-
PDF
- Split View
-
Views
-
Cite
Cite
Benjamin Emmanuel, Samer S El-Kamary, Laurence S Magder, Kristen A Stafford, Man E Charurat, Cheryl Chairez, Mary McLaughlin, Colleen Hadigan, Ludmila Prokunina-Olsson, Thomas R O’Brien, Henry Masur, Shyam Kottilil, Metabolic Changes in Chronic Hepatitis C Patients Who Carry IFNL4-ΔG and Achieve Sustained Virologic Response With Direct-Acting Antiviral Therapy, The Journal of Infectious Diseases, Volume 221, Issue 1, 1 January 2020, Pages 102–109, https://doi.org/10.1093/infdis/jiz435
Close - Share Icon Share
Abstract
Clearance of hepatitis C virus (HCV) results in rapid changes in metabolic parameters early in direct-acting antiviral (DAA) therapy. Long-term changes after sustained virologic response (SVR) remain unknown.
We investigated longitudinal changes in metabolic and inflammatory outcomes in chronic hepatitis C (CHC) patients: low-density lipoprotein (LDL), high-density lipoprotein, triglycerides, alanine aminotransferase (ALT), and aspartate aminotransferase (AST) using a general linear model for repeated measurements at 5 clinical time points and by human immunodeficiency virus (HIV) coinfection and IFNL4 genotype.
The mean LDL increased markedly during DAA therapy (pre-DAA, 86.6 to DAA, 107.4 mg/dL; P < .0001), but then it decreased to 97.7 mg/dL by post-SVR year 1 (P < .001 compared with DAA; P = .0013 compared with SVR). In patients who carry the IFNL4-ΔG allele, mean LDL increased during treatment, then decreased at post-SVR year 1; however, in patients with TT/TT, genotype did not change during and after DAA treatment. The mean ALT and AST normalized rapidly between pre-DAA and DAA, whereas only mean ALT continued to decrease until post-SVR. Metabolic and inflammatory outcomes were similar by HIV-coinfection status.
Changes in LDL among CHC patients who achieved SVR differed by IFNL4 genotype, which implicates the interferon-λ4 protein in metabolic changes observed in HCV-infected patients.
Hepatitis C virus (HCV) utilizes and changes the host’s lipid metabolic pathways during viral replication [1, 2]. Hepatitis C virus virions circulating in the blood within lipoproteins, known as lipoviroparticles, are associated with low-density lipoproteins (LDLs) and very LDLs, which may shield HCV from neutralization [3]. Chronic hepatitis C (CHC) is associated with metabolic complications that act in synergy with HCV to increase mortality [4], including insulin resistance that may progress to type 2 diabetes, hepatic steatosis, hypobetalipoproteinemia, and hypocholesterolemia [1, 2, 5, 6].
Hepatitis C virus treatment has progressed from less effective interferon (IFN)-based regimens to highly successful IFN-free regimens of direct-acting antiviral (DAA) drugs with almost 100% HCV clearance by end of 12 weeks posttreatment, known as sustained virologic response (SVR) [7]. Rapid changes in peripheral and intrahepatic metabolic pathways have been shown among HCV-monoinfected patients who achieved SVR with DAA therapy [8]. Levels of serum LDLs increase significantly from start of DAA therapy to week 4, likely reflecting a shift in lipid metabolism due to inhibition of HCV replication [8]. Similar LDL increase has been shown in DAA-based therapy [9, 10] and IFN-based therapy [11, 12], suggesting a direct effect of viral suppression [8].
Host genetic variants within or near IFNL4 have been associated with achievement of SVR with both IFN-based [13, 14] and DAA-based regimens [15]. The rs12979860 single-nucleotide polymorphism, which is commonly referred to as an IL28B marker, actually lies within intron 1 of the IFNL4 gene and is in linkage disequilibrium with the IFNL4 rs368234815 dinucleotide polymorphism that controls generation of the IFN-λ4 protein. Individuals who carry the IFNL4 rs368234815-ΔG allele (ΔG/ΔG or ΔG/TT genotypes) produce IFN-λ4, whereas those with the IFNL4 rs368234815-TT/TT genotype cannot make this protein [13]. Both IFNL4 polymorphisms have been associated with changes in LDL levels in response to IFN-based therapy in CHC patients [2, 5, 16–19]. With an estimated 25% of human immunodeficiency virus (HIV) patients having CHC [20], whether the change in LDL and other metabolic parameters differ by HIV-coinfection status is important to explore. Alanine aminotransferase (ALT) and aspartate aminotransferase (AST), surrogate markers for hepatic inflammation, typically normalized with viral suppression on IFN-therapy; however, any continuing elevations in aminotransferases after SVR had implications on HCV management and care [21]. In today’s era of highly effective DAA therapy, long-term changes in metabolic and inflammatory parameters after SVR remain unknown.
Given the evidence of lipid metabolism and inflammatory response with HCV, understanding the posttherapeutic changes in metabolic and inflammatory parameters is crucial. If metabolic and inflammatory abnormalities persist after achieving SVR, further therapeutic interventions may be needed with long-term follow-up and care. Furthermore, this would potentially expand the focus of long-term CHC care beyond complications like cirrhosis, liver decompensation, and hepatocellular carcinoma to include extrahepatic morbidity associated with HCV such as cardiovascular disease and dyslipidemia. Successful HCV clearance has been shown to improve some extrahepatic complications including diabetes mellitus [22]. There are no longitudinal studies on metabolic and inflammatory parameters after successful treatment with DAA therapy. In this study, we used a longitudinal cohort of CHC patients treated with DAA therapy to investigate the longitudinal changes in metabolic and inflammatory outcomes from pre-DAA therapy to post-SVR in an urban population of the District of Columbia.
PATIENTS AND METHODS
Source Population
A retrospective, longitudinal cohort of CHC patients was drawn from 4 clinical trials of DAA therapy conducted at the National Institute of Health/National Institute of Allergy and Infectious Diseases (NIH/NIAID) that are known as SPARE (www.clinicaltrials.gov no. NCT01441180), SYNERGY (www.clinicaltrials.gov no. NCT01805882), ERADICATE (www.clinicaltrials.gov no. NCT01878799), and CONQUER (www.clinicaltrials.gov no. NCT02124044) [23–30]. All patients from the clinical trials were enrolled from the same HCV clinics in Washington, District of Columbia [23–30]. In brief, the clinical trial inclusion criteria were as follows: at least 18 years of age, documented HCV genotype 1 (1a or 1b), documented fibrosis staging, and confirmed CHC before enrollment. Human immunodeficiency virus-coinfected patients had to be on stable, protocol-approved, antiretroviral therapy (ART) regimen for at least 8 weeks before starting DAA therapy with HIV-ribonucleic acid values ≤50 copies/mL and a CD4 T-lymphocyte count of ≥100 cells/μL. If not receiving ART, HIV-coinfected patients had to have a CD4 T-lymphocyte count of ≥500 cells/mm3 (regardless of HIV viral load) and a stable CD4 count with HIV viral load less than 500 copies/mL [23–30]. The exclusion criteria were as follows: pregnancy or breastfeeding, abnormal hematological and biochemical parameters at screening, and clinically significant illness other than HCV or a major medical disorder that may interfere with treatment and compliance, including any chronic liver disease or hepatocellular carcinoma [23–30]. DAA regimen and duration varied within each clinical trial [23–30]. Baseline demographics and clinical characteristics, including repeated laboratory data from pre-DAA therapy to post-SVR, were previously collected in the clinical trials. Additional post-SVR laboratory data were collected from patients who enrolled after the clinical trials into a NIH longitudinal study known as HEPPRO (NCT01350648). Partial data used in the current study were reported previously [31]. All patients provided written informed consent, and all protocols were approved by the NIH/NIAID Institutional Review Board.
Study Population
The study population consisted of HCV-monoinfected and HIV/HCV-coinfected patients treated in 1 of the 4 DAA clinical trials and with the following inclusion criteria: patients achieved SVR (including SVR after retreatment due to viral relapse with initial DAA therapy) and at least 1 pre-DAA therapy and 1 post-SVR laboratory measurement for each metabolic and inflammatory outcome through May 5, 2017.
Metabolic and Aminotransferase Measurements
Patients were instructed to fast before serum blood draws during the clinical trials. Measurements for LDL, high-density lipoprotein (HDL), triglycerides (TG), ALT, and AST were performed in the NIH Clinical Research Center laboratory on the day of collection [23–30].
IFNL4 Genotyping
Genotyping of the IFNL4 polymorphisms rs368234815 and rs12979860 was performed with TaqMan genotype assays and the ABI7500 instrument (Applied Biosystems), as previously described [13].
Statistical Analysis
Repeated measurements of metabolic and inflammatory outcomes after pre-DAA therapy were restricted to the treatment duration that led to achievement of SVR. The following are the clinical time points: pre-DAA therapy, during DAA therapy, end of treatment (EOT), SVR, and post-SVR. Pre-DAA therapy was the single, closest measurement within 6 months before the start of any DAA therapy regardless of DAA therapy retreatment. Patients had repeated measurements during the time points of DAA therapy and EOT (12 weeks before achievement of SVR). The SVR time point was the single measurement at or within 6 months of achieving SVR. The post-SVR year 1 time point were any repeated measurements during the time period after the SVR date to 1.5 years post-SVR.
A general linear model for repeated measurements was used for the clinical time points with unstructured variance/covariance matrices to generate restricted maximum likelihood estimates. The metabolic and inflammatory mean estimate and 95% confidence interval (CI) for each clinical time point and the difference in the mean change between time points were reported. The overall model was also stratified by HIV-coinfection status (HCV-monoinfected and HIV/HCV-coinfected) and IFNL4 rs368234815 genotype (TT/TT and IFNL4-ΔG carriers [ΔG/TT or ΔG/ΔG]) to determine mean difference at each clinical time point and difference in the mean change by time points between HIV-coinfection and IFNL4 rs368234815, respectively. SAS version 9.4 (SAS Institute, Cary, NC) was used for all statistical analyses. All P values were 2-sided and P < .05 was considered statistically significant.
RESULTS
A total of 301 CHC patients enrolled into 4 clinical trials and 269 (89%) achieved SVR with DAA therapy, 195 HCV-monoinfected and 75 HIV/HCV-coinfected. For this study, the CHC patients who achieved SVR were predominantly middle aged (median 57 years), male (67%), black (79%), and noncirrhotic (93%). Table 1 shows the baseline demographics and clinical characteristics of CHC patients overall and by HIV-coinfection and IFNL4-rs368234815 status.
Baseline Characteristics of 269 Chronic Hepatitis C Patients Treated With DAA Therapy and Achieved SVR, Stratified by HIV-Coinfection Status and IFNL4 Genotype
| . | Overall . | HCV . | HIV/HCV . | IFNL4 TT/TT . | IFNL4-ΔG Carriers . |
|---|---|---|---|---|---|
| Characteristic . | (n = 269) . | (n = 194) . | (n = 75) . | (n = 46) . | (n = 221) . |
| Median age (IQR), years | 57 (52–61) | 57 (52–61) | 57 (51–62) | 56 (50–60) | 57 (53–61) |
| Men, n (%) | 181 (67) | 129 (66) | 52 (70) | 31 (67) | 149 (67) |
| Race, n (%) | |||||
| White | 54 (20) | 38 (20) | 16 (21) | 19 (41) | 35 (16) |
| Black | 212 (79) | 153 (79) | 59 (79) | 26 (57) | 184 (83) |
| Other | 3 (1) | 3 (1) | 0 (0) | 1 (2) | 2 (1) |
| Hispanic, n (%)a | 9 (5) | 4 (3) | 5 (20) | 5 (16) | 4 (3) |
| Median body mass index (IQR), kg/m2 | 28 (24–31) | 28 (25–32) | 27 (23–29) | 27 (24–30) | 28 (24–32) |
| Fibrosis Stage, n (%)b | |||||
| 0 | 43 (16) | 28 (14) | 15 (20) | 8 (17) | 35 (16) |
| 1 | 116 (43) | 81 (42) | 35 (47) | 24 (52) | 90 (41) |
| 2 | 20 (7) | 17 (9) | 3 (4) | 3 (7) | 17 (8) |
| 3 | 71 (27) | 53 (27) | 18 (25) | 9 (20) | 62 (28) |
| 4 | 18 (7) | 15 (8) | 3 (4) | 2 (4) | 16 (7) |
| Cirrhosis, n (%) | 18 (7) | 15 (8) | 3 (4) | 2 (4) | 16 (7) |
| Median HCV RNA log10 IU/mL (IQR) | 6.2 (5.7–6.5) | 6.2 (5.8–6.6) | 6.1 (5.4–6.5) | 6.1 (5.7–6.5) | 6.2 (5.7–6.5) |
| HCV genotype 1a, n (%) | 187 (70) | 136 (70) | 51 (68) | 29 (63) | 157 (71) |
| HCV treatment naive, n (%) | 238 (88) | 174 (90) | 64 (85) | 40 (87) | 197 (89) |
| Retreated with DAA therapy, n (%) | 44 (16) | 44 (23) | 0 (0) | 2 (4) | 41 (19) |
| DAA Regimen for SVR, n (%) | |||||
| ASV/DCV | 8 (2) | 0 (0) | 8 (11) | 3 (6) | 4 (1) |
| ASV/DCV/BCV | 18 (7) | 0 (0) | 18 (24) | 12 (26) | 6 (3) |
| LDV/SOF | 114 (42) | 65 (34) | 49 (65) | 13 (28) | 100 (45) |
| LDV/SOF/GS-9451 | 67 (25) | 67 (35) | 0 (0) | 8 (17) | 59 (27) |
| LDV/SOF/GS-9669 | 19 (7) | 19 (9) | 0 (0) | 3 (6) | 16 (7) |
| LDV/SOF/GS-9669/GS-9451 | 5 (1) | 5 (2) | 0 (0) | 0 (0) | 5 (2) |
| SOF/RBV | 38 (14) | 38 (20) | 0 (0) | 7 (15) | 31 (14) |
| DAA Duration for SVR, n (%) | |||||
| 4 weeks | 15 (6) | 15 (8) | 0 (0) | 2 (4) | 13 (6) |
| 6 weeks | 76 (28) | 76 (39) | 0 (0) | 9 (20) | 67 (30) |
| 12 weeks | 132 (49) | 65 (33) | 67 (89) | 25 (54) | 106 (48) |
| 24 weeks | 46 (17) | 38 (20) | 8 (11) | 10 (22) | 35 (16) |
| HIV-coinfected, n (%) | 75 (28) | 0 (0) | 75 (100) | 23 (50) | 51 (23) |
| ART, n (%) | 62 (23) | N/A | 62 (82) | 21 (46) | 40 (18) |
| ART, Regimen, n (%) | |||||
| ABC/3TC+RAL | 10 (16) | 10 (16) | 6 (28) | 4 (10) | |
| TDF/FTC+RAL | 26 (42) | 26 (42) | 11 (52) | 14 (35) | |
| TDF/FTC/EFV | 15 (24) | 15 (24) | 3 (14) | 12 (30) | |
| TDF/FTC/EFV+RAL | 1 (1) | 1 (1) | 0 (0) | 1 (3) | |
| TDF/FTC/RPV | 7 (11) | 7 (11) | 1 (4) | 6 (15) | |
| TDF/FTC/RPV+RAL | 3 (4) | 3 (4) | 0 (0) | 3 (7) | |
| IFNL4-rs368234815, n (%)b | |||||
| IFNL4-TT/TT | 46 (17) | 23 (12) | 23 (31) | 46 (100) | 0 (0) |
| IFNL4-ΔG carriers (ΔG/TT or ΔG/ΔG) | 221 (83) | 170 (88) | 51 (69) | 0 (0) | 221 (100) |
| Median Baseline Metabolic Parameters (IQR)c | |||||
| LDL, mg/dL | 83 (65–106) | 84 (67–106) | 79 (62–107) | 91 (78–116) | 82 (64–102) |
| HDL, md/dL | 46 (37–64) | 47 (37–64) | 44 (37–60) | 43.5 (34–53.5) | 48.0 (37–64) |
| TG, mg/dL | 109 (79–150) | 106 (76–151) | 115 (88–144) | 100.5 (73–130) | 114 (81–151) |
| Median Baseline Inflammatory Parameters (IQR)c | |||||
| ALT, U/L | 57 (39–83) | 60 (40–88) | 47 (32–72) | 80.5 (43–109) | 51.0 (36–72) |
| AST, U/L | 48 (34–76) | 50 (34–78) | 44 (32–68) | 56.0 (30–81) | 45.0 (32–65) |
| . | Overall . | HCV . | HIV/HCV . | IFNL4 TT/TT . | IFNL4-ΔG Carriers . |
|---|---|---|---|---|---|
| Characteristic . | (n = 269) . | (n = 194) . | (n = 75) . | (n = 46) . | (n = 221) . |
| Median age (IQR), years | 57 (52–61) | 57 (52–61) | 57 (51–62) | 56 (50–60) | 57 (53–61) |
| Men, n (%) | 181 (67) | 129 (66) | 52 (70) | 31 (67) | 149 (67) |
| Race, n (%) | |||||
| White | 54 (20) | 38 (20) | 16 (21) | 19 (41) | 35 (16) |
| Black | 212 (79) | 153 (79) | 59 (79) | 26 (57) | 184 (83) |
| Other | 3 (1) | 3 (1) | 0 (0) | 1 (2) | 2 (1) |
| Hispanic, n (%)a | 9 (5) | 4 (3) | 5 (20) | 5 (16) | 4 (3) |
| Median body mass index (IQR), kg/m2 | 28 (24–31) | 28 (25–32) | 27 (23–29) | 27 (24–30) | 28 (24–32) |
| Fibrosis Stage, n (%)b | |||||
| 0 | 43 (16) | 28 (14) | 15 (20) | 8 (17) | 35 (16) |
| 1 | 116 (43) | 81 (42) | 35 (47) | 24 (52) | 90 (41) |
| 2 | 20 (7) | 17 (9) | 3 (4) | 3 (7) | 17 (8) |
| 3 | 71 (27) | 53 (27) | 18 (25) | 9 (20) | 62 (28) |
| 4 | 18 (7) | 15 (8) | 3 (4) | 2 (4) | 16 (7) |
| Cirrhosis, n (%) | 18 (7) | 15 (8) | 3 (4) | 2 (4) | 16 (7) |
| Median HCV RNA log10 IU/mL (IQR) | 6.2 (5.7–6.5) | 6.2 (5.8–6.6) | 6.1 (5.4–6.5) | 6.1 (5.7–6.5) | 6.2 (5.7–6.5) |
| HCV genotype 1a, n (%) | 187 (70) | 136 (70) | 51 (68) | 29 (63) | 157 (71) |
| HCV treatment naive, n (%) | 238 (88) | 174 (90) | 64 (85) | 40 (87) | 197 (89) |
| Retreated with DAA therapy, n (%) | 44 (16) | 44 (23) | 0 (0) | 2 (4) | 41 (19) |
| DAA Regimen for SVR, n (%) | |||||
| ASV/DCV | 8 (2) | 0 (0) | 8 (11) | 3 (6) | 4 (1) |
| ASV/DCV/BCV | 18 (7) | 0 (0) | 18 (24) | 12 (26) | 6 (3) |
| LDV/SOF | 114 (42) | 65 (34) | 49 (65) | 13 (28) | 100 (45) |
| LDV/SOF/GS-9451 | 67 (25) | 67 (35) | 0 (0) | 8 (17) | 59 (27) |
| LDV/SOF/GS-9669 | 19 (7) | 19 (9) | 0 (0) | 3 (6) | 16 (7) |
| LDV/SOF/GS-9669/GS-9451 | 5 (1) | 5 (2) | 0 (0) | 0 (0) | 5 (2) |
| SOF/RBV | 38 (14) | 38 (20) | 0 (0) | 7 (15) | 31 (14) |
| DAA Duration for SVR, n (%) | |||||
| 4 weeks | 15 (6) | 15 (8) | 0 (0) | 2 (4) | 13 (6) |
| 6 weeks | 76 (28) | 76 (39) | 0 (0) | 9 (20) | 67 (30) |
| 12 weeks | 132 (49) | 65 (33) | 67 (89) | 25 (54) | 106 (48) |
| 24 weeks | 46 (17) | 38 (20) | 8 (11) | 10 (22) | 35 (16) |
| HIV-coinfected, n (%) | 75 (28) | 0 (0) | 75 (100) | 23 (50) | 51 (23) |
| ART, n (%) | 62 (23) | N/A | 62 (82) | 21 (46) | 40 (18) |
| ART, Regimen, n (%) | |||||
| ABC/3TC+RAL | 10 (16) | 10 (16) | 6 (28) | 4 (10) | |
| TDF/FTC+RAL | 26 (42) | 26 (42) | 11 (52) | 14 (35) | |
| TDF/FTC/EFV | 15 (24) | 15 (24) | 3 (14) | 12 (30) | |
| TDF/FTC/EFV+RAL | 1 (1) | 1 (1) | 0 (0) | 1 (3) | |
| TDF/FTC/RPV | 7 (11) | 7 (11) | 1 (4) | 6 (15) | |
| TDF/FTC/RPV+RAL | 3 (4) | 3 (4) | 0 (0) | 3 (7) | |
| IFNL4-rs368234815, n (%)b | |||||
| IFNL4-TT/TT | 46 (17) | 23 (12) | 23 (31) | 46 (100) | 0 (0) |
| IFNL4-ΔG carriers (ΔG/TT or ΔG/ΔG) | 221 (83) | 170 (88) | 51 (69) | 0 (0) | 221 (100) |
| Median Baseline Metabolic Parameters (IQR)c | |||||
| LDL, mg/dL | 83 (65–106) | 84 (67–106) | 79 (62–107) | 91 (78–116) | 82 (64–102) |
| HDL, md/dL | 46 (37–64) | 47 (37–64) | 44 (37–60) | 43.5 (34–53.5) | 48.0 (37–64) |
| TG, mg/dL | 109 (79–150) | 106 (76–151) | 115 (88–144) | 100.5 (73–130) | 114 (81–151) |
| Median Baseline Inflammatory Parameters (IQR)c | |||||
| ALT, U/L | 57 (39–83) | 60 (40–88) | 47 (32–72) | 80.5 (43–109) | 51.0 (36–72) |
| AST, U/L | 48 (34–76) | 50 (34–78) | 44 (32–68) | 56.0 (30–81) | 45.0 (32–65) |
Abbreviations: ABC, abacavir; ALT, alanine aminotransferase; ART, antiretroviral therapy; AST, aspartate aminotransferase; ASV, asunaprevir; BCV, beclabuvir; DAA, direct-acting antiviral; DCV, daclatasvir; EFV, efavirenz; FTC, emtricitabine; HCV, hepatitis C virus; HDL, high-density lipoprotein; HIV, human immunodeficiency virus; IFNL4, interferon lambda 4; IFNL4-ΔG carriers, ΔG/TT or ΔG/ΔG; IQR, interquartile range; LDL, low-density lipoprotein; LDV, ledipasvir; N/A, not applicable; RAL, raltegravir; RBV, ribavirin; RNA, ribonucleic acid; RPV, rilpivirine; SOF, sofosbuvir; SVR, sustained virologic response; TDF, tenofovir; TG, triglycerides; 3TC, lamivudine.
aMissing for 88 patients.
bMissing for 2 patients.
cPatients included for analysis only.
Baseline Characteristics of 269 Chronic Hepatitis C Patients Treated With DAA Therapy and Achieved SVR, Stratified by HIV-Coinfection Status and IFNL4 Genotype
| . | Overall . | HCV . | HIV/HCV . | IFNL4 TT/TT . | IFNL4-ΔG Carriers . |
|---|---|---|---|---|---|
| Characteristic . | (n = 269) . | (n = 194) . | (n = 75) . | (n = 46) . | (n = 221) . |
| Median age (IQR), years | 57 (52–61) | 57 (52–61) | 57 (51–62) | 56 (50–60) | 57 (53–61) |
| Men, n (%) | 181 (67) | 129 (66) | 52 (70) | 31 (67) | 149 (67) |
| Race, n (%) | |||||
| White | 54 (20) | 38 (20) | 16 (21) | 19 (41) | 35 (16) |
| Black | 212 (79) | 153 (79) | 59 (79) | 26 (57) | 184 (83) |
| Other | 3 (1) | 3 (1) | 0 (0) | 1 (2) | 2 (1) |
| Hispanic, n (%)a | 9 (5) | 4 (3) | 5 (20) | 5 (16) | 4 (3) |
| Median body mass index (IQR), kg/m2 | 28 (24–31) | 28 (25–32) | 27 (23–29) | 27 (24–30) | 28 (24–32) |
| Fibrosis Stage, n (%)b | |||||
| 0 | 43 (16) | 28 (14) | 15 (20) | 8 (17) | 35 (16) |
| 1 | 116 (43) | 81 (42) | 35 (47) | 24 (52) | 90 (41) |
| 2 | 20 (7) | 17 (9) | 3 (4) | 3 (7) | 17 (8) |
| 3 | 71 (27) | 53 (27) | 18 (25) | 9 (20) | 62 (28) |
| 4 | 18 (7) | 15 (8) | 3 (4) | 2 (4) | 16 (7) |
| Cirrhosis, n (%) | 18 (7) | 15 (8) | 3 (4) | 2 (4) | 16 (7) |
| Median HCV RNA log10 IU/mL (IQR) | 6.2 (5.7–6.5) | 6.2 (5.8–6.6) | 6.1 (5.4–6.5) | 6.1 (5.7–6.5) | 6.2 (5.7–6.5) |
| HCV genotype 1a, n (%) | 187 (70) | 136 (70) | 51 (68) | 29 (63) | 157 (71) |
| HCV treatment naive, n (%) | 238 (88) | 174 (90) | 64 (85) | 40 (87) | 197 (89) |
| Retreated with DAA therapy, n (%) | 44 (16) | 44 (23) | 0 (0) | 2 (4) | 41 (19) |
| DAA Regimen for SVR, n (%) | |||||
| ASV/DCV | 8 (2) | 0 (0) | 8 (11) | 3 (6) | 4 (1) |
| ASV/DCV/BCV | 18 (7) | 0 (0) | 18 (24) | 12 (26) | 6 (3) |
| LDV/SOF | 114 (42) | 65 (34) | 49 (65) | 13 (28) | 100 (45) |
| LDV/SOF/GS-9451 | 67 (25) | 67 (35) | 0 (0) | 8 (17) | 59 (27) |
| LDV/SOF/GS-9669 | 19 (7) | 19 (9) | 0 (0) | 3 (6) | 16 (7) |
| LDV/SOF/GS-9669/GS-9451 | 5 (1) | 5 (2) | 0 (0) | 0 (0) | 5 (2) |
| SOF/RBV | 38 (14) | 38 (20) | 0 (0) | 7 (15) | 31 (14) |
| DAA Duration for SVR, n (%) | |||||
| 4 weeks | 15 (6) | 15 (8) | 0 (0) | 2 (4) | 13 (6) |
| 6 weeks | 76 (28) | 76 (39) | 0 (0) | 9 (20) | 67 (30) |
| 12 weeks | 132 (49) | 65 (33) | 67 (89) | 25 (54) | 106 (48) |
| 24 weeks | 46 (17) | 38 (20) | 8 (11) | 10 (22) | 35 (16) |
| HIV-coinfected, n (%) | 75 (28) | 0 (0) | 75 (100) | 23 (50) | 51 (23) |
| ART, n (%) | 62 (23) | N/A | 62 (82) | 21 (46) | 40 (18) |
| ART, Regimen, n (%) | |||||
| ABC/3TC+RAL | 10 (16) | 10 (16) | 6 (28) | 4 (10) | |
| TDF/FTC+RAL | 26 (42) | 26 (42) | 11 (52) | 14 (35) | |
| TDF/FTC/EFV | 15 (24) | 15 (24) | 3 (14) | 12 (30) | |
| TDF/FTC/EFV+RAL | 1 (1) | 1 (1) | 0 (0) | 1 (3) | |
| TDF/FTC/RPV | 7 (11) | 7 (11) | 1 (4) | 6 (15) | |
| TDF/FTC/RPV+RAL | 3 (4) | 3 (4) | 0 (0) | 3 (7) | |
| IFNL4-rs368234815, n (%)b | |||||
| IFNL4-TT/TT | 46 (17) | 23 (12) | 23 (31) | 46 (100) | 0 (0) |
| IFNL4-ΔG carriers (ΔG/TT or ΔG/ΔG) | 221 (83) | 170 (88) | 51 (69) | 0 (0) | 221 (100) |
| Median Baseline Metabolic Parameters (IQR)c | |||||
| LDL, mg/dL | 83 (65–106) | 84 (67–106) | 79 (62–107) | 91 (78–116) | 82 (64–102) |
| HDL, md/dL | 46 (37–64) | 47 (37–64) | 44 (37–60) | 43.5 (34–53.5) | 48.0 (37–64) |
| TG, mg/dL | 109 (79–150) | 106 (76–151) | 115 (88–144) | 100.5 (73–130) | 114 (81–151) |
| Median Baseline Inflammatory Parameters (IQR)c | |||||
| ALT, U/L | 57 (39–83) | 60 (40–88) | 47 (32–72) | 80.5 (43–109) | 51.0 (36–72) |
| AST, U/L | 48 (34–76) | 50 (34–78) | 44 (32–68) | 56.0 (30–81) | 45.0 (32–65) |
| . | Overall . | HCV . | HIV/HCV . | IFNL4 TT/TT . | IFNL4-ΔG Carriers . |
|---|---|---|---|---|---|
| Characteristic . | (n = 269) . | (n = 194) . | (n = 75) . | (n = 46) . | (n = 221) . |
| Median age (IQR), years | 57 (52–61) | 57 (52–61) | 57 (51–62) | 56 (50–60) | 57 (53–61) |
| Men, n (%) | 181 (67) | 129 (66) | 52 (70) | 31 (67) | 149 (67) |
| Race, n (%) | |||||
| White | 54 (20) | 38 (20) | 16 (21) | 19 (41) | 35 (16) |
| Black | 212 (79) | 153 (79) | 59 (79) | 26 (57) | 184 (83) |
| Other | 3 (1) | 3 (1) | 0 (0) | 1 (2) | 2 (1) |
| Hispanic, n (%)a | 9 (5) | 4 (3) | 5 (20) | 5 (16) | 4 (3) |
| Median body mass index (IQR), kg/m2 | 28 (24–31) | 28 (25–32) | 27 (23–29) | 27 (24–30) | 28 (24–32) |
| Fibrosis Stage, n (%)b | |||||
| 0 | 43 (16) | 28 (14) | 15 (20) | 8 (17) | 35 (16) |
| 1 | 116 (43) | 81 (42) | 35 (47) | 24 (52) | 90 (41) |
| 2 | 20 (7) | 17 (9) | 3 (4) | 3 (7) | 17 (8) |
| 3 | 71 (27) | 53 (27) | 18 (25) | 9 (20) | 62 (28) |
| 4 | 18 (7) | 15 (8) | 3 (4) | 2 (4) | 16 (7) |
| Cirrhosis, n (%) | 18 (7) | 15 (8) | 3 (4) | 2 (4) | 16 (7) |
| Median HCV RNA log10 IU/mL (IQR) | 6.2 (5.7–6.5) | 6.2 (5.8–6.6) | 6.1 (5.4–6.5) | 6.1 (5.7–6.5) | 6.2 (5.7–6.5) |
| HCV genotype 1a, n (%) | 187 (70) | 136 (70) | 51 (68) | 29 (63) | 157 (71) |
| HCV treatment naive, n (%) | 238 (88) | 174 (90) | 64 (85) | 40 (87) | 197 (89) |
| Retreated with DAA therapy, n (%) | 44 (16) | 44 (23) | 0 (0) | 2 (4) | 41 (19) |
| DAA Regimen for SVR, n (%) | |||||
| ASV/DCV | 8 (2) | 0 (0) | 8 (11) | 3 (6) | 4 (1) |
| ASV/DCV/BCV | 18 (7) | 0 (0) | 18 (24) | 12 (26) | 6 (3) |
| LDV/SOF | 114 (42) | 65 (34) | 49 (65) | 13 (28) | 100 (45) |
| LDV/SOF/GS-9451 | 67 (25) | 67 (35) | 0 (0) | 8 (17) | 59 (27) |
| LDV/SOF/GS-9669 | 19 (7) | 19 (9) | 0 (0) | 3 (6) | 16 (7) |
| LDV/SOF/GS-9669/GS-9451 | 5 (1) | 5 (2) | 0 (0) | 0 (0) | 5 (2) |
| SOF/RBV | 38 (14) | 38 (20) | 0 (0) | 7 (15) | 31 (14) |
| DAA Duration for SVR, n (%) | |||||
| 4 weeks | 15 (6) | 15 (8) | 0 (0) | 2 (4) | 13 (6) |
| 6 weeks | 76 (28) | 76 (39) | 0 (0) | 9 (20) | 67 (30) |
| 12 weeks | 132 (49) | 65 (33) | 67 (89) | 25 (54) | 106 (48) |
| 24 weeks | 46 (17) | 38 (20) | 8 (11) | 10 (22) | 35 (16) |
| HIV-coinfected, n (%) | 75 (28) | 0 (0) | 75 (100) | 23 (50) | 51 (23) |
| ART, n (%) | 62 (23) | N/A | 62 (82) | 21 (46) | 40 (18) |
| ART, Regimen, n (%) | |||||
| ABC/3TC+RAL | 10 (16) | 10 (16) | 6 (28) | 4 (10) | |
| TDF/FTC+RAL | 26 (42) | 26 (42) | 11 (52) | 14 (35) | |
| TDF/FTC/EFV | 15 (24) | 15 (24) | 3 (14) | 12 (30) | |
| TDF/FTC/EFV+RAL | 1 (1) | 1 (1) | 0 (0) | 1 (3) | |
| TDF/FTC/RPV | 7 (11) | 7 (11) | 1 (4) | 6 (15) | |
| TDF/FTC/RPV+RAL | 3 (4) | 3 (4) | 0 (0) | 3 (7) | |
| IFNL4-rs368234815, n (%)b | |||||
| IFNL4-TT/TT | 46 (17) | 23 (12) | 23 (31) | 46 (100) | 0 (0) |
| IFNL4-ΔG carriers (ΔG/TT or ΔG/ΔG) | 221 (83) | 170 (88) | 51 (69) | 0 (0) | 221 (100) |
| Median Baseline Metabolic Parameters (IQR)c | |||||
| LDL, mg/dL | 83 (65–106) | 84 (67–106) | 79 (62–107) | 91 (78–116) | 82 (64–102) |
| HDL, md/dL | 46 (37–64) | 47 (37–64) | 44 (37–60) | 43.5 (34–53.5) | 48.0 (37–64) |
| TG, mg/dL | 109 (79–150) | 106 (76–151) | 115 (88–144) | 100.5 (73–130) | 114 (81–151) |
| Median Baseline Inflammatory Parameters (IQR)c | |||||
| ALT, U/L | 57 (39–83) | 60 (40–88) | 47 (32–72) | 80.5 (43–109) | 51.0 (36–72) |
| AST, U/L | 48 (34–76) | 50 (34–78) | 44 (32–68) | 56.0 (30–81) | 45.0 (32–65) |
Abbreviations: ABC, abacavir; ALT, alanine aminotransferase; ART, antiretroviral therapy; AST, aspartate aminotransferase; ASV, asunaprevir; BCV, beclabuvir; DAA, direct-acting antiviral; DCV, daclatasvir; EFV, efavirenz; FTC, emtricitabine; HCV, hepatitis C virus; HDL, high-density lipoprotein; HIV, human immunodeficiency virus; IFNL4, interferon lambda 4; IFNL4-ΔG carriers, ΔG/TT or ΔG/ΔG; IQR, interquartile range; LDL, low-density lipoprotein; LDV, ledipasvir; N/A, not applicable; RAL, raltegravir; RBV, ribavirin; RNA, ribonucleic acid; RPV, rilpivirine; SOF, sofosbuvir; SVR, sustained virologic response; TDF, tenofovir; TG, triglycerides; 3TC, lamivudine.
aMissing for 88 patients.
bMissing for 2 patients.
cPatients included for analysis only.
At pre-DAA therapy, patients with the IFNL4-TT/TT genotype had higher LDL (P = .01) and ALT (P = .0009) and lower HDL (P = .02) values than IFNL4-ΔG carriers, whereas there was no difference in TG, total cholesterol, and AST values (Table 2).
Pre-DAA Therapy Results (Median [IQR]) for All Lipid Measurements by IFNL4 rs368234815 Genotype Among Chronic Hepatitis C Patients Treated With DAA Therapy and Achieved SVR
| Metabolic and Hepatic Inflammatory Outcomes . | IFNL4 TT/TT . | IFNL4-ΔG Carriersb . | P Valuea . |
|---|---|---|---|
| LDL, mg/dL | 91 (78–116) | 82 (64–102) | .01 |
| HDL, mg/dL | 43.5 (34–53.5) | 48.0 (37–64) | .02 |
| TG, mg/dL | 100.5 (73–130) | 114 (81–151) | .52 |
| TC, mg/dL | 154.5 (133–195) | 161.5 (139–186) | .23 |
| ALT, U/L | 80.5 (43–109) | 51.0 (36–72) | .0009 |
| AST, U/L | 56.0 (30–81) | 45.0 (32–65) | .38 |
| Metabolic and Hepatic Inflammatory Outcomes . | IFNL4 TT/TT . | IFNL4-ΔG Carriersb . | P Valuea . |
|---|---|---|---|
| LDL, mg/dL | 91 (78–116) | 82 (64–102) | .01 |
| HDL, mg/dL | 43.5 (34–53.5) | 48.0 (37–64) | .02 |
| TG, mg/dL | 100.5 (73–130) | 114 (81–151) | .52 |
| TC, mg/dL | 154.5 (133–195) | 161.5 (139–186) | .23 |
| ALT, U/L | 80.5 (43–109) | 51.0 (36–72) | .0009 |
| AST, U/L | 56.0 (30–81) | 45.0 (32–65) | .38 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; DAA, direct-acting antiviral; IQR, interquartile range; HCV, hepatitis C virus; HDL, high-density lipoprotein; HIV, human immunodeficiency virus; LDL, low-density lipoprotein; SVR, sustained virologic response; TG, triglycerides; TC, total cholesterol.
aAdjusted model: age, sex, race, fibrosis stage, HCV viral load, and HIV status.
bIFNL4-ΔG carriers: ΔG/TT or ΔG/ΔG.
Pre-DAA Therapy Results (Median [IQR]) for All Lipid Measurements by IFNL4 rs368234815 Genotype Among Chronic Hepatitis C Patients Treated With DAA Therapy and Achieved SVR
| Metabolic and Hepatic Inflammatory Outcomes . | IFNL4 TT/TT . | IFNL4-ΔG Carriersb . | P Valuea . |
|---|---|---|---|
| LDL, mg/dL | 91 (78–116) | 82 (64–102) | .01 |
| HDL, mg/dL | 43.5 (34–53.5) | 48.0 (37–64) | .02 |
| TG, mg/dL | 100.5 (73–130) | 114 (81–151) | .52 |
| TC, mg/dL | 154.5 (133–195) | 161.5 (139–186) | .23 |
| ALT, U/L | 80.5 (43–109) | 51.0 (36–72) | .0009 |
| AST, U/L | 56.0 (30–81) | 45.0 (32–65) | .38 |
| Metabolic and Hepatic Inflammatory Outcomes . | IFNL4 TT/TT . | IFNL4-ΔG Carriersb . | P Valuea . |
|---|---|---|---|
| LDL, mg/dL | 91 (78–116) | 82 (64–102) | .01 |
| HDL, mg/dL | 43.5 (34–53.5) | 48.0 (37–64) | .02 |
| TG, mg/dL | 100.5 (73–130) | 114 (81–151) | .52 |
| TC, mg/dL | 154.5 (133–195) | 161.5 (139–186) | .23 |
| ALT, U/L | 80.5 (43–109) | 51.0 (36–72) | .0009 |
| AST, U/L | 56.0 (30–81) | 45.0 (32–65) | .38 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; DAA, direct-acting antiviral; IQR, interquartile range; HCV, hepatitis C virus; HDL, high-density lipoprotein; HIV, human immunodeficiency virus; LDL, low-density lipoprotein; SVR, sustained virologic response; TG, triglycerides; TC, total cholesterol.
aAdjusted model: age, sex, race, fibrosis stage, HCV viral load, and HIV status.
bIFNL4-ΔG carriers: ΔG/TT or ΔG/ΔG.
Metabolic Outcomes
The mean LDL increased markedly during DAA therapy (pre-DAA, 86.6 to DAA, 107.4 mg/dL; P < .0001) (Figure 1), but then it decreased to 97.7 mg/dL by post-SVR year 1 (P < .001 compared with DAA; P = .0013 compared with SVR). This pattern was seen for both HCV-monoinfected and HCV/HIV-coinfected patients, and mean LDL did not differ between these groups at any time point (Supplemental Table 1). These changes in LDL were largely driven by individuals who carried the IFNL4-ΔG allele (Figure 2). Among the 185 IFNL4-ΔG carriers, LDL increased from 85.1 mg/dL pre-DAA to 109.6 mg/dL during DAA treatment (P < .0001) and then fell to 98.8 mg/dL at post-SVR year 1 (P < .0001 compared with DAA). Among 36 TT/TT patients, LDL levels were as follows: pre-DAA, 93.8; DAA, 97.0 (P = .41 compared with pre-DAA); post-SVR year 1, 92.9 mg/dL (P = .38 compared with DAA). There was a significant difference in mean LDL change between TT/TT and IFNL4-ΔG patients at pre-DAA to DAA therapy (P < .0001) but not DAA therapy to post-SVR year 1 (P = .78).
![The mean ± 95% confidence interval (CI) for low-density lipoprotein ([LDL] n = 223), high-density lipoprotein ([HDL] n = 234), triglycerides ([TG] n = 235) at pre-direct-acting antiviral (DAA) therapy (PRE), DAA therapy (DAA), end of treatment (EOT), sustained virologic response (SVR), and post-SVR year 1 (YR 1) among chronic hepatitis C patients treated with DAA therapy and achieved SVR based on the longitudinal regression model.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jid/221/1/10.1093_infdis_jiz435/3/m_jiz435f0001.jpeg?Expires=1750045672&Signature=YLJsu8c52QbwNhcmnK8Awziy-CQuy2lz8SjXZKhGghl3kG~CgaDKCDT0vLiMlylAJf4CqP0jsROcxc2uHCyj7Tvh65HGVzXEM~tQejEZ6uUc5zE-KgBiEGnpyonSkNtWCcppZZer4xKZcV7hKAsGSNzEm45s47xqxWk3AZZMawtzwZ3maiTprDjDg~yzINwMRYSfW9mhmX2NuTosLVopqoPm5SF~kXQK9E9GiZ~hg3IN5cm-3Z8~AE8j3saXzdTMLs7ZqwuxxYbhsch~KhrWV7MNZBBZd4IP-hWD837-rIQi6UrdwGDNreHPm7VbqPSUf2tPnxbaTGOAXAt2JCrDpQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
The mean ± 95% confidence interval (CI) for low-density lipoprotein ([LDL] n = 223), high-density lipoprotein ([HDL] n = 234), triglycerides ([TG] n = 235) at pre-direct-acting antiviral (DAA) therapy (PRE), DAA therapy (DAA), end of treatment (EOT), sustained virologic response (SVR), and post-SVR year 1 (YR 1) among chronic hepatitis C patients treated with DAA therapy and achieved SVR based on the longitudinal regression model.
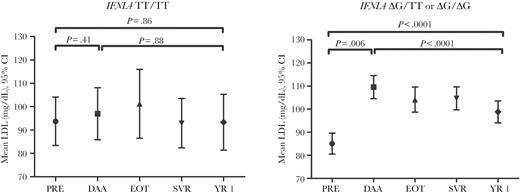
The mean ± 95% confidence interval (CI) for low-density lipoprotein (LDL) for IFNL4 genotype (TT/TT, n = 36; ΔG/TT or ΔG/ΔG, n = 185) at pre-direct-acting antiviral (DAA) therapy (PRE), DAA therapy (DAA), end of treatment (EOT), sustained virologic response (SVR), and post-SVR year 1 (YR 1) among chronic hepatitis C patients treated with DAA therapy and achieved SVR based on the longitudinal regression model.
In contrast to LDL, HDL levels did not change in response to DAA therapy (Figure 1), including by IFNL4 genotype. At each time point, mean HDL was similar in HCV-monoinfected and HIV/HCV-coinfected patients (Supplemental Table 1).
The mean TG decreased markedly during DAA therapy (pre-DAA, 126.2 to DAA, 109.3 mg/dL, P < .0001) (Figure 1), but then it increased to 117.2 mg/dL at post-SVR year 1; however, there was no statistically significant increase between pre-DAA therapy and post-SVR year 1 (P = .08). The initial decrease during DAA therapy was seen among both HCV-monoinfected (P < .0001) and HIV/HCV-coinfected patients (P = .01) (Supplemental Table 1). There was no significant difference in mean TG at each clinical time point (except DAA therapy, P = .05) or difference in the mean change (pre-DAA therapy and post-SVR year 1, P = .65) between HCV-monoinfected and HIV/HCV-coinfected patients. There was a significant decrease during DAA therapy in mean TG among IFNL4-ΔG (128.7 to 110.1 mg/dL, P < .0001), but not TT/TT patients (114.6 to 101.8 mg/dL, P = .11). There was no difference in the mean change from pre-DAA and DAA therapy (P = .51) or DAA therapy to post-SVR year 1 (P = .82) by IFNL4 genotype, respectively.
Hepatic Inflammatory Outcomes
The mean ALT decreased from 70.0 U/L at pre-DAA therapy to 26.8 U/L during DAA therapy (P < .0001) and then to 20.9 U/L at post-SVR year 1 (P < .0001 compared with DAA; P = .001 compared with SVR) (Figure 3). The mean ALT decreased from pre-DAA to DAA therapy and DAA therapy to post-SVR year 1 was seen in both HCV-monoinfected (P < .001) and HIV/HCV-coinfected (P < .001) patients. There was no significant difference in mean ALT between HCV-monoinfected and HIV/HCV-coinfected patients, except at post-SVR year 1, and no difference in the mean ALT change (Supplemental Table 2). Similar outcome for mean ALT was seen when stratified by IFNL4 genotype. Between pre-DAA and DAA therapy, ALT decreased in patients with TT/TT (84.0 to 25.5 U/L, P < .0001) and IFNL4-ΔG carriers (67.4 to 27.1 U/L, P < .0001). Alanine aminotransferase continued to decrease until post-SVR year 1 in TT/TT (21.2 U/L, P = .03 compared with DAA therapy) and IFNL4-ΔG carriers (20.9 U/L, P < .0001 compared with DAA therapy).
![The mean ± 95% confidence interval (CI) for alanine aminotransferase ([ALT] n = 262) and aspartate aminotransferase ([AST] n = 262) at pre-direct-acting antiviral (DAA) therapy (PRE), DAA therapy (DAA), end of treatment (EOT), sustained virologic response (SVR), and post-SVR year 1 (YR 1) among chronic hepatitis C patients treated with DAA therapy and achieved SVR based on the longitudinal regression model.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jid/221/1/10.1093_infdis_jiz435/3/m_jiz435f0003.jpeg?Expires=1750045673&Signature=b8UdI-G6Y9BQf9MuPDtZolZ8Y4vPAo9E3HnQTjNW60D34A8ybtBQAusUouX67sfM0unVfSYHlvuWe6fcqQElOn9MmonhUHAGgt1Z-UGnzNOoBEYQaUKACrBsTcWuEG6wuH5b3dI6mJIUFUmZQ8lt6VJpRWTe4LVyG8QmbSQ88zet~hpb2bxVLPzLYG2MfJtqW0n~a1hjUipsAfwfOxne-N2yShNYXnpyGLzS0WnXb7PYbG~nQbGBhqv6tDwhKUAiATcjRAbf7WTcqRoPYk7Yx2FmGglhnyiFqHeN4cxDyOkGmgFTnHZYfeBhzqEXnGjKdJ7y1S5qdYwA2coYGMjdCg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
The mean ± 95% confidence interval (CI) for alanine aminotransferase ([ALT] n = 262) and aspartate aminotransferase ([AST] n = 262) at pre-direct-acting antiviral (DAA) therapy (PRE), DAA therapy (DAA), end of treatment (EOT), sustained virologic response (SVR), and post-SVR year 1 (YR 1) among chronic hepatitis C patients treated with DAA therapy and achieved SVR based on the longitudinal regression model.
Similar to our findings for ALT, mean AST decreased from 60.1 U/L pre-DAA therapy to 26.4 U/L during DAA therapy (P < .0001). However, in contrast to the ALT results, we observed no subsequent decrease because mean AST was 25.6 U/L at the post-SVR year 1 time point (P = .37 compared with DAA therapy) (Figure 3). The initial mean AST decrease from pre-DAA therapy to DAA therapy was seen in both HCV-monoinfected (P < .001) and HIV/HCV-coinfected patients (P < .001), with no change between DAA therapy and post-SVR year 1 for HCV (P = .51) and HIV/HCV (P = .57) patients. There was no significant difference in mean AST at each clinical time point between HCV-monoinfected and HIV/HCV-coinfected patients, except at EOT (Supplemental Table 2). The mean AST change (pre-DAA therapy and DAA therapy, P = .06; DAA therapy and post-SVR year 1, P = .89) did not differ between HCV-monoinfected and HIV/HCV-coinfected patients. Similar outcome for mean AST was seen when stratified by IFNL4 genotype. Between pre-DAA and DAA therapy, AST decreased in patients with TT/TT (60.6 to 24.6 U/L, P < .0001) and IFNL4-ΔG carriers (60.1 to 26.7 U/L, P < .0001). Aspartate aminotransferase did not significantly change between DAA therapy and post-SVR year 1 in TT/TT (P = .45) and IFNL4-ΔG carriers (P = .18).
DISCUSSION
To our knowledge, this longitudinal study is the first to report the association of IFNL4 genotype with metabolic changes during DAA therapy. Changes in LDL were limited to patients who carry the IFNL4-ΔG allele and, therefore, are able to generate the IFN-λ4 protein. If LDL changes in CHC patients are due to HCV lipoviroparticles secreted using LDL exosomes, our data suggest this process is likely to be affected by IFN-λ4. Alternatively, IFN-λ4 induced by HCV infection may have a more direct role in regulating LDL levels; this regulation would be absent in the TT/TT patients who do not produce IFN-λ4. Levels of both ALT and AST decreased and improved even DAA therapy, yet ALT continued improving beyond SVR. There was no significant difference by HIV-coinfection status for all metabolic and inflammatory outcomes, except that change in ALT lagged behind in HIV/HCV-coinfected patients at year 1.
Hepatitis C virus hijacks the host lipid metabolism for HCV replication and circulates in the blood encased in lipoviroparticles [3]. Hepatitis C virus viral suppression results in a reversal of the lipoviroparticles, which likely causes the initial LDL increase early during DAA therapy. The rapid increase in LDL has been shown in previous IFN and DAA based therapy, presumably as a reflection of a shift in the lipid metabolism with inhibition of HCV replication [9–12, 32]. Because HCV hijacks LDL to export virions, this increase in LDL is likely due to a release of LDL from the Golgi due to suppression of HCV. Although LDL improved after DAA therapy by decreasing over time until post-SVR, it did not reach the pre-DAA therapy level, which needs further evaluation in future long-term studies. The results in this study support the evidence that rapid normalization of ALT and AST is sustained until post-SVR therapy, primarily with ALT as a surrogate of hepatic inflammation.
Previous studies have shown strong associations between IFNL4 rs12979860 genotype and lipid levels (total cholesterol, TG, LDL, and apolipoproteins) at pre-IFN-based therapy [16, 17]. At pre-DAA therapy, our study showed significant difference by LDL, HDL, and ALT when comparing IFNL4 TT/TT and IFNL4-ΔG patients. The LDL increase between pre-DAA and DAA therapy was seen in patients with ΔG/TT or ΔG/ΔG genotypes (ie, those who can generate IFN-λ4 protein), but not patients with the IFNL4 TT/TT genotype, who cannot generate IFN-λ4 protein. These findings suggest that generation of IFN-λ4 may explain these genotype differences. The mechanism for this LDL restriction over time with HCV viral clearance suggest that in patients with IFNL4 TT/TT, HCV excretion may involve a different mechanism than LDL excretory pathway. Clark et al [19] noted that the mechanism for LDL being associated with SVR by peginterferon/ribavirin in heterozygous IL28B patients must be immune-related. The authors also reported that among patients achieving SVR on peginterferon/ribavirin, LDL decreased from pre-IFN to IFN therapy and then increased at SVR [19], which is opposite to the results in our study with DAA therapy. Further studies are needed to confirm and understand the association between of IFNL4 genotype and LDL with DAA therapy.
Limitations of the study include using secondary data from previous clinical trials with inclusion criteria in the current study that could have led to potential selection bias for an observational cohort. There was no difference in baseline demographic and clinical characteristics among patients who had longer follow-up compared to those who had shorter follow-up within the HEPPRO data; except for HIV-coinfected patients likely as a result of many of them connected to the research clinic for their HIV. The study population from the clinical trials is representative of a high-risk HIV and HCV population in the District of Columbia so there is likely no biological difference in HCV and HIV infection among patients enrolled compared with those who did not enroll. However, patients with gross metabolic abnormalities or uncontrolled diabetes were excluded from the clinical trials, which could have resulted in different long-term results for a more severe or less well controlled diabetic population had they been enrolled into the clinical trials. Second, although there were different DAA drugs and treatment duration, patients were selected based on SVR achievement, and there is no evidence in the literature of a biological effect of DAA drugs or the DAA duration treatment on metabolic and inflammatory parameters. Third, we do not have data on use of lipid-lowering therapy and therefore do not control for this potential exposure in our analyses. Fourth, during clinical study visits, there were no objective measures used to verify fasting before serum blood draws, which could have impacted lipid levels. In addition, estimated LDL levels could be affected by changes in other lipids, although TG changes are valid. Although repeated laboratory measurements were at nonuniform time periods, the use of general linear models allowed for analyzing unbalanced repeated measurements. Our primary analysis of change over time for the overall CHC population was well powered; however, the secondary analysis stratified by IFNL4 genotype would need a larger sample size to detect a difference with confidence.
The study has several strengths and innovations including the enrollment of an at-risk urban study population, the use of a longitudinal study design, and the evaluation of metabolic and inflammatory outcomes. This study used an established infrastructure of 4 clinical trials to design an observational, retrospective cohort with repeated metabolic and inflammatory outcomes among CHC patients who achieved SVR with DAA therapy and with longer post-SVR follow-up than prior studies. The study population included a large proportion of African Americans with both HCV-monoinfected and HIV/HCV-coinfected patients treated with DAA therapy. African Americans have significantly higher frequency of the IFNL4-ΔG allele and lower linkage disequilibrium with another IFNL4 polymorphism, rs12979860, which is less informative in this population compared with European Americans. The homogenous population in the community-based clinics of Washington, District of Columbia strengthens our external validity to similar urban cohorts to persons living with HCV in other parts of the United States.
CONCLUSIONS
For the first time, we have a disease model in which chronic viral infection that persisted for over 30 years in patients is suddenly eliminated in 12 weeks with DAA therapy. It is not well understood how the body reacts to this change and resets the metabolic homeostasis. The results from our study provide a preliminary step in our understanding of viral-host interactions during DAA therapy, at SVR, and, more importantly, after long-term, post-SVR follow-up, which has not been assessed in the current literature. This longitudinal study showed that CHC patients who achieved SVR with DAA therapy had LDL increased during DAA therapy, worsening the LDL profile, then decreased in IFNL4-ΔG carriers, but not in IFNL4 TT/TT patients, which implicates the IFN-λ4 protein in metabolic changes observed in HCV-infected patients.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Note
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- alleles
- low-density lipoproteins
- hiv
- triglycerides
- hepatitis c, chronic
- drug clearance
- high density lipoproteins
- alanine transaminase
- antiviral agents
- aspartate aminotransferases
- community health centers
- genotype
- interferons
- transaminases
- low density lipoprotein increased
- human leukocyte interferon
- hepatitis c virus
- coinfection
- metabolic disturbance
- sustained virologic response




