arm C (2 IPV) | … | … | 95 (119/125) | 100 (122/122) | 100 (117/117) |
| Arm D (1 IPV + 1fIPV) | … | … | 99 (124/125) | 100 (118/118) | 100 (113/113) |
Abbreviation: fIPV, fractional inactivated poliovirus vaccine.
| Poliovirus Vaccine . | Seroprevalence, % (No. Seropositive/Total No.) . | . | . | . | . |
|---|---|---|---|---|---|
| . | 14 weeks (n = 250) . | 18 weeks (n = 224) . | 9 months (n = 250) . | 10 months (n = 462) . | 21 months (n = 445) . |
| Poliovirus type 1 | |||||
| Arm A (2 IPV) | 94 (117/125) | 98 (106/108) | … | 100 (108/108) | 100 (104/104) |
| Arm B (2 fIPV) | 96 (120/125) | 97 (113/116) | … | 100 (114/114) | 100 (111/111) |
| Arm C (2 IPV) | … | … | 100 (125/125) | 100 (122/122) | 100 (117/117) |
| Arm D (1 IPV + 1fIPV) | … | … | 99 (124/125) | 100 (118/118) | 100 (113/113) |
| Poliovirus type 3 | … | … | … | … | … |
| Arm A (2 IPV) | 88 (110/125) | 95 (103/108) | … | 100 (108/108) | 100 (104/104) |
| Arm B (2 fIPV) | 86 (108/125) | 94 (109/116) | … | 98 (112/114) | 99 (110/111) |
| Arm C (2 IPV) | … | … | 95 (119/125) | 100 (122/122) | 100 (117/117) |
| Arm D (1 IPV + 1fIPV) | … | … | 99 (124/125) | 100 (118/118) | 100 (113/113) |
| Poliovirus Vaccine . | Seroprevalence, % (No. Seropositive/Total No.) . | . | . | . | . |
|---|---|---|---|---|---|
| . | 14 weeks (n = 250) . | 18 weeks (n = 224) . | 9 months (n = 250) . | 10 months (n = 462) . | 21 months (n = 445) . |
| Poliovirus type 1 | |||||
| Arm A (2 IPV) | 94 (117/125) | 98 (106/108) | … | 100 (108/108) | 100 (104/104) |
| Arm B (2 fIPV) | 96 (120/125) | 97 (113/116) | … | 100 (114/114) | 100 (111/111) |
| Arm C (2 IPV) | … | … | 100 (125/125) | 100 (122/122) | 100 (117/117) |
| Arm D (1 IPV + 1fIPV) | … | … | 99 (124/125) | 100 (118/118) | 100 (113/113) |
| Poliovirus type 3 | … | … | … | … | … |
| Arm A (2 IPV) | 88 (110/125) | 95 (103/108) | … | 100 (108/108) | 100 (104/104) |
| Arm B (2 fIPV) | 86 (108/125) | 94 (109/116) | … | 98 (112/114) | 99 (110/111) |
| Arm C (2 IPV) | … | … | 95 (119/125) | 100 (122/122) | 100 (117/117) |
| Arm D (1 IPV + 1fIPV) | … | … | 99 (124/125) | 100 (118/118) | 100 (113/113) |
Abbreviation: fIPV, fractional inactivated poliovirus vaccine.
We have not found any significant associations between serological outcomes and sex, age at enrollment, nutritional status, family size, history of breastfeeding, or family income (P > .1 for all factors).
There were 693 adverse events including 13 serious adverse events (SEA) reported among enrolled participants. Among SAE, there were 2 deaths: 1 was 7 weeks after enrolment and the second was 11 weeks after enrollment. All serious adverse events were reported to the DSMB; none were associated with the trial intervention based on the evaluation of the principal investigator (WHO) and the DSMB.
DISCUSSION
This is the first study to compare the decline in titers of poliovirus antibodies following administration of full and fIPV doses in human subjects. We demonstrated that antibody titers decline rapidly in an area without environmental poliovirus circulation.
In arms A and B, both fIPV and full IPV achieved almost 100% seroprevalence to poliovirus type 2 after 2 doses and, as expected, the median antibody titer achieved with full IPV was higher than with fIPV. We cannot fully assess the magnitude of this difference because the maximum reported titer reported by the CDC laboratory was censored to 1:1448. A significant decline in seroprevalence as well as in median titers was observed in both full and fIPV recipients over a 11-month period.
The slope of antibody decline to poliovirus type 2 in our study suggests similar decline for fIPV recipients and full IPV recipients. This is despite different final median titers and proportion of seroprevalence in arms A and B; we believe that this observation was due to the fact that we were unable to determine the true upper limit of the titers for arm A. On the other hand, none of the median titers in arms C or D reached the maximum level and consequently the slope of decline in seroprevalence and antibody titers was not significantly different between arms C and D.
The duration of immunity induced by the full series of IPV is considered to be long, possibly lasting for life [16]. However, it was previously demonstrated that the antibody titers decline rapidly with time since the last IPV dose [17]. Memory B cells are considered to maintain immunological protection in IPV recipients whose antibody titers fall below detectable levels [18]. Memory B cells persisted in macaque models despite absence of poliovirus neutralizing antibodies after 2 doses of fIPV or IPV [19].
Study arms C and D enrolled 9-month-old children with proof of full IPV receipt at 14 weeks of age. These children reached lower seroprevalence and lower median titers than those in arms A and B. This may be because some of the children in arms C and D did not, in reality, receive IPV dose despite having it documented in a vaccination card. Our study pointed to the fact that official vaccination records in this area may not be accurate.
Our data demonstrated a very high seroprevalence of type 1 poliovirus antibodies. This provides evidence that in the study area, the immunization program was able to achieve a very high coverage with poliovirus vaccines even in very young infants (14-week seroprevalence approximately 95%). Regular campaigns with bivalent OPV (types 1 and 3) have been conducted in this area as a response to ongoing transmission of wild poliovirus type 1 in Pakistan; therefore, we were unable to document decline in type 1 and 3 antibodies.
Our data showed maternal antibodies against type 2 at 14 weeks of about 19%. This is somewhat lower than the finding from Vietnam, which was 27% [20]. Our result provides some evidence that there was no circulation of type 2 in the study area during the study period.
Our study had limitations. The upper bound of reported titers limited our ability to more precisely evaluate the rate of titer decline. In addition, a total of 96 subjects had to be excluded from the analysis, as they received an extra dose of IPV as part of the IPV outbreak response campaign in the study area. Further, the study population is very well followed up by the Aga Khan University research staff and therefore the generalizability of type 1 seroprevalence data to other areas of Karachi or Pakistan may be limited.
We plan to further follow up this cohort of children to demonstrate whether the same rate of decline in antibody titers would be observed in the future. Despite falling antibody titers, we hypothesize that the IPV vaccinated children that seroconverted (irrespective of full or fIPV administration) continue to be protected against paralytic poliomyelitis through memory B-cell immunity even if their antibody titers fall below detectable levels, as has been demonstrated previously [21].
Our study provides solid evidence that a 2-dose fIPV schedule with doses given at age 14 weeks and 9 months induces a similar immunogenicity profile to that induced by a 2 full-dose IPV schedule, and that the slope of neutralizing antibody decay appears to be similar between the study groups. This study adds to a growing body of literature that confirms fIPV is a viable option as a dose-sparing strategy for inclusion in routine immunization programs or for outbreak control response.
Notes
Acknowledgments. We thank Dr M. Steven Oberste, and Deborah Moore, Yiting Zhang, Sharla McDonald, William Hendley, Kathryn Manly, and Mario Nicolas, Division of Viral Diseases, Centers for Disease Control and Prevention (CDC), for conducting the neutralization assays; Ms Shahida Qureshi and Aneeta Hotwani, Infectious Disease Research Laboratory, Aga Khan University, for the storage and shipment of the samples; and Mr Najeeb Ahmed (Data Management Unit, Aga Khan University), Dr Usman Chachar (Coordinator, Emergency Operation Centre, Sindh), Dr Temesgen Demeke (Team Leader, World Health Organization (WHO), Poverty Eradication Initiative, Sindh), and the Government of Sindh in Bin Qasim and Landhi Town for constant support during the project.
Disclaimer. The findings and conclusions in this report are those of the author(s) and do not necessarily represent the views of CDC and other contributing agencies.
Financial support. This work was supported by the WHO from a grant from International PolioPlus Committee, Rotary International, Evanston, IL; and the National Institutes of Health, Fogarty International Center (grant number D43 TW007585-01 research training support to A. F. S.). The CDC supported the project in-kind by provision of laboratory testing and expertise in interpretation.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.




