-
PDF
- Split View
-
Views
-
Cite
Cite
Leyi Lin, Michael A Koren, Kristopher M Paolino, Kenneth H Eckels, Rafael De La Barrera, Heather Friberg, Jeffrey R Currier, Gregory D Gromowski, Naomi E Aronson, Paul B Keiser, Marvin J Sklar, Erica L Sondergaard, Louis E Jasper, Timothy P Endy, Richard G Jarman, Stephen J Thomas, Immunogenicity of a Live-Attenuated Dengue Vaccine Using a Heterologous Prime-Boost Strategy in a Phase 1 Randomized Clinical Trial, The Journal of Infectious Diseases, Volume 223, Issue 10, 15 May 2021, Pages 1707–1716, https://doi.org/10.1093/infdis/jiaa603
Close - Share Icon Share
Abstract
Dengue is a global health problem and the development of a tetravalent dengue vaccine with durable protection is a high priority. A heterologous prime-boost strategy has the advantage of eliciting immune responses through different mechanisms and therefore may be superior to homologous prime-boost strategies for generating durable tetravalent immunity.
In this phase 1 first-in-human heterologous prime-boost study, 80 volunteers were assigned to 4 groups and received a tetravalent dengue virus (DENV-1–4) purified inactivated vaccine (TDENV-PIV) with alum adjuvant and a tetravalent dengue virus (DENV-1–4) live attenuated vaccine (TDENV-LAV) in different orders and dosing schedules (28 or 180 days apart).
All vaccination regimens had acceptable safety profiles and there were no vaccine-related serious adverse events. TDEN-PIV followed by TDEN-LAV induced higher neutralizing antibody titers and a higher rate of tetravalent seroconversions compared to TDEN-LAV followed by TDEN-PIV. Both TDEN-PIV followed by TDEN-LAV groups demonstrated 100% tetravalent seroconversion 28 days following the booster dose, which was maintained for most of these subjects through the day 180 measurement.
A heterologous prime-boost vaccination strategy for dengue merits additional evaluation for safety, immunogenicity, and potential for clinical benefit.
NCT02239614.
Dengue remains the world’s leading mosquito borne illness with an estimated 390 million infections occurring annually, of which over 95 million result in clinically apparent disease [1]. Having an effective tetravalent dengue vaccine is a high priority and the dengue vaccine field is robust with numerous candidates in preclinical and clinical development [2]. Several excellent reviews on the status of dengue vaccine development have been published [3, 4]. Dengvaxia, a tetravalent dengue vaccine with a yellow fever virus backbone, is the only vaccine that has been licensed and has been registered in over 20 countries. It is a case study on the current difficulties in developing dengue vaccines due to the lack of an optimal animal model that reliably translates to human efficacy and the lack of a biomarker that indicates protection [5]. For Dengvaxia, a safety signal observed in the youngest recipients and those who were dengue seronegative at baseline prompted a change in the original indication. Currently, the vaccine is licensed by the US Food and Drug Administration for individuals 9 through 16 years of age with laboratory-confirmed previous dengue infection and who live in endemic areas [6]. Recently, Takeda Pharmaceuticals published the initial results of their tetravalent live-attenuated vaccine (TAK-003) in 20 071 participants through 1 year post vaccination [7]. The overall vaccine efficacy in the safety population was 80.9% (95% confidence interval [CI], 75.2–85.3). However, wide ranging confidence intervals for dengue virus (DENV) serotypes 1 and 3 along with indeterminable efficacy for serotype 4 suggests tetravalent protection may not be induced by this vaccine.
All the current dengue vaccines in clinical trials are live-attenuated tetravalent vaccines requiring utilizing 1 or multiple doses (homologous prime-boost strategy) to achieve adequate tetravalent neutralizing antibody titers. A heterologous prime-boost strategy, which involves consecutive doses of different vaccine platforms, has the advantage of improving both humoral and cellular immunity to a specific antigen [8–10]. For dengue vaccines, a prime-boost immunization strategy has been proposed to induce or boost a cell mediated immune response while avoiding the induction of antibodies that potentially mediate antibody-dependent enhancement [10]. This technique was employed in rhesus macaques by priming with a tetravalent purified inactivated dengue vaccine followed by boosting with a tetravalent live-attenuated dengue vaccine [11]. This strategy resulted in high titers of neutralizing antibody and protection upon challenge.
We describe the results of a phase 1 clinical trial testing heterologous prime-boost vaccine administration strategies using different dosing schedules of tetravalent live and inactivated dengue vaccine candidates (ClinicalTrials.gov NCT02239614). The objective of the trial was to explore whether a heterologous prime-boost vaccination strategy could improve reactogenicity profiles, compress dosing schedules, and improve the breadth of immunologic responses when compared to historic experiments testing homologous prime-boost administration strategies.
METHODS
Study Objectives and Design
The primary study objectives were to evaluate the safety and immunogenicity of 4 different vaccine administration strategies from day 0 through 28 days after each dose. This study was a phase 1, randomized, open-label, study with 4 treatment groups consisting of 2 tetravalent dengue vaccine candidates administered using a heterologous prime-boost administration strategy and 2 dosing schedules (day 0 and day 28 vs day 0 and day 180). The study was conducted under an Investigational New Drug application filed with the US Food and Drug Administration. The study was conducted at the Walter Reed Army Institute of Research (WRAIR), Clinical Trials Center, Silver Spring, MD (ClinicalTrials.gov NCT02239614). The WRAIR Institutional Review Board and United States Army Medical Research and Materiel Command Office of Research Protections reviewed and approved the protocol. The investigators have adhered to the policies for protection of human subjects as prescribed in Army Regulation 70-25.
Vaccines
Two investigational products were studied, a tetravalent dengue virus (DENV-1–4) purified formalin-inactivated vaccine (TDENV-PIV) with alum adjuvant and a tetravalent dengue virus (DENV-1–4) live attenuated vaccine (TDENV-LAV) described previously [11–17]. TDENV-PIV consists of a tetravalent formulation of the following nonattenuated dengue virus strains: West Pac 74 (DENV-1), S16803 (DENV-2), CH53489 (DENV-3), and TVP360 (DENV-4), propagated in Vero cells, purified, inactivated with formalin, and formulated with 4 µg per DENV serotype per dose. TDENV-PIV was adjuvanted with alum (Alhydrogel 2%, 10.38 mg of Al3+/mL; Brenntag Biosector); after dilution, each 0.5-mL vaccine dose contained 500 μg of Al3+. The TDENV-LAV vaccine was made using the following attenuated virus seeds: DENV-1 (45AZ5, PDK-27), DENV-2 (S16803, PDK-50), DENV-3 (CH53489, PDK-20), and DENV-4 (341750, PDK-6). Clinical vaccine lots were prepared at the WRAIR Pilot Bioproduction Facility, Silver Spring, MD. Each vaccine was delivered in a 0.5-mL dose volume using a needle and syringe; TDENV-PIV was administered intramuscularly while TDENV-LAV was administered subcutaneously.
Study Volunteers and Enrollment
Healthy male and female adults between 18 and 49 years of age were recruited. Female participants had to be of nonchildbearing potential, abstinent, or had to use adequate contraceptive precautions for 30 days prior to vaccination, had a negative pregnancy test on the day of vaccination, and agreed to continue such precautions for 90 days after completion of the vaccination series. Exclusion criteria were seropositivity for hepatitis B, hepatitis C, human immunodeficiency virus, or a history of chronic disease, chronic alcohol consumption and/or drug abuse, receipt of immunoglobulins and/or any blood products within 90 days preceding vaccination or planned administration during the study period, as well as clinically significant laboratory abnormalities. Participants were not screened for flavivirus antibodies at enrollment but the according-to-protocol (ATP) analysis of immunogenicity included only participants who were seronegative for all 4 DENV serotypes at baseline (50% microneutralization assay end point [MN50] titer <1:10). Serostatus for DENV was determined after enrollment based on prevaccination blood samples.
A total of 80 volunteers were enrolled and randomized to 1 of 4 groups in a 1:1:1:1 distribution. Block randomization was used to allocate volunteers to each study group divided into 20 blocks of 4 by the order of study enrollment. Group 1 received a single dose of TDEN-LAV at day 0 and a boost of TDENV-PIV at day 28; group 2 received TDENV-PIV at day 0 and TDENV-LAV at day 28; group 3 received TENV-LAV at day 0 and TDENV-PIV at day 180; and group 4 received TDENV-PIV at day 0 and TDENV-LAV at day 180.
Safety Evaluation
Solicited injection site and general adverse events (AEs) were followed for 21 days following each dose using both a memory aid completed by the volunteer and clinical visits with an investigator. Unsolicited AEs were recorded for 28 days following each dose and were coded with the use of the “Medical Dictionary for Regulatory Activities.” Serious adverse events (SAEs), potential immune-mediated diseases, and medically attended events were recorded throughout the entire study period. Each group was split into 2 subgroups (A or B) each corresponding to a blood collection schedule. Splitting the groups allowed greater breadth of sampling over time while reducing the overall blood donation burden for each individual volunteer.
Viremia
Assessment for viremia was conducted at WRAIR using quantitative reverse transcription polymerase chain reaction (RT-PCR) as previously described. Viremia after TDENV-LAV vaccination typically occurs 5–14 days post vaccination based on previous data [12, 13, 15, 16]. Viremia was not expected after TDENV-PIV vaccination. Viremia was measured at 5 time points per volunteer following TDENV-LAV.
Neutralizing Antibody Assay
DENV neutralizing antibodies were measured using a microneutralization assay to estimate MN50 as previously described [14–17]. The parental virus strains to be neutralized were DENV-1 WP74, DENV-2 S16803, DENV-3 CH53489, and DENV-4 341750. Seropositivity was defined as a titer ≥1:10.
Blood Sampling
Blood sampling is detailed in Supplementary Figure 1.
Immunogenicity Assessment
Cell-mediated immunity responses to vaccination were assessed by interferon-γ enzyme-linked immunospot (IFN-γ ELISPOT) assay prevaccination (study day 0) and at 28 days and 180 days post dose 2 in all groups using methods as previously described [18]. Briefly, cryopreserved peripheral blood mononuclear cells (PBMCs) were thawed and rested overnight before plating at 100 000–200 000 cells per well with 1 μg/mL/peptide of the applicable peptide pool or medium plus 0.5% DMSO (negative control). Peptide pools included overlapping peptides spanning the C/prM, E, NS1, NS3, and NS5 protein regions of all 4 DENV types, as previously described [18]. Data were normalized to spot-forming cells (SFC) per million PBMC for analysis and reporting purposes.
Statistical Analysis
The total vaccinated population includes all volunteers who received both vaccine doses. The ATP immunogenicity cohort includes all volunteers who received both doses of study vaccine and were seronegative to all 4 DENV types at baseline. The percentages of volunteers reporting any AE within 28 days (0–27) following vaccination were computed by study group according to the type of AE, intensity, and relationship to vaccination. The percentages of volunteers with measurable viremia by DENV type were tabulated after each dose and for each study group. Seropositivity rates for each DENV type and tetravalent antibody responses as determined by MN50 titer were calculated with 95% CI using Wald method for each vaccination group. Antibody titers were summarized as geometric mean titers (GMT), with the 95% CI, and reverse cumulative curves. A DENV-primed volunteer was defined as having a MN50 titer of ≥10 in the prevaccination (day 0) sample. Tetravalent seropositivity rates were defined as the percentage of volunteers who were seropositive to all 4 DENV types. The GMT calculations were performed by taking the antilog of the mean of the log 10 titer transformations. Antibody titers below the cutoff of the assay were given an arbitrary value of 5 (half the cutoff value) for the purpose of GMT calculation. For a given volunteer and a given immunogenicity measurement, missing or nonevaluable measurements were not replaced. Therefore, ATP analysis excluded volunteers with missing or nonevaluable measurements.
RESULTS
Study Population
Eighty volunteers were enrolled in the study and divided among the 4 different groups as shown in Figure 1; 94% (75/80) of volunteers received their second vaccination. Five volunteers were withdrawn prior to the second vaccination. The ATP cohort included 17 subjects in groups 1 and 2, 20 subjects in group 3, and 12 subjects in group 4. Two subjects were withdrawn from group 4 due to consistent failure to present for scheduled study visits. The mean age of volunteers enrolled in the study was 34.9 years (range 19 to 50). There were 28 women (35%) and 52 men (65%). Most volunteers were either Caucasian (40%) or African American (49%).
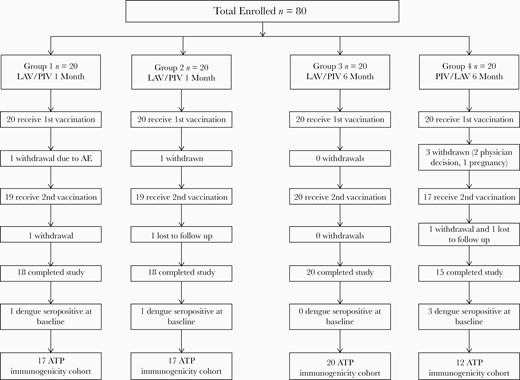
Enrollment scheme: group 1, LAV day 0/PIV day 28; group 2, PIV day 0/LAV day 28; group 3, LAV day 0/PIV day 180; group 4, PIV day 0/LAV day 180. Abbreviations: ATP, according to protocol; LAV, live attenuated vaccine; PIV, purified inactivated vaccine.
Safety and Reactogenicity
The frequencies of solicited local AEs and systemic AEs are presented in Table 1 and Table 2. Injection site pain was generally higher following administration of PIV compared to LAV (0%–40% vs 0%–20%) across all groups. No grade 3 solicited local AEs occurred in any group with either vaccination. Overall, most solicited systemic AEs were mild or moderate. The most commonly reported systemic AEs were fatigue (15%–40% with LAV and 10%–20% with PIV) and headache (15%–40% with LAV and 5%–25% with PIV). Myalgias and arthralgias were also commonly reported with generally higher rates in groups 2 and 4 compared to groups 1 and 3 but not reaching statistical significance. No fevers were recorded following PIV administration, but 20% of volunteers in group 2 and 25% of volunteers in group 4 reported fever following administration of LAV. No volunteers in groups 1 or 3 reported any fevers following LAV. A maculopapular rash was observed in 8 volunteers following LAV vaccination. Three occurred following LAV prime (2 in group 1 and 1 in group 3) and 5 occurred following LAV boost (2 in group 2 and 3 in group 4). Several volunteers reported transient grade 3 solicited systemic AEs: abdominal pain and nausea/vomiting following PIV; headache, fever, nausea/vomiting, myalgia, arthralgia, and fatigue rarely reported following LAV. There were no study-related SAEs. Related laboratory abnormalities occurred rarely and included grade 1 leukopenia in 1 volunteer in group 1, grade 2 neutropenia in 1 volunteer in group 3, grade 2 hyperbilirubinemia in 1 volunteer in group 3, and grade 2 neutropenia and lymphopenia in 1 volunteer in group 4. All of these laboratory abnormalities were transient and resolved without sequelae.
Incidence of Solicited Local Symptoms During the 21 Days Following Each Vaccination
| Symptom . | Vaccine . | Grade . | Group 1 (n = 20) . | . | Group 2 (n = 20) . | . | Group 3 (n = 20) . | . | Group 4 (n = 20) . | . |
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | n (%) . | 95% CI . | n (%) . | 95% CI . | n (%) . | 95% CI . | n (%) . | 95% CI . |
| Pain | LAV | Any | 1 (5.0) | .1–24.9 | 2 (10.0) | 1.2–31.7 | 4 (20.0) | 5.7–43.7 | 0 (0.0) | .0–16.8 |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | ||
| PIV | Any | 3 (15.0) | 3.2–37.9 | 5 (25.0) | 8.7–49.1 | 7 (35.0) | 15.4–59.2 | 8 (40.0) | 19.1–63.9 | |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | ||
| Redness | LAV | Any | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | ||
| PIV | Any | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | ||
| Swelling | LAV | Any | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | ||
| PIV | Any | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 |
| Symptom . | Vaccine . | Grade . | Group 1 (n = 20) . | . | Group 2 (n = 20) . | . | Group 3 (n = 20) . | . | Group 4 (n = 20) . | . |
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | n (%) . | 95% CI . | n (%) . | 95% CI . | n (%) . | 95% CI . | n (%) . | 95% CI . |
| Pain | LAV | Any | 1 (5.0) | .1–24.9 | 2 (10.0) | 1.2–31.7 | 4 (20.0) | 5.7–43.7 | 0 (0.0) | .0–16.8 |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | ||
| PIV | Any | 3 (15.0) | 3.2–37.9 | 5 (25.0) | 8.7–49.1 | 7 (35.0) | 15.4–59.2 | 8 (40.0) | 19.1–63.9 | |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | ||
| Redness | LAV | Any | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | ||
| PIV | Any | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | ||
| Swelling | LAV | Any | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | ||
| PIV | Any | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 |
Abbreviations: CI, confidence interval; LAV, live attenuated vaccine; PIV, purified inactivated vaccine.
Incidence of Solicited Local Symptoms During the 21 Days Following Each Vaccination
| Symptom . | Vaccine . | Grade . | Group 1 (n = 20) . | . | Group 2 (n = 20) . | . | Group 3 (n = 20) . | . | Group 4 (n = 20) . | . |
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | n (%) . | 95% CI . | n (%) . | 95% CI . | n (%) . | 95% CI . | n (%) . | 95% CI . |
| Pain | LAV | Any | 1 (5.0) | .1–24.9 | 2 (10.0) | 1.2–31.7 | 4 (20.0) | 5.7–43.7 | 0 (0.0) | .0–16.8 |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | ||
| PIV | Any | 3 (15.0) | 3.2–37.9 | 5 (25.0) | 8.7–49.1 | 7 (35.0) | 15.4–59.2 | 8 (40.0) | 19.1–63.9 | |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | ||
| Redness | LAV | Any | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | ||
| PIV | Any | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | ||
| Swelling | LAV | Any | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | ||
| PIV | Any | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 |
| Symptom . | Vaccine . | Grade . | Group 1 (n = 20) . | . | Group 2 (n = 20) . | . | Group 3 (n = 20) . | . | Group 4 (n = 20) . | . |
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | n (%) . | 95% CI . | n (%) . | 95% CI . | n (%) . | 95% CI . | n (%) . | 95% CI . |
| Pain | LAV | Any | 1 (5.0) | .1–24.9 | 2 (10.0) | 1.2–31.7 | 4 (20.0) | 5.7–43.7 | 0 (0.0) | .0–16.8 |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | ||
| PIV | Any | 3 (15.0) | 3.2–37.9 | 5 (25.0) | 8.7–49.1 | 7 (35.0) | 15.4–59.2 | 8 (40.0) | 19.1–63.9 | |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | ||
| Redness | LAV | Any | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | ||
| PIV | Any | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | ||
| Swelling | LAV | Any | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | ||
| PIV | Any | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 |
Abbreviations: CI, confidence interval; LAV, live attenuated vaccine; PIV, purified inactivated vaccine.
Incidence of Solicited Systemic Adverse Events During the 21 Days Following Each Vaccination
| Symptom . | Vaccine . | Grade . | Group 1 (n = 20) . | . | Group 2 (n = 20) . | . | Group 3 (n = 20) . | . | Group 4 (n = 20) . | . |
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | n (%) . | 95% CI . | n (%) . | 95% CI . | n (%) . | 95% CI . | n (%) . | 95% CI . |
| Abdominal Pain | LAV | Any | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | 2 (10.0) | 1.2–31.7 | 0 (0.0) | .0–16.8 |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | ||
| PIV | Any | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | |
| Grade 3 | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | ||
| Arthralgia | LAV | Any | 2 (10.0) | 1.2–31.7 | 5 (25.0) | 8.7–49.1 | 2 (10.0) | 1.2–31.7 | 4 (20.0) | 5.7–43.7 |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | ||
| PIV | Any | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | 2 (10.0) | 1.2–31.7 | 0 (0.0) | .0–16.8 | |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | ||
| Diarrhea | LAV | Any | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | 1 (5.0) | .1–24.9 | 1 (5.0) | .1–24.9 |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | ||
| PIV | Any | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | ||
| Fatigue | LAV | Any | 3 (15.0) | 3.2–37.9 | 8 (40.0) | 19.1–63.9 | 5 (25.0) | 8.7–49.1 | 5 (25.0) | 8.7–49.1 |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 2 (10.0) | 1.2–31.7 | ||
| PIV | Any | 2 (10.0) | 1.2–31.7 | 2 (10.0) | 1.2–31.7 | 3 (15.0) | 3.2–37.9 | 4 (20.0) | 5.7–43.7 | |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | ||
| Fever | LAV | Any | 0 (0.0) | .0–16.8 | 5 (25.0) | 8.7–49.1 | 0 (0.0) | .0–16.8 | 5 (25.0) | 8.7–49.1 |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | ||
| PIV | Any | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | ||
| Headache | LAV | Any | 3 (15.0) | 3.2–37.9 | 8 (40.0) | 19.1–63.9 | 8 (40.0) | 19.1–63.9 | 6 (30.0) | 11.9–54.3 |
| Grade 3 | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | 0 (0.0) | .0–16.8 | 2 (10.0) | 1.2–31.7 | ||
| PIV | Any | 1 (5.0) | .1–24.9 | 3 (15.0) | 3.2–37.9 | 5 (25.0) | 8.7–49.1 | 3 (15.0) | 3.2–37.9 | |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | ||
| Myalgia | LAV | Any | 1 (5.0) | .1–24.9 | 5 (25.0) | 8.7–49.1 | 2 (10.0) | 1.2–31.7 | 6 (30.0) | 11.9–54.3 |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | ||
| PIV | Any | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | 4 (20.0) | 5.7–43.7 | 3 (15.0) | 3.2–37.9 | |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | ||
| Nausea | LAV | Any | 1 (5.0) | .1–24.9 | 1 (5.0) | .1–24.9 | 3 (15.0) | 3.2–37.9 | 1 (5.0) | .1–24.9 |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | 0 (0.0) | .0–16.8 | ||
| PIV | Any | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | 1 (5.0) | .1–24.9 | 1 (5.0) | .1–24.9 | |
| Grade 3 | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | 1 (5.0) | .1–24.9 | 0 (0.0) | .0–16.8 | ||
| Vomiting | LAV | Any | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | 1 (5.0) | .1–24.9 |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | 0 (0.0) | .0–16.8 | ||
| PIV | Any | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | 0 (0.0) | .0–16.8 | |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | 0 (0.0) | .0–16.8 | ||
| Overall | LAV | Any | 5 (25.0) | 8.7–49.1 | 10 (50.0) | 27.2–72.8 | 8 (40.0) | 19.1–63.9 | 8 (40.0) | 19.1–63.9 |
| Grade 3 | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | 1 (5.0) | .1–24.9 | 4 (20.0) | 5.7–43.7 | ||
| PIV | Any | 2 (10.0) | 1.2–31.7 | 4 (20.0) | 5.7–43.7 | 5 (25.0) | 8.7–49.1 | 8 (40.0) | 19.1–63.9 | |
| Grade 3 | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | 1 (5.0) | .1–24.9 | 0 (0.0) | .0–16.8 |
| Symptom . | Vaccine . | Grade . | Group 1 (n = 20) . | . | Group 2 (n = 20) . | . | Group 3 (n = 20) . | . | Group 4 (n = 20) . | . |
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | n (%) . | 95% CI . | n (%) . | 95% CI . | n (%) . | 95% CI . | n (%) . | 95% CI . |
| Abdominal Pain | LAV | Any | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | 2 (10.0) | 1.2–31.7 | 0 (0.0) | .0–16.8 |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | ||
| PIV | Any | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | |
| Grade 3 | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | ||
| Arthralgia | LAV | Any | 2 (10.0) | 1.2–31.7 | 5 (25.0) | 8.7–49.1 | 2 (10.0) | 1.2–31.7 | 4 (20.0) | 5.7–43.7 |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | ||
| PIV | Any | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | 2 (10.0) | 1.2–31.7 | 0 (0.0) | .0–16.8 | |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | ||
| Diarrhea | LAV | Any | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | 1 (5.0) | .1–24.9 | 1 (5.0) | .1–24.9 |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | ||
| PIV | Any | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | ||
| Fatigue | LAV | Any | 3 (15.0) | 3.2–37.9 | 8 (40.0) | 19.1–63.9 | 5 (25.0) | 8.7–49.1 | 5 (25.0) | 8.7–49.1 |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 2 (10.0) | 1.2–31.7 | ||
| PIV | Any | 2 (10.0) | 1.2–31.7 | 2 (10.0) | 1.2–31.7 | 3 (15.0) | 3.2–37.9 | 4 (20.0) | 5.7–43.7 | |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | ||
| Fever | LAV | Any | 0 (0.0) | .0–16.8 | 5 (25.0) | 8.7–49.1 | 0 (0.0) | .0–16.8 | 5 (25.0) | 8.7–49.1 |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | ||
| PIV | Any | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | ||
| Headache | LAV | Any | 3 (15.0) | 3.2–37.9 | 8 (40.0) | 19.1–63.9 | 8 (40.0) | 19.1–63.9 | 6 (30.0) | 11.9–54.3 |
| Grade 3 | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | 0 (0.0) | .0–16.8 | 2 (10.0) | 1.2–31.7 | ||
| PIV | Any | 1 (5.0) | .1–24.9 | 3 (15.0) | 3.2–37.9 | 5 (25.0) | 8.7–49.1 | 3 (15.0) | 3.2–37.9 | |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | ||
| Myalgia | LAV | Any | 1 (5.0) | .1–24.9 | 5 (25.0) | 8.7–49.1 | 2 (10.0) | 1.2–31.7 | 6 (30.0) | 11.9–54.3 |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | ||
| PIV | Any | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | 4 (20.0) | 5.7–43.7 | 3 (15.0) | 3.2–37.9 | |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | ||
| Nausea | LAV | Any | 1 (5.0) | .1–24.9 | 1 (5.0) | .1–24.9 | 3 (15.0) | 3.2–37.9 | 1 (5.0) | .1–24.9 |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | 0 (0.0) | .0–16.8 | ||
| PIV | Any | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | 1 (5.0) | .1–24.9 | 1 (5.0) | .1–24.9 | |
| Grade 3 | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | 1 (5.0) | .1–24.9 | 0 (0.0) | .0–16.8 | ||
| Vomiting | LAV | Any | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | 1 (5.0) | .1–24.9 |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | 0 (0.0) | .0–16.8 | ||
| PIV | Any | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | 0 (0.0) | .0–16.8 | |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | 0 (0.0) | .0–16.8 | ||
| Overall | LAV | Any | 5 (25.0) | 8.7–49.1 | 10 (50.0) | 27.2–72.8 | 8 (40.0) | 19.1–63.9 | 8 (40.0) | 19.1–63.9 |
| Grade 3 | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | 1 (5.0) | .1–24.9 | 4 (20.0) | 5.7–43.7 | ||
| PIV | Any | 2 (10.0) | 1.2–31.7 | 4 (20.0) | 5.7–43.7 | 5 (25.0) | 8.7–49.1 | 8 (40.0) | 19.1–63.9 | |
| Grade 3 | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | 1 (5.0) | .1–24.9 | 0 (0.0) | .0–16.8 |
Abbreviations: CI, confidence interval; LAV, live attenuated vaccine; PIV, purified inactivated vaccine.
Incidence of Solicited Systemic Adverse Events During the 21 Days Following Each Vaccination
| Symptom . | Vaccine . | Grade . | Group 1 (n = 20) . | . | Group 2 (n = 20) . | . | Group 3 (n = 20) . | . | Group 4 (n = 20) . | . |
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | n (%) . | 95% CI . | n (%) . | 95% CI . | n (%) . | 95% CI . | n (%) . | 95% CI . |
| Abdominal Pain | LAV | Any | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | 2 (10.0) | 1.2–31.7 | 0 (0.0) | .0–16.8 |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | ||
| PIV | Any | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | |
| Grade 3 | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | ||
| Arthralgia | LAV | Any | 2 (10.0) | 1.2–31.7 | 5 (25.0) | 8.7–49.1 | 2 (10.0) | 1.2–31.7 | 4 (20.0) | 5.7–43.7 |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | ||
| PIV | Any | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | 2 (10.0) | 1.2–31.7 | 0 (0.0) | .0–16.8 | |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | ||
| Diarrhea | LAV | Any | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | 1 (5.0) | .1–24.9 | 1 (5.0) | .1–24.9 |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | ||
| PIV | Any | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | ||
| Fatigue | LAV | Any | 3 (15.0) | 3.2–37.9 | 8 (40.0) | 19.1–63.9 | 5 (25.0) | 8.7–49.1 | 5 (25.0) | 8.7–49.1 |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 2 (10.0) | 1.2–31.7 | ||
| PIV | Any | 2 (10.0) | 1.2–31.7 | 2 (10.0) | 1.2–31.7 | 3 (15.0) | 3.2–37.9 | 4 (20.0) | 5.7–43.7 | |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | ||
| Fever | LAV | Any | 0 (0.0) | .0–16.8 | 5 (25.0) | 8.7–49.1 | 0 (0.0) | .0–16.8 | 5 (25.0) | 8.7–49.1 |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | ||
| PIV | Any | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | ||
| Headache | LAV | Any | 3 (15.0) | 3.2–37.9 | 8 (40.0) | 19.1–63.9 | 8 (40.0) | 19.1–63.9 | 6 (30.0) | 11.9–54.3 |
| Grade 3 | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | 0 (0.0) | .0–16.8 | 2 (10.0) | 1.2–31.7 | ||
| PIV | Any | 1 (5.0) | .1–24.9 | 3 (15.0) | 3.2–37.9 | 5 (25.0) | 8.7–49.1 | 3 (15.0) | 3.2–37.9 | |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | ||
| Myalgia | LAV | Any | 1 (5.0) | .1–24.9 | 5 (25.0) | 8.7–49.1 | 2 (10.0) | 1.2–31.7 | 6 (30.0) | 11.9–54.3 |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | ||
| PIV | Any | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | 4 (20.0) | 5.7–43.7 | 3 (15.0) | 3.2–37.9 | |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | ||
| Nausea | LAV | Any | 1 (5.0) | .1–24.9 | 1 (5.0) | .1–24.9 | 3 (15.0) | 3.2–37.9 | 1 (5.0) | .1–24.9 |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | 0 (0.0) | .0–16.8 | ||
| PIV | Any | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | 1 (5.0) | .1–24.9 | 1 (5.0) | .1–24.9 | |
| Grade 3 | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | 1 (5.0) | .1–24.9 | 0 (0.0) | .0–16.8 | ||
| Vomiting | LAV | Any | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | 1 (5.0) | .1–24.9 |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | 0 (0.0) | .0–16.8 | ||
| PIV | Any | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | 0 (0.0) | .0–16.8 | |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | 0 (0.0) | .0–16.8 | ||
| Overall | LAV | Any | 5 (25.0) | 8.7–49.1 | 10 (50.0) | 27.2–72.8 | 8 (40.0) | 19.1–63.9 | 8 (40.0) | 19.1–63.9 |
| Grade 3 | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | 1 (5.0) | .1–24.9 | 4 (20.0) | 5.7–43.7 | ||
| PIV | Any | 2 (10.0) | 1.2–31.7 | 4 (20.0) | 5.7–43.7 | 5 (25.0) | 8.7–49.1 | 8 (40.0) | 19.1–63.9 | |
| Grade 3 | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | 1 (5.0) | .1–24.9 | 0 (0.0) | .0–16.8 |
| Symptom . | Vaccine . | Grade . | Group 1 (n = 20) . | . | Group 2 (n = 20) . | . | Group 3 (n = 20) . | . | Group 4 (n = 20) . | . |
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | n (%) . | 95% CI . | n (%) . | 95% CI . | n (%) . | 95% CI . | n (%) . | 95% CI . |
| Abdominal Pain | LAV | Any | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | 2 (10.0) | 1.2–31.7 | 0 (0.0) | .0–16.8 |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | ||
| PIV | Any | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | |
| Grade 3 | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | ||
| Arthralgia | LAV | Any | 2 (10.0) | 1.2–31.7 | 5 (25.0) | 8.7–49.1 | 2 (10.0) | 1.2–31.7 | 4 (20.0) | 5.7–43.7 |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | ||
| PIV | Any | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | 2 (10.0) | 1.2–31.7 | 0 (0.0) | .0–16.8 | |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | ||
| Diarrhea | LAV | Any | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | 1 (5.0) | .1–24.9 | 1 (5.0) | .1–24.9 |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | ||
| PIV | Any | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | ||
| Fatigue | LAV | Any | 3 (15.0) | 3.2–37.9 | 8 (40.0) | 19.1–63.9 | 5 (25.0) | 8.7–49.1 | 5 (25.0) | 8.7–49.1 |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 2 (10.0) | 1.2–31.7 | ||
| PIV | Any | 2 (10.0) | 1.2–31.7 | 2 (10.0) | 1.2–31.7 | 3 (15.0) | 3.2–37.9 | 4 (20.0) | 5.7–43.7 | |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | ||
| Fever | LAV | Any | 0 (0.0) | .0–16.8 | 5 (25.0) | 8.7–49.1 | 0 (0.0) | .0–16.8 | 5 (25.0) | 8.7–49.1 |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | ||
| PIV | Any | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | ||
| Headache | LAV | Any | 3 (15.0) | 3.2–37.9 | 8 (40.0) | 19.1–63.9 | 8 (40.0) | 19.1–63.9 | 6 (30.0) | 11.9–54.3 |
| Grade 3 | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | 0 (0.0) | .0–16.8 | 2 (10.0) | 1.2–31.7 | ||
| PIV | Any | 1 (5.0) | .1–24.9 | 3 (15.0) | 3.2–37.9 | 5 (25.0) | 8.7–49.1 | 3 (15.0) | 3.2–37.9 | |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | ||
| Myalgia | LAV | Any | 1 (5.0) | .1–24.9 | 5 (25.0) | 8.7–49.1 | 2 (10.0) | 1.2–31.7 | 6 (30.0) | 11.9–54.3 |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | ||
| PIV | Any | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | 4 (20.0) | 5.7–43.7 | 3 (15.0) | 3.2–37.9 | |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | ||
| Nausea | LAV | Any | 1 (5.0) | .1–24.9 | 1 (5.0) | .1–24.9 | 3 (15.0) | 3.2–37.9 | 1 (5.0) | .1–24.9 |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | 0 (0.0) | .0–16.8 | ||
| PIV | Any | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | 1 (5.0) | .1–24.9 | 1 (5.0) | .1–24.9 | |
| Grade 3 | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | 1 (5.0) | .1–24.9 | 0 (0.0) | .0–16.8 | ||
| Vomiting | LAV | Any | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | 1 (5.0) | .1–24.9 |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | 0 (0.0) | .0–16.8 | ||
| PIV | Any | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | 0 (0.0) | .0–16.8 | |
| Grade 3 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | 0 (0.0) | .0–16.8 | ||
| Overall | LAV | Any | 5 (25.0) | 8.7–49.1 | 10 (50.0) | 27.2–72.8 | 8 (40.0) | 19.1–63.9 | 8 (40.0) | 19.1–63.9 |
| Grade 3 | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | 1 (5.0) | .1–24.9 | 4 (20.0) | 5.7–43.7 | ||
| PIV | Any | 2 (10.0) | 1.2–31.7 | 4 (20.0) | 5.7–43.7 | 5 (25.0) | 8.7–49.1 | 8 (40.0) | 19.1–63.9 | |
| Grade 3 | 0 (0.0) | .0–16.8 | 1 (5.0) | .1–24.9 | 1 (5.0) | .1–24.9 | 0 (0.0) | .0–16.8 |
Abbreviations: CI, confidence interval; LAV, live attenuated vaccine; PIV, purified inactivated vaccine.
RNAemia
Levels of peripheral RNAemia detected per visit and per volunteer by group are displayed in Supplementary Table 1. RNAemia was detected in all groups, with DENV-2 and DENV-4 the most frequently detected. Overall, DENV-2 was detected in 63% of positive samples, DENV-4 in 31% of samples, and DENV-1 in 6%. DENV-3 RNAemia was not detected in any sample at any of the tested time points. Detectable RNAemia occurred most frequently between days 7 and 14 post LAV vaccination, while days 10 and 11 were the 2 days with the highest detection rate. A significantly greater number of volunteers in group 4 had detectable RNAemia compared to other groups (P = .023). However, all volunteers in group 4 had detectable RNAemia at only 1 time point while in group 1, 3 volunteers had detectable RNAemia at 2 time points. Group 2 similarly had 3 volunteers with detectable RNAemia at 2 time points and group 3 had 1 volunteer with detectable RNAemia at 3 time points. An analysis of frequency of detectable RNAemia days by group using a Fisher exact test failed to demonstrate any significant difference (P = .547). Levels of RNAemia were higher for DENV-4 compared to DENV-2 across all groups (Supplementary Figure 2).
Immunogenicity
Humoral Immunogenicity
Seropositivity rates based on neutralizing antibody titers by group and measured at baseline and 28 and 180 days following booster vaccination are presented in Table 3. PIV priming was superior to LAV priming when measured by the percent of the cohort with tetravalent seroconversion following a complete vaccination series. PIV priming followed by LAV boost resulted in 100% tetravalent seroconversion at 28 days post vaccination when LAV was administered either 28 or 180 days later. In contrast, only 35% of LAV recipients developed a tetravalent response when boosted with PIV at 28 days and 75% when boosted at 180 days. The distribution of neutralizing antibody responses across the 4 serotypes was also more balanced in the PIV primed groups versus the LAV primed groups. At 28 days following a LAV-PIV prime-boost regimen, DENV-1–4 seroconversion rates were 71%, 82%, 41%, and 47% in the 0–28 day group and 90%, 90%, 85%, and 75% for the 0–180 day group. In contrast, the PIV-prime LAV-boost regimen resulted in seroconversion rates of 100% for each serotype for both 0–28 and 0–180 dosing regimens. At 180 days following a LAV-PIV prime-boost regimen, DENV-1–4 seroconversion rates were 47%, 77%, 41%, and 29% for the 0–28 day dosing and 60%, 80%, 45%, and 30% for the 0–180 day dosing. When the prime-boost regimen was PIV-LAV the seroconversion rates for DENV-1–4 were 94%, 94%, 88%, and 82% for the 0–28 dosing and 100% for all serotypes for the 0–180 regimen.
Seropositivity Rates by Group at Baseline and 28 and 180 Days Following Booster Vaccination
| Serotype/Time point . | Group 1 (n = 17) . | . | Group 2 (n = 17) . | . | Group 3 (n = 20) . | . | Group 4 (n = 12) . | . |
|---|---|---|---|---|---|---|---|---|
| . | n (%) . | 95% CI . | n (%) . | 95% CI . | n (%) . | 95% CI . | n (%) . | 95% CI . |
| DENV-1 | ||||||||
| Baseline | 0 (0.0) | .0–19.5 | 0 (0.0) | .0–19.5 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–26.5 |
| 28 days | 12 (70.6) | 44.0–89.7 | 17 (100.0) | 80.5–1.0 | 18 (90.0) | 68.3–98.8 | 12 (100.0) | 73.5–1.0 |
| 180 days | 8 (47.1) | 23.0–72.2 | 16 (94.1) | 71.3–99.9 | 12 (60.0) | 36.1–80.9 | 12 (100.0) | 73.5–1.0 |
| DENV-2 | ||||||||
| Baseline | 0 (0.0) | .0–19.5 | 0 (0.0) | .0–19.5 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–26.5 |
| 28 days | 14 (82.4) | 56.6–96.2 | 17 (100.0) | 80.5–1.0 | 18 (90.0) | 68.3–98.8 | 12 (100.0) | 73.5–1.0 |
| 180 days | 13 (76.5) | 50.1–93.2 | 16 (94.1) | 71.3–99.9 | 16 (80.0) | 56.3–94.3 | 12 (100.0) | 73.5–1.0 |
| DENV-3 | ||||||||
| Baseline | 0 (0.0) | .0–19.5 | 0 (0.0) | .0–19.5 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–26.5 |
| 28 days | 7 (41.2) | 18.4–67.1 | 17 (100.0) | 80.5–1.0 | 17 (85.0) | 62.1–96.8 | 12 (100.0) | 73.5–1.0 |
| 180 days | 7 (41.2) | 18.4–67.1 | 15 (88.2) | 63.6–98.5 | 9 (45.0) | 23.1–68.5 | 12 (100.0) | 73.5–1.0 |
| DENV-4 | ||||||||
| Baseline | 0 (0.0) | .0–19.5 | 0 (0.0) | .0–19.5 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–26.5 |
| 28 days | 8 (47.1) | 23.0–72.2 | 17 (100.0) | 80.5–1.0 | 15 (75.0) | 50.9–91.3 | 12 (100.0) | 73.5–1.0 |
| 180 days | 5 (29.4) | 10.3–56.0 | 14 (82.4) | 56.6–96.2 | 6 (30.0) | 11.9–54.3 | 12 (100.0) | 73.5–1.0 |
| Tetravalent seropositivity | ||||||||
| Baseline | 0 (0.0) | .0–19.5 | 0 (0.0) | .0–19.5 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–26.5 |
| 28 days | 6 (35.3) | 14.2–61.7 | 17 (100.0) | 80.5–1.0 | 15 (75.0) | 50.9–91.3 | 12 (100.0) | 73.5–1.0 |
| 180 days | 5 (29.4) | 10.3–56.0 | 12 (70.6) | 44.0–89.7 | 5 (25.0) | 8.7–49.1 | 12 (100.0) | 73.5–1.0 |
| Serotype/Time point . | Group 1 (n = 17) . | . | Group 2 (n = 17) . | . | Group 3 (n = 20) . | . | Group 4 (n = 12) . | . |
|---|---|---|---|---|---|---|---|---|
| . | n (%) . | 95% CI . | n (%) . | 95% CI . | n (%) . | 95% CI . | n (%) . | 95% CI . |
| DENV-1 | ||||||||
| Baseline | 0 (0.0) | .0–19.5 | 0 (0.0) | .0–19.5 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–26.5 |
| 28 days | 12 (70.6) | 44.0–89.7 | 17 (100.0) | 80.5–1.0 | 18 (90.0) | 68.3–98.8 | 12 (100.0) | 73.5–1.0 |
| 180 days | 8 (47.1) | 23.0–72.2 | 16 (94.1) | 71.3–99.9 | 12 (60.0) | 36.1–80.9 | 12 (100.0) | 73.5–1.0 |
| DENV-2 | ||||||||
| Baseline | 0 (0.0) | .0–19.5 | 0 (0.0) | .0–19.5 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–26.5 |
| 28 days | 14 (82.4) | 56.6–96.2 | 17 (100.0) | 80.5–1.0 | 18 (90.0) | 68.3–98.8 | 12 (100.0) | 73.5–1.0 |
| 180 days | 13 (76.5) | 50.1–93.2 | 16 (94.1) | 71.3–99.9 | 16 (80.0) | 56.3–94.3 | 12 (100.0) | 73.5–1.0 |
| DENV-3 | ||||||||
| Baseline | 0 (0.0) | .0–19.5 | 0 (0.0) | .0–19.5 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–26.5 |
| 28 days | 7 (41.2) | 18.4–67.1 | 17 (100.0) | 80.5–1.0 | 17 (85.0) | 62.1–96.8 | 12 (100.0) | 73.5–1.0 |
| 180 days | 7 (41.2) | 18.4–67.1 | 15 (88.2) | 63.6–98.5 | 9 (45.0) | 23.1–68.5 | 12 (100.0) | 73.5–1.0 |
| DENV-4 | ||||||||
| Baseline | 0 (0.0) | .0–19.5 | 0 (0.0) | .0–19.5 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–26.5 |
| 28 days | 8 (47.1) | 23.0–72.2 | 17 (100.0) | 80.5–1.0 | 15 (75.0) | 50.9–91.3 | 12 (100.0) | 73.5–1.0 |
| 180 days | 5 (29.4) | 10.3–56.0 | 14 (82.4) | 56.6–96.2 | 6 (30.0) | 11.9–54.3 | 12 (100.0) | 73.5–1.0 |
| Tetravalent seropositivity | ||||||||
| Baseline | 0 (0.0) | .0–19.5 | 0 (0.0) | .0–19.5 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–26.5 |
| 28 days | 6 (35.3) | 14.2–61.7 | 17 (100.0) | 80.5–1.0 | 15 (75.0) | 50.9–91.3 | 12 (100.0) | 73.5–1.0 |
| 180 days | 5 (29.4) | 10.3–56.0 | 12 (70.6) | 44.0–89.7 | 5 (25.0) | 8.7–49.1 | 12 (100.0) | 73.5–1.0 |
Abbreviations: CI, confidence interval; DENV, dengue virus.
Seropositivity Rates by Group at Baseline and 28 and 180 Days Following Booster Vaccination
| Serotype/Time point . | Group 1 (n = 17) . | . | Group 2 (n = 17) . | . | Group 3 (n = 20) . | . | Group 4 (n = 12) . | . |
|---|---|---|---|---|---|---|---|---|
| . | n (%) . | 95% CI . | n (%) . | 95% CI . | n (%) . | 95% CI . | n (%) . | 95% CI . |
| DENV-1 | ||||||||
| Baseline | 0 (0.0) | .0–19.5 | 0 (0.0) | .0–19.5 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–26.5 |
| 28 days | 12 (70.6) | 44.0–89.7 | 17 (100.0) | 80.5–1.0 | 18 (90.0) | 68.3–98.8 | 12 (100.0) | 73.5–1.0 |
| 180 days | 8 (47.1) | 23.0–72.2 | 16 (94.1) | 71.3–99.9 | 12 (60.0) | 36.1–80.9 | 12 (100.0) | 73.5–1.0 |
| DENV-2 | ||||||||
| Baseline | 0 (0.0) | .0–19.5 | 0 (0.0) | .0–19.5 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–26.5 |
| 28 days | 14 (82.4) | 56.6–96.2 | 17 (100.0) | 80.5–1.0 | 18 (90.0) | 68.3–98.8 | 12 (100.0) | 73.5–1.0 |
| 180 days | 13 (76.5) | 50.1–93.2 | 16 (94.1) | 71.3–99.9 | 16 (80.0) | 56.3–94.3 | 12 (100.0) | 73.5–1.0 |
| DENV-3 | ||||||||
| Baseline | 0 (0.0) | .0–19.5 | 0 (0.0) | .0–19.5 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–26.5 |
| 28 days | 7 (41.2) | 18.4–67.1 | 17 (100.0) | 80.5–1.0 | 17 (85.0) | 62.1–96.8 | 12 (100.0) | 73.5–1.0 |
| 180 days | 7 (41.2) | 18.4–67.1 | 15 (88.2) | 63.6–98.5 | 9 (45.0) | 23.1–68.5 | 12 (100.0) | 73.5–1.0 |
| DENV-4 | ||||||||
| Baseline | 0 (0.0) | .0–19.5 | 0 (0.0) | .0–19.5 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–26.5 |
| 28 days | 8 (47.1) | 23.0–72.2 | 17 (100.0) | 80.5–1.0 | 15 (75.0) | 50.9–91.3 | 12 (100.0) | 73.5–1.0 |
| 180 days | 5 (29.4) | 10.3–56.0 | 14 (82.4) | 56.6–96.2 | 6 (30.0) | 11.9–54.3 | 12 (100.0) | 73.5–1.0 |
| Tetravalent seropositivity | ||||||||
| Baseline | 0 (0.0) | .0–19.5 | 0 (0.0) | .0–19.5 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–26.5 |
| 28 days | 6 (35.3) | 14.2–61.7 | 17 (100.0) | 80.5–1.0 | 15 (75.0) | 50.9–91.3 | 12 (100.0) | 73.5–1.0 |
| 180 days | 5 (29.4) | 10.3–56.0 | 12 (70.6) | 44.0–89.7 | 5 (25.0) | 8.7–49.1 | 12 (100.0) | 73.5–1.0 |
| Serotype/Time point . | Group 1 (n = 17) . | . | Group 2 (n = 17) . | . | Group 3 (n = 20) . | . | Group 4 (n = 12) . | . |
|---|---|---|---|---|---|---|---|---|
| . | n (%) . | 95% CI . | n (%) . | 95% CI . | n (%) . | 95% CI . | n (%) . | 95% CI . |
| DENV-1 | ||||||||
| Baseline | 0 (0.0) | .0–19.5 | 0 (0.0) | .0–19.5 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–26.5 |
| 28 days | 12 (70.6) | 44.0–89.7 | 17 (100.0) | 80.5–1.0 | 18 (90.0) | 68.3–98.8 | 12 (100.0) | 73.5–1.0 |
| 180 days | 8 (47.1) | 23.0–72.2 | 16 (94.1) | 71.3–99.9 | 12 (60.0) | 36.1–80.9 | 12 (100.0) | 73.5–1.0 |
| DENV-2 | ||||||||
| Baseline | 0 (0.0) | .0–19.5 | 0 (0.0) | .0–19.5 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–26.5 |
| 28 days | 14 (82.4) | 56.6–96.2 | 17 (100.0) | 80.5–1.0 | 18 (90.0) | 68.3–98.8 | 12 (100.0) | 73.5–1.0 |
| 180 days | 13 (76.5) | 50.1–93.2 | 16 (94.1) | 71.3–99.9 | 16 (80.0) | 56.3–94.3 | 12 (100.0) | 73.5–1.0 |
| DENV-3 | ||||||||
| Baseline | 0 (0.0) | .0–19.5 | 0 (0.0) | .0–19.5 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–26.5 |
| 28 days | 7 (41.2) | 18.4–67.1 | 17 (100.0) | 80.5–1.0 | 17 (85.0) | 62.1–96.8 | 12 (100.0) | 73.5–1.0 |
| 180 days | 7 (41.2) | 18.4–67.1 | 15 (88.2) | 63.6–98.5 | 9 (45.0) | 23.1–68.5 | 12 (100.0) | 73.5–1.0 |
| DENV-4 | ||||||||
| Baseline | 0 (0.0) | .0–19.5 | 0 (0.0) | .0–19.5 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–26.5 |
| 28 days | 8 (47.1) | 23.0–72.2 | 17 (100.0) | 80.5–1.0 | 15 (75.0) | 50.9–91.3 | 12 (100.0) | 73.5–1.0 |
| 180 days | 5 (29.4) | 10.3–56.0 | 14 (82.4) | 56.6–96.2 | 6 (30.0) | 11.9–54.3 | 12 (100.0) | 73.5–1.0 |
| Tetravalent seropositivity | ||||||||
| Baseline | 0 (0.0) | .0–19.5 | 0 (0.0) | .0–19.5 | 0 (0.0) | .0–16.8 | 0 (0.0) | .0–26.5 |
| 28 days | 6 (35.3) | 14.2–61.7 | 17 (100.0) | 80.5–1.0 | 15 (75.0) | 50.9–91.3 | 12 (100.0) | 73.5–1.0 |
| 180 days | 5 (29.4) | 10.3–56.0 | 12 (70.6) | 44.0–89.7 | 5 (25.0) | 8.7–49.1 | 12 (100.0) | 73.5–1.0 |
Abbreviations: CI, confidence interval; DENV, dengue virus.
GMTs were also higher in PIV primed groups (Figure 2). When measured 28 days following the booster dose, LAV-PIV regimens had GMTs across the DENV-1–4 serotypes of 37, 280, 22, and 25 for the 0–28 dosing regimen and 107, 308, 89, and 48 for the 0–180 regimen. The PIV-LAV regimens had DENV-1–4 GMTs of 915, 2370, 772, and 1052 for the 0–28 dosing regimen and 3459, 4472, 4043, and 4141 for the 0–180 dosing regimen (Supplementary Table 2). These data indicated the PIV-prime LAV-boost regimen induced higher GMTs overall, and that this was more pronounced in the group that received a later boost (0–180) as compared to an earlier boost (0–28) as measured 180 days following the final vaccination.
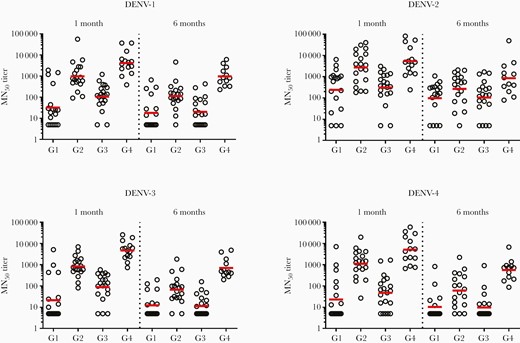
Neutralizing antibody titers by group, serotype, and measurement at 28 and 180 days following booster vaccination. Abbreviations: DENV, dengue virus; G1, group 1; G2, group 2; G3, group 3; G4, group 4; MN50, 50% microneutralization end point.
Cell-Mediated Immunogenicity
Response rates to vaccination as assessed using an IFN-γ ELISpot assay for each serotype are shown in Supplementary Table 3. Only DENV-2–derived peptides elicited response rates that were statistically greater than prevaccination for all groups at both 28 days and 180 days postvaccination. In all groups DENV-2–derived peptides were the most frequently recognized antigens at both time points post vaccination. There were 9 subjects (3 from group 1; 1 from group 2; 4 from group 3; and 1 from group 4) who demonstrated no ELISpot response post vaccination. However, there was no diminution of the response rates from days 28 to 180 post vaccination among responders. The valency of the response post vaccination (as determined by overlapping peptide pools representing the C/prM, E, NS1, NS3, and NS5 protein regions of each DENV type) was predominantly multivalent with combined tri- and tetravalent response rates ranging from 20% to 75% (Figure 3). There was no diminution of response valency from day 28 to day 180 post vaccination, with group 4 displaying the greatest breadth of response (75% tetravalent) at 180 days post vaccination. Response magnitudes for positive responder subjects are shown in Figure 4 and Supplementary Table 4. Responses were robust and frequently exceeded 1000 SFC/106 PBMC. For all peptide sets and all groups there was a significant increase in ELISpot response magnitude at both postvaccination time points compared to prevaccination.
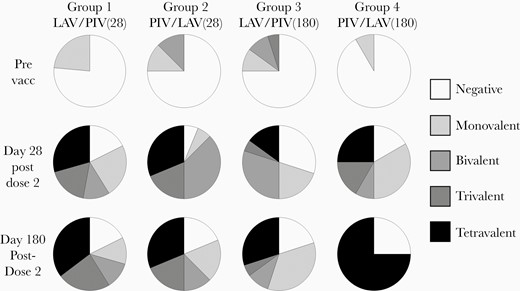
ELISpot response valency. Pie chart segments represent the fraction of subjects responding to at least 1 peptide pool from 0, 1, 2, 3, or 4 DENV serotypes. Abbreviations: ELISpot, enzyme-linked immunospot; DENV, dengue virus; LAV, live attenuated vaccine; PIV, purified inactivated vaccine; Prevacc, prevaccination.
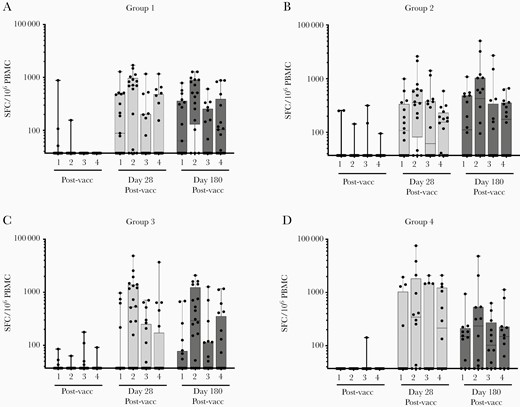
Total ELISpot response frequencies. Each serotype (1 through 4) is designated for each time point tested. Total frequencies were calculated by summing the SFC/106 PBMC value for each of the 5 peptide pools matching a given serotype (C/preM, E, NS1, NS3, and NS5). Each dot represents the response frequency of a single subject to a given serotype. Abbreviations: ELISpot, enzyme-linked immunospot; PBMC, peripheral blood mononuclear cells; Postvacc, postvaccination; Prevacc, prevaccination; SFC, spot-forming cells.
Discussion
A heterologous prime-boost vaccination strategy combining live and inactivated vaccine products has the potential to generate durable humoral and cellular immune responses to each of the 4 dengue serotypes. Our results demonstrated that priming with TDEN-PIV and boosting with TDEN-LAV resulted in higher tetravalent seroconversion rates and higher neutralizing antibody titers than priming with TDEN-LAV and boosting with TDEN-PIV. Neutralizing antibody titers and seroconversion rates for the PIV followed by LAV prime-boost regimen exceeded those achieved following 2 doses of LAV or 2 doses of PIV with alum administration in previous studies [15, 19]. Two doses of PIV with ASO1E or ASO3B adjuvants significantly improved humoral responses compared to alum-adsorbed PIV; however, all 2-dose PIV regimens resulted in rapidly waning neutralizing antibody titers by 6 months post dose 2 [19].
In our study, PIV followed by LAV at 1 month generated significantly higher titers compared to the best performing PIV adjuvanted 2-dose homologous prime-boost vaccination. Spacing the LAV booster dose to 6 months following PIV priming further increased the humoral response in magnitude. Future trials will address whether a schedule in between 1 and 6 months will induce a comparable antibody profile while minimizing the time between doses. Additionally, human challenge studies are planned to address the potential for protective efficacy.
IFN-γ–mediated T-cell responses induced by the 4 different vaccination regimens tested in this study were variable overall, but comparable among the 4 groups. Each group contained some very high responders (>1000 SFC/106 PBMC) for all serotypes and some nonresponders (<38 SFC/106 PBMC) against any serotype. DENV-2 was the most frequently recognized serotype and induced the highest magnitude IFN-γ response in all groups at both time points. This observation is consistent with the RNAemia data and the notion that T-cell immunity is driven by actively replicating vaccine virus. Importantly, T-cell responses did not wane significantly in frequency or magnitude up to 180 days post vaccination. Of the 4 groups, group 4 in particular induced T-cell responses that best met 3 important qualities of a DENV vaccine: balanced, durable, and tetravalent.
Overall, the heterologous prime-boost strategy as utilized in this study resulted in an acceptable safety profile. In general, it was noted that there were higher rates of multiple solicited systemic AEs in the PIV primed groups along with a notably higher rate of grade 3 solicited systemic AEs in group 4. It was also noted that unprimed LAV recipients had no recorded fevers, in contrast to PIV-primed LAV recipients. This observation stands in contrast to previous studies with this same TDEN-LAV formulation, wherein recorded fever rates of 9.1%–18.2% were observed [15]. Overall, given the small numbers available from this phase 1 study, confidence intervals between the observed rates of these AEs overlap significantly suggesting that further study is warranted to confirm whether or not reactogenicity is, in fact, increased by PIV priming or that this differential rate was observed by chance alone.
The mechanisms underlying the improvement in antibody titers along with the trend towards greater reactogenicity observed in the PIV/LAV groups are not yet fully characterized by this study. The antibody titers generated by 1 dose of PIV alone were overall very low or undetectable and so instead of neutralizing vaccine strains from the LAV, it is possible that these low levels of antibody actually enhanced vaccine strain replication through an antibody-dependent enhancement mechanism. Further studies to evaluate the immune mechanisms driving the differential responses observed in this study are warranted and currently ongoing.
This study had a number of limitations. The open-label design may have impacted the unbiased assessment of AEs. The vaccines have distinct appearances and are administered differently (intramuscular vs subcutaneous), making a double-blinded study design difficult. There was also an unexpectedly high number of subject withdrawals, particularly in group 4, which were unrelated to study events. Lastly, sampling of RNAemia at a limited number of time points on an alternating day schedule may have resulted in missed detection for some subjects with viremia on 1 day only.
In summary, this first-in-human dengue heterologous prime-boost trial had an acceptable safety profile. This trial established the superior humoral immunogenicity of priming with an inactivated vaccine candidate followed by an attenuated live virus vaccine. This trial supports continued testing of a heterologous prime-boost strategy against dengue.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We acknowledge the staff of the Walter Reed Army Institute of Research (WRAIR) Clinical Trial Center, the WRAIR Quality Management Unit, and specimen processing teams, along with all of the study volunteers who made this study possible. The study was sponsored by the Surgeon General, Department of the Army. The US Army Medical Materiel Development Activity, as the Office of the Surgeon General’s sponsor representative, monitored and reported on volunteer safety.
Disclaimer. Material has been reviewed by the WRAIR. There is no objection to its presentation and/or publication. The opinions or assertions contained herein are the private views of the authors, and are not to be construed as official, or as reflecting true views of the Department of the Army or the Department of Defense. All authors have reviewed the manuscript and vouch for its accuracy and completeness.
Financial support. This work was supported by the Military Infectious Diseases Research Program.
Potential conflicts of interest. S. J. T. has been compensated as an advisory board member for Sanofi-Pasteur, Takeda, Merck, PrimeVax, Chugai, Tremau, and Janssen; and is a patent holder on combination flavivirus vaccine strategy patent number US10086061B2. K. H. E. is a patent holder on the tetravalent live attenuated dengue vaccine and tetravalent purified inactivated vaccine products utilized in this study. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.




