-
PDF
- Split View
-
Views
-
Cite
Cite
Yiu-Wing Kam, Juliana Almeida Leite, Siti Naqiah Amrun, Fok-Moon Lum, Wearn-Xin Yee, Farhana Abu Bakar, Kai Er Eng, David C Lye, Yee-Sin Leo, Chia-Yin Chong, Andre Ricardo Ribas Freitas, Guilherme Paier Milanez, Jose Luiz Proença-Modena, Laurent Rénia, Fabio T M Costa, Lisa F P Ng, Zika-Unicamp Network , ZIKV-Specific NS1 Epitopes as Serological Markers of Acute Zika Virus Infection, The Journal of Infectious Diseases, Volume 220, Issue 2, 15 July 2019, Pages 203–212, https://doi.org/10.1093/infdis/jiz092
Close - Share Icon Share
Abstract
Zika virus (ZIKV) infections have reemerged as a global health issue due to serious clinical complications. Development of specific serological assays to detect and differentiate ZIKV from other cocirculating flaviviruses for accurate diagnosis remains a challenge.
We investigated antibody responses in 51 acute ZIKV-infected adult patients from Campinas, Brazil, including 7 pregnant women who later delivered during the study. Using enzyme-linked immunosorbent assays, levels of antibody response were measured and specific epitopes identified.
Several antibody-binding hot spots were identified in ZIKV immunogenic antigens, including membrane, envelope (E) and nonstructural protein 1 (NS1). Interestingly, specific epitopes (2 from E and 2 from NS1) strongly recognized by ZIKV-infected patients’ antibodies were identified and were not cross-recognized by dengue virus (DENV)-infected patients’ antibodies. Corresponding DENV peptides were not strongly recognized by ZIKV-infected patients’ antibodies. Notably, ZIKV-infected pregnant women had specific epitope recognition for ZIKV NS1 (amino acid residues 17–34), which could be a potential serological marker for early ZIKV detection.
This study identified 6 linear ZIKV-specific epitopes for early detection of ZIKV infections. We observed differential epitope recognition between ZIKV-infected and DENV-infected patients. This information will be useful for developing diagnostic methods that differentiate between closely related flaviviruses.
ZIKV virus (ZIKV) was first identified in Uganda and isolated from infected monkeys in 1947 [1]. ZIKV has emerged as an important flavivirus that has caused several Zika fever (ZIKF) epidemics worldwide. Since 2007, 76 countries and territories have reported mosquito-borne local ZIKV transmission, 29 of which have reported congenital anomalies such as microcephaly possibly associated with ZIKV infection [2, 3]. ZIKV is mainly transmitted by Aedes mosquitoes and the majority of patients remain asymptomatic or exhibits mild symptoms that normally last for less than 7 days. These symptoms include fever, arthritis/arthralgia, skin rash, conjunctivitis, joint pain, and headache [4]. However, reports have indicated the role of ZIKV infection in Guillain-Barré syndrome and congenital central nervous system (CNS) abnormalities [5–8]. Therefore, ZIKV infection poses a global public health emergency. Treatment is usually symptomatic and pain-relief medicine is the only available option during disease onset. Brazil, hit by the ZIKV epidemics between 2014 and 2016, accounted for more than 200 000 probable cases of ZIKV with almost 2000 cases of microcephaly in 2015–2016 [9].
Serologic testing is an important tool for the diagnosis of ZIKV infection in patients living in areas where circulation of multiple pathogens causes similar disease symptoms. Currently, there is a lack of an accurate diagnostic system available on the market due to high cross-reactivity between ZIKV and other closely related flaviviruses [10, 11]. Several approaches using inactivated whole virus or full-length antigens have been reported in the development of new diagnostic kits with better sensitivity and specificity [12–15]. Therefore, alternative approaches that can offer an accurate yet inexpensive way to differentiate ZIKV from other closely related flaviviruses infections are highly desired. While antibody cross-reactivity against different flaviviruses exists, it is not known whether protein regions with lower sequence similarity may generate less cross-reactive antibodies and improve the differentiation performance in serological detection.
In this study, humoral immune response was characterized by analyzing the antibody profiles of ZIKV-infected patients from a major economic and travel hub, Campinas metropolitan area, in Brazil. Patients were classified according to the in-house diagnostic test result and type of clinical observation during hospitalization [16]. Virion-based enzyme-linked immunosorbent assays (ELISAs) were performed to detect the specific antibody profiles of ZIKV-infected adult patients and pregnant women who later gave birth to babies with or without congenital CNS deformities. Peptide-based ELISAs were performed to identify differential epitopes that distinguished ZIKV-infected and dengue virus (DENV)-infected adult patients. Interestingly, specific epitopes were recognized by the antibodies of ZIKV-infected nonpregnant adults and ZIKV-infected pregnant women. These findings provide critical differential markers of ZIKV infection and serve to improve current practices in early diagnostic management.
MATERIALS AND METHODS
Ethical Approval
Written informed consent was obtained from all participants and the study was conducted according to Declaration of Helsinki principles. All procedures in this study were approved by the Research Ethics Committee of the University of Campinas (CAAE: 56793516.0.0000.5404), the National Healthcare Group Domain-Specific Review Board (DSRB/E/09/00432), SingHealth Centralized Institutional Review Board (CIRB Ref: 2016/2219), and National Healthcare Group Domain-Specific Review Board (DSRB Ref: 2015/00528).
Patients and Serum Collection
Acute-phase serum specimens were collected from the Brazil ZIKV cohort, including 51 ZIKV-infected patients at median 3 days after illness onset. Within this cohort, 7 were pregnant women who also provided serum samples between 1 and 3 months (convalescent phase) after the first sampling. Serum samples were obtained from 10 mL of peripheral blood collected in dry tubes after peripheral venipuncture. All samples were transported on ice within 6 hours to the Laboratory for Study of Emerging Viruses at the Biology Institute of the University of Campinas. All samples were processed and tested for ZIKV on real-time reverse transcription polymerase chain reaction (RT-PCR) [17], and for DENV by rapid diagnostic tests, that is a solid-phase immunochromatography test for DENV IgG/IgM detection (Biopix, São Paulo, Brazil). All ZIKV-positive patients were DENV IgM negative. Healthy samples from 4 donors were included and prescreened for presence of ZIKV viral RNA and ZIKV-specific antibodies.
Three pregnant women from the Singapore ZIKV cohort were enrolled into the study upon admission into the KK Women’s and Children’s Hospital, Singapore. Hematological and biochemistry laboratory tests were performed in parallel with ZIKV-specific RT-PCR upon admission [17]. ZIKV infection was confirmed by positive ZIKV RT-PCR result in whole blood. Whole blood specimens were obtained at 2 collection time points: acute (0–7 days after illness onset) and convalescent (>10 days after illness onset) phases. Plasma samples were obtained from 2 mL of whole blood collected in ethylenediaminetetraacetic acid Vacutainer tubes (Becton Dickinson) after peripheral venipuncture.
Twelve DENV-infected patients, selected from a group of 400 patients recruited at the Communicable Disease Centre, Tan Tock Seng Hospital, Singapore between January 2010 and September 2012, were included in this study for epitope screening comparison [18, 19]. Febrile patients were diagnosed to be DENV-infected by positive detection of DENV nonstructural protein 1 (NS1) antigen and DENV RNA. Plasma specimens were collected from 12 DENV-infected patients at median 3.5 days after illness onset [19], aliquoted and stored at −80°C until use.
Immunological Analyses
ZIKV virion-based ELISA was performed as described previously with modifications [20]. Briefly, Polystyrene 96-well microtiter plates (MaxiSorp, Nunc) were coated with purified ZIKV (20 000 virions per µL in PBS; 50 µL per well). Wells were blocked with phosphate-buffered saline (PBS) containing 0.05% Tween-20 and 5% nonfat milk (PBST-milk), and incubated for 1.5 hours at 37°C. Serum samples were then diluted 1:200 with PBST-milk and incubated for 1 hour at 37°C. Horseradish peroxidase-conjugated goat antihuman IgG and IgM (Invitrogen) were used to detect human antibodies bound to virus-coated wells. Reactions were developed using 3,3',5,5'-tetramethylbenzidine substrate (Sigma-Aldrich) and terminated by Stop reagent (Sigma-Aldrich). Absorbance was measured at 450 nm. The healthy donors sample pool was used as control. ELISA readings were done in duplicates.
Peptide-based ELISA for epitope screening was first performed on synthesized biotinylated peptides library (Mimotopes) consisting of 18-mer overlapping peptides generated from ZIKV Polynesian isolate (KJ776791) and the consensus sequence of DENV1 strains (KP406801, KJ649286, JQ675358, JN697058, JF459993, GQ398255, KC762654, KJ726664, KJ189347, HG316481, and JX669475) as previously described [20]. Due to a nation-wide vaccination program against yellow fever in Brazil [21], cross-reactive antibodies induced by yellow fever vaccination are expected to cause high levels of background signal in our study. Therefore, a biotinylated peptide library generated from yellow fever virus (YFV strain 17D) was synthesized for background signal detection against ZIKV-infected patient samples from Brazil in this study. After the full library screening, 13 peptide sequences were selected from the full peptide library and resynthesized for further validation screening (EMC microcollections GmbH).
Computational Modeling
Structural data of the envelope (E) glycoproteins and NS1 protein were retrieved from Protein Data Bank (PDB) (identifiers 5IZ7 and 5K6K) and visualized using the University of California San Francisco (UCSF) CHIMERA software as described [22]. Structures of membrane (prM) sequences were predicted separately using individual I-TASSER queries, and visualized using UCSF Chimera software as described [22]. Sequence similarity was calculated using DNASTAR Lasergene 15 software.
Data Analysis and Statistical Analysis
All data are presented as either mean or mean ± standard deviation. Differences in specific antibody recognition responses among patient groups between different peptides were analyzed using Mann-Whitney U tests. Differences were considered statistically significant at P < .05. The post hoc power analysis was done in G*Power version 3.1.9.3 using Mann-Whitney U test as the test of interest with an alpha of 0.05. The effect sizes were computed from the results in this study and used to estimate the power achieved. Heat-map and plots were done using GraphPad Prism version 7.
RESULTS
Antibody Profiles of ZIKV-Infected Patients in Campinas, Brazil
Between February and August 2016, 51 patients were recruited in this study based on their clinical symptoms during hospital admission (Table 1 and Figure 1A) [16]. All febrile patients’ sera were screened by quantitative RT-PCR (qRT-PCR) and/or an in-house anti-ZIKV serology ELISA (Supplementary Table 1) [16]. The majority of the nonpregnant adult patients (32 out of 44) were anti-ZIKV IgG positive during the first 10 days after illness onset (Figure 1A). In line with the observation that ZIKV is generally self-limiting with mild symptoms, the majority of the nonpregnant adult patients (42 out of 44) were observed to display mild symptoms with no neurological complications during the acute phase of the infection. All ZIKV-positive patients included in this study were negative for DENV-IgM in a solid-phase immunochromatography test (Biopix, São Paulo, Brazil).
Demographics and Characteristics of ZIKV-Infected Patients Admitted to University of Campinas Hospital and Women’s Comprehensive Healthcare Center and Enrolled in This Study (February 2016–August 2016)
| Characteristics . | Patients (n = 51)a . |
|---|---|
| Age, median (range), years | 35 (18–66) |
| Sex ratio, male/female | 0.5 (17 M / 34 F) |
| No. of pregnant women | 7 |
| Newborns with fetal growth restriction, n (%) | 1 (14.3)b |
| Length of admission, median (range), days | 3 (1–10) |
| Viral load in urine, mean ± SD, CT | 36.83 ± 0.67 |
| Viral load in blood, mean ± SD, CT | 36.65 ± 0.95 |
| Fever, n (%) | 34 (66.6) |
| Body temperature, range, °C | 38–40 |
| Skin rash, n (%) | 42 (82.4) |
| Conjunctivitis, n (%) | 21 (41.2) |
| Neurological syndrome, n (%) | 2 (3.9) |
| Anti-ZIKV IgG positivity, n (%) | 39 (76.5) |
| Characteristics . | Patients (n = 51)a . |
|---|---|
| Age, median (range), years | 35 (18–66) |
| Sex ratio, male/female | 0.5 (17 M / 34 F) |
| No. of pregnant women | 7 |
| Newborns with fetal growth restriction, n (%) | 1 (14.3)b |
| Length of admission, median (range), days | 3 (1–10) |
| Viral load in urine, mean ± SD, CT | 36.83 ± 0.67 |
| Viral load in blood, mean ± SD, CT | 36.65 ± 0.95 |
| Fever, n (%) | 34 (66.6) |
| Body temperature, range, °C | 38–40 |
| Skin rash, n (%) | 42 (82.4) |
| Conjunctivitis, n (%) | 21 (41.2) |
| Neurological syndrome, n (%) | 2 (3.9) |
| Anti-ZIKV IgG positivity, n (%) | 39 (76.5) |
Abbreviation: CT, cycle threshold; ZIKV, Zika virus.
aZIKV positivity were confirmed by reverse transcription polymerase chain reaction as described previously [16].
bPercentage is based on the number of pregnant women enrolled in this study.
Demographics and Characteristics of ZIKV-Infected Patients Admitted to University of Campinas Hospital and Women’s Comprehensive Healthcare Center and Enrolled in This Study (February 2016–August 2016)
| Characteristics . | Patients (n = 51)a . |
|---|---|
| Age, median (range), years | 35 (18–66) |
| Sex ratio, male/female | 0.5 (17 M / 34 F) |
| No. of pregnant women | 7 |
| Newborns with fetal growth restriction, n (%) | 1 (14.3)b |
| Length of admission, median (range), days | 3 (1–10) |
| Viral load in urine, mean ± SD, CT | 36.83 ± 0.67 |
| Viral load in blood, mean ± SD, CT | 36.65 ± 0.95 |
| Fever, n (%) | 34 (66.6) |
| Body temperature, range, °C | 38–40 |
| Skin rash, n (%) | 42 (82.4) |
| Conjunctivitis, n (%) | 21 (41.2) |
| Neurological syndrome, n (%) | 2 (3.9) |
| Anti-ZIKV IgG positivity, n (%) | 39 (76.5) |
| Characteristics . | Patients (n = 51)a . |
|---|---|
| Age, median (range), years | 35 (18–66) |
| Sex ratio, male/female | 0.5 (17 M / 34 F) |
| No. of pregnant women | 7 |
| Newborns with fetal growth restriction, n (%) | 1 (14.3)b |
| Length of admission, median (range), days | 3 (1–10) |
| Viral load in urine, mean ± SD, CT | 36.83 ± 0.67 |
| Viral load in blood, mean ± SD, CT | 36.65 ± 0.95 |
| Fever, n (%) | 34 (66.6) |
| Body temperature, range, °C | 38–40 |
| Skin rash, n (%) | 42 (82.4) |
| Conjunctivitis, n (%) | 21 (41.2) |
| Neurological syndrome, n (%) | 2 (3.9) |
| Anti-ZIKV IgG positivity, n (%) | 39 (76.5) |
Abbreviation: CT, cycle threshold; ZIKV, Zika virus.
aZIKV positivity were confirmed by reverse transcription polymerase chain reaction as described previously [16].
bPercentage is based on the number of pregnant women enrolled in this study.
![Antibody profiles of Zika virus (ZIKV)-infected patients. A, ZIKV IgG antibody detection in ZIKV-infected nonpregnant adult serum samples (n = 44) during the acute phase of disease, at a dilution of 1:200 were determined by ZIKV virion-based enzyme-linked immunosorbent assay (ELISA). Dotted line represents the mean value of the healthy donors and data are presented as mean. Serum samples were then pooled from 32 anti-ZIKV IgG-positive nonpregnant adults (solid circle) and were subjected to ZIKV and dengue virus (DENV) peptide-based ELISAs corresponding to the antigens (B) membrane (prM) protein, (C) envelope (E) glycoprotein, and (D) nonstructural protein 1 (NS1) protein. Data were normalized and expressed as specific antibody recognition against ZIKV and DENV peptides from the same region, as described in Supplementary Figure 1. Data were further expressed as antibody recognition fold difference between ZIKV and DENV peptides from the same region (fold = OD from ZIKV peptide − OD from yellow fever virus [YFV] peptide / OD from DENV peptide − OD from YFV peptide) and are presented in heat-map format with red representing stronger antibody recognition on ZIKV peptides (ZIKV prefer) and yellow representing stronger antibody recognition on DENV peptides (DENV prefer). The regions of protein found to be important for antibody recognition against ZIKV peptide are indicated in red below the heat map. The numbers correspond to the amino acid positions along the ZIKV antigens and the first amino acid from each antigen is annotated as 1. Results are means from 2 independent experiments.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jid/220/2/10.1093_infdis_jiz092/1/m_jiz092f0001.jpeg?Expires=1750192152&Signature=KtLWm11vVhTolX4OSFqmU-OH~hUJHyf55F3Xu8L7Bgy6mLCv0IlKzUZwYSCSZRebzyf5QhQYr~o-P30Y0wqRMQkD-jgshthhjlzI3qmcKh8DbKhJixIOVy7oXqO0w7vAxkwA45o6zFOE0--9UkMK9xLNvRh7S~cy0C3HFgp1i4Kw49LjcyN1X6wZUqfK6JTmlZWLR95rSjozCA35KoMs7kvBV-SbB8hhwpdIDgFqpahqJn-z1l7NnyvANnTVql4WJ8AyRxvuZ8H6AmRPw~mK45Zj0-VNwd5YDPCb8YQCEiwdBFpdthgsudaWNjnBXCk6WR3kCvmEun5~XwP9VEaiug__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Antibody profiles of Zika virus (ZIKV)-infected patients. A, ZIKV IgG antibody detection in ZIKV-infected nonpregnant adult serum samples (n = 44) during the acute phase of disease, at a dilution of 1:200 were determined by ZIKV virion-based enzyme-linked immunosorbent assay (ELISA). Dotted line represents the mean value of the healthy donors and data are presented as mean. Serum samples were then pooled from 32 anti-ZIKV IgG-positive nonpregnant adults (solid circle) and were subjected to ZIKV and dengue virus (DENV) peptide-based ELISAs corresponding to the antigens (B) membrane (prM) protein, (C) envelope (E) glycoprotein, and (D) nonstructural protein 1 (NS1) protein. Data were normalized and expressed as specific antibody recognition against ZIKV and DENV peptides from the same region, as described in Supplementary Figure 1. Data were further expressed as antibody recognition fold difference between ZIKV and DENV peptides from the same region (fold = OD from ZIKV peptide − OD from yellow fever virus [YFV] peptide / OD from DENV peptide − OD from YFV peptide) and are presented in heat-map format with red representing stronger antibody recognition on ZIKV peptides (ZIKV prefer) and yellow representing stronger antibody recognition on DENV peptides (DENV prefer). The regions of protein found to be important for antibody recognition against ZIKV peptide are indicated in red below the heat map. The numbers correspond to the amino acid positions along the ZIKV antigens and the first amino acid from each antigen is annotated as 1. Results are means from 2 independent experiments.
Differential Epitopes Recognition in ZIKV-Infected Adult Patients
To identify the epitopes recognized in ZIKV-infected patients, 3 immunodominant antigens (prM, E, and NS1) were selected because they have been found to elicit host adaptive immune responses during viral infection and have been demonstrated to be the targets of humoral immunity in humans [23, 24]. Epitope recognition profiles of ZIKV-infected patients were first assessed using pooled serum samples from 32 anti-ZIKV IgG-positive patient samples collected during the acute phase of disease (Figure 1B–1D, Supplementary Figure 1, and Supplementary Table 2). Although low levels of background reactivity to YFV were detected, results did not change the epitope identification of this study (Supplementary Table 2). Multiple linear epitopes were identified from several immunodominant antigens: 6 peptide regions from prM, 3 peptide regions from E, and 2 peptide regions from NS1 (Figure 1B–1D). These ZIKV peptide regions were recognized by pooled serum samples with intensity stronger than the recognition of corresponding peptide regions on DENV. Therefore, these peptide regions were specifically recognized by antibodies of ZIKV-infected patients.
Next, specific epitope-containing peptides were resynthesized and tested against the current ZIKV-infected patient cohort and compared with DENV-infected patient samples that were collected at a similar disease phase after illness onset (median 3.5 days) between 2010 and 2012, more than 4 years before the emergence of the ZIKF epidemic [19]. Three peptide sequences (ZIKV E peptide 27, ZIKV NS1 peptide 86, and ZIKV NS1 peptide 117, amino acid residues 49–66, 17–34, and 257–274, respectively) were specifically recognized by ZIKV-infected patient samples but not DENV-infected patient samples (Figure 2A and 2B, and Supplementary Table 3). ZIKV-infected patient samples showed a higher level of recognition on another peptide sequence, ZIKV E peptide 29 (amino acid residues 65–82) relative to the corresponding DENV peptide sequence (Figure 2A and Supplementary Table 3). Interestingly, DENV-infected patients’ samples specifically recognized 2 peptide regions (DENV prM peptide 8 and ZIKV prM peptide 13, amino acid residues 57–73 and 97–114, respectively) from the prM protein but not from the other antigens (Figure 2C and Supplementary Table 3). Importantly, peptide region ZIKV prM peptide 8 with amino acid residues 57–74 (corresponding peptide of DENV prM peptide 8 with amino acid residues 57–73) was specifically recognized by ZIKV-infected patient samples (Figure 2C and Supplementary Table 3). This suggests that these 6 regions could contain differential epitopes to distinguish between ZIKV and DENV infections. In summary, our results showed that ZIKV-infected and DENV-infected patients can be serologically differentiated using specific epitopes from various immunodominant antigens.

Differential pattern of epitope recognition between Zika virus (ZIKV)-infected and dengue virus (DENV)-infected patients. Selected peptides were resynthesized and used as indicated to perform enzyme-linked immunosorbent assays (ELISAs). Individual ZIKV-infected nonpregnant adult serum samples (n = 32) and DENV-infected nonpregnant adult serum samples (n = 12) were subjected to ZIKV and DENV peptide-based ELISAs corresponding to the antigens: (A) envelope (E) glycoprotein, (B) nonstructural protein 1 (NS1) protein, and (C) membrane (prM) protein. Due to possible noise signal from yellow fever virus (YFV) vaccination, data from ZIKV-infected nonpregnant adult serum samples were first subtracted with signal against corresponding YFV peptides (OD from ZIKV peptide − OD from YFV peptide) and expressed as specific antibody recognition to identify the specific response without noise signal due to YFV vaccination. Next, data from DENV-infected nonpregnant adults and data from subtracted ZIKV-infected nonpregnant adults were normalized as fold change relative to the healthy controls and are presented as mean ± SEM. Yellow bars represent signal against DENV peptides and blue bars represent signal against ZIKV peptides. Dotted line represents the antibody recognition level from healthy donors. Results are averages of 2 independent experiments. Statistical significance was measured using Mann–Whitney U test. *, P < .05; **, P < .01; ***, P < .001.
Specific Epitope Recognition by Antibodies from ZIKV-Infected Pregnant Women
Fetal development malformations have been reported in a significant proportion of ZIKV-infected pregnant women and, hence, early diagnostic research is imperative before the next wave of ZIKV outbreaks [7, 8, 16]. In this study, 7 ZIKV-infected pregnant women were enrolled. The median age of this patient group was 25 years (interquartile range, 20–30 years), and the difference in age of patients between this patient group and the nonpregnant adults cohort (median age, 35; interquartile range, 18–66 years) was not significant. Out of the 7 pregnant woman, 1 woman was later found to carry a baby with fetal growth associated malformations [16]. All pregnant women were anti-ZIKV IgG positive at acute and convalescent phases (Figure 3A).
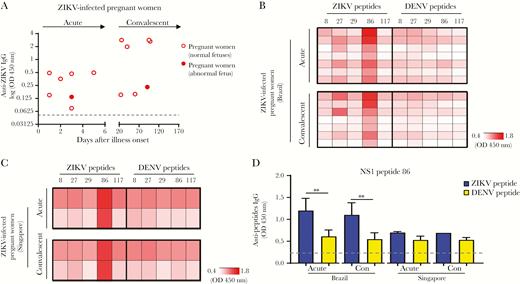
Specific epitope recognition in Zika virus (ZIKV)-infected pregnant women from different geographic locations. A, ZIKV IgG antibody detection in Brazil ZIKV-infected pregnant women (6 ZIKV-infected pregnant women with normal fetuses and 1 ZIKV-infected pregnant woman whose fetus had congenital anomalies), during the acute and convalescent phases of disease, at a dilution of 1:200 were determined by ZIKV virion-based enzyme-linked immunosorbent assay (ELISA). Dotted line represents the mean value of the healthy donors and data are means. B, Serum samples from 7 ZIKV-infected pregnant women from the Brazil ZIKV cohort, collected during the acute and convalescent phases of disease, were subjected to ZIKV and dengue virus (DENV) peptide-based ELISAs corresponding to the antigens membrane (prM) protein, envelope (E) glycoprotein, and nonstructural protein 1 (NS1) protein. C, Serum samples from 2 ZIKV-infected pregnant women from the Singapore ZIKV cohort, collected during acute and convalescent phases of disease, were subjected to the same peptide-based ELISAs. Due to the possible noise signal from yellow fever virus (YFV) vaccination, data from Brazil ZIKV-infected pregnant women serum samples were first subtracted with signal against corresponding YFV peptides (OD from ZIKV peptide − OD from YFV peptide) and expressed as specific antibody recognition in order to identify the specific response without the noise signal due to YFV vaccination. Next, data are presented in heat-map format, with white representing low signal and red representing high signal against the peptides. Results are averages of 2 independent experiments. D, Specific ZIKV epitope recognized by ZIKV-infected pregnant women was identified along the NS1 protein region. Abbreviation: Con, convalescent. Antipeptide antibody responses are presented as mean ± SD. Statistical significance was measured using Mann–Whitney U test. ***P < .001.
ZIKV epitopes that were detected exclusively by ZIKV-infected adult patients were selected (Figure 1B–1D) and screened with samples from ZIKV-infected pregnant women with positive anti-ZIKV IgG antibody response (Figure 3A). Interestingly, only 1 specific epitope (ZIKV NS1 peptide 86 with amino acid residues 17–34) was recognized by antibodies from ZIKV-infected pregnant women (Figure 3B and Supplementary Table 3), at a higher level of recognition than the other selected epitopes. To further validate the versatility of this specific epitope as an early serological target, we screened plasma samples from ZIKV-infected pregnant women collected from a separate cohort in Singapore during the ZIKF outbreaks in 2016 (Supplementary Figure 2A) [25]. Two out of 3 ZIKV-infected pregnant women from the Singapore ZIKV cohort showed positive anti-ZIKV IgG antibody response during the acute and convalescent phase of disease and samples from these 2 anti-ZIKV IgG-positive pregnant women were used for subsequent peptide screening (Supplementary Figure 2B and 2C). Peptide screening assays further demonstrated that ZIKV-infected pregnant women from both the Brazil and Singapore ZIKV cohorts showed a higher level of recognition for the ZIKV NS1 peptide 86 (amino acid residues 17–34), as compared to the corresponding peptide from DENV (Figure 3C–3D). Similar levels of antibodies that recognized this epitope were present at both the acute and convalescent phase of disease (Figure 3D). Thus, our results suggested that antibodies against ZIKV NS1 peptide 86 appear to be a common early marker for ZIKV infections, especially in ZIKV-infected pregnant women. The reason for a difference in epitope recognition between nonpregnant adults and pregnant women is currently unknown. There is limited information about the effect of pregnancy on the profiles of epitope-specific antibody expression during infections. This observation may be related to the immunologic alternations and modulation of immune status locally or systemically during pregnancy [26–28].
DISCUSSION
According to the World Health Organization research and development blueprint 2018, ZIKF is a prioritized disease as it has the potential to cause serious international epidemics [29]. Brazil has declared an end to the recent ZIKV epidemic as the number of reported cases has decreased significantly since the later part of 2016, and our previous study has reported the immune profiles of ZIKV-infected patients, including pregnant women carrying fetuses with congenital CNS abnormalities [16]. In this study, 51 patient samples (44 adult patients and 7 pregnant women) collected from the same cohort were screened against an overlapping peptide library, in order to determine the potential ZIKV epitopes in ZIKV-infected patients.
This study showed that all 3 immunodominant antigens (prM, E, and NS1) carry distinct epitopes that were recognized by the sera of ZIKV-infected and DENV-infected patient cohorts (Figure 4A and Supplementary Figure 3). To locate the spatial position of the identified epitopes, epitope-containing sequences were then mapped onto predicted 3-dimensional structures of the ZIKV prM proteins or the available 3-dimensional crystal structures of the ZIKV E glycoprotein and NS1 protein (PDB identifier 5IZ7 and 5K6K) [22]. Using different approaches, other epitopes have also been reported [30]. Based on the available information, the 3 immunodominant antigens are structurally similar between the 2 closely related flaviviruses [31–33]. The sequence similarity of identified epitopes between ZIKV and DENV is at relatively moderate level (Figure 4A) and mapping results showed that all the epitope sequences are located in a solvent-exposed region (Figure 4B–4D). Moreover, a separate study using computational approaches also identified epitopes from the E glycoprotein and NS1 protein located at the surface exposed sites [34]. This complements the results where epitopes identified in this study had a sufficient level of exposure for antibody interaction.
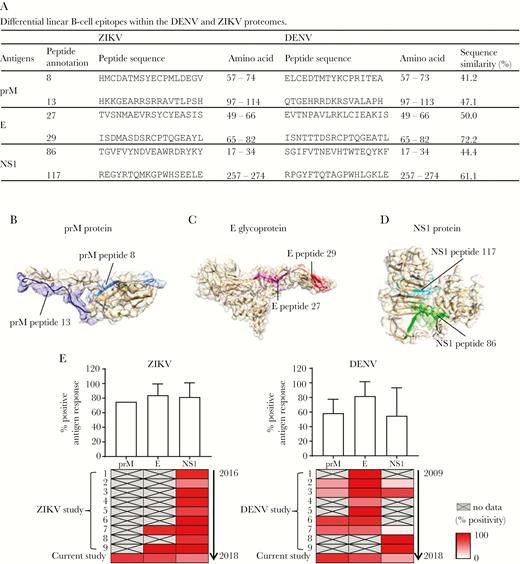
Mapping of epitopes onto Zika virus (ZIKV) membrane (prM), envelope (E), and nonstructural protein 1 (NS1) structures. A, Differential linear B-cell epitopes within the dengue virus (DENV) and ZIKV proteomes are listed. Amino acid numbers correspond to the amino acid positions along the antigens and the first amino acid from each antigen is annotated as 1. B–D, Schematic representations of identified epitope position determined by peptide enzyme-linked immunosorbent assay (ELISA) in the ZIKV prM protein, E glycoprotein, and NS1 protein. Epitopes in the prM protein were located based on structures predicted by the I-TASSER. Epitopes in E glycoprotein and NS1 protein were located based on structural data retrieved from Protein Data Bank records 5IZ7 and 5K6K, respectively. E, Antigenic response profiles in ZIKV-infected and DENV-infected patients from 18 independent published cohort studies and the current Brazil ZIKV-infected patients and Singapore DENV-infected patients. The average percentage of patients showing positive antibody response against the respective antigens are represented by the bar chart (upper panel). The heat map shows the percentage of patients with positive antibody response against the respective antigens reported from the individual study, with data arranged in chronological order (lower panel).
ZIKV infections in geographically different areas have been widely reported in recent years. Therefore, we sought to collate the data from studies reporting antibody response in different ZIKV cohorts, and compared the data with the findings from our current study (Supplementary Table 4). Studies reporting antibody response in different DENV cohorts were also collated for comparison (Supplementary Table 4). The pooled data sets suggested that all 3 antigens gave a high level of positive antibody response even from different patient cohorts (Figure 4E). A similar observation was obtained between ZIKV and DENV cohorts. A similar pattern of antigen recognition was also observed from ZIKV-infected patients in the Singapore cohort (S. N. Amrun, W. X. Yee, F. A. Bakar, et al, manuscript submitted).
At present, there is no published report demonstrating the presence of antibody response against ZIKV prM in ZIKV-infected patients (Figure 4E). In contrast, similar studies reported the presence of antibodies against DENV prM proteins in DENV-infected patients, based on immune sera and monoclonal antibody generation from the B-cell population of DENV-infected patients [35, 36]. Our study also identified 1 peptide region (ZIKV prM peptide 8/DENV prM peptide 8) from prM proteins, strongly and specifically recognized by ZIKV-infected patients and DENV-infected patients, respectively (Figure 2C). Virus neutralizing and infection enhancing activities mediated by anti-prM antibody in DENV-infected patients have also been demonstrated, thus suggesting the complexity of the role of anti-prM antibody in flavivirus disease. In addition, a single mutation in the prM, apparently present in noninfectious virions, contributes to fetal microcephaly, indicating that anti-prM antibodies can contribute to ZIKV pathogenesis [37]. Thus, further studies need to be carried out to understand the differential pattern of anti-prM antibody response between closely related flavivirus infections.
In this serological analysis, anti-NS1 antibody was detected in our patient cohort, in both ZIKV-infected nonpregnant adults and ZIKV-infected pregnant women (Figures 2, 3, and 4E). This is consistent with findings reported from other cohorts, which also claim that anti-NS1 antibodies have a protective effect against flavivirus infections (Supplementary Table 4). However, a recent study using the B cells of DENV-infected patients demonstrates the relationship between the presence of anti-NS1 antibody and increased disease severity [38]. This could be due to the cross-reactivity of DENV NS1 antibody with endothelial cells, which may in turn affect the integrity of the vascular system [39]. The phenomenon of cross-reactivity with the endothelial cells can be explained by a previous study that suggested this interaction is due to the presence of common epitopes between the flavivirus NS1 antigen and human cellular proteins, including integrin/adhesion proteins on endothelial cells [40]. Although we do not observe a similar correlation between anti-NS1 antibody and disease severity in our ZIKV cohort, further studies carried out with more cohorts will be important in identifying the pathogenic/protective role of anti-NS1 antibody in ZIKV (or flavivirus) pathogenesis. This will provide important information to facilitate vaccine development against ZIKV.
ZIKV-infected nonpregnant adults from Brazil ZIKV cohorts recognized multiple epitopes from the 3 immunodominant antigens with differential recognition capacities for ZIKV and other closely related flaviviruses (Figure 2). However, ZIKV-infected pregnant women from both the Brazil and Singapore ZIKV cohorts showed a higher level of recognition only for the ZIKV NS1 peptide 86, as compared to the corresponding peptide from DENV (Figure 3C–3D). This suggests that the pattern of epitope-specific antibody expression changes under specific physiological conditions. This observation may be related to the immunologic alternations and modulation of host serum components (for example blood lipoprotein) during pregnancy [26, 27, 41]. Although little is known about the effect of lipoprotein expression in patients infected by flaviviruses, the association between the immunodominant antigens and lipoprotein might affect the accessibility of epitopes [41]. Alternatively, a recent study shows that an Asian strain of ZIKV infection leads to an exacerbate immunosuppression during pregnancy by a phenomenon dependent of polarization of M2 macrophages [28], This differential immunomodulatory response of blood monocytes after strain-specific ZIKV infection during pregnancy could bias the epitope-specific antibody production in pregnant women. Establishing the effect of pregnancy on immunological status during ZIKV infections will be of interest in the future.
While the present study presents interesting information on the identification of specific peptide regions for differentiation between dengue and Zika infection, some limitations remain due to the small patient population. Moving forward, independent patient cohorts will further strengthen the robustness of the identified viral epitopes for virus differentiation. Moreover, modification of peptide-based formats could further improve ELISAs in the future.
In conclusion, antibodies against ZIKV NS1 peptide 86 could serve as a common early marker for ZIKV infections, especially in ZIKV-infected pregnant women. This epitope screening results can be used to improve diagnostic efficiency and clinical management in the face of new outbreaks.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank the study participants and healthy volunteers for their participation and clinical staff from University of Campinas Hospital (HC Unicamp) and Women’s Comprehensive Healthcare Center, Brazil for assistance in patient enrollment and care, blood sample preparation, study coordination, and data entry. The authors acknowledge the clinical and research staff from the Communicable Disease Centre/Tan Tock Seng Hospital and KK Women’s and Children’s Hospital, Singapore for assistance in blood sample preparation, for patient enrollment and care, study coordination, and data entry. We acknowledge Bernett Lee for critical discussion of the manuscript.
Disclaimer. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author contributions. Y. W. K. and J. A. L. performed immunological peptide-based assays. L. F. P. N., L. R., J. L. P. M., and F. T. M. C. conceptualized the study. D. C. L., Y. S. L., C. Y. C., A. R. R. F., G. P. M., J. L. P. M., L. F. P. N., F. T. M. C., and Z. U. N. contributed materials. Y. W. K., L. R., L. F. P. N., and F. T. M. C. analyzed the data. Y. W. K., J. A. L., S. N. A., F. M. L., W. X. Y., F. A. B., K. E. E., J. L. P. M., L. F. P. N., and F. T. M. C. wrote the manuscript. All authors read and approved of the manuscript.
Zika-Unicamp Network. Eliana Amaral, Renato Passini Junior, Helaine Maria Besteti Pires Mayer-Milanez, Carolina C. Ribeiro-do-Valle, Roseli Calil, Maria Laura Costa, João Renato Bennini Junior, Giuliane Jesus Lajos, Marcia Teixeira Garcia, Kleber Yotsumoto Fertrin, Maria Luiza Moretti, Mariangela Ribeiro Resende, Rodrigo Angerami, Gabriela Mansano do Nascimento, Leonardo Cardia Caserta, Carla Cristina Judice, Ana Lucia Rodrigues Soledade, Matheus Martini, Carla C Judice, Daniel Augusto de Toledo-Teixeira, Pierina Lorencini Parise, and Mariene Ribeiro Amorim.
Financial support. This work is supported by the Biomedical Research Council (BMRC) core research grants to the Singapore Immunology Network and the Zika Virus Consortium Fund, led by BMRC A*Star (project number 15/1/82/27/001); Agency for Science, Technology and Research, Singapore; Fundação de Amparo à Pesquisa do Estado de São Paulo (grant numbers 2016/00194-8 and 2013/25807-4 fellowship to J. A. L.); and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (research fellowships to G. P. M. and F. T. M. C.).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
Author notes
F. T. M. C and L. F. P. N. contributed equally to this report.




