-
PDF
- Split View
-
Views
-
Cite
Cite
Ali F Saleem, Ondrej Mach, Mohammad T Yousafzai, Asia Khan, William C Weldon, M Steven Oberste, Syed S Zaidi, Muhammad M Alam, Farheen Quadri, Roland W Sutter, Anita K M Zaidi, Immunogenicity of Different Routine Poliovirus Vaccination Schedules: A Randomized, Controlled Trial in Karachi, Pakistan, The Journal of Infectious Diseases, Volume 217, Issue 3, 1 February 2018, Pages 443–450, https://doi.org/10.1093/infdis/jix577
Close - Share Icon Share
Abstract
We assessed immunity against polioviruses induced with a new Pakistani poliovirus immunization schedule and compared it to alternative poliovirus immunization schedules.
Newborns were randomized to undergo vaccination based on 1 of 5 vaccination schedules, with doses administered at birth and at 6, 10, and 14 weeks of age. Arm A received inactivated poliovirus vaccine (IPV) at all time points. Arm B received bivalent oral poliovirus vaccine (bOPV) at all time points. Arms C and D received bOPV at the first 3 time points and bOPV plus IPV at the final time point (the current schedule). Arm E received trivalent OPV (tOPV) at all time points. At 22 weeks of age, all children received 1 challenge dose of tOPV, and children in arm D received 1 additional IPV dose. Sera were analyzed for the presence of poliovirus neutralizing antibodies at birth and 14 and 22 weeks of age.
Seroconversion for poliovirus type 1 (PV1) at 22 weeks of age was observed in 80% of individuals in arm A, 97% in arm B, 94% in arm C, 96% in arm D, and 94% in arm E; for PV2, seroconversion frequencies were 84%, 19%, 53%, 49%, and 93%, respectively; and for PV3, seroconversion frequencies were 93%, 94%, 98%, 94%, and 85%, respectively.
The current immunization schedule in Pakistan induced high seroconversion rates for PV1 and PV3; however, it induced PV2 seroconversion in only half of study subjects. There is a growing cohort of young children in Pakistan who are unprotected against PV2; and this creates an increasing risk of a large-scale outbreak of poliomyelitis caused by circulating vaccine-derived PV2.
During January–September 2017, 11 cases of poliomyelitis caused by wild poliovirus type 1 (WPV1) were reported in Pakistan (5 cases) and Afghanistan (6 cases). There were no cases reported in 2017 from the third remaining polio-endemic country, Nigeria [1]. Complete poliovirus eradication, however, requires the disappearance not only of WPVs but of all polioviruses from human populations, including those resulting from use of oral poliovirus vaccine (OPV). The Polio Eradication and Endgame Strategic Plan 2013–2018 provides a framework for interruption of WPV transmission in remaining polio-endemic foci and lays out a plan for the new polio endgame, which includes the withdrawal of Sabin virus strains contained in OPV, starting with poliovirus type 2 (PV2), and the introduction of at least 1 dose of inactivated poliovirus vaccine (IPV) in every country’s routine immunization schedule, for risk mitigation purposes [2, 3]. The switch from trivalent OPV (tOPV) to bivalent OPV (bOPV) without PV2 was conducted in a globally synchronized manner in April 2016. As of May 2016, there were no countries still using PV2-containing OPV in routine immunization; however, the World Health Organization maintains a reserved stock of PV2-monovalent OPV (mOPV2) for response to outbreaks of circulating vaccine-derived PV2 (cVDPV2) infection or accidental release of WPV2 [4].
A new routine immunization schedule was introduced in Pakistan after the switch from tOPV to bOPV. The schedule consists of 4 bOPV doses, administered at birth and 6, 10, and 14 weeks of age, and 1 IPV dose, administered concomitantly with the last bOPV dose. It is important to assess the immunogenicity of the new immunization schedule in Pakistan to better understand the risk of a large-scale epidemic of poliomyelitis in case of VDPV2 emergence and subsequent spread, as well as to assess whether seroconversion for PV1 and PV3 is maintained at high levels.
This report summarizes data obtained from a large study that was conducted to assess the immunogenicity achieved with different routine poliovirus immunization schedules and to assess different poliovirus outbreak response strategies. Here, we present the data comparing different routine poliovirus immunization schedules, including the current Pakistani routine immunization schedule.
METHODS
The study was conducted in 4 low-income areas in and around Karachi (4 peri-urban, contiguous coastal villages: Rehri Goth, Bhains Colony, Ali Akber Shah, and Ibrahim Hydri), where the Aga Khan University’s Department of Pediatrics and Child Health has a well-established demographic surveillance system that captures population size, pregnancies, and births. The population of the study area according to the last census, from 2015, was 294171. Each area has a primary health center operated by the Department of Pediatrics and Child Health Research Program of Aga Khan University, which also provides Expanded Programme on Immunizations services. The neonatal mortality rate in the study area is estimated at 34.4 cases/1000 live births, compared with the overall rate in Pakistan of 55 cases/1000 live births; the mortality rate among children aged <5 years in the study population was estimated at 77 cases/1000 live births, compared with the overall rate in Pakistan of 89 cases/1000 live births [5].
Expectant women were approached at home during pregnancy or immediately after delivery by health center staff, and information about the trial and an invitation to participate in the study were given. Inclusion criteria included healthy newborns with a birth weight of ≥2.0 kg, informed consent from parent or guardian, and residence in the service area of the study clinic for at least the prior 3 months, with no plans to move during the study period. Selected newborns were examined by a study physician at enrollment, and those who had any clinical sign of illness, based on the World Health Organization (WHO) Integrated Management of Neonatal and Childhood Illness guideline, who required hospitalization, or who were at risk of immunodeficiency (as determined by a family history screen) were excluded from the trial.
After enrollment, children were randomly assigned to 1 of 5 study arms (Table 1). Randomization was performed using sealed envelopes that contained arm assignments prepared by the Clinical Trial Unit of Aga Khan University, using block randomization. Poliovirus vaccines were administered at birth and at weeks 6, 10, and 14 of life. Arm A received inactivated poliovirus vaccine (IPV) at all time points. Arm B received bivalent oral poliovirus vaccine (bOPV) at all time points. Arms C and D received bOPV at the first 3 time points and bOPV plus IPV at the final time point (the current schedule). Arm E received trivalent OPV (tOPV) at all time points. At 22 weeks of age, all children received 1 challenge dose of tOPV, and children in arm D received 1 additional IPV dose.
| Variable . | Vaccine Dose Received, by Age . | Challenge Dose Received, Age 22 wk . | Follow-up, Age 23 wk . | |||
|---|---|---|---|---|---|---|
| Birth . | 6 wk . | 10 wk . | 14 wk . | |||
| Study arm | ||||||
| A | IPV | IPV | IPV | IPV | tOPV | … |
| B | bOPV | bOPV | bOPV | bOPV | tOPV | … |
| C | bOPV | bOPV | bOPV | bOPV and IPV | tOPV | … |
| D | bOPV | bOPV | bOPV | bOPV and IPV | tOPV and IPV | … |
| E | tOPV | tOPV | tOPV | tOPV | tOPV | … |
| Sample(s) collected | Cord or peripheral blood | Peripheral blood | Peripheral blood | Blood | Blood and stool | Stool |
| Variable . | Vaccine Dose Received, by Age . | Challenge Dose Received, Age 22 wk . | Follow-up, Age 23 wk . | |||
|---|---|---|---|---|---|---|
| Birth . | 6 wk . | 10 wk . | 14 wk . | |||
| Study arm | ||||||
| A | IPV | IPV | IPV | IPV | tOPV | … |
| B | bOPV | bOPV | bOPV | bOPV | tOPV | … |
| C | bOPV | bOPV | bOPV | bOPV and IPV | tOPV | … |
| D | bOPV | bOPV | bOPV | bOPV and IPV | tOPV and IPV | … |
| E | tOPV | tOPV | tOPV | tOPV | tOPV | … |
| Sample(s) collected | Cord or peripheral blood | Peripheral blood | Peripheral blood | Blood | Blood and stool | Stool |
Abbreviations: bOPV, bivalent oral poliovirus vaccine; IPV, inactivated poliovirus vaccine; tOPV, trivalent oral poliovirus vaccine.
| Variable . | Vaccine Dose Received, by Age . | Challenge Dose Received, Age 22 wk . | Follow-up, Age 23 wk . | |||
|---|---|---|---|---|---|---|
| Birth . | 6 wk . | 10 wk . | 14 wk . | |||
| Study arm | ||||||
| A | IPV | IPV | IPV | IPV | tOPV | … |
| B | bOPV | bOPV | bOPV | bOPV | tOPV | … |
| C | bOPV | bOPV | bOPV | bOPV and IPV | tOPV | … |
| D | bOPV | bOPV | bOPV | bOPV and IPV | tOPV and IPV | … |
| E | tOPV | tOPV | tOPV | tOPV | tOPV | … |
| Sample(s) collected | Cord or peripheral blood | Peripheral blood | Peripheral blood | Blood | Blood and stool | Stool |
| Variable . | Vaccine Dose Received, by Age . | Challenge Dose Received, Age 22 wk . | Follow-up, Age 23 wk . | |||
|---|---|---|---|---|---|---|
| Birth . | 6 wk . | 10 wk . | 14 wk . | |||
| Study arm | ||||||
| A | IPV | IPV | IPV | IPV | tOPV | … |
| B | bOPV | bOPV | bOPV | bOPV | tOPV | … |
| C | bOPV | bOPV | bOPV | bOPV and IPV | tOPV | … |
| D | bOPV | bOPV | bOPV | bOPV and IPV | tOPV and IPV | … |
| E | tOPV | tOPV | tOPV | tOPV | tOPV | … |
| Sample(s) collected | Cord or peripheral blood | Peripheral blood | Peripheral blood | Blood | Blood and stool | Stool |
Abbreviations: bOPV, bivalent oral poliovirus vaccine; IPV, inactivated poliovirus vaccine; tOPV, trivalent oral poliovirus vaccine.
Cord or peripheral blood specimens (2 mL) were collected at birth or within 72 hours of birth, and peripheral blood specimens were collected at 14, 22, 23, and 26 weeks of age. Blood specimens collected at the clinical sites were allowed to clot and were centrifuged to separate serum. Specimens were then transported to the Infectious Disease Research Laboratory (IDRL) of Aga Khan University, where they were stored at −20°C until shipment to the Centers for Disease Control and Prevention (CDC; Atlanta, GA), where sera were tested for presence of poliovirus-neutralizing antibodies by using standard neutralization assays [6].
For each serotype, seropositivity was defined as a reciprocal titer of poliovirus neutralizing antibodies of ≥8. Seroconversion in children with no maternal antibodies at birth was defined as the change from seronegativity to seropositivity (ie, from a reciprocal titer of <8 to one of ≥8); in those with maternal antibodies at birth, seroconversion was defined as a 4-fold increase in reciprocal titer over the expected decline in maternal antibody titer, with an estimated half-life of 28 days. The analysis of seroconversion was restricted to infants with an initial maternal antibody titer of <1448, to demonstrate a 4-fold increase over the expected decline. The maternal antibody titer was classified as high when the antibody titer at birth was ≥64 [7].
Stool specimens were collected at the primary care clinic or at children’s homes on week 22 (before study vaccine administration) and on week 23. Specimens were stored at the IDRL at 4°C until shipment to the WHO regional reference laboratory for polio in Pakistan (National Institute of Health, Islamabad), where they were tested for the presence of poliovirus, using standard poliovirus detection methods [8]. Presence or absence of serotype-specific poliovirus in stool samples was reported.
Adverse events following vaccination were identified by site investigators and reviewed by the principal investigator. Children were observed for 30 minutes following the administration of the vaccine, for immediate adverse events; parents were instructed to immediately report back to the health centers if adverse events occurred. Serious adverse events were reported for review to the data and safety monitoring board and the ethical review committee. Additional monitoring of infants between birth and 3 months of age was instituted during the trial implementation.
IPV was produced by Bilthoven Biologicals (Bilthoven, the Netherlands) and presented in 1-dose vials (0.5 mL), tOPV was produced by Sanofi Pasteur (Lyon, France), and bOPV was produced by GlaxoSmithKline (Brussels, Belgium).
The target sample size for each arm was calculated to be 190 newborns, with a minimum analyzable sample size of 110 per arm, based on an α of 0.05 and a power of 80% and the assumption of a 20% difference in seroconversion frequencies between arms.
Data were analyzed using Stata, version 11 (StataCorp, College Station, TX). The proportion of seroconverting individuals in different study arms was compared by the χ2 test for quantitative variables. P values were calculated to assess differences in immune response between study arms. k sample tests on the equality of median values were performed to compare the median titers across the study arms, and 95% confidence intervals for median titers were calculated.
The study was approved by the Ethical Review Committee of Aga Khan University, the National Bioethics Committee of Pakistan, and the Ethical Review Committee of the WHO. All activities followed the guidelines of good clinical practice; the trial protocol was registered at ClinicalTrials.gov (identifier NCT02189811). The WHO assisted in study design, trial implementation, and monitoring and contributed to the writing of the report. Aga Khan University conducted the trial.
RESULTS
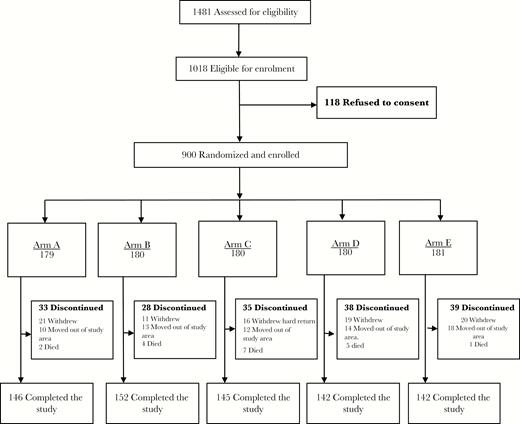
A total of 1481 newborns were assessed for eligibility, and 1018 (69%) were found eligible. Of these 1018, 118 (12%) were not enrolled, owing to a lack of consent. There were 900 newborns enrolled in the study and randomly assigned to 1 of 5 study arms. A total of 727 (81%) completed the study procedures and were included in the final analysis. A total of 173 newborns (19%) were lost to follow-up because of refusal to continue (87 [9.7%]), relocation away from the study area (67 [7.4%]), and death (19 [2.1%]; Figure 1).
Baseline demographic indicators and seroprevalences of antipoliovirus maternal antibodies are shown in Table 2. There were no significant differences between arms in any demographic indicators except for the seroprevalence of maternal antibody against PV2, with values of 90% in arm A and 77% in arm E (P = .032). There was no significant difference in the median maternal antibody titer for any serotype.
Baseline Demographic Indicators and Seroprevalence of Maternal Antibodies (Abs) Against Poliovirus at Birth
| Variable . | Value . |
|---|---|
| Demographic indicator | |
| Male sex | 439/900 (49) |
| No formal education for mother | 423/900 (47) |
| Place of birth other than home | 624/900 (69) |
| Birth weight, kg, median (IQR) | 2.81 (2.53–3.1) |
| Family size, individuals, no. | |
| <6 | 304/900 (34) |
| 6–9 | 294/900 (33) |
| >9 | 302/900 (34) |
| Monthly household income, $ | |
| <50 | 168/896 (18) |
| 50–100 | 418/896 (47) |
| >100 | 310/896 (35) |
| Exclusive breastfeeding at age 22 wk | 447/713 (63) |
| Maternal Ab seroprevalence, by poliovirus type | |
| Type 1 | |
| Seroprevalence | |
| Proportion | 634/713 |
| Percentage (95% CI) | 89 (87–91) |
| Titer, median (95% CI) | 90.5 (72–113.8) |
| Type 2 | |
| Seroprevalence | |
| Proportion | 590/713 |
| Percentage (95% CI) | 83a (80–86) |
| Titer, median (95% CI) | 36 (28.4–45.3) |
| Type 3 | |
| Seroprevalence | |
| Proportion | 440/713 |
| Percentage (95% CI) | 62 (58–65) |
| Titer, median (95% CI) | 11.3 (11.3–14.2) |
| Variable . | Value . |
|---|---|
| Demographic indicator | |
| Male sex | 439/900 (49) |
| No formal education for mother | 423/900 (47) |
| Place of birth other than home | 624/900 (69) |
| Birth weight, kg, median (IQR) | 2.81 (2.53–3.1) |
| Family size, individuals, no. | |
| <6 | 304/900 (34) |
| 6–9 | 294/900 (33) |
| >9 | 302/900 (34) |
| Monthly household income, $ | |
| <50 | 168/896 (18) |
| 50–100 | 418/896 (47) |
| >100 | 310/896 (35) |
| Exclusive breastfeeding at age 22 wk | 447/713 (63) |
| Maternal Ab seroprevalence, by poliovirus type | |
| Type 1 | |
| Seroprevalence | |
| Proportion | 634/713 |
| Percentage (95% CI) | 89 (87–91) |
| Titer, median (95% CI) | 90.5 (72–113.8) |
| Type 2 | |
| Seroprevalence | |
| Proportion | 590/713 |
| Percentage (95% CI) | 83a (80–86) |
| Titer, median (95% CI) | 36 (28.4–45.3) |
| Type 3 | |
| Seroprevalence | |
| Proportion | 440/713 |
| Percentage (95% CI) | 62 (58–65) |
| Titer, median (95% CI) | 11.3 (11.3–14.2) |
Data are proportion (%) of children, unless otherwise indicated.
Abbreviations: CI, confidence interval; IQR, interquartile range.
Statistically significant variation between arms.
Baseline Demographic Indicators and Seroprevalence of Maternal Antibodies (Abs) Against Poliovirus at Birth
| Variable . | Value . |
|---|---|
| Demographic indicator | |
| Male sex | 439/900 (49) |
| No formal education for mother | 423/900 (47) |
| Place of birth other than home | 624/900 (69) |
| Birth weight, kg, median (IQR) | 2.81 (2.53–3.1) |
| Family size, individuals, no. | |
| <6 | 304/900 (34) |
| 6–9 | 294/900 (33) |
| >9 | 302/900 (34) |
| Monthly household income, $ | |
| <50 | 168/896 (18) |
| 50–100 | 418/896 (47) |
| >100 | 310/896 (35) |
| Exclusive breastfeeding at age 22 wk | 447/713 (63) |
| Maternal Ab seroprevalence, by poliovirus type | |
| Type 1 | |
| Seroprevalence | |
| Proportion | 634/713 |
| Percentage (95% CI) | 89 (87–91) |
| Titer, median (95% CI) | 90.5 (72–113.8) |
| Type 2 | |
| Seroprevalence | |
| Proportion | 590/713 |
| Percentage (95% CI) | 83a (80–86) |
| Titer, median (95% CI) | 36 (28.4–45.3) |
| Type 3 | |
| Seroprevalence | |
| Proportion | 440/713 |
| Percentage (95% CI) | 62 (58–65) |
| Titer, median (95% CI) | 11.3 (11.3–14.2) |
| Variable . | Value . |
|---|---|
| Demographic indicator | |
| Male sex | 439/900 (49) |
| No formal education for mother | 423/900 (47) |
| Place of birth other than home | 624/900 (69) |
| Birth weight, kg, median (IQR) | 2.81 (2.53–3.1) |
| Family size, individuals, no. | |
| <6 | 304/900 (34) |
| 6–9 | 294/900 (33) |
| >9 | 302/900 (34) |
| Monthly household income, $ | |
| <50 | 168/896 (18) |
| 50–100 | 418/896 (47) |
| >100 | 310/896 (35) |
| Exclusive breastfeeding at age 22 wk | 447/713 (63) |
| Maternal Ab seroprevalence, by poliovirus type | |
| Type 1 | |
| Seroprevalence | |
| Proportion | 634/713 |
| Percentage (95% CI) | 89 (87–91) |
| Titer, median (95% CI) | 90.5 (72–113.8) |
| Type 2 | |
| Seroprevalence | |
| Proportion | 590/713 |
| Percentage (95% CI) | 83a (80–86) |
| Titer, median (95% CI) | 36 (28.4–45.3) |
| Type 3 | |
| Seroprevalence | |
| Proportion | 440/713 |
| Percentage (95% CI) | 62 (58–65) |
| Titer, median (95% CI) | 11.3 (11.3–14.2) |
Data are proportion (%) of children, unless otherwise indicated.
Abbreviations: CI, confidence interval; IQR, interquartile range.
Statistically significant variation between arms.
Seroconversion was measured at weeks 14 and 22 of life, following completion of different schedules of primary polio vaccination series (Table 3). Final frequencies (at week 22) of seroconversion ranged from 79% to 96% for PV1, from 20% to 93% for PV2, and from 85% to 98% for PV3. Seroconversion at week 14 was lower than at week 22, with values ranging from 70% to 91% for PV1, from 15% to 89% for PV2, and from 75% to 91% for PV3. For all 3 serotypes, there were significant differences between study arms both at week 14 and week 22 (P < .001). For PV1, the seroconversion frequency at 22 weeks in arms B, C, D, and E did not significantly differ; the seroconversion frequency in arm A was significantly lower than in any of the other arms (P < .001). For PV2, the seroconversion frequency at 22 weeks was significantly higher in arm E than in arm A (P = .01), and for PV3, it was significantly lower in arm E (P = .01) than in any of the other arms. Reverse cumulative distributions of reciprocal antibody titers are shown in Figure 2 and demonstrate similar trends as observed in the analysis of seroconversion.
Seroconversion to Poliovirus Types 1, 2, and 3 at 14 and 22 Weeks of Life, by Study Arm
| Age, Type, Measure . | Arm A . | Arm B . | Arm C . | Arm D . | Arm E . |
|---|---|---|---|---|---|
| 14 wk | |||||
| Type 1 | |||||
| Seroconversion | |||||
| Proportion | 96/138 | 130/144 | 121/139 | 119/138 | 110/134 |
| Percentage (95% CI) | 70 (63–79) | 90 (85–94) | 87 (83–94) | 86 (69–91) | 82 (76–88) |
| Titer,a median (95% CI) | 831 (455 to ≥1448) | ≥1448 (≥1448 to ≥1448) | ≥1448 (≥1448 to ≥1448) | ≥1448 (≥1448 to ≥1448) | 1152 (1152 to ≥1448) |
| Type 2 | |||||
| Seroconversion | |||||
| Proportion | 98/138 | 26/144 | 19/139 | 28/138 | 119/134 |
| Percentage (95% CI) | 71 (66–78) | 18 (13–25) | 14 (9–21) | 20 (13–23) | 89 (82–93) |
| Titer,a median (95% CI) | 181 (113–288) | 362 (228–724) | 724 (455–910) | 416 (228–724) | 1152 (724 to ≥1448) |
| Type 3 | |||||
| Seroconversion | |||||
| Proportion | 126/138 | 133/144 | 120/139 | 119/138 | 100/134 |
| Percentage (95% CI) | 91 (86–96) | 92 (86–95) | 86 (80–92) | 86 (80–92) | 75 (67–82) |
| Titer,a median (95% CI) | 724 (576–1152) | 588 (455–724) | 588 (455–910) | 446 (408–724) | 362 (455–724) |
| 22 wk | |||||
| Type 1 | |||||
| Seroconversion | |||||
| Proportion | 111/138 | 139/144 | 131/139 | 132/138 | 126/134 |
| Percentage (95% CI) | 80 (72–86) | 97 (93–100) | 94 (90–98) | 96 (92–99) | 94 (90–98) |
| Titer,a median (95% CI) | 445 (227–910) | ≥1448 (≥1448 to ≥1448) | ≥1448 (≥1448 to ≥1448) | ≥1448 (≥1448 to ≥1448) | 1152 (910 to ≥1448) |
| Type 2 | |||||
| Seroconversion | |||||
| Proportion | 116/138 | 28/144 | 74/139 | 68/138 | 125/134 |
| Percentage (95% CI) | 84 (78–90) | 19 (13–27) | 53 (44–61) | 49 (40–57) | 93 (89–98) |
| Titer,a median (95% CI) | 111 (90–181) | 181 (113–288) | 32 (28–57) | 39 (23–114) | 724 (650–1152) |
| Type 3 | |||||
| Seroconversion | |||||
| Proportion | 129/138 | 135/144 | 136/139 | 130/138 | 114/134 |
| Percentage (95% CI) | 93 (89–98) | 94 (90–98) | 98 (95–100) | 94 (90–98) | 85 (79–91) |
| Titer,a median (95% CI) | 446 (362–910) | 588 (455–910) | 1261 (910 to ≥1448) | 1176 (1031 to ≥1448) | 294 (288–515) |
| Age, Type, Measure . | Arm A . | Arm B . | Arm C . | Arm D . | Arm E . |
|---|---|---|---|---|---|
| 14 wk | |||||
| Type 1 | |||||
| Seroconversion | |||||
| Proportion | 96/138 | 130/144 | 121/139 | 119/138 | 110/134 |
| Percentage (95% CI) | 70 (63–79) | 90 (85–94) | 87 (83–94) | 86 (69–91) | 82 (76–88) |
| Titer,a median (95% CI) | 831 (455 to ≥1448) | ≥1448 (≥1448 to ≥1448) | ≥1448 (≥1448 to ≥1448) | ≥1448 (≥1448 to ≥1448) | 1152 (1152 to ≥1448) |
| Type 2 | |||||
| Seroconversion | |||||
| Proportion | 98/138 | 26/144 | 19/139 | 28/138 | 119/134 |
| Percentage (95% CI) | 71 (66–78) | 18 (13–25) | 14 (9–21) | 20 (13–23) | 89 (82–93) |
| Titer,a median (95% CI) | 181 (113–288) | 362 (228–724) | 724 (455–910) | 416 (228–724) | 1152 (724 to ≥1448) |
| Type 3 | |||||
| Seroconversion | |||||
| Proportion | 126/138 | 133/144 | 120/139 | 119/138 | 100/134 |
| Percentage (95% CI) | 91 (86–96) | 92 (86–95) | 86 (80–92) | 86 (80–92) | 75 (67–82) |
| Titer,a median (95% CI) | 724 (576–1152) | 588 (455–724) | 588 (455–910) | 446 (408–724) | 362 (455–724) |
| 22 wk | |||||
| Type 1 | |||||
| Seroconversion | |||||
| Proportion | 111/138 | 139/144 | 131/139 | 132/138 | 126/134 |
| Percentage (95% CI) | 80 (72–86) | 97 (93–100) | 94 (90–98) | 96 (92–99) | 94 (90–98) |
| Titer,a median (95% CI) | 445 (227–910) | ≥1448 (≥1448 to ≥1448) | ≥1448 (≥1448 to ≥1448) | ≥1448 (≥1448 to ≥1448) | 1152 (910 to ≥1448) |
| Type 2 | |||||
| Seroconversion | |||||
| Proportion | 116/138 | 28/144 | 74/139 | 68/138 | 125/134 |
| Percentage (95% CI) | 84 (78–90) | 19 (13–27) | 53 (44–61) | 49 (40–57) | 93 (89–98) |
| Titer,a median (95% CI) | 111 (90–181) | 181 (113–288) | 32 (28–57) | 39 (23–114) | 724 (650–1152) |
| Type 3 | |||||
| Seroconversion | |||||
| Proportion | 129/138 | 135/144 | 136/139 | 130/138 | 114/134 |
| Percentage (95% CI) | 93 (89–98) | 94 (90–98) | 98 (95–100) | 94 (90–98) | 85 (79–91) |
| Titer,a median (95% CI) | 446 (362–910) | 588 (455–910) | 1261 (910 to ≥1448) | 1176 (1031 to ≥1448) | 294 (288–515) |
Analysis was performed on subjects who provided sera at birth and at 14 and 22 weeks of age and completed all vaccinations.
Abbreviation: CI, confidence interval.
Data are for children who seroconverted.
Seroconversion to Poliovirus Types 1, 2, and 3 at 14 and 22 Weeks of Life, by Study Arm
| Age, Type, Measure . | Arm A . | Arm B . | Arm C . | Arm D . | Arm E . |
|---|---|---|---|---|---|
| 14 wk | |||||
| Type 1 | |||||
| Seroconversion | |||||
| Proportion | 96/138 | 130/144 | 121/139 | 119/138 | 110/134 |
| Percentage (95% CI) | 70 (63–79) | 90 (85–94) | 87 (83–94) | 86 (69–91) | 82 (76–88) |
| Titer,a median (95% CI) | 831 (455 to ≥1448) | ≥1448 (≥1448 to ≥1448) | ≥1448 (≥1448 to ≥1448) | ≥1448 (≥1448 to ≥1448) | 1152 (1152 to ≥1448) |
| Type 2 | |||||
| Seroconversion | |||||
| Proportion | 98/138 | 26/144 | 19/139 | 28/138 | 119/134 |
| Percentage (95% CI) | 71 (66–78) | 18 (13–25) | 14 (9–21) | 20 (13–23) | 89 (82–93) |
| Titer,a median (95% CI) | 181 (113–288) | 362 (228–724) | 724 (455–910) | 416 (228–724) | 1152 (724 to ≥1448) |
| Type 3 | |||||
| Seroconversion | |||||
| Proportion | 126/138 | 133/144 | 120/139 | 119/138 | 100/134 |
| Percentage (95% CI) | 91 (86–96) | 92 (86–95) | 86 (80–92) | 86 (80–92) | 75 (67–82) |
| Titer,a median (95% CI) | 724 (576–1152) | 588 (455–724) | 588 (455–910) | 446 (408–724) | 362 (455–724) |
| 22 wk | |||||
| Type 1 | |||||
| Seroconversion | |||||
| Proportion | 111/138 | 139/144 | 131/139 | 132/138 | 126/134 |
| Percentage (95% CI) | 80 (72–86) | 97 (93–100) | 94 (90–98) | 96 (92–99) | 94 (90–98) |
| Titer,a median (95% CI) | 445 (227–910) | ≥1448 (≥1448 to ≥1448) | ≥1448 (≥1448 to ≥1448) | ≥1448 (≥1448 to ≥1448) | 1152 (910 to ≥1448) |
| Type 2 | |||||
| Seroconversion | |||||
| Proportion | 116/138 | 28/144 | 74/139 | 68/138 | 125/134 |
| Percentage (95% CI) | 84 (78–90) | 19 (13–27) | 53 (44–61) | 49 (40–57) | 93 (89–98) |
| Titer,a median (95% CI) | 111 (90–181) | 181 (113–288) | 32 (28–57) | 39 (23–114) | 724 (650–1152) |
| Type 3 | |||||
| Seroconversion | |||||
| Proportion | 129/138 | 135/144 | 136/139 | 130/138 | 114/134 |
| Percentage (95% CI) | 93 (89–98) | 94 (90–98) | 98 (95–100) | 94 (90–98) | 85 (79–91) |
| Titer,a median (95% CI) | 446 (362–910) | 588 (455–910) | 1261 (910 to ≥1448) | 1176 (1031 to ≥1448) | 294 (288–515) |
| Age, Type, Measure . | Arm A . | Arm B . | Arm C . | Arm D . | Arm E . |
|---|---|---|---|---|---|
| 14 wk | |||||
| Type 1 | |||||
| Seroconversion | |||||
| Proportion | 96/138 | 130/144 | 121/139 | 119/138 | 110/134 |
| Percentage (95% CI) | 70 (63–79) | 90 (85–94) | 87 (83–94) | 86 (69–91) | 82 (76–88) |
| Titer,a median (95% CI) | 831 (455 to ≥1448) | ≥1448 (≥1448 to ≥1448) | ≥1448 (≥1448 to ≥1448) | ≥1448 (≥1448 to ≥1448) | 1152 (1152 to ≥1448) |
| Type 2 | |||||
| Seroconversion | |||||
| Proportion | 98/138 | 26/144 | 19/139 | 28/138 | 119/134 |
| Percentage (95% CI) | 71 (66–78) | 18 (13–25) | 14 (9–21) | 20 (13–23) | 89 (82–93) |
| Titer,a median (95% CI) | 181 (113–288) | 362 (228–724) | 724 (455–910) | 416 (228–724) | 1152 (724 to ≥1448) |
| Type 3 | |||||
| Seroconversion | |||||
| Proportion | 126/138 | 133/144 | 120/139 | 119/138 | 100/134 |
| Percentage (95% CI) | 91 (86–96) | 92 (86–95) | 86 (80–92) | 86 (80–92) | 75 (67–82) |
| Titer,a median (95% CI) | 724 (576–1152) | 588 (455–724) | 588 (455–910) | 446 (408–724) | 362 (455–724) |
| 22 wk | |||||
| Type 1 | |||||
| Seroconversion | |||||
| Proportion | 111/138 | 139/144 | 131/139 | 132/138 | 126/134 |
| Percentage (95% CI) | 80 (72–86) | 97 (93–100) | 94 (90–98) | 96 (92–99) | 94 (90–98) |
| Titer,a median (95% CI) | 445 (227–910) | ≥1448 (≥1448 to ≥1448) | ≥1448 (≥1448 to ≥1448) | ≥1448 (≥1448 to ≥1448) | 1152 (910 to ≥1448) |
| Type 2 | |||||
| Seroconversion | |||||
| Proportion | 116/138 | 28/144 | 74/139 | 68/138 | 125/134 |
| Percentage (95% CI) | 84 (78–90) | 19 (13–27) | 53 (44–61) | 49 (40–57) | 93 (89–98) |
| Titer,a median (95% CI) | 111 (90–181) | 181 (113–288) | 32 (28–57) | 39 (23–114) | 724 (650–1152) |
| Type 3 | |||||
| Seroconversion | |||||
| Proportion | 129/138 | 135/144 | 136/139 | 130/138 | 114/134 |
| Percentage (95% CI) | 93 (89–98) | 94 (90–98) | 98 (95–100) | 94 (90–98) | 85 (79–91) |
| Titer,a median (95% CI) | 446 (362–910) | 588 (455–910) | 1261 (910 to ≥1448) | 1176 (1031 to ≥1448) | 294 (288–515) |
Analysis was performed on subjects who provided sera at birth and at 14 and 22 weeks of age and completed all vaccinations.
Abbreviation: CI, confidence interval.
Data are for children who seroconverted.
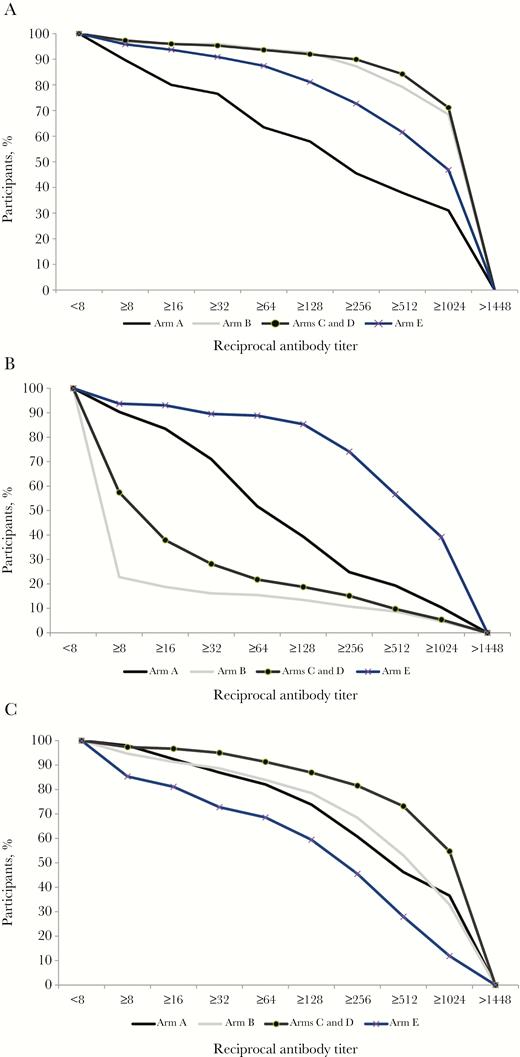
Reverse cumulative antibody titer distribution at 22 weeks of age (arms C and D were combined because children in these two arms received the same vaccines until week 22).
For arm A (IPV only), a lower seroconversion rate was associated with a high maternal antibody titer (ie, ≥64; Figure 3). Specifically, 70% with a high maternal antibody titer and 95% with a low titer seroconverted to PV1 (P < .001); 70% and 96%, respectively, seroconverted to PV2 (P < .001); and 71% and 98%, respectively, seroconverted to PV3 (P < .001). The association between high maternal antibody titers and seroconversion did not reach statistical significance for any other study arm.
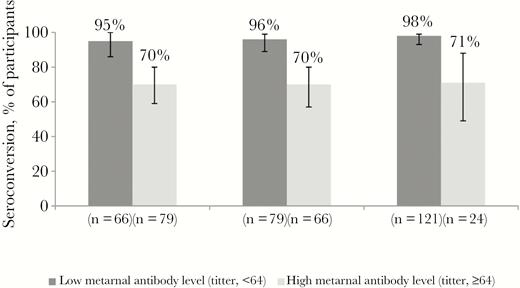
Seroconversion to poliovirus type 1 (PV1), PV2, and PV3, by maternal antibody level.
One IPV dose was administered at 14 weeks of age to children in arms C and D. To isolate the immunogenicity of this IPV dose on PV2 seroconversion, we tried to eliminate the effect of exposure to environmental PV2 by restricting this analysis to the 230 children who did not seroconvert to PV2 between birth and 14 weeks of age. Among those children, 102 (44%; 95% confidence interval, 38%–51%) seroconverted to PV2 after receiving 1 IPV dose at 22 weeks of age, and there was no statistically significant difference between arms C and D (P = .2). The frequency of seroconversion to PV2 was significantly lower among children with a high maternal antibody titer, compared with those who had a low titer (29% vs 54%; P < .001).
Excretion of polioviruses was measured in stool specimens collected 1 week after OPV challenge (Figure 4). For this analysis, we excluded children (7 of 402 [2%] for PV1, 10 of 402 [2%] for PV2, and 11 of 402 [3%] for PV3) who excreted the corresponding poliovirus serotype before challenge. The percentage of children in arm E who shed PV2 was significantly lower than in any of the other study arms (P < .001); in arm D, it was lower than in arms A, B, and C (P < .02). In arm A, excretion of both PV1 and PV3 was detected at significantly higher frequencies than in the other arms (P < .05).
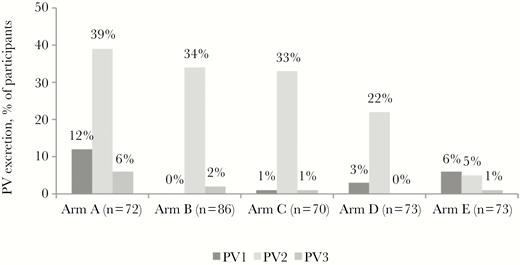
Prevalence of poliovirus type 1 (PV1), PV2, and PV3 excretion, by study arm.
DISCUSSION
We evaluated the previous, current, and possible future routine immunization schedules for poliomyelitis prevention in Pakistan. Our study confirmed the excellent immunogenicity of the current schedule (4 doses of bOPV and 1 dose of IPV) against PV1 and PV3 and its ability to provide a moderate base of immunity against PV2.
The performance of the 4-dose IPV schedule, with doses given at birth and at 6, 10, and 14 weeks of age, was lower than expected, and substantial gaps in immunity to each serotype remained. The stratification by maternally derived antibody level confirmed the relationship between low maternally derived antibody titers and a high seroconversion frequency. This observation was in line with conclusions from trials in Puerto Rico and Cuba that demonstrated that a schedule in which IPV is given at 6, 10, and 14 weeks of age remains inferior to a schedule in which IPV is administered later in life, such as at 2, 4, and 6 months of age [9, 10]. This study highlighted again that the immunogenicity of IPV is greatly dependent on the age at receipt of the first dose and the interval between doses. It is very apparent that 2 doses of IPV (whether a fractional or full dose) can achieve seroconversion rates of >90% against all 3 poliovirus serotypes if administered later in life [11]. In addition to the age at first dose receipt and the interval between doses, the quantity of antigen in IPV affects seroconversion: monovalent high-dose IPV2 had a seroconversion rate superior to that for PV2 when compared to regular IPV [12].
The lower than previously observed frequency of PV2 following tOPV challenge in arm A suggested that a proportion of the study subjects had been exposed secondarily to Sabin PV2 from contacts with infants who received tOPV [10]. In our study, 14%–20% of children enrolled in arms with no PV2 vaccines administered before week 14 of life (ie, those in arms B, C, and D) seroconverted to PV2, most likely because of secondary exposure to PV2. This is in line with previous observations from Pakistan [7], but it is higher than observed in Bangladesh [13], possibly because the frequency of vaccination campaigns with tOPV conducted in Pakistan was greater than that in Bangladesh.
The current routine immunization schedule implemented since April 2016 induced >90% seroconversion against PV1 and PV3 and approximately 50% against PV2. The low frequency of poliovirus excretion following challenge with tOPV indicated that excellent PV1 and PV3 mucosal immunity was achieved. The immunogenicity achieved in Pakistan with this schedule was similar to that reported from India and Latin American countries [14, 15]. Unlike in a previous study, we did not observe superiority of bOPV over tOPV for PV1 seroconversion in our study [16].
Our study had limitations. The resistance to excretion following tOPV demonstrated that a proportion of children were exposed secondarily to Sabin polioviruses [17–19]. Participant retention rate was 80%; however, dropout was evenly distributed among study arms and likely did not significantly bias the results. We found a higher maternal anti-PV2 antibody seroprevalence in arm A than in arm E, but we do not believe that this difference biased the seroconversion rates, because the median antibody titer at birth was not significantly different and because we accounted for natural antibody decay when calculating seroconversion. The vaccines were well tolerated. Nineteen deaths were reported among study subjects during the study period. However, when fatalities were investigated and reported to the data and safety monitoring board, none were attributed to the study procedures; rather, they were attributed to the poor socioeconomic and sanitation conditions in this area, where the baseline infant mortality rate is high.
The routine immunization coverage in Pakistan is quite low, especially in areas where the risk of polio is high. Therefore, there is a growing cohort of young children with no anti-PV2 antibodies [20]. Solutions to better protect the population against potential VDPV2 outbreaks should be considered. Based on the recent recommendations of the Strategic Advisory Group of Experts on immunizations, the introduction of a more immunogenic 2-dose schedule with fractional intradermal IPV into the routine immunization schedule instead of 1 full IPV dose is one such option [11, 21].
Notes
Acknowledgments. We thank Dr Mark A Pallansch (director, Division of Viral Diseases, CDC) and the team conducting the neutralization assays at the CDC laboratory, including Deborah Moore, Yiting Zhang, Sharla McDonald, Larin McDuffie, Will Hendley, Patricia Mitchell, and Mario Nicolas; Ms Shahida Qureshi, Aneeta Hotwani, from the Infectious Disease Research Laboratory, Aga Khan University, for the storage and shipment of the samples; Mr Najeeb Ahmed (Data Management Unit, Aga Khan University), Dr Usman Chachar (coordinator, Emergency Operation Center Sindh), Dr Temesgen Demeke (team leader, WHO Expanded Program on Immunization Sindh, Government of Sindh, Bin Qasim and Landhi Town), for constant support during the project; GlaxoSmithKline, Sanofi Pasteur, and Bilthoven Biologicals, for donation of their vaccines and permission to use the vaccine in a clinical trial; and the staff, particularly Mr Shahzad Shaukat, of the Regional Reference Laboratory for Polio at the National Institute of Health, Islamabad, Pakistan, for analyzing the stool samples from this study.
Disclaimer. The findings and conclusions in this report are those of the author(s) and do not necessarily represent the views of the Centers for Disease Control and Prevention and other contributing agencies.
Financial support. This work was supported the World Health Organization, National Institute of Health’s Fogarty International Center (support 1 D43 TW007585-01 to A. F. S.), and the Centers for Disease Control and Prevention.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References




