-
PDF
- Split View
-
Views
-
Cite
Cite
Zhenyu Wu, Yuzi Tian, Hasan B Alam, Patrick Li, Xiuzhen Duan, Aaron M Williams, Baoling Liu, Jianjie Ma, Yongqing Li, Peptidylarginine Deiminases 2 Mediates Caspase-1-Associated Lethality in Pseudomonas aeruginosa Pneumonia-Induced Sepsis, The Journal of Infectious Diseases, Volume 223, Issue 6, 15 March 2021, Pages 1093–1102, https://doi.org/10.1093/infdis/jiaa475
Close - Share Icon Share
Abstract
Pseudomonas aeruginosa (PA) is a pathogenic bacterium that causes severe pneumonia in critically ill and immunocompromised patients. Peptidylarginine deiminase (PAD) 2, PAD4, and caspase-1 are important enzymes in mediating host response to infection. The goal of this study was to determine the interplay between PAD2, PAD4, and caspase-1 in PA pneumonia-induced sepsis.
Pneumonia was produced in wild-type, Pad2−/−, and Pad4−/− mice by intranasal inoculation of PA (2.5 × 106 colony-forming units per mouse), and survival (n = 15/group) was monitored for 10 days. Bone marrow-derived macrophages (BMDMs) were isolated for in vitro studies. Samples were collected at specific timepoints for Western blot, bacterial load determination, and flow cytometry analysis.
Caspase-1-dependent inflammation was diminished in PA-inoculated Pad2−/− mice, contributing to reduced macrophage death and enhanced bacterial clearance. In addition, Pad2−/− mice exhibited improved survival and attenuated acute lung injury after PA infection. In contrast, Pad4−/− mice did not display diminished caspase-1 activation, altered bacterial loads, or improved survival.
Peptidylarginine deiminase 2 plays an essential role in the pathogenesis of pulmonary sepsis by mediating caspase-1 activation. This goes against previous findings of PAD4 in sepsis. Our study suggests that PAD2 is a potential therapeutic target of PA pneumonia-induced sepsis.
Pseudomonas aeruginosa (PA) is a highly pathogenic opportunistic Gram-negative bacterium that can affect many different organs and systems [1]. It commonly infects the lungs of hospitalized patients, especially those in the intensive care unit, and is associated with a high mortality rate [2, 3]. Pseudomonas aeruginosa is one of the leading causes of hospital-acquired pneumonia due to its high pathogenicity in patients with weakened immune systems [4]. In addition to the microbe’s intrinsic and acquired defense mechanisms that allow it to resist current antibiotic treatment, PA can also trigger a dysregulated innate host inflammatory response that can promote further tissue damage [4, 5]. It is difficult to eradicate PA infection because it has a type III secretion system (T3SS), which allows it to easily evade host defense and breach blood-gas barrier [5]. In addition, PA is resistant to a wide range of antibiotics, which makes it challenging to find effective therapies [6].
The host innate immune system plays an important role in the response to PA. In the lower airways, there is inflammasome-driven activation of alveolar macrophages, with resultant release of proinflammatory cytokines interleukin (IL)-1β and IL-18 [7, 8]. A type of inflammatory programmed cell death, termed pyroptosis, is critical to this process. Pyroptosis can be mediated by caspase-1 (canonical pathway) and/or caspase-11 (noncanonical pathway). Canonical pyroptosis usually consists of 3 key steps: inflammasome and caspase-1 activation, membrane rupture and cell death, and IL-1β/IL-18 release. The overall effects of these inflammatory caspases are various and still need further investigation. The increased IL-1β and IL-18 activity is beneficial in recruiting neutrophils and aiding in bacterial clearance in studies with Salmonella typhimurium, Legionella pneumophila, and Burkholderia thailandensis [9, 10]. However, in other cases, especially in PA infections, excessive inflammasome activity can be detrimental to the host because it can hamper host defense and exaggerate lung injury [11, 12].
Peptidylarginine deiminase (PAD) enzymes are a family of 5 enzymes (PAD1, 2, 3, 4, and 6) that play a role in host defense, inflammation, and autoimmunity [13–16]. Their function is to convert arginine residues into citrullines [17]. Peptidylarginine deiminases 2 and 4 have been shown to have abundant expression in the immune cells [13–16]. Our previous work revealed that dual inhibition of PAD2 and PAD4 by Cl-amidine significantly improved survival and decreased bacterial loads in a murine model of lethal cecal ligation and puncture [18]. An interesting finding of recent study reported that dual inhibition of PAD2 and PAD4 by Cl-amidine can notably decrease caspase-1-dependent pyroptosis in vitro, suggesting an association between PAD activity and caspase-dependent inflammasome activity. However, the clinical significance of this is unknown because this has not been studied in any disease models.
In this study, our goal was to understand the effects of PAD activity on pyroptosis in PA pneumonia. We discovered that Pad2 knockout decreases mortality and increases bacterial clearance in a PA pneumonia mouse model, likely through decreased caspase-1-dependent inflammasome activity in macrophages.
MATERIALS AND METHODS
Animals
Wild-type (WT) mice and Pad2−/− mice [15] with an FVB background were obtained from Dr. Scott Coonrod (Cornell University) and bred at University of Michigan. Pad4−/− mice and the WT littermates (8–10 weeks) were developed in-house (Supplemental Figure S1) and backcrossed for 7 times with WT C57B6/J mice (Jackson Laboratory, Bar Harbor, ME). All animal experiments were conducted in compliance with the animal protocol (protocol no. PRO00008861) approved by the University of Michigan Institutional Animal Care and Use Committee.
Reagents
Reagents used in this study are listed in Supplemental Table 1.
In Vitro Bone Marrow-Derived Macrophage Infection
Bone marrow-derived macrophages (BMDMs) were plated in 6-well plates at a density of 1 × 106 cells/well and cultured overnight at 37°C. Then, the media were replaced with fresh opti-MEM containing or not containing PA (multiplicity of infection = 10). After media replacement, BMDMs were cultured at 37°C for an additional hour. Then, cell lysates and culture supernatants were harvested for analysis. All of the experiments were performed 3–5 times with 4 replicates.
In Vitro Lactate Dehydrogenase Release Assay
Lactate dehydrogenase (LDH) release by BMDMs after 3-hour PA infection was determined by a cytotoxicity detection kit following the manufacturer’s instructions.
Pulmonary Infection Model
Mice (8–10 weeks old) were subject to intranasal PA administration to develop PA pneumonia-induced sepsis as previously described [19]. In brief, PA solution was prepared at the concentration of 8.25 × 107 colony-forming units [CFU]/mL or 6.67 × 106 CFU/mL in phosphate-buffered saline (PBS). The mice were anesthetized with ketamine (100 mg/kg body weight) and xylazine (20 mg/kg). Then, they were held vertically, and 15 µL PA solution was dripped into each nostril (totally 30 µL to reach a final bacterial load of 2.5 × 106 CFU). The animals inoculated with sterile PBS served as sham controls. In nonsurvival studies, the mice were euthanized by CO2 at specific timepoints (9 hours and 24 hours) after inoculation. Serum, bronchoalveolar lavage fluid (BALF), and organs were harvested and stored at −80°C. In survival studies, mice (n = 15/group) were monitored for 10 days and then euthanized with CO2 at the endpoint of observation or whenever they were found moribund.
Acute Lung Injury Assessment
The alterations of lung permeability were evaluated by determining the concentrations of total proteins in BALF [20]. Lung tissue sections stained with hematoxylin and eosin were graded by a board-certified pathologist who was blinded to the experimental assignment. At least 20 vision fields under ×400 magnification were analyzed.
Bacterial Load Determination
Homogenized tissues of lung and spleen in PBS (1 mL) and samples of blood and BALF were serially diluted by 10-fold in sterile PBS. Ten microliters of each sample were plated on nutrient agar plates and incubated at 37°C for 15 hours. The numbers of bacterial colonies were counted from the plates.
Cytokine Determination
The levels of IL-1β and IL-18 in cell culture supernatants, BALF, and lung were determined using corresponding enzyme-linked immunosorbent assay kit and following the manufacturer’s instructions.
Flow Cytometry
Macrophage numbers in BALF were determined by flow cytometry. After red blood cell lysis, cells in BALF were pelleted by centrifugation at 1000 ×g for 5 minutes and resuspended in FACS buffer (PBS supplemented with 4% fetal bovine serum and 0.05% sodium azide). Then, cell concentrations were adjusted to 1 × 106 cells/mL. Cells were incubated with Alexa Fluor 488-cojugated anti-CD45 antibody and APC-conjugated anti-F4/80 antibody to label BALF macrophages, which show double-positive signals during flow cytometry. Data obtained from flow cytometry were analyzed by FCS Express Software.
Statistical Analysis
Analysis was performed using GraphPad Prism 7. Kaplan-Meier curves and log-rank test was used to analyze the survival curve. For comparisons among 3 or more groups, one-way analysis of variance with Bonferroni’s multiple comparison test was performed. Mann-Whitney U tests were used to make comparisons between 2 groups. All the data are presented as mean ± standard error of mean. All of the experiments were conducted at least 3 independent times with 4 replicates. P < .05 was considered statistically significant.
RESULTS
Pad2 Deficiency Improves Survival in Pseudomonas aeruginosa Pneumonia-Induced Sepsis
First, we assessed 10-day survival in a mouse model of pulmonary sepsis induced by PA. Almost all Pad2−/− mice (13 of 15) survived the 10-day observation period (P < .0001; Figure 1), whereas all WT mice (n = 15) and Pad4−/− mice (n = 15) died at approximately 50 hours post-PA inoculation. These findings demonstrate that Pad2 but not Pad4 deficiency protects the animals against PA pneumonia-induced sepsis.
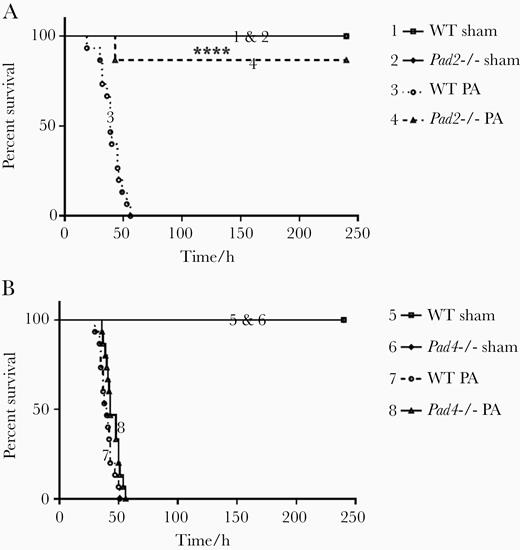
Pad2 deficiency improves survival in Pseudomonas aeruginosa (PA) pneumonia-induced sepsis. Mice were intranasally inoculated with PA (2.5 × 106 colony-forming units per mouse), and the survival of mice was monitored over the subsequent 10 days (n = 15 per group). (A) Survival of wild-type (WT) and Pad2−/− mice were monitored post-PA inoculation; (B) Survival of WT and Pad4−/− mice were monitored post-PA inoculation. Data were presented as percentages of survival at observational timepoints. Data were analyzed using log rank tests. ****, P < .0001 compared with WT PA. Pad, peptidylarginine deiminase.
Pad2 Deficiency Attenuates Acute Lung Injury and Protects Alveolar Macrophages
Because lung was the primary focus of infection, we next examined the functional and histological alterations in the lung. First, we determined the concentrations of total proteins in BALF, which were leaked into alveoli after PA infection and considered a parameter reflecting lung permeability [20]. We found that lower protein levels were detected in BALF from Pad2−/− mice in comparison with WT mice, whereas the Pad4−/− mice were similar to the WT mice (Supplemental Figure S2). Then, the levels of proinflammatory cytokines in the lung were measured to compare the inflammatory conditions in WT mice, Pad2−/− mice, and Pad4−/− mice. We noted that Pad2−/− mice produced lower levels of IL-1β and IL-18 in the lung compared with WT mice. Whereas the levels of IL-1β and IL-18 in the lung of Pad4−/− mice were as high as those of WT mice (Supplemental Figure S3). Although IL-1β is reported to cause lung injury and worsen prognosis, the protective effects of IL-1β blockage were only observed in the sickest patients [21]. Thus, we wondered whether IL-1β production in a less severe disease model would still be decreased by Pad2 deficiency. We compared the survival and IL-1β/IL-18 production in the mice challenged with a low PA dose (2 × 105 CFU/mouse) with those challenged with a high PA dose (2.5 × 106 CFU). Consequently, Pad2−/− mice showed improved survival and decreased IL-1β production both in low-dose and high-dose challenge (Supplemental Figure S4A and B). However, in the low-dose group, IL-18 production in Pad2−/− mice was not different from that in WT mice (Supplemental Figure S4C). This was probably because the regulatory mechanisms controlling IL-1β and IL-18 are different [22]. A low PA load was not enough to induce a notable upregulation of pro-IL-18.
The histopathological changes in lung were graded by a blinded pathologist. Pad2 knockout animals displayed significantly attenuated acute lung injury ([ALI] P = .0038), whereas Pad4 knockout did not (Figure 2A and B). Specifically, WT mice showed more inflammatory cell infiltration in lung interstitials, pulmonary edema, and alveolar hemorrhage compared with Pad2−/− mice. Moreover, more macrophages were observed in alveolar of PA-challenged Pad2−/− mice (indicated by black arrows in Figure 2A). Next, we quantified the macrophages in alveoli with flow cytometry. Anti-CD45 antibody were applied to categorize hematopoietic cells, and anti-F4/80 antibody were used to label macrophages among these cells. Consistent with histological findings, results of flow cytometry analysis showed that more macrophages were present in BALF of PA insulted Pad2−/− mice than that of PA-insulted WT mice and Pad4−/− mice (Figure 2C and D). These findings suggested that improved survival and alleviated ALI in Pad2−/− mice were possibly related to the differences in macrophage numbers.
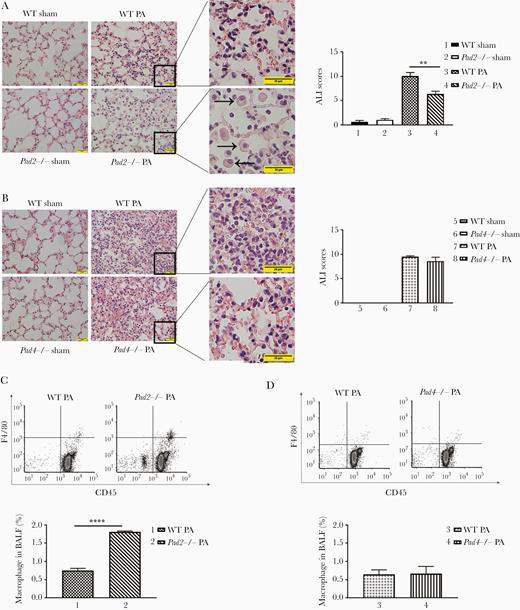
Pad2 deficiency ameliorates acute lung injury (ALI) in a mouse model of Pseudomonas aeruginosa (PA)-induced sepsis. Mice were intranasally inoculated with PA (2.5 × 106 colony-forming units per mouse). (A, left) Representative images show hematoxylin and eosin-stained lung sections of wild-type (WT) and Pad2−/− mice at 24 hours after PA inoculation; (right) ALI scores were quantified for the groups shown at left (scale bar, 20 μm; black arrows were pointed to macrophages); (B, left) Representative images show hematoxylin and eosin-stained lung sections of WT and Pad4−/− mice at 24 hours after PA inoculation; (right) ALI scores were quantified for the groups shown at left (scale bar, 20 μm); (C, top) Flow cytometry show macrophage numbers in alveoli of WT mice, Pad2−/− mice, and Pad4−/− mice at 24 hours after PA inoculation; (bottom) quantification of flow cytometry data were performed. All experiments were conducted at least 3 independent times with 4 replicates. Data in A and B were analyzed by one-way analysis of variance followed by Tukey’s multiple comparison tests. Data in C were analyzed by unpaired Student t tests. All the data are presented as mean ± standard error of mean. **, P < .01; ****, P < .0001. BALF, bronchoalveolar lavage fluid; Pad, peptidylarginine deiminase.
Pad2 Knockout Decreases Pyroptosis in Lung
To determine whether the differences in alveolar macrophage numbers were caused by pyroptosis, we investigated caspase-1 activation in lung and IL-1β/IL-18 release in BALF. Because the cleavage of caspase-1 occurs before cell death and cytokine release, the levels of caspase-1 activation in lung were determined at an earlier timepoint (9 hours postinfection), whereas cytokine levels were measured at the timepoint of 24 hours. We found that caspase-1 cleavage was reduced in lung of PA-inoculated Pad2−/− mice when compared with PA-inoculated WT mice (P = .0002) (Figure 3A). Likewise, the levels of IL-1β (P = .0001) and IL-18 (P = .0007) were also significantly lower in Pad2−/− mice after PA insult (compared with WT mice) (Figure 3B). However, Pad4 deficiency did not affect the activation of caspase-1 and the release of IL-1β and IL-18 (Figure 3C and D). These results indicated that Pad2 might mediate PA-induced caspase-1 activation and downstream macrophage death.
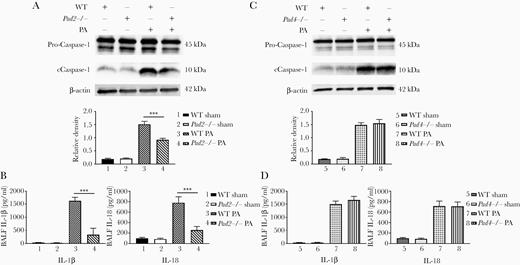
Pad2 deficiency decreases caspase-1-dependent pyroptosis in the lungs of Pseudomonas aeruginosa (PA)-inoculated mice. (A, top) Western blot was performed to detect PA-induced caspase-1 activation in the lungs of wild-type (WT) and Pad2−/− mice at 9 hours after PA inoculation (all of the bands at approximately 45 kDa represent procaspase-1). (Bottom) Relative densities of corresponding Western blots were quantified; (B) enzyme-linked immunosorbent assay (ELISA) data show concentrations of interleukin (IL)-1β and IL-18 in bronchoalveolar lavage fluid (BALF) of WT mice, Pad2−/− mice, and Pad4−/− mice at 24 hours after PA inoculation; (C, top) Western blot was performed to detect PA-induced caspase-1 activation in lung of WT and Pad4−/− mice at 9 hours after PA inoculation. (Bottom) Relative densities of corresponding Western blots were quantified; (D) ELISA data show concentrations of IL-1β and IL-18 in BALF of WT mice, Pad2−/− mice, and Pad4−/− mice at 24 hours after PA inoculation; all experiments were conducted at least 3 independent times with 4 replicates. Data were analyzed by one-way analysis of variance followed by Tukey’s multiple comparison tests. Data are presented as mean ± standard error of mean. ***, P < .001; ****, P < .0001. cCaspase-1, cleaved caspase-1; Pad, peptidylarginine deiminase.
Peptidylarginine Deiminase 2 Deficiency Reduces Caspase-1-Dependent Pyroptosis in Pseudomonas aeruginosa-Treated Bone Marrow-Derived Macrophages
To further explore whether the protective effects of Pad2 deficiency were directly associated with macrophages, BMDMs were isolated for mechanistic studies. Isolated BMDMs were subject to 1 hours or 3 hours PA infection. Results showed that 1-hour PA infection significantly promoted caspase-1 cleavage in WT BMDMs. However, Pad2−/− BMDMs exhibited a decrease in caspase-1 activation (cell lysates: P = .001, supernatants: P = .029), whereas Pad4−/− BMDMs had comparable levels of caspase-1 activation as WT BMDMs (Figure 4A). The levels of IL-1β and IL-18 in cell culture supernatants were also measured to assess the effects of caspase-1 inhibition on downstream cytokine release. Pseudomonas aeruginosa-infected Pad2−/− BMDMs released less IL-1β and IL-18 when compared with PA-infected WT BMDMs (P < .0001). In contrast, PAD4 deficiency in BMDMs did not affect IL-1β and IL-18 secretion (Figure 4B). Meanwhile, we used LDH release assay to investigate whether the knockout of Pad2 was associated with protecting BMDMs from PA-induced cell death. As expected, Pad2−/− BMDMs displayed higher viability than WT BMDMs and Pad4−/− BMDMs after cultured with PA for 3 hours (P < .0001), which is indicated by lower LDH levels in supernatants (Figure 4C). These findings suggested that it was PAD2 instead of PAD4 that was involved in PA-induced pyroptosis in macrophages and that Pad2 deficiency might promote bacterial clearance via reducing PA-induced pyroptotic macrophage death.
![Pad2 deficiency diminishes casaspe-1-dependent pyroptosis in Pseudomonas aeruginosa (PA)-infected bone marrow-derived macrophages (BMDMs). (A) Western blot was performed to detect PA-induced caspase-1 activation in wild-type (WT) BMDMs, Pad2−/− BMDMs, and Pad4−/− BMDMs (multiplicity of infection [MOI] = 10 for PA 1-hour infection) (all of the bands at approximately 45 kDa represent pro-caspase-1); (B) enzyme-linked immunosorbent assay results show the levels of interleukin (IL)-1β and IL-18 in cell culture supernatants (SP) of WT BMDMs, Pad2−/− BMDMs, and Pad4−/− BMDMs (MOI = 10 for PA 1-hour infection); (C) lactate dehydrogenase (LDH) assay was conducted to detect PA-induced macrophage death in WT BMDMs, Pad2−/− BMDMs, and Pad4−/− BMDMs (MOI = 10 for PA 3-hour infection). All experiments were conducted at least 3 independent times with 4 replicates. Data in A and B were analyzed by one-way analysis of variance followed by Tukey’s multiple comparison tests. Data in C were analyzed by unpaired Student t tests. Data are presented as mean ± standard error of mean. *, P < .05; ***, P < .001; ****, P < .0001. cCaspase-1, cleaved Caspase-1; CL, cell lysates; Pad, peptidylarginine deiminase.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jid/223/6/10.1093_infdis_jiaa475/1/m_jiaa475_fig4.jpeg?Expires=1750274074&Signature=wtfTxz4EuGLdoCTA7a-LmftlGOAj7SRJspW3TB1av8ukg4mz3Ms~sgqm0yKGIuQeY~N3yvFMnTD7rpRiWMiz97bvMWFc~cKsOhrHVeYLEkXVM3DPAiB-9G-Lg8Qz-KowWCe4RjppBrYldwKWsEl8ME5kwAoxmhXhRymxY21u0AYSVKB5uG-bPVs~1XnV9yOgMnKJgwudlJHIvIQJHle3X6vc6HwRkR6WAcC5TZiLVm1oHqIBpHEYJ62zqwFEqZlv1e6bH~gCFmgoq5EIn095svIcux2NARx5BQE8jHALe975y58CCEYB4LgAw2eVPIkgYVZafkGCUjx6ylsdZbSInQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Pad2 deficiency diminishes casaspe-1-dependent pyroptosis in Pseudomonas aeruginosa (PA)-infected bone marrow-derived macrophages (BMDMs). (A) Western blot was performed to detect PA-induced caspase-1 activation in wild-type (WT) BMDMs, Pad2−/− BMDMs, and Pad4−/− BMDMs (multiplicity of infection [MOI] = 10 for PA 1-hour infection) (all of the bands at approximately 45 kDa represent pro-caspase-1); (B) enzyme-linked immunosorbent assay results show the levels of interleukin (IL)-1β and IL-18 in cell culture supernatants (SP) of WT BMDMs, Pad2−/− BMDMs, and Pad4−/− BMDMs (MOI = 10 for PA 1-hour infection); (C) lactate dehydrogenase (LDH) assay was conducted to detect PA-induced macrophage death in WT BMDMs, Pad2−/− BMDMs, and Pad4−/− BMDMs (MOI = 10 for PA 3-hour infection). All experiments were conducted at least 3 independent times with 4 replicates. Data in A and B were analyzed by one-way analysis of variance followed by Tukey’s multiple comparison tests. Data in C were analyzed by unpaired Student t tests. Data are presented as mean ± standard error of mean. *, P < .05; ***, P < .001; ****, P < .0001. cCaspase-1, cleaved Caspase-1; CL, cell lysates; Pad, peptidylarginine deiminase.
Pad2 Deficiency Enhances Bacterial Clearance
To evaluate the effects of Pad2 deficiency and reduced pyroptosis on bacterial clearance, the bacterial loads in lung, BALF, blood, and spleen were determined at 24 hours after PA inoculation. We found that the numbers of bacteria were remarkably decreased in lung homogenates and BALF of PA-inoculated Pad2−/− mice compared with WT mice (P < .0001 and P = .0006, respectively). It is interesting to note that no bacteria were detected in blood and spleen homogenates of Pad2−/− mice after PA challenge (both P < .0001), which indicated that Pad2 knockout prevented PA infection from disseminating (Figure 5A). Nevertheless, WT mice and Pad4−/− mice showed comparable bacterial numbers at these 4 locations (Figure 5B).
![Pad2 deficiency enhances bacterial clearance. Mice were intranasally inoculated with Pseudomonas aeruginosa (PA) (2.5 × 106 colony-forming units [CFU] per mouse). (A) Bacterial loads in lung, bronchoalveolar lavage fluid (BALF), blood and spleen of wild-type (WT) and Pad2−/− mice were determined at 24 hours after PA inoculation; (B) Bacterial loads in lung, BALF, blood and spleen of WT mice and Pad4−/− mice were determined at 24 hours after PA inoculation. All experiments were conducted at least 3 independent times with 4 replicates. All the data were analyzed by unpaired Student t tests. Data are presented as mean ± standard error of mean. ***, P < .001; ****, P < .0001. Pad, peptidylarginine deiminase.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jid/223/6/10.1093_infdis_jiaa475/1/m_jiaa475_fig5.jpeg?Expires=1750274074&Signature=1rAe6vcwnZtBdhIYlhpGnF501b-DK2mSUzq2OhC-c2Woz4zt-pABa1F0~PN53g4SdU5dumIyozImkt0wCJZ06rPG6QVQ2kNawzgCZZwsL719wODGuXOeroxY12NavineeMfnBzgaD-9mWhq9i1hT5Et9VviA-F1ZISdunRnykW-b4MjKkP4sv8jvkqEbPW-O7QWPseLmsbHCwWYNFIvNiuaK-5fNhkfyu6JFjs-WnIbFkeGpfg4EG5BaOupGKuhgX4HgVRCvsU5GgqmOg3TRcXWdEiDPT~xjxsFtTaHmcEBKbxtsZUKD0cAfnS32ZYuAt0NGV6oBFC42qvpvNNxL0g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Pad2 deficiency enhances bacterial clearance. Mice were intranasally inoculated with Pseudomonas aeruginosa (PA) (2.5 × 106 colony-forming units [CFU] per mouse). (A) Bacterial loads in lung, bronchoalveolar lavage fluid (BALF), blood and spleen of wild-type (WT) and Pad2−/− mice were determined at 24 hours after PA inoculation; (B) Bacterial loads in lung, BALF, blood and spleen of WT mice and Pad4−/− mice were determined at 24 hours after PA inoculation. All experiments were conducted at least 3 independent times with 4 replicates. All the data were analyzed by unpaired Student t tests. Data are presented as mean ± standard error of mean. ***, P < .001; ****, P < .0001. Pad, peptidylarginine deiminase.
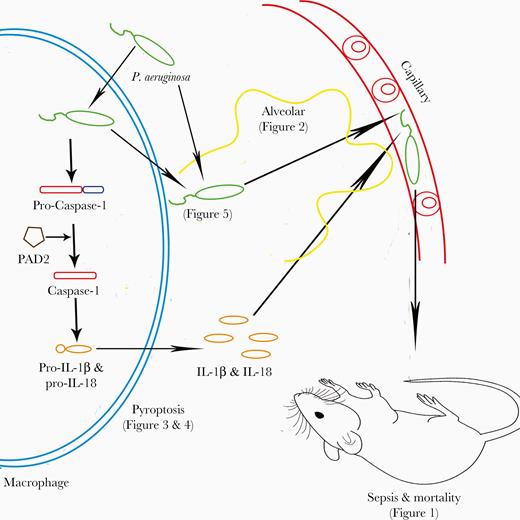
Schematic diagram shows a possible pathway of peptidylarginine deiminase (PAD2)-mediated caspase-1 activation, macrophage pyroptosis, Pseudomonas aeruginosa dissemination, and mouse mortality. IL, interleukin.
DISCUSSION
In this study, we discovered the novel role of PAD2 in mediating ALI in PA pneumonia. We reported for the first time that Pad2 deficiency can improve survival in a mouse model of PA pneumonia. We also introduced a potential mechanism for lung injury in which PAD2 can activate caspase-1-dependent pyroptosis, resulting in increased macrophage death and decreased bacterial clearance.
Our data suggest that PAD2 mediates development of ALI during PA pneumonia. Prior studies involving PAD2 were related to breast cancer, neurodegenerative diseases, and autoimmune diseases [15, 16, 23]. Our studies have previously shown that PAD2 and PAD4 dual inhibitors such as Cl-amidine and YW3-56 can improve survival in septic models or endotoxic models [18, 24]. More recently, we confirmed that the survival benefits are primarily associated with inhibition of PAD2 with specific inhibitor AFM32a [25]. Therefore, in the present study, we used a more clinically relevant PA pneumonia mouse model to further explore the roles of PAD2 during sepsis. In line with previous findings, Pad2 deficiency dramatically improved survival of PA-challenged mice. No bacteria were detected in the spleen homogenates and blood of PA-inoculated Pad2−/− mice, suggesting that Pad2 deficiency is related to enhanced bacterial clearance. Pad2−/− mice also had decreased ALI and improved survival compared with WT mice. These results, taken together, identified PAD2 as a detrimental factor to the host in PA infection.
Peptidylarginine deiminase 2 mediates PA-induced ALI through caspase-1-mediated pyroptosis in alveolar macrophages. The role of caspase-1-dependent inflammasome activity in PA infection has recently been an active area of investigation [7]. Inflammasomes, multimolecular signaling platforms in macrophages that recognize patterns from bacterial products, cleave and activate procaspase-1 to caspase-1, resulting in a signaling cascade that secretes the proinflammatory cytokines IL-1β and IL-18, and ultimately results in pyroptotic cell death. NLRP3 and NLRC4 inflammasomes have been primarily implicated in PA infections. Previously, caspase-1-dependent pyroptosis was believed to play an essential role in bacterial clearance by exposing intracellular bacteria to subsequent recognition and killing by neutrophils [26, 27]. However, it has also been shown that a hyperinflammatory response mediated by overactive inflammasome activity impairs bacterial clearance, resulting in poor host outcomes [28]. We now offer further support that caspase-1-dependent pyroptosis can be detrimental to PA-infected host through a PAD2-mediated process. By showing cleavage of caspase-1, IL-1β, and IL-18, we have demonstrated that pyroptosis in Pad2−/− mice were significantly reduced. This suggests that PAD2 serves as a regulator in mediating PA-induced activation of caspase-1 and downstream signaling pathways. The mechanism behind the destruction is likely multifactorial. Interleukin-1β and IL-18 released during pyroptosis has been shown to impair host defense against PA and result in tissue injury [29–31]. They are also related to higher bacterial loads in the lung during PA pneumonia [29, 31]. Moreover, IL-1β can directly induce ALI [32, 33]. These findings add to the growing understanding of the association between PAD and the pathogenesis of PA-induced pneumonia.
This study, for the first time, highlights the role of PAD2 in the development of pulmonary sepsis, which differs from previous studies that implicated primarily PAD4 in extrapulmonary sepsis. Peptidylarginine deiminase 4 has been associated with the deployment of neutrophil extracellular traps (NETosis) after systemic infection. Neutrophil extracellular traps are web-like structures that can capture, sequester, and kill various microbes [34]. Peptidylarginine deiminase 4 has been shown to be essential in forming NETs [14, 35]. McDonald et al [14] reported that the ablation of Pad4 could diminish multiple organ damage and coagulopathy during sepsis owing to decreased NET formation. However, Claushuis et al [36] demonstrated that Pad4 deficiency does not affect bacterial growth or dissemination during Klebsiella pneumoniae-induced pulmonary sepsis because NETosis can still occur without PAD4. Interestingly enough, our data demonstrate that Pad4 deficiency did not confer survival benefit nor enhanced the bacterial clearance. Pad4−/− mice exhibited 100% mortality after PA challenge, which was identical to the WT mice. The bacterial loads in Pad4−/− mice were comparably high. However, our results implicate PAD2 as being a more essential player in PA pneumonia. The disparate outcomes might be attributed to varying activation of NLRP3 over NLRC4 inflammasomes. Mishra et al [37] demonstrated that PAD2 and PAD4 dual inhibition leads to NLRP3 inflammasome signaling pathway suppression. It is possible that PAD2 alone results in NLRC4 inflammasome activity, which has been associated with more overexuberant and detrimental inflammatory activity [38]. More research needs to be done to elucidate the differing and interconnected roles of PAD2, PAD4, NETosis, and pyroptosis in PA ALI.
Peptidylarginine deiminases 2 and PAD4 are constitutively expressed in different cells. The expression of PAD2 or PAD4 can change with cell differentiation or in response to pathogenic stimulation [39, 40]. Protein citrullination catalyzed by PAD2 and PAD4 has significant effects on diverse activities in vivo. Whereas dysregulated citrullination of proteins is associated with many diseases such as multiple sclerosis, rheumatoid arthritis, and Alzheimer’s diseases [41]. The 2 PADs citrullinate a wide range of protein substrates. Most substrates of PAD2 and PAD4 are different: PAD2s are known to citrullinate myelin basic protein, vimentin, and actin, whereas PAD4 catalyzes the citrullination of nucleophosmin and nuclear lamin C [42]. However, both PAD2 and PAD4 can citrullinate histone H3 and fibrinogen [42]. Although the mechanism through which PAD2 regulates caspase-1 activation remains unclear, it may be associated with ASC speck citrullination [37]. ASC specks participate in the assembly of inflammasomes, triggering caspase-1 activation. With regards to the regulatory effects of protein citrullination on NET formation, it has been demonstrated that histone hypercitrullination renders chromatins susceptible to decondensation, which is a key step during NETosis [43]. Although we previously revealed that treatment with PAD2 inhibitor decreases the production of NETs [25], we later found that the effects of PAD2 on macrophages are more predominant than NETosis during sepsis (Y. Tian, submitted). Thus, we focused on investigating the PAD2/caspase-1/pyroptosis pathway in the present study.
This study has some limitations. For example, we failed to identify Gasdermin D (GSDMD), a key regulator in pyroptosis, across all study groups. As part of the pyroptosis pathway, GSDMD is cleaved and converted into active forms by caspase-1 and caspase-11. Cleaved GSDMD can then insert into plasma membrane, create pores, and cause cell rupture. In our study, however, the levels of GSDMD cleavage were similarly low in WT and Pad2−/− BMDMs after PA infection (data not shown). A possible explanation is that GSDMD plays an indispensable role only in caspase-11-mediated pyroptosis. In contrast, caspase-1-mediated pyroptosis can still occur in the absence of GSDMD, which suggests that there are some undefined alternative pathways [44]. Moreover, it remains unknown how PA induced PAD2-catalyzed citrullination and how the citrullinated proteins could participate in the pathway. A previous study reported that ATP can induce PAD2-dependent citrullination in mast cells via P2X7 receptors [45]. Is this pathway also applied to PA-infected macrophages? Citrullinomics will be carried out after PAD2/caspase-1 immunoprecipitation to identify specific proteins associated with the pyroptosis in the future.
CONCLUSIONS
In conclusion, in this experimental model of PA pneumonia-induced sepsis, we discovered that PAD2 promotes caspase-1 activation in response to PA pulmonary inoculation and thus leads to enhanced pyroptosis, reduced bacterial clearance, resulting in worsening ALI and increased mortality. This differs from the existing understanding of the role that PAD4 plays in systemic infections. More studies are warranted to further elucidate the mechanism of PAD2 in PA-triggered pyroptosis. These results identify PAD2 as a potential therapeutic target of PA pneumonia-induced sepsis.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. Y. L. and H. B. A. designed the study. Z. W. carried out the experiments. Z. W. and P. L. wrote the manuscript, and Y. L., P. L., and H. B. A. made a critical revision. Y. T., A. M. W., B. L., and J. M. reviewed and revised the manuscript. Y. T. and X. D. provided experimental support. All authors have read and approved the final manuscript.
Financial support. This work was funded by grants from the Joint-of-Institute (U068874) and MCubed (U064088) to Y. L. and Professorship to H. B. A.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Presented in part: 43rd Annual Conference on Shock.
References
Author notes
Y. L. and H. B. A. are cocorresponding authors.




