-
PDF
- Split View
-
Views
-
Cite
Cite
Rong Fan, Jie Peng, Qing Xie, Deming Tan, Min Xu, Junqi Niu, Hao Wang, Hong Ren, Xinyue Chen, Maorong Wang, Jifang Sheng, Hong Tang, Xuefan Bai, Yaobo Wu, Bin Zhou, Jian Sun, Jinlin Hou, for the Chronic Hepatitis B Study Consortium , Combining Hepatitis B Virus RNA and Hepatitis B Core–Related Antigen: Guidance for Safely Stopping Nucleos(t)ide Analogues in Hepatitis B e Antigen–Positive Patients With Chronic Hepatitis B, The Journal of Infectious Diseases, Volume 222, Issue 4, 15 August 2020, Pages 611–618, https://doi.org/10.1093/infdis/jiaa136
Close - Share Icon Share
Abstract
Safe nucleos(t)ide analogue discontinuation in chronic hepatitis B (CHB) is an unmet need. We aimed to investigate whether combining hepatitis B virus (HBV) RNA and hepatitis B core–related antigen (HBcrAg) could perform satisfactorily in predicting off-treatment outcomes.
The evaluation cohort included 127 hepatitis B e antigen (HBeAg)–positive patients from a multicenter prospective trial who stopped telbivudine-based therapy after achieving HBeAg seroconversion and HBV DNA < 50 IU/mL for > 48 weeks. As validation, 59 patients treated with entecavir or tenofovir before discontinuation were analyzed.
At the end of treatment (EOT), HBV RNA and HBcrAg were significant independent predictors of the clinical relapse risk. In the evaluation cohort, no clinical relapse occurred among patients with negative HBV RNA and HBcrAg < 4 log10 U/mL at EOT (low-risk group), whereas 46.8% patients with positive HBV RNA and HBcrAg ≥ 4 log10 U/mL (high-risk group) experienced clinical relapse during 4-year posttreatment follow-up (P < .001); the corresponding incidences in the validation cohort were 0% and 69.4% (P < .001), respectively. More patients in the low-risk group achieved HBsAg loss than the other patients after treatment cessation (16.1% vs 1.3%, P = .002).
Combining HBV RNA and HBcrAg performed satisfactorily in predicting clinical relapse and HBsAg loss after treatment cessation in HBeAg-positive patients with CHB.
The combination of hepatitis B virus RNA and hepatitis B core–related antigen performed satisfactorily in predicting clinical relapse and hepatitis B surface antigen loss after stopping nucleos(t)ide analogue treatment among noncirrhotic hepatitis B e antigen–positive patients with chronic hepatitis B and could be used to guide safe discontinuation.
Long-term nucleos(t)ide analogue (NA) treatment can suppress hepatitis B virus (HBV) replication, reverse liver fibrosis, and reduce the risk of hepatocellular carcinoma (HCC) [1–3]. Because NAs are viral polymerase inhibitors and inhibit only viral replication, they could not achieve the complete eradication of stably existing covalently closed circular DNA (cccDNA). Therefore, lifelong NA therapy is theoretically necessary but causes a substantial burden on the medical system as well as safety concerns for women of childbearing age.
HBV RNA is considered a potential biomarker for cccDNA activity. We and others have demonstrated that HBV RNA levels at the end of treatment (EOT) could predict off-treatment relapse [4–6]. With these findings, we further determined that negative results for both HBV DNA and RNA were strongly associated with off-treatment durability [6].
Hepatitis B core–related antigen (HBcrAg), another potentially useful biomarker for predicting off-treatment relapse, includes the hepatitis B core antigen (HBcAg), hepatitis B e antigen (HBeAg), and a truncated precore protein (p22Cr). HBcrAg is also considered to be correlated with cccDNA activity. Previous studies demonstrated that HBcrAg levels could predict HBeAg seroconversion, sustained response to NA, the risk of HCC development, and postoperative HCC recurrence [7]. However, whether combining HBV RNA and HBcrAg could perform better in predicting off-treatment outcomes is still not clear.
In the present study, we aimed to evaluate the performance of the combination of HBV RNA and HBcrAg in predicting off-treatment relapse and HBsAg loss in a prospective multicenter cohort, with validation in another independent prospective cohort.
METHODS
Patients
Evaluation Cohort
The patients in the evaluation cohort were enrolled from a series of trials lasting 7 years, consisting of a 2-year randomized controlled trial (EFFicacy Optimization of Response to Telbivudine [EFFORT] 1 study, NCT00962533), its 3-year extension trial (EFFORT 2 study, NCT01529255), and its 2-year further extension trial (EFFORT 3 study, NCT02826070). The main findings and detailed eligibility criteria of the initial trial have been reported previously [8]. In brief, 599 adult patients with HBV DNA ≥ 20 000 IU/mL, alanine aminotransferase (ALT) 2–10 times the upper limit of normal (ULN), and compensated naive HBeAg-positive CHB received telbivudine-based antiviral treatment for up to 7 years. Since the beginning of year 3, patients who met the stopping criteria [9], defined as achieving HBeAg seroconversion and HBV DNA < 50 IU/mL for at least 48 weeks, were advised to stop treatment and were monitored for the occurrence of relapse (Figure 1). Once clinical relapse occurred, antiviral treatment was restarted.
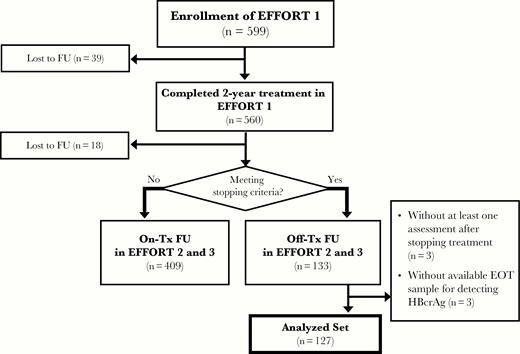
Flowchart of patients included in the evaluation cohort. The stopping criteria were defined as hepatitis B e antigen seroconversion and hepatitis B virus DNA < 50 IU/mL for at least 48 weeks. Abbreviations: EFFORT, EFFicacy Optimization of Response to Telbivudine; EOT, end of treatment; FU, follow-up; HBcrAg, hepatitis B core–related antigen; Tx, treatment.
Validation Cohort
The patients in the validation cohort were enrolled from another prospective trial conducted in Nanfang Hospital (Guangzhou, China) [10]. Patients discontinued entecavir or tenofovir therapy following the similar stopping criteria described above.
Patients in the 2 cohorts who stopped treatment per the protocol with at least 1 off-treatment assessment and available EOT serum samples were included in the analysis. The primary off-treatment outcome was clinical relapse, defined as HBV DNA > 2000 IU/mL plus ALT > 2 × ULN. The secondary outcomes included virologic relapse and HBeAg reversion, defined as HBV DNA > 2000 IU/mL and the reappearance of HBeAg at 2 consecutive protocol-defined visits, respectively.
Clinical and Laboratory Evaluation
Clinical and laboratory assessments were performed prospectively every 12 or 16 weeks during off-treatment follow-up. HBV DNA and serological markers were measured with the Roche COBAS TaqMan HBV Test and ARCHITECT I2000SR, respectively, in the central laboratory located in Nanfang Hospital, as established by the research group. Serum ALT levels were assessed at local laboratories according to standard procedures.
Serum HBV RNA Evaluation
HBV RNA was isolated from 200 µL of serum using the QIAamp MinElute Virus Spin Kit (Qiagen, Hilden, Germany), according to the manufacturer’s protocol and subsequently treated with DNase I (Thermo Fisher Scientific) to remove any DNA present. DNase I–digested HBV RNA was quantified by 1-step reverse transcription real-time quantitative polymerase chain reaction (RT-qPCR) in a LightCycler 480 Instrument II system (Roche) with the TaqMan probe method. The pair of primers was designed to determine the HBV RNA levels in the present study (S-F: 5′-GCG GGG TTT TTC TTG AC-3′ and S-R: 3’-GCG ATA ACC AGG ACA AAT TG-5′). In addition, to ensure that no HBV DNA was measured, qPCR was performed in parallel on DNase I–treated RNA samples without a reverse transcription step (“no-RT controls”). Negative HBV RNA was defined as targets not detected by RT-qPCR. For more information on the HBV RNA assay, see the Supplementary Materials and Supplementary Figure 1.
Serum HBcrAg Measurements
The HBcrAg levels at EOT were measured using the Lumipulse G HBcrAg assay (Fujirebio Europe) in serum samples stored at –80°C on a LUMIPULSE G1200 analyzer (Fujirebio) following the manufacturer’s instructions, using a lower limit of quantification of 3 log10 U/mL.
Statistical Analysis
The data are expressed as counts and percentages for categorical variables and as the median and interquartile range (IQR) for continuous variables. Qualitative and quantitative differences between subgroups were analyzed using the χ 2 test or Fisher exact test for categorical parameters and Student t test or the Mann–Whitney test for continuous parameters, as appropriate. The cumulative rate of off-treatment relapse was calculated using the Kaplan–Meier method, with the log-rank test for comparisons. Cox proportional hazards regression analysis was used to determine predictors of off-treatment relapse. Statistical analysis was performed using IBM SPSS software version 20.0. A P value < .05, based on a 2-tailed test, was considered statistically significant.
The study was conducted in accordance with the guidelines of the Declaration of Helsinki and the principles of good clinical practice. All the patients provided written informed consent to have their data used (anonymously) for research purposes.
RESULTS
Clinical Characteristics
A total of 127 and 59 patients in the evaluation and validation cohorts, respectively, were included in the analysis. Table 1 shows the clinical characteristics of these patients at EOT. In the evaluation and validation cohorts, the proportions of patients with negative HBV RNA were 31.5% (40/127) and 35.6% (21/59), respectively, and the median HBcrAg levels were 4.3 (IQR, 4.0–4.7) and 3.9 (IQR, 3.3–4.5) log10 U/mL. The 4-year cumulative rates of clinical relapse, virologic relapse, and HBeAg reversion in the evaluation cohort were 31.5%, 55.8%, and 17.2%, respectively, and the corresponding rates in the validation cohort were 35.7%, 73.9%, and 16.2%.
| Characteristics . | Evaluation Cohort(n = 127) . | Validation Cohort (n = 59) . |
|---|---|---|
| Age, y | 30 (25–35) | 36 (30–43) |
| Male sex | 92 (72.4) | 46 (78.0) |
| HBV genotype | ||
| B | 55 (43.3) | NA |
| C | 72 (56.7) | NA |
| HBsAg level at EOT, log10 IU/mL | 3.3 (2.9–3.7) | 2.6 (1.4–3.0) |
| < 2 log10 IU/mL | 11 (8.7) | 22 (37.3) |
| HBcrAg level at EOT, log10 U/mL | 4.3 (4.0–4.7) | 3.9 (3.3–4.5) |
| < 4 log10 U/mL | 30 (23.6) | 31 (52.5) |
| HBV RNA level at EOT, log10 copies/mL | 3.0 (0–3.2) | 3.8 (0–4.5) |
| Negative HBV RNA | 40 (31.5) | 21 (35.6) |
| Negative HBV DNA at EOTa | 62 (48.8) | 43 (72.9) |
| Treatment duration, y | 2.9 (2.1–3.9) | 4.5 (3.2–6.0) |
| Consolidation treatment durationb, y | 1.5 (1.2–2.1) | 2.4 (1.3–3.5) |
| Characteristics . | Evaluation Cohort(n = 127) . | Validation Cohort (n = 59) . |
|---|---|---|
| Age, y | 30 (25–35) | 36 (30–43) |
| Male sex | 92 (72.4) | 46 (78.0) |
| HBV genotype | ||
| B | 55 (43.3) | NA |
| C | 72 (56.7) | NA |
| HBsAg level at EOT, log10 IU/mL | 3.3 (2.9–3.7) | 2.6 (1.4–3.0) |
| < 2 log10 IU/mL | 11 (8.7) | 22 (37.3) |
| HBcrAg level at EOT, log10 U/mL | 4.3 (4.0–4.7) | 3.9 (3.3–4.5) |
| < 4 log10 U/mL | 30 (23.6) | 31 (52.5) |
| HBV RNA level at EOT, log10 copies/mL | 3.0 (0–3.2) | 3.8 (0–4.5) |
| Negative HBV RNA | 40 (31.5) | 21 (35.6) |
| Negative HBV DNA at EOTa | 62 (48.8) | 43 (72.9) |
| Treatment duration, y | 2.9 (2.1–3.9) | 4.5 (3.2–6.0) |
| Consolidation treatment durationb, y | 1.5 (1.2–2.1) | 2.4 (1.3–3.5) |
Categorical variables are expressed as counts (percentage). Continuous variables are expressed as median values (interquartile range).
Abbreviations: ALT, alanine aminotransferase; EOT, end of treatment; HBsAg, hepatitis B surface antigen; HBcrAg, hepatitis B core–related antigen; HBV, hepatitis B virus; NA, not available; ULN, upper limit of normal.
aNegative HBV DNA was defined as HBV DNA “target not detected.”
bConsolidation treatment duration was defined as the treatment duration after achieving HBV DNA < 50 IU/mL and hepatitis B e antigen seroconversion.
| Characteristics . | Evaluation Cohort(n = 127) . | Validation Cohort (n = 59) . |
|---|---|---|
| Age, y | 30 (25–35) | 36 (30–43) |
| Male sex | 92 (72.4) | 46 (78.0) |
| HBV genotype | ||
| B | 55 (43.3) | NA |
| C | 72 (56.7) | NA |
| HBsAg level at EOT, log10 IU/mL | 3.3 (2.9–3.7) | 2.6 (1.4–3.0) |
| < 2 log10 IU/mL | 11 (8.7) | 22 (37.3) |
| HBcrAg level at EOT, log10 U/mL | 4.3 (4.0–4.7) | 3.9 (3.3–4.5) |
| < 4 log10 U/mL | 30 (23.6) | 31 (52.5) |
| HBV RNA level at EOT, log10 copies/mL | 3.0 (0–3.2) | 3.8 (0–4.5) |
| Negative HBV RNA | 40 (31.5) | 21 (35.6) |
| Negative HBV DNA at EOTa | 62 (48.8) | 43 (72.9) |
| Treatment duration, y | 2.9 (2.1–3.9) | 4.5 (3.2–6.0) |
| Consolidation treatment durationb, y | 1.5 (1.2–2.1) | 2.4 (1.3–3.5) |
| Characteristics . | Evaluation Cohort(n = 127) . | Validation Cohort (n = 59) . |
|---|---|---|
| Age, y | 30 (25–35) | 36 (30–43) |
| Male sex | 92 (72.4) | 46 (78.0) |
| HBV genotype | ||
| B | 55 (43.3) | NA |
| C | 72 (56.7) | NA |
| HBsAg level at EOT, log10 IU/mL | 3.3 (2.9–3.7) | 2.6 (1.4–3.0) |
| < 2 log10 IU/mL | 11 (8.7) | 22 (37.3) |
| HBcrAg level at EOT, log10 U/mL | 4.3 (4.0–4.7) | 3.9 (3.3–4.5) |
| < 4 log10 U/mL | 30 (23.6) | 31 (52.5) |
| HBV RNA level at EOT, log10 copies/mL | 3.0 (0–3.2) | 3.8 (0–4.5) |
| Negative HBV RNA | 40 (31.5) | 21 (35.6) |
| Negative HBV DNA at EOTa | 62 (48.8) | 43 (72.9) |
| Treatment duration, y | 2.9 (2.1–3.9) | 4.5 (3.2–6.0) |
| Consolidation treatment durationb, y | 1.5 (1.2–2.1) | 2.4 (1.3–3.5) |
Categorical variables are expressed as counts (percentage). Continuous variables are expressed as median values (interquartile range).
Abbreviations: ALT, alanine aminotransferase; EOT, end of treatment; HBsAg, hepatitis B surface antigen; HBcrAg, hepatitis B core–related antigen; HBV, hepatitis B virus; NA, not available; ULN, upper limit of normal.
aNegative HBV DNA was defined as HBV DNA “target not detected.”
bConsolidation treatment duration was defined as the treatment duration after achieving HBV DNA < 50 IU/mL and hepatitis B e antigen seroconversion.
Performance of HBV RNA and HBcrAg in Predicting Clinical Relapse in the Evaluation Cohort
At EOT, patients with negative HBV RNA had significantly lower levels of HBcrAg than those with positive HBV RNA (4.1 vs 4.5 log10 U/mL, P < .001). These patients also had a significantly lower risk of clinical relapse during the 4-year off-treatment follow-up (12.9% vs 40.1%, P = .004; Figure 2A). The Youden index (rounded to the nearest integer), that is 4 log10 U/mL, was used as the optimal cutoff value of EOT HBcrAg for predicting clinical relapse. The cumulative incidence of clinical relapse at year 4 was significantly lower in patients with lower HBcrAg levels (< 4 log10 U/mL) than in those with higher HBcrAg levels (≥ 4 log10 U/mL) (7.3% vs 39.5%, P < .001; Figure 2B). Patients with either negative HBV RNA or lower HBcrAg levels also had lower incidences of virological relapse and HBeAg reversion, respectively (Supplementary Figures 2 and 3).

Cumulative incidences of clinical relapse stratified by hepatitis B virus RNA (A) and hepatitis B core–related antigen (B) status at the end of treatment in the evaluation cohort. Abbreviations: EOT, end of treatment; HBcrAg, hepatitis B core–related antigen; HBV, hepatitis B virus.
Predictors for Clinical Relapse in the Evaluation Cohort
To further evaluate variables at EOT in predicting clinical relapse, a Cox regression analysis was conducted. The univariable regression analysis showed that sex (male vs female), HBV DNA (positive vs negative), HBV RNA (positive vs negative), and HBcrAg level (higher vs lower) were significantly associated with the occurrence of clinical relapse (Table 2). In the multivariable analysis (with stepwise selection), the following 2 variables were found to be independently significant in predicting the risk of clinical relapse: HBV RNA (hazard ratio [HR], 3.580; 95% confidence interval [CI], 1.264–10.135; P = .017) and HBcrAg (HR, 5.696; 95% CI, 1.371–23.670; P = .017). In addition, HBV RNA and HBcrAg were also independent predictors for virologic relapse and HBeAg reversion (Supplementary Tables 2 and 3).
Association Between Clinical Characteristics and Clinical Relapse in the Evaluation Cohort
| Factors . | Univariable analysis . | . | . | Multivariable analysis . | . | . |
|---|---|---|---|---|---|---|
| . | HR . | (95% CI) . | P Value . | HR . | (95% CI) . | P Value . |
| Age (≥ 30 vs < 30 y) | 1.514 | (.767–2.988) | .234 | … | … | |
| Sex (male vs female) | 2.988 | (1.058–8.437) | .040 | … | … | |
| Genotype (B vs C) | 1.115 | (.570–2.180) | .752 | … | … | |
| Treatment duration (< 3 vs ≥ 3 y) | 1.196 | (.606–2.363) | .607 | … | … | |
| Consolidation duration (< 2 vs ≥ 2 y) | 1.201 | (.545–2.647) | .651 | … | … | |
| EOT HBsAg level (≥ 2 vs < 2 log10 IU/mL) | 1.461 | (.353–6.052) | .603 | … | … | |
| EOT HBV DNA (positive vs negative) | 2.642 | (1.267–5.510) | .010 | … | … | |
| EOT HBV RNA (positive vs negative) | 4.116 | (1.457–11.630) | .008 | 3.580 | (1.264–10.135) | .017 |
| EOT HBcrAg level (≥ 4 vs < 4 log10 U/mL) | 6.548 | (1.580–27.142) | .010 | 5.696 | (1.371–23.670) | .017 |
| Factors . | Univariable analysis . | . | . | Multivariable analysis . | . | . |
|---|---|---|---|---|---|---|
| . | HR . | (95% CI) . | P Value . | HR . | (95% CI) . | P Value . |
| Age (≥ 30 vs < 30 y) | 1.514 | (.767–2.988) | .234 | … | … | |
| Sex (male vs female) | 2.988 | (1.058–8.437) | .040 | … | … | |
| Genotype (B vs C) | 1.115 | (.570–2.180) | .752 | … | … | |
| Treatment duration (< 3 vs ≥ 3 y) | 1.196 | (.606–2.363) | .607 | … | … | |
| Consolidation duration (< 2 vs ≥ 2 y) | 1.201 | (.545–2.647) | .651 | … | … | |
| EOT HBsAg level (≥ 2 vs < 2 log10 IU/mL) | 1.461 | (.353–6.052) | .603 | … | … | |
| EOT HBV DNA (positive vs negative) | 2.642 | (1.267–5.510) | .010 | … | … | |
| EOT HBV RNA (positive vs negative) | 4.116 | (1.457–11.630) | .008 | 3.580 | (1.264–10.135) | .017 |
| EOT HBcrAg level (≥ 4 vs < 4 log10 U/mL) | 6.548 | (1.580–27.142) | .010 | 5.696 | (1.371–23.670) | .017 |
Abbreviations: CI, confidence interval; EOT, end of treatment; HBcrAg, hepatitis B core–related antigen; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; HR, hazard ratio.
Association Between Clinical Characteristics and Clinical Relapse in the Evaluation Cohort
| Factors . | Univariable analysis . | . | . | Multivariable analysis . | . | . |
|---|---|---|---|---|---|---|
| . | HR . | (95% CI) . | P Value . | HR . | (95% CI) . | P Value . |
| Age (≥ 30 vs < 30 y) | 1.514 | (.767–2.988) | .234 | … | … | |
| Sex (male vs female) | 2.988 | (1.058–8.437) | .040 | … | … | |
| Genotype (B vs C) | 1.115 | (.570–2.180) | .752 | … | … | |
| Treatment duration (< 3 vs ≥ 3 y) | 1.196 | (.606–2.363) | .607 | … | … | |
| Consolidation duration (< 2 vs ≥ 2 y) | 1.201 | (.545–2.647) | .651 | … | … | |
| EOT HBsAg level (≥ 2 vs < 2 log10 IU/mL) | 1.461 | (.353–6.052) | .603 | … | … | |
| EOT HBV DNA (positive vs negative) | 2.642 | (1.267–5.510) | .010 | … | … | |
| EOT HBV RNA (positive vs negative) | 4.116 | (1.457–11.630) | .008 | 3.580 | (1.264–10.135) | .017 |
| EOT HBcrAg level (≥ 4 vs < 4 log10 U/mL) | 6.548 | (1.580–27.142) | .010 | 5.696 | (1.371–23.670) | .017 |
| Factors . | Univariable analysis . | . | . | Multivariable analysis . | . | . |
|---|---|---|---|---|---|---|
| . | HR . | (95% CI) . | P Value . | HR . | (95% CI) . | P Value . |
| Age (≥ 30 vs < 30 y) | 1.514 | (.767–2.988) | .234 | … | … | |
| Sex (male vs female) | 2.988 | (1.058–8.437) | .040 | … | … | |
| Genotype (B vs C) | 1.115 | (.570–2.180) | .752 | … | … | |
| Treatment duration (< 3 vs ≥ 3 y) | 1.196 | (.606–2.363) | .607 | … | … | |
| Consolidation duration (< 2 vs ≥ 2 y) | 1.201 | (.545–2.647) | .651 | … | … | |
| EOT HBsAg level (≥ 2 vs < 2 log10 IU/mL) | 1.461 | (.353–6.052) | .603 | … | … | |
| EOT HBV DNA (positive vs negative) | 2.642 | (1.267–5.510) | .010 | … | … | |
| EOT HBV RNA (positive vs negative) | 4.116 | (1.457–11.630) | .008 | 3.580 | (1.264–10.135) | .017 |
| EOT HBcrAg level (≥ 4 vs < 4 log10 U/mL) | 6.548 | (1.580–27.142) | .010 | 5.696 | (1.371–23.670) | .017 |
Abbreviations: CI, confidence interval; EOT, end of treatment; HBcrAg, hepatitis B core–related antigen; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; HR, hazard ratio.
Combining HBV RNA and HBcrAg in Predicting Clinical Relapse
To further evaluate the performance of HBV RNA, HBcrAg, and their combination in predicting clinical relapse, the area under the receiver operating characteristic (AUROC) values of each parameter were calculated. The results showed that the AUROC value of the combination of HBV RNA and HBcrAg was 0.696, which was higher than either parameter alone (HBV RNA: 0.635, P = .058; HBcrAg: 0.621, P = .007; Figure 3A).
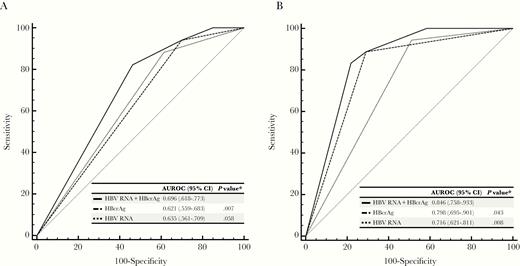
Area under the receiver operating characteristic curves of hepatitis B virus (HBV) RNA, hepatitis B core–related antigen (HBcrAg), and their combination for predicting clinical relapse in the evaluation (A) and validation (B) cohorts. *Comparison with HBV RNA plus HBcrAg. Abbreviations: AUROC, area under the receiver operating characteristic curve; CI, confidence interval; HBcrAg, hepatitis B core–related antigen; HBV, hepatitis B virus.
According to the statuses of HBV RNA and HBcrAg at EOT, the patients were categorized into low‐risk (negative HBV RNA and lower HBcrAg, n = 14), medium‐risk (positive HBV RNA and lower HBcrAg or negative HBV RNA and higher HBcrAg, n = 42), and high‐risk (positive HBV RNA and higher HBcrAg, n = 71) groups. The 4‐year cumulative incidences of clinical relapse were 0%, 17.3%, and 46.8% in the low-, medium-, and high-risk groups, respectively (P < .001; Figure 4A).
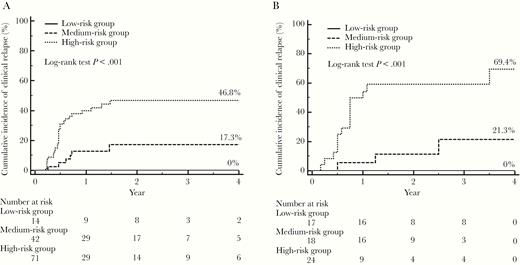
Cumulative incidences of clinical relapse in the low-, medium- and high-risk groups in the evaluation (A) and validation (B) cohorts. The low-risk group included patients with negative hepatitis B virus (HBV) RNA and lower hepatitis B core–related antigen (HBcrAg) (< 4 log10 U/mL) levels at the end of treatment (EOT); the medium-risk group included patients with negative HBV RNA and higher HBcrAg (≥ 4 log10 U/mL) levels or positive HBV RNA and lower HBcrAg levels at EOT; and the high-risk group included patients with positive HBV RNA and higher HBcrAg levels at EOT.
External Validation of Combining HBV RNA and HBcrAg in Predicting Clinical Relapse
In the validation cohort, the combination of HBV RNA and HBcrAg offered a similarly good predictability of clinical relapse (AUROC: 0.846 [HBV RNA + HBcrAg] vs 0.716 [HBV RNA] vs 0.798 [HBcrAg]) (Figure 3B). The 4‐year cumulative incidences of clinical relapse in patients in the low-risk (n = 17), medium-risk (n = 18), and high-risk (n = 24) risk groups were 0%, 21.3% and 69.4%, respectively (P < .001; Figure 4B).
Association of HBcrAg and HBV RNA With HBsAg Loss After Treatment Cessation
In the present study, the number of patients achieving HBsAg loss in each cohort was small (n = 1 and 6 in the evaluation and validation cohorts, respectively), so we combined the 2 cohorts to conduct the related analysis. At EOT, patients with negative HBV RNA had significantly lower levels of quantitative HBsAg (qHBsAg) than those with positive HBV RNA (2.2 vs 3.1 log10 IU/mL, P < .001). Similarly, the qHBsAg level was significantly lower in patients with lower HBcrAg levels than in those with higher HBcrAg levels (2.3 vs 3.0 log10 IU/mL, P = .002). During the off-treatment follow-up, the incidence of HBsAg loss was significantly higher in the low-risk patients than in the other patients (16.1% [5/31] vs 1.3% [2/155], P = .002).
DISCUSSION
To our knowledge, this is the first study that evaluated the role of the combination of HBV RNA and HBcrAg in predicting off-treatment relapse and HBsAg loss in 2 independent, prospective, multicenter, well-characterized cohorts of CHB patients with close monitoring and comprehensive off-treatment data collection. Our findings showed that the combination of HBV RNA and HBcrAg, as nucleic acid and protein biomarkers that reflect the activity of cccDNA, performed satisfactorily in predicting off-treatment outcomes.
HBV RNA at EOT could predict clinical relapse after NA treatment cessation, which has been evaluated in several studies [5, 11]. The results of this study also demonstrated that patients with negative HBV RNA had better off-treatment durability than those with positive HBV RNA. In addition, our team comprehensively assessed the HBV RNA profile after stopping NA treatment in a previous study and found that the AUROC value of EOT HBV RNA was higher than those of HBV DNA and HBsAg for predicting clinical relapse. Patients with clinical relapse had an elevation in HBV RNA after drug cessation. In contrast, among patients without clinical relapse, the level of HBV RNA remained very low [6]. All of the above results support the important role of HBV RNA in guiding the termination of NA therapy associated with off-treatment durability. Jung et al reported that an EOT HBcrAg level > 3.7 log10 IU/mL predicted virological relapse within 1 year of the cessation of NA [12]. The Japan Society of Hepatology (JSH) suggested combining HBsAg < 80 IU/mL and HBcrAg < 3 log10 U/mL to guide NA cessation in patients with long‐standing viral suppression [13]. In the current study, the value of HBcrAg at EOT in predicting clinical relapse was further confirmed. Considering that HBV RNA and HBcrAg at EOT both performed well in predicting clinical relapse, we proposed another prediction combination for clinical relapse: negative HBV RNA plus HBcrAg < 4 log10 U/mL. By using our prediction combination, the discrimination for predicting clinical relapse could be significantly improved. Because there were no patients with HBsAg < 80 IU/mL and HBcrAg < 3 log10 U/mL at EOT in the evaluation cohort, we were unable to compare the accuracy of our prediction combination with the JSH score. However, our data showed that the prediction combination performed better than the combination of HBcrAg < 4 log10 U/mL and HBsAg < 200 IU/mL for predicting clinical relapse (Supplementary Figure 4).
Recently, a growing number of studies showed that HBsAg could be cleared after stopping NA treatment due to the activation of the host immune response along with off-treatment relapse in CHB patients [14, 15]. In this study, we found that the qHBsAg level was positively associated with HBV RNA and HBcrAg. Patients with negative HBV RNA and lower HBcrAg at EOT had a higher possibility of HBsAg loss than the other patients after stopping treatment. This finding further supports the use of the combination of HBV RNA and HBcrAg to guide the cessation of NA treatment. Furthermore, we also suggested that negative HBV RNA and/or lower HBcrAg should be considered as one of the main subject inclusion criteria in future clinical trials evaluating HBsAg clearance after treatment discontinuation.
Based on the above findings, we proposed that patients with negative HBV RNA and lower HBcrAg levels, accounting for approximately 20% of the total study population, could try to stop NA treatment, as no clinical relapse and > 15% of HBsAg loss occurred during the 4-year discontinuation follow-up. In contrast, for patients with positive HBV RNA and higher HBcrAg levels, it was strongly recommended not to stop NA therapy due to the risk of relapse being as high as 50% and due to rare HBsAg loss. Obviously, the final decision to (dis)continue therapy is at the discretion of the treating physician, who should take into account other factors such as the economic burden of patients and family history as well.
It should be mentioned that the average level of EOT HBcrAg in our study was higher than the previous reported. The overall undetectable rate of EOT HBcrAg was only 4.8%. Carey et al reported that 67% patients could achieve undetectable HBcrAg after 3-year treatment among 66 HBeAg-negative patients, most of whom were non-Asians and infected with genotype A or D [16]. Another Chinese study showed that only 21.3% of patients (65% were HBeAg positive) could achieve undetectable HBcrAg after 8 years of antiviral treatment, and the HBeAg-negative patients had higher possibility to achieve undetectable HBcrAg than HBeAg-positive patients [17]. Therefore, we considered that the discrepant results may be explained by different clinical characteristics of patients, including HBeAg status, infection genotype, and ethnicity.
The EOT HBsAg level has been demonstrated to be associated with off-treatment durability in previous studies. In the present study, although EOT HBsAg was not significantly associated with clinical relapse in the evaluation cohort, the patients with EOT HBsAg < 100 IU/mL had lower clinical relapse rates than those with EOT HBsAg ≥ 100 IU/mL (18.2% [2/11] vs 27.6% [32/116], P = .726), which was consistent with the findings of previous reports. The nonsignificant difference was possibly due to the relatively small number of patients with lower EOT HBsAg levels.
Our study has several strengths, including the use of 2 prospective, well-characterized cohorts with comprehensive data collection, which increased the reliability of the results. Nonetheless, our study also has a few limitations. First, the HBcrAg level in the evaluation cohort was relatively higher, and no patient achieved HBcrAg < 3 log10 U/mL at EOT. Further investigation is needed to explore a more optimal HBcrAg threshold to predict clinical relapse. Second, the patients in the 2 cohorts were all HBeAg-positive Asians. Whether the study results could be applicable to HBeAg-negative patients or other ethnic groups merits further investigation. Third, the HBV RNA assay used in the study had not been widely validated and commercialized, which will influence the application of HBV RNA in clinical practice.
In conclusion, the current study showed that the combination of HBV RNA and HBcrAg performed satisfactorily in predicting clinical relapse and HBsAg loss after treatment discontinuation among noncirrhotic HBeAg-positive CHB patients and could be used to guide safe NA discontinuation in clinical practice.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Chronic Hepatitis B Study Consortium. Qin Ning (Department and Institute of Infectious Disease, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan), Guangfeng Shi (Department of Infectious Diseases, Huashan Hospital, Fudan University, Shanghai), Mobin Wan (Department of Infectious Diseases, Changhai Hospital, Shanghai), Shijun Chen (Ji’nan Infectious Diseases Hospital, Ji’nan), Yanyan Yu (Department of Infectious Diseases, First Hospital of Peking University, Beijing), Hong Ma (Liver Research Center, Beijing Friendship Hospital, Capital Medical University, Beijing, China), Jun Cheng (Beijing Ditan Hospital, Beijing), Hongfei Zhang (302nd PLA Hospital, Beijing), Huimin Liu (6th People’s Hospital, Hangzhou), Zhiliang Gao (Department of Infectious Diseases, Sun Yat-Sen University Third Affiliated Hospital, Guangzhou).
Author contributions. R. F., J. S., and J. L. H. were involved in the study design. R. F., Q. X., D. M. T., M. X., J. Q. N., H. W., H. R., X. Y. C., M. R. W., J. F. S., H. T., and X. F. B. collected data of the evaluation cohort. J. P. collected data of the validation cohort. Y. B. W. and B. Z. conducted the tests of hepatitis B virus RNA. R. F., J. S., and J. L. H. analyzed and interpreted the data. R. F. drafted the manuscript. J. P., Q. X., J. S., and J. L. H. reviewed and revised the manuscript. All of the authors had full access to the final version of the manuscript and agreed to submit it for publication.
Financial support. This work was supported by the National Natural Science Foundation of China (grant numbers 81700530, 81772187, 81971949); the National Science and Technology Major Project of China (grant numbers 2017ZX10202202, 2018ZX10301202, 2017ZX10302201); and the Local Innovative and Research Teams Project of Guangdong Pearl River Talents Program (grant number 2017BT01S131).
Acknowledgments. The authors thank the study investigators, coordinators, nurses, patients, and their families for their contributions, and Novartis for providing free study drug and financially supported monitoring service.
Potential conflicts of interest. Q. X. has received consulting fees from Roche, Novartis, GlaxoSmithKline, and Bristol-Myers Squibb (BMS) and has received grant/research support from Roche. J. L. H. has received consulting fees from AbbVie, Arbutus, BMS, Gilead Sciences, Johnson & Johnson, and Roche and has received grants from BMS and Johnson & Johnson. All other authors report no potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
Author notes
R. F., J. P., and Q. X. contributed equally to this work.
J. S. and J. H. contributed equally to this work as the corresponding authors.
Members of the Chronic Hepatitis B Study Consortium are listed in the Notes.




