-
PDF
- Split View
-
Views
-
Cite
Cite
Yehudit Michelson, Yaniv Lustig, Shira Avivi, Eli Schwartz, Amos Danielli, Highly Sensitive and Specific Zika Virus Serological Assays Using a Magnetic Modulation Biosensing System, The Journal of Infectious Diseases, Volume 219, Issue 7, 1 April 2019, Pages 1035–1043, https://doi.org/10.1093/infdis/jiy606
Close - Share Icon Share
Abstract
Zika virus has created global alarm because it has been associated with catastrophic fetal abnormalities, including microcephaly, spontaneous abortion, and intrauterine growth restriction. Current serological assays that detect antiviral antibodies suffer from low sensitivity and high cross-reactivity among different flaviviruses.
In this study, utilizing a novel magnetic modulation biosensing (MMB) system and the Zika nonstructural 1 protein, we show highly sensitive and specific Zika serological assays. We blindly tested 60 reverse-transcription polymerase chain reaction Zika-positive samples and healthy patients’ serum samples, as well as 44 serum samples from enzyme-linked immunosorbent assay (ELISA) West Nile- and dengue-positive patients. The Zika-positive samples were collected from Israeli travelers returning from Zika-endemic areas.
The MMB Zika assays have 88%–97% sensitivity, much higher than the current state-of-the-art EUROIMMUN ELISA assays (38%–74%). In addition, the specificity is 100%, and the cross-reactivity with West Nile and dengue viruses is minimal (0%–4%). Furthermore, the MMB assays detected Zika IgM antibodies as early as 5 days and as late as 180 days postsymptoms onset, significantly extending the number of days that the antibodies are detectable.
The sensitivity, specificity, and simplicity of the MMB assays may significantly improve Zika diagnosis and provide accurate results for public health agencies.
Zika virus (ZIKV)—a Flavivirus in the Flaviviridae family—was first introduced into Brazil during 2014 from the Pacific Islands and spread rapidly throughout the Americas [1]. Zika infection in pregnant women is a major concern, because it is linked to catastrophic fetal abnormalities [1–3]. Hence, the ability to accurately diagnose ZIKV, especially in pregnant women, is highly important [1]. Zika virus is most commonly transmitted by mosquitos, but recently it was shown to also be spread through sexual transmission [4], and therefore accurate diagnosis is important for both spouses.
Current ZIKV diagnostic tests detect either the viral components in the body fluids [5] or the antiviral antibodies, namely, immunoglobulin (Ig)M and IgG. Viral components can be detected by virus isolation [6] or quantitative reverse-transcription polymerase chain reaction (qRT-PCR) [7]. These methods are highly specific and indicate acute-phase infections [1, 8]. Nevertheless, the tests are laborious and time consuming, and they are not applicable in low-resource settings. In addition, ZIKV usually disappears from the blood a few days after the onset of symptoms [7], and therefore these tests are effective only within a short period of time. For these reasons, indirect serologic tests were developed, such as an enzyme-linked immunosorbent assay (ELISA) and plaque-reduction neutralization test that detects virus-specific neutralization antibodies [9]. These methods are not limited to the period in which the virus is still in the blood stream, and they constitute the bulk of the workload of many virology laboratories [10].
The major drawbacks of serological tests, such as IgM/IgG ELISA and neutralization assays, are reduced clinical sensitivity [10], high cross-reactivity [11], and lengthy and laborious protocols. They are considered unreliable in identifying ZIKV infections for several reasons. First, antibody concentrations can be very low and difficult to detect by standard ELISA tests. In particular, previous exposure to other Flaviviruses [10] or the presence of secondary infections [12] can result in low concentrations of IgM antibodies. For example, the IgM sensitivity of standard ELISA tests in Zika-infected patients from nonendemic areas is 87.5%, which is much higher than the IgM sensitivity in Zika-infected patients who reside in endemic areas (31.6%) [11]. Second, extensive cross-reactivity among Flaviviruses causes false-positive results [13]. Third, the ongoing use of vaccines for dengue, yellow fever, and Japanese encephalitis increases the level of antibodies to these viruses in the blood, which may also increase the number of false-positive results [7].
Several commercial Zika serological tests are now available, including the IgM antibody capture ELISA (MAC-ELISA) (eg, InBios), which utilizes the envelope protein of the virus as a capture antigen [14]. Due to the similarity between the envelope proteins of the Flavivirus family members, false-positive outcomes are common [9]. To overcome this challenge, researchers are using the nonstructural 1 (NS1) protein as a capture antigen [11, 15], which is more specific [11, 16]. However, low levels of IgG/IgM antibodies against the NS1 protein are produced, which can result in a higher rate of false-negative results [17]. Moreover, the above-mentioned tests must be performed in a laboratory setting. Currently, there are no commercial in vitro diagnostic products to detect the ZIKV at the point of care in low-resource settings [18]. To increase access to ZIKV testing and enhance clinically based diagnosis in regions without access to well equipped laboratories, new technologies must be developed.
In this study, we evaluate novel ZIKV serological assays, utilizing the highly specific NS1 antigen and a novel and highly sensitive detection technology—named magnetic modulation biosensing (MMB)—that significantly improves clinical performance. Magnetic modulation biosensing utilizes magnetic beads that are conjugated to NS1 protein, which specifically captures the Zika IgM/IgG antibodies. A second, fluorescently labeled antibody is then added to form a “sandwich” assay with the analyte and the capture protein (Supplementary Material, Supplementary Figure S1). Although the principles of the MMB technology have been described before [19–21], it has not been applied to serological assays, nor has it been used in clinical applications.
To demonstrate the clinical utility of the MMB technology, the MMB Zika IgM and IgG assays were utilized to blindly test serum samples from qRT-PCR Zika-positive patients and healthy patients. Cross-reactivity was evaluated by testing serum samples from dengue virus (DENV)- and West Nile virus (WNV)-positive patients.
MATERIAL AND METHODS
Sample Collection
A total of 36 serum samples from 20 qRT-PCR or neutralization Zika-positive patients, 22 serum samples from WNV-positive patients, 22 serum samples from dengue-positive patients, and 24 serum samples from healthy patients were obtained from the National Center for Zoonotic Viruses at the Central Virology Laboratory of the Ministry of Health at Sheba Medical Center, Israel. The Zika-positive samples were collected from Israeli travelers presenting at the Institute of Tropical Medicine at Sheba Medical Center after returning from Zika-endemic areas. Zika virus qRT-PCR was adopted from the method established during the ZIKV outbreak in Micronesia [22]. For neutralization, the samples were subjected to a microneutralization assay (with 100TCID50 Zika MR-677 and a cutoff value of 1:10), which was shown to be comparable to PRNT90 [23, 24]. The dengue-positive samples were collected from either Israeli or German travelers returning from dengue-endemic areas. Dengue-positive samples that were collected in Israel were diagnosed using DENV IgM and IgG antibody capture ELISA (Panbio, Brisbane, Australia) and dengue qRT-PCR [25] or DENV NS1 antigen (Panbio). Dengue-positive samples that were collected in Germany were diagnosed using PCR (altona Diagnostics), NS1-ELISA (Bio-Rad), and IgM- and IgG-IIFA (in-house indirect immunofluorescence assay) [25]. West Nile virus-positive samples were diagnosed according to the WNV disease definition (ELISA; WNV IgM capture DxSelect ELISA and IgG DxSelect ELISA kits by Focus Diagnostics Inc., Cypress, CA) [23]. Patient information—including age, gender, country of origin, country of acquisition, and the day the sample was taken after the onset of symptoms—was obtained from the electronic medical record. All of the samples (50 µL each) were stored at −20ºC, delivered to Bar-Ilan University on dry ice, and then thawed once for the MMB assay. The study was approved by IRB of Sheba Medical Center.
Serological Assays
Magnetic Modulation Biosensing Immunoglobulin (Ig)M and IgG Anti-Zika Nonstructural 1 Dose Response
To determine the analytical sensitivity, limit of detection (LoD), and dynamic range of the human IgM and IgG anti-Zika NS1 assays, we measured increasing concentrations of recombinant ZIKV antibodies (human IgM and IgG anti-Zika NS1 monoclonal antibodies, MAB12123 and MAB12122, native antigen) in the MMB system. Each assay consisted of ~25000 tosylactivated magnetic beads (M-280; Thermo Fisher Scientific), which were preconjugated to the Zika NS1-Suriname strain (The Native Antigen Company, UK) using Thermo Fisher’s standard coupling procedures. The beads were incubated for 1 hour at room temperature with human IgG or human IgM anti Zika NS1 in concentrations of 0, 101, 102, 103, 104, 105, 106, and 107 ng/L. The initial incubation was followed by incubation with a fluorescently labeled detection antibody. For IgM, we used Anti-Human IgM (µ-chain specific)-Biotin (Sigma-Aldrich Ltd., Rehovot, Israel). After a second wash, the beads were incubated with R-Phycoerythrin-Streptavidin (The Jackson Laboratory). For IgG, we used donkey F(ab’)2 Anti-Human IgG H&L (phycoerythrin [PE]) (Abcam plc., UK). To remove unbound detection antibodies, a single buffer replacement was done after the final incubation. The final solution was then loaded into a borosilicate glass cuvette and measured in the MMB system. The number of repetitions of the blank measurement was 6 (n = 6). The number of repetitions for all other concentrations was 3 (n = 3). The LoD for each set of experiments was calculated as 3 standard deviations over the blank measurement. The reaction buffer solution was a mixture of phosphate-buffered saline (×1), 1 mg/mL bovine serum albumin (VWR), and 0.05% of Tween 20 (Sigma-Aldrich). To compare the analytical performance of the MMB system to the EUROIMMUN ZIKV ELISA, we measured the same concentrations of recombinant ZIKV antibodies using EUROIMMUN ZIKV ELISA (EUROIMMUN, Luebeck, Germany) according to the manufacturer’s recommendations.
Magnetic Modulation Biosensing Zika Immunoglobulin G Clinical Assay
To determine the receiver operating characteristic (ROC) cutoff for Zika IgG assays, we blindly tested 34 Zika-positive samples that were taken from day 7 onward and 22 serum samples from healthy patients. The IgG assay consisted of 25000 beads that were incubated for 1 hour at room temperature with 2 µL of serum sample diluted in buffer. After a washing step, the detection antibody (donkey F(ab’)2 Anti-Human IgG H&L [PE]) was added to the beads, followed by another washing step. Triplicates from the final solution were measured using the MMB system.
Magnetic Modulation Biosensing Zika Immunoglobulin M Clinical Assay
To determine the ROC cutoff for Zika IgM assays, we blindly tested 26 Zika-positive samples that were taken from days 1–60 and 24 serum samples from healthy patients. For the IgM assay, to absorb the interfering IgG antibodies, 3 µL of each serum sample was first diluted in liquid rheumatoid factor (RF) absorbant (EUROIMMUN), incubated at room temperature, and centrifuged at 2000 RPM. Then, the unabsorbed solution was added to the capture beads and incubated at room temperature. The beads were washed and incubated with Anti-Human IgM (μ-chain specific)-Biotin (Sigma-Aldrich Ltd.). After a second wash, the beads were incubated with R-Phycoerythrin-Streptavidin (The Jackson Laboratory). To remove unbound PE from the solution, a single buffer replacement was done after the final incubation. Triplicates from the final solution were then measured in the MMB system.
Zika Virus Nonstructural 1-Based Enzyme-Linked Immunosorbent Assay
Zika-positive patients were subjected to EUROIMMUN ZIKV ELISA (EUROIMMUN) according to the manufacturer’s recommendations. In brief, serum samples were diluted 1:101 in sample buffer and incubated at 37°C for 60 minutes. Before IgM detection, the samples were preincubated with sample buffer containing RF absorbant, as recommended. After several incubation and washing steps, the optical density was measured in a Tecan Sunrise system (Tecan Austria, GMBH). Dengue virus- and WNV-positive samples were tested by DENV NS1 antigen, IgM/IgG antibody capture ELISA (Panbio), and WNV IgM capture DxSelect ELISA and WNV IgG capture DxSelect ELISA kits (Focus Diagnostics Inc., Cypress, CA), respectively.
Data Analysis
The clinical sensitivity of the MMB and EUROIMMUN assays for ZIKV was calculated as the percentage of ZIKV-positive patients that were identified as positive by each assay. This should be clearly distinguished from the analytical sensitivity of a detection system, which is the ability of the system to produce a change in signal for a defined change of the quantity being measured, and LoD, which is the minimal target molecules that can be detected by the detection system [26]. Specificity was calculated as the percentage of healthy patients that were identified as negative by the assay. Cross-reactivity levels were calculated as the percentage of the WNV- or DENV-positive patients that were wrongly identified as ZIKV positive by the assay [27]. Statistical analyses were performed using GraphPad Prism 7 (GraphPad Software, Inc.).
RESULTS
Immunoglobulin (Ig)M and IgG Anti-Zika Nonstructural 1 Dose Response
The dose-response curves of the MMB IgM and IgG antibodies are depicted in Figure 1A. The limits of detection, calculated as 3 standard deviations from the blank measurement, are 99 and 102 ng/L, with dynamic ranges of 3 logs and 2 logs, respectively. The dose-response curves of EUROIMMUN ELISA are shown in Figure 1B. In the EUROIMMUN ELISA, only values above 1.1 are regarded as positive [11], and therefore it can positively detect IgM and IgG concentrations of 6 × 105 and 5 × 104 ng/L, respectively. In comparison, the ROC cutoffs of the MMB assays are 19.6 (MMB normalized signal) for IgM and 10.85 for IgG. Therefore, the MMB assays can detect IgM and IgG concentrations of 7 × 103 ng/L (an ~85-fold improvement over EUROIMMUN ELISA) and 9 × 103 (a ~5-fold improvement over EUROIMMUN ELISA), respectively.
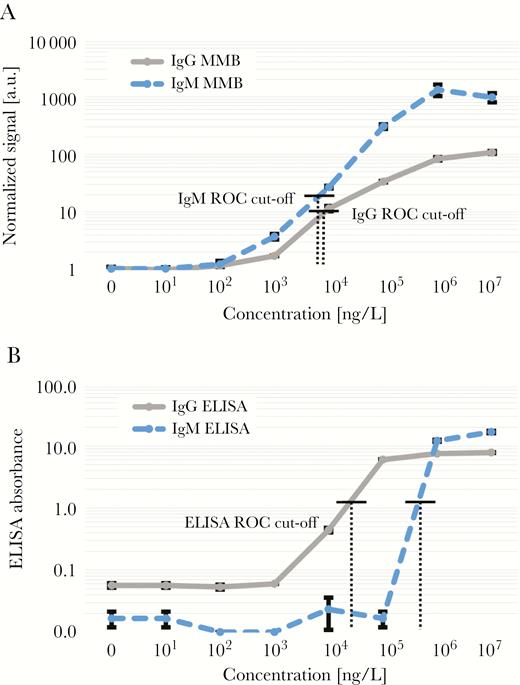
Dose responses of recombinant human immunoglobulin (Ig)M and human IgG anti-Zika nonstructural 1. (a) Magnetic modulation biosensing (MMB)-based assays and (b) EUROIMMUN enzyme-linked immunosorbent assays (ELISAs). Error bars represent the standard deviation of blank measurements (n = 6) and all other samples (n = 3). The receiver operating characteristic (ROC) cutoffs for MMB-based Zika IgG and IgM are 10.85 and 19.6, respectively. The ROC cutoff for EUROIMMUN ELISA Zika IgG and Zika IgM is 1.1.
Clinical Sensitivity, Specificity, and Cross-Reactivity of the Magnetic Modulation Biosensing Zika Immunoassays
Results of the MMB IgM and IgG assays of clinical samples are depicted in Figure 2. The information for each sample, including the results of the EUROIMMUN ELISA and the MMB assays, is listed in Table 1. The MMB IgM assay was able to detect 23 of 26 Zika-positive samples (88% sensitivity), whereas the EUROIMMUN IgM ELISA was able to detect 10 of 26 (38% sensitivity). The MMB IgG assay was able to detect 33 of 34 Zika-positive samples (97% sensitivity), whereas the EUROIMMUN IgG ELISA was able to detect 25 of 34 samples (74% sensitivity). The combined sensitivity of the MMB assay was 100% (ie, all the Zika-positive samples were either positive for IgM or IgG), whereas the combined sensitivity of the EUROIMMUN ELISA was 86%. The specificity of the MMB assay for both IgM and IgG was 100%. In addition, the MMB IgM assay was negative for all WNV and DENV samples (Supplementary Material, Supplementary Table S1, Supplementary Table S2). Thus, the cross-reactivity to WNV and DENV was 0%. In the MMB IgG assay, all the WNV samples were detected as negative (the cross-reactivity to WNV was 0%), but the cross-reactivity to DENV was 4%, with 1 of 24 DENV samples detected as positive.
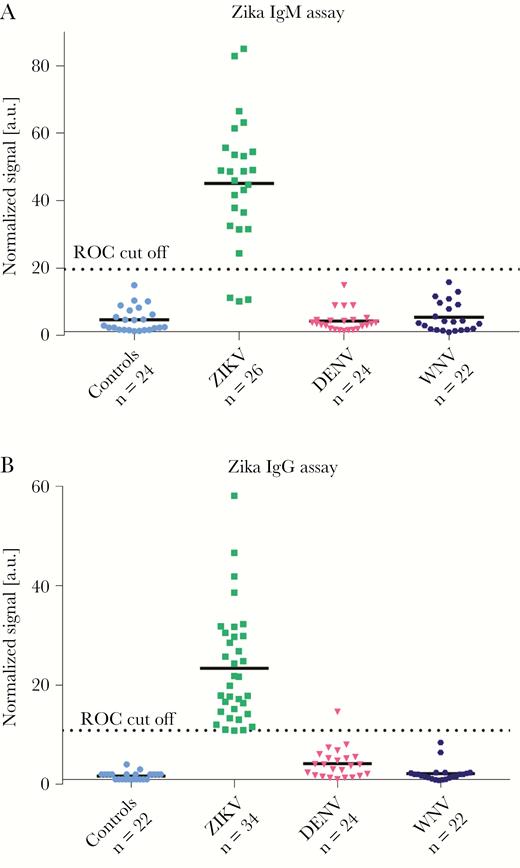
Sensitivity, specificity, and cross-reactivity of the magnetic modulation biosensing Zika immunoglobulin (Ig)G and IgM serological assays. (a) Zika IgM and (b) Zika IgG assays’ results. All of the Zika-positive samples were taken from quantitative reverse-transcription polymerase chain reaction/neutralization-positive patients. The IgM and IgG samples were obtained on days 1–60 and 7–180 postsymptom onset, respectively. Dengue virus (DENV)- and West Nile virus (WNV)-positive samples were taken from enzyme-linked immunosorbent assay-positive patients. a.u., arbitrary units; ROC, receiver operating characteristic; ZIKV, Zika virus.
Characteristics of 20 Israeli Patients Returning from Zika-Endemic Countries and Having qRT-PCR/Neutralization-Positive Serum Tests, Confirmed ZIKV Infection (Total Number of Samples n = 36)a
| Patient No. . | Country of Possible Exposure . | Gender/ Age (Year) . | qRT-PCR Result . | Neutralization Result (Titer) . | Dengueb . | Sample Number . | Time From Onset (Days) . | Zika IgM . | Zika IgG . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IgM . | IgG . | ELISA . | MMB . | ELISA . | MMB . | |||||||
| 1 | Colombia | F/50 | Pos | Pos (640) | Neg | Pos | 1st | 20 | Neg | Pos | Pos | Pos |
| 2nd | 92 | Neg | Neg | Pos | Pos | |||||||
| 2 | Colombia | F/32 | Pos | Pos (1280) | Neg | Pos | 1st | 53 | Equ | Pos | Pos | Pos |
| 3 | Dominican Republic | M/30 | Pos | Pos (1280) | Neg | Pos | 1st | 26 | Pos | Pos | Pos | Pos |
| 2nd | 46 | Neg | Pos | Pos | Pos | |||||||
| 4 | Colombia | M/38 | Neg | Pos (1280) | Neg | Pos | 1st | 34 | Equ | Pos | Pos | Pos |
| 5 | Jamaica | M/23 | Pos | Pos (640) | Neg | Neg | 1st | 11 | Pos | Pos | Equ | Pos |
| 2nd | 25 | Pos | Pos | Pos | Pos | |||||||
| 3rd | 49 | Neg | Pos | Pos | Pos | |||||||
| 6 | United States (Miami) | F/30 | Pos | Pos (160) | Neg | Pos | 1st | 8 | Pos | Pos | Neg | Pos |
| 7 | Costa Rica | M/27 | Pos | Pos (1280) | Neg | Pos | 1st | 7 | Pos | Pos | Neg | Pos |
| 2nd | 18 | Equ | Pos | Equ | Pos | |||||||
| 3rd | 37 | Neg | Pos | Pos | Pos | |||||||
| 8 | Mexico | M/26 | Pos | Pos (640) | Neg | Pos | 1st | 27 | Equ | Pos | Pos | Pos |
| 9 | Mexico | F/30 | Pos | Pos (1280) | Neg | Neg | 1st | 12 | Pos | Pos | Equ | Pos |
| 2nd | 29 | Equ | Pos | Pos | Pos | |||||||
| 3rd | 50 | Neg | Pos | Pos | Pos | |||||||
| 10 | Mexico | M/37 | Pos | Pos (1280) | Neg | Pos | 1st | 5 | Equ | Pos | Neg | Neg |
| 2nd | 14 | Pos | Pos | Neg | Pos | |||||||
| 3rd | 59 | Neg | Pos | Neg | Pos | |||||||
| 4st | 95 | Neg | Neg | Pos | Pos | |||||||
| 11 | Costa Rica | M/26 | Pos | Pos (160) | Neg | Pos | 1st | 12 | Pos | Pos | Neg | Neg |
| 12 | Costa Rica | M/20 | Neg | Pos (1280) | Neg | Pos | 1st | 29 | Pos | Pos | Pos | Pos |
| 13 | Mexico/Cuba | F/21 | Pos | Pos (1280) | Neg | Neg | 1st | 6 | Neg | Pos | Neg | Neg |
| 2nd | 16 | Pos | Pos | Pos | Pos | |||||||
| 14 | Colombia | F/22 | Neg | Pos (40) | Neg | Pos | 1st | 59 | Neg | Neg | Pos | Pos |
| 2nd | 61 | Neg | Neg | Pos | Pos | |||||||
| 15 | Honduras | F/22 | Neg | Pos (160) | Neg | Pos | 1st | ~60 | Neg | Neg | Pos | Pos |
| 16 | Central America | M/29 | Neg | Pos (320) | Neg | Neg | 1st | 105 | Neg | Neg | Pos | Pos |
| 17 | Panama | M/30 | Neg | Pos (40) | Neg | Pos | 1st | 100 | Neg | Pos | Pos | Pos |
| 2nd | 108 | Neg | Pos | Pos | Pos | |||||||
| 3rd | 180 | Neg | Pos | Equ | Pos | |||||||
| 18 | Cuba/ Mexico | M/29 | Neg | Pos (320) | Neg | Neg | 1st | ~75 | Neg | Neg | Pos | Pos |
| 19 | Thailand/ Philippines | M/26 | Neg | Pos (160) | Neg | Pos | 1st | ~30 | Neg | Neg | Pos | Pos |
| 2nd | ~40 | Neg | Neg | Pos | Pos | |||||||
| 20 | Puerto Rico | M/35 | Neg | Pos (20) | Neg | Pos | 1st | ~70 | Neg | Neg | Pos | Pos |
| Patient No. . | Country of Possible Exposure . | Gender/ Age (Year) . | qRT-PCR Result . | Neutralization Result (Titer) . | Dengueb . | Sample Number . | Time From Onset (Days) . | Zika IgM . | Zika IgG . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IgM . | IgG . | ELISA . | MMB . | ELISA . | MMB . | |||||||
| 1 | Colombia | F/50 | Pos | Pos (640) | Neg | Pos | 1st | 20 | Neg | Pos | Pos | Pos |
| 2nd | 92 | Neg | Neg | Pos | Pos | |||||||
| 2 | Colombia | F/32 | Pos | Pos (1280) | Neg | Pos | 1st | 53 | Equ | Pos | Pos | Pos |
| 3 | Dominican Republic | M/30 | Pos | Pos (1280) | Neg | Pos | 1st | 26 | Pos | Pos | Pos | Pos |
| 2nd | 46 | Neg | Pos | Pos | Pos | |||||||
| 4 | Colombia | M/38 | Neg | Pos (1280) | Neg | Pos | 1st | 34 | Equ | Pos | Pos | Pos |
| 5 | Jamaica | M/23 | Pos | Pos (640) | Neg | Neg | 1st | 11 | Pos | Pos | Equ | Pos |
| 2nd | 25 | Pos | Pos | Pos | Pos | |||||||
| 3rd | 49 | Neg | Pos | Pos | Pos | |||||||
| 6 | United States (Miami) | F/30 | Pos | Pos (160) | Neg | Pos | 1st | 8 | Pos | Pos | Neg | Pos |
| 7 | Costa Rica | M/27 | Pos | Pos (1280) | Neg | Pos | 1st | 7 | Pos | Pos | Neg | Pos |
| 2nd | 18 | Equ | Pos | Equ | Pos | |||||||
| 3rd | 37 | Neg | Pos | Pos | Pos | |||||||
| 8 | Mexico | M/26 | Pos | Pos (640) | Neg | Pos | 1st | 27 | Equ | Pos | Pos | Pos |
| 9 | Mexico | F/30 | Pos | Pos (1280) | Neg | Neg | 1st | 12 | Pos | Pos | Equ | Pos |
| 2nd | 29 | Equ | Pos | Pos | Pos | |||||||
| 3rd | 50 | Neg | Pos | Pos | Pos | |||||||
| 10 | Mexico | M/37 | Pos | Pos (1280) | Neg | Pos | 1st | 5 | Equ | Pos | Neg | Neg |
| 2nd | 14 | Pos | Pos | Neg | Pos | |||||||
| 3rd | 59 | Neg | Pos | Neg | Pos | |||||||
| 4st | 95 | Neg | Neg | Pos | Pos | |||||||
| 11 | Costa Rica | M/26 | Pos | Pos (160) | Neg | Pos | 1st | 12 | Pos | Pos | Neg | Neg |
| 12 | Costa Rica | M/20 | Neg | Pos (1280) | Neg | Pos | 1st | 29 | Pos | Pos | Pos | Pos |
| 13 | Mexico/Cuba | F/21 | Pos | Pos (1280) | Neg | Neg | 1st | 6 | Neg | Pos | Neg | Neg |
| 2nd | 16 | Pos | Pos | Pos | Pos | |||||||
| 14 | Colombia | F/22 | Neg | Pos (40) | Neg | Pos | 1st | 59 | Neg | Neg | Pos | Pos |
| 2nd | 61 | Neg | Neg | Pos | Pos | |||||||
| 15 | Honduras | F/22 | Neg | Pos (160) | Neg | Pos | 1st | ~60 | Neg | Neg | Pos | Pos |
| 16 | Central America | M/29 | Neg | Pos (320) | Neg | Neg | 1st | 105 | Neg | Neg | Pos | Pos |
| 17 | Panama | M/30 | Neg | Pos (40) | Neg | Pos | 1st | 100 | Neg | Pos | Pos | Pos |
| 2nd | 108 | Neg | Pos | Pos | Pos | |||||||
| 3rd | 180 | Neg | Pos | Equ | Pos | |||||||
| 18 | Cuba/ Mexico | M/29 | Neg | Pos (320) | Neg | Neg | 1st | ~75 | Neg | Neg | Pos | Pos |
| 19 | Thailand/ Philippines | M/26 | Neg | Pos (160) | Neg | Pos | 1st | ~30 | Neg | Neg | Pos | Pos |
| 2nd | ~40 | Neg | Neg | Pos | Pos | |||||||
| 20 | Puerto Rico | M/35 | Neg | Pos (20) | Neg | Pos | 1st | ~70 | Neg | Neg | Pos | Pos |
Abbreviations: ELISA, enzyme-linked immunosorbent assay; Equ, equivocal; F, female; Ig, immunoglobulin; M, male; MMB, magnetic modulation biosensing; Neg, negative; Pos, positive; qRT-PCR, quantitative reverse-transcription polymerase chain reaction; ROC, receiver operating characteristic.
aThe ELISA is the EUROIMMUN ZIKV ELISA serological test. According to the EUROIMMUN guidelines, values below 0.8 (ELISA absorbance units) are regarded as negative, values in the range of 0.8–1.1 are regarded as equivocal (which are clinically regarded as negative, but require further monitoring), and values above 1.1 are regarded as positive. The MMB is the MMB serological test. Dengue IgG and IgM results were the ROC cutoffs for MMB-based Zika IgG and IgM and are 10.85 and 19.6, respectively.
bDengue IgG and IgM results were obtained using DENV IgG and IgM antibody capture ELISA.
Characteristics of 20 Israeli Patients Returning from Zika-Endemic Countries and Having qRT-PCR/Neutralization-Positive Serum Tests, Confirmed ZIKV Infection (Total Number of Samples n = 36)a
| Patient No. . | Country of Possible Exposure . | Gender/ Age (Year) . | qRT-PCR Result . | Neutralization Result (Titer) . | Dengueb . | Sample Number . | Time From Onset (Days) . | Zika IgM . | Zika IgG . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IgM . | IgG . | ELISA . | MMB . | ELISA . | MMB . | |||||||
| 1 | Colombia | F/50 | Pos | Pos (640) | Neg | Pos | 1st | 20 | Neg | Pos | Pos | Pos |
| 2nd | 92 | Neg | Neg | Pos | Pos | |||||||
| 2 | Colombia | F/32 | Pos | Pos (1280) | Neg | Pos | 1st | 53 | Equ | Pos | Pos | Pos |
| 3 | Dominican Republic | M/30 | Pos | Pos (1280) | Neg | Pos | 1st | 26 | Pos | Pos | Pos | Pos |
| 2nd | 46 | Neg | Pos | Pos | Pos | |||||||
| 4 | Colombia | M/38 | Neg | Pos (1280) | Neg | Pos | 1st | 34 | Equ | Pos | Pos | Pos |
| 5 | Jamaica | M/23 | Pos | Pos (640) | Neg | Neg | 1st | 11 | Pos | Pos | Equ | Pos |
| 2nd | 25 | Pos | Pos | Pos | Pos | |||||||
| 3rd | 49 | Neg | Pos | Pos | Pos | |||||||
| 6 | United States (Miami) | F/30 | Pos | Pos (160) | Neg | Pos | 1st | 8 | Pos | Pos | Neg | Pos |
| 7 | Costa Rica | M/27 | Pos | Pos (1280) | Neg | Pos | 1st | 7 | Pos | Pos | Neg | Pos |
| 2nd | 18 | Equ | Pos | Equ | Pos | |||||||
| 3rd | 37 | Neg | Pos | Pos | Pos | |||||||
| 8 | Mexico | M/26 | Pos | Pos (640) | Neg | Pos | 1st | 27 | Equ | Pos | Pos | Pos |
| 9 | Mexico | F/30 | Pos | Pos (1280) | Neg | Neg | 1st | 12 | Pos | Pos | Equ | Pos |
| 2nd | 29 | Equ | Pos | Pos | Pos | |||||||
| 3rd | 50 | Neg | Pos | Pos | Pos | |||||||
| 10 | Mexico | M/37 | Pos | Pos (1280) | Neg | Pos | 1st | 5 | Equ | Pos | Neg | Neg |
| 2nd | 14 | Pos | Pos | Neg | Pos | |||||||
| 3rd | 59 | Neg | Pos | Neg | Pos | |||||||
| 4st | 95 | Neg | Neg | Pos | Pos | |||||||
| 11 | Costa Rica | M/26 | Pos | Pos (160) | Neg | Pos | 1st | 12 | Pos | Pos | Neg | Neg |
| 12 | Costa Rica | M/20 | Neg | Pos (1280) | Neg | Pos | 1st | 29 | Pos | Pos | Pos | Pos |
| 13 | Mexico/Cuba | F/21 | Pos | Pos (1280) | Neg | Neg | 1st | 6 | Neg | Pos | Neg | Neg |
| 2nd | 16 | Pos | Pos | Pos | Pos | |||||||
| 14 | Colombia | F/22 | Neg | Pos (40) | Neg | Pos | 1st | 59 | Neg | Neg | Pos | Pos |
| 2nd | 61 | Neg | Neg | Pos | Pos | |||||||
| 15 | Honduras | F/22 | Neg | Pos (160) | Neg | Pos | 1st | ~60 | Neg | Neg | Pos | Pos |
| 16 | Central America | M/29 | Neg | Pos (320) | Neg | Neg | 1st | 105 | Neg | Neg | Pos | Pos |
| 17 | Panama | M/30 | Neg | Pos (40) | Neg | Pos | 1st | 100 | Neg | Pos | Pos | Pos |
| 2nd | 108 | Neg | Pos | Pos | Pos | |||||||
| 3rd | 180 | Neg | Pos | Equ | Pos | |||||||
| 18 | Cuba/ Mexico | M/29 | Neg | Pos (320) | Neg | Neg | 1st | ~75 | Neg | Neg | Pos | Pos |
| 19 | Thailand/ Philippines | M/26 | Neg | Pos (160) | Neg | Pos | 1st | ~30 | Neg | Neg | Pos | Pos |
| 2nd | ~40 | Neg | Neg | Pos | Pos | |||||||
| 20 | Puerto Rico | M/35 | Neg | Pos (20) | Neg | Pos | 1st | ~70 | Neg | Neg | Pos | Pos |
| Patient No. . | Country of Possible Exposure . | Gender/ Age (Year) . | qRT-PCR Result . | Neutralization Result (Titer) . | Dengueb . | Sample Number . | Time From Onset (Days) . | Zika IgM . | Zika IgG . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IgM . | IgG . | ELISA . | MMB . | ELISA . | MMB . | |||||||
| 1 | Colombia | F/50 | Pos | Pos (640) | Neg | Pos | 1st | 20 | Neg | Pos | Pos | Pos |
| 2nd | 92 | Neg | Neg | Pos | Pos | |||||||
| 2 | Colombia | F/32 | Pos | Pos (1280) | Neg | Pos | 1st | 53 | Equ | Pos | Pos | Pos |
| 3 | Dominican Republic | M/30 | Pos | Pos (1280) | Neg | Pos | 1st | 26 | Pos | Pos | Pos | Pos |
| 2nd | 46 | Neg | Pos | Pos | Pos | |||||||
| 4 | Colombia | M/38 | Neg | Pos (1280) | Neg | Pos | 1st | 34 | Equ | Pos | Pos | Pos |
| 5 | Jamaica | M/23 | Pos | Pos (640) | Neg | Neg | 1st | 11 | Pos | Pos | Equ | Pos |
| 2nd | 25 | Pos | Pos | Pos | Pos | |||||||
| 3rd | 49 | Neg | Pos | Pos | Pos | |||||||
| 6 | United States (Miami) | F/30 | Pos | Pos (160) | Neg | Pos | 1st | 8 | Pos | Pos | Neg | Pos |
| 7 | Costa Rica | M/27 | Pos | Pos (1280) | Neg | Pos | 1st | 7 | Pos | Pos | Neg | Pos |
| 2nd | 18 | Equ | Pos | Equ | Pos | |||||||
| 3rd | 37 | Neg | Pos | Pos | Pos | |||||||
| 8 | Mexico | M/26 | Pos | Pos (640) | Neg | Pos | 1st | 27 | Equ | Pos | Pos | Pos |
| 9 | Mexico | F/30 | Pos | Pos (1280) | Neg | Neg | 1st | 12 | Pos | Pos | Equ | Pos |
| 2nd | 29 | Equ | Pos | Pos | Pos | |||||||
| 3rd | 50 | Neg | Pos | Pos | Pos | |||||||
| 10 | Mexico | M/37 | Pos | Pos (1280) | Neg | Pos | 1st | 5 | Equ | Pos | Neg | Neg |
| 2nd | 14 | Pos | Pos | Neg | Pos | |||||||
| 3rd | 59 | Neg | Pos | Neg | Pos | |||||||
| 4st | 95 | Neg | Neg | Pos | Pos | |||||||
| 11 | Costa Rica | M/26 | Pos | Pos (160) | Neg | Pos | 1st | 12 | Pos | Pos | Neg | Neg |
| 12 | Costa Rica | M/20 | Neg | Pos (1280) | Neg | Pos | 1st | 29 | Pos | Pos | Pos | Pos |
| 13 | Mexico/Cuba | F/21 | Pos | Pos (1280) | Neg | Neg | 1st | 6 | Neg | Pos | Neg | Neg |
| 2nd | 16 | Pos | Pos | Pos | Pos | |||||||
| 14 | Colombia | F/22 | Neg | Pos (40) | Neg | Pos | 1st | 59 | Neg | Neg | Pos | Pos |
| 2nd | 61 | Neg | Neg | Pos | Pos | |||||||
| 15 | Honduras | F/22 | Neg | Pos (160) | Neg | Pos | 1st | ~60 | Neg | Neg | Pos | Pos |
| 16 | Central America | M/29 | Neg | Pos (320) | Neg | Neg | 1st | 105 | Neg | Neg | Pos | Pos |
| 17 | Panama | M/30 | Neg | Pos (40) | Neg | Pos | 1st | 100 | Neg | Pos | Pos | Pos |
| 2nd | 108 | Neg | Pos | Pos | Pos | |||||||
| 3rd | 180 | Neg | Pos | Equ | Pos | |||||||
| 18 | Cuba/ Mexico | M/29 | Neg | Pos (320) | Neg | Neg | 1st | ~75 | Neg | Neg | Pos | Pos |
| 19 | Thailand/ Philippines | M/26 | Neg | Pos (160) | Neg | Pos | 1st | ~30 | Neg | Neg | Pos | Pos |
| 2nd | ~40 | Neg | Neg | Pos | Pos | |||||||
| 20 | Puerto Rico | M/35 | Neg | Pos (20) | Neg | Pos | 1st | ~70 | Neg | Neg | Pos | Pos |
Abbreviations: ELISA, enzyme-linked immunosorbent assay; Equ, equivocal; F, female; Ig, immunoglobulin; M, male; MMB, magnetic modulation biosensing; Neg, negative; Pos, positive; qRT-PCR, quantitative reverse-transcription polymerase chain reaction; ROC, receiver operating characteristic.
aThe ELISA is the EUROIMMUN ZIKV ELISA serological test. According to the EUROIMMUN guidelines, values below 0.8 (ELISA absorbance units) are regarded as negative, values in the range of 0.8–1.1 are regarded as equivocal (which are clinically regarded as negative, but require further monitoring), and values above 1.1 are regarded as positive. The MMB is the MMB serological test. Dengue IgG and IgM results were the ROC cutoffs for MMB-based Zika IgG and IgM and are 10.85 and 19.6, respectively.
bDengue IgG and IgM results were obtained using DENV IgG and IgM antibody capture ELISA.
Zika Virus Magnetic Modulation Biosensing Assay Extends EUROIMMUN Nonstructural 1 Enzyme-Linked Immunosorbent Assay’s Detection Limits
Figure 3 presents the percentage of Zika-positive samples that were identified as positive using the MMB IgM and IgG assays and the EUROIMMUN ELISA assay. Using the MMB IgM assay, IgM antibodies were detected as early as day 5 and as late as day 180 postsymptoms onset (PSO). In comparison, the EUROIMMUN IgM ELISA assay detected IgM antibodies from days 7 to 29 PSO. In addition, using the MMB IgG assay, IgG antibodies were detected as early as day 7 PSO, whereas the EUROIMMUN IgG ELISA assay detected IgG antibodies from day 16 PSO onward (Table 1).
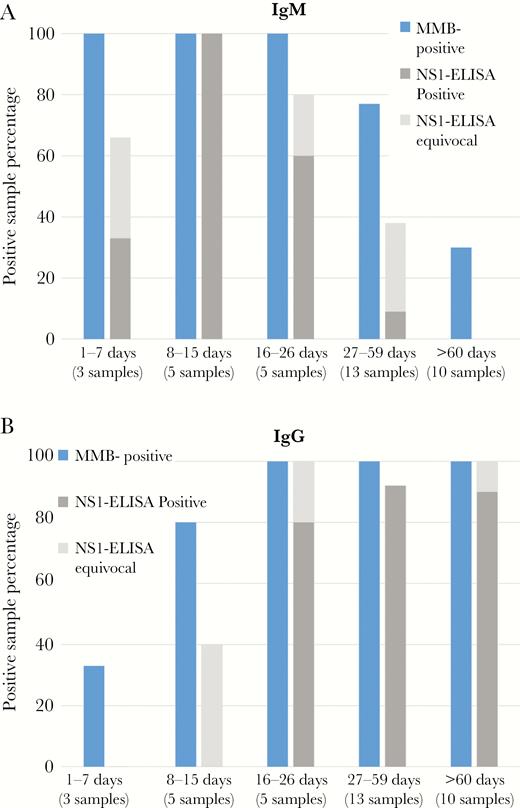
Comparison between the magnetic modulation biosensing (MMB) Zika immunoassay and the EUROIMMUN enzyme-linked immunosorbent assay (ELISA). Percentage of Zika-positive (a) immunoglobulin (Ig)M and (b) IgG samples detected by the MMB and EUROIMMUN ELISA assays. The tested groups included a total of 36 samples from quantitative reverse-transcription polymerase chain reaction/neutralization-positive tests of Israeli patients, who had returned from areas where the Zika virus is endemic. The samples were obtained 1–7, 8–15, 16–26, 27–59, and >60 days after the onset of symptoms.
The positive samples were divided into 5 groups according to the day of the sample retrieval: 1−7, 8−15, 16−26, 27–59, and ≥60 days PSO. The MMB IgM assay identified 100% of the positive samples for days 1–7, 8−15, and 16−26 PSO and 77% for days 27–59. The EUROIMMUN IgM assay identified 33%, 100%, 60%, and 9% of the same samples, respectively. For samples that were retrieved ≥60 days PSO, the MMB IgM assay identified 30% of the positive samples, whereas the EUROIMMUN IgM assay identified 0% for the same period (Figure 3A). When the “equivocal” results are combined with the positive results, the sensitivity of the EUROIMMUN IgM assay improves to 66%, 100%, 80%, 38%, and 0%, respectively for each group.
The MMB IgG assay identified 33% of the positive samples for days 1–7, 80% of the positive samples for days 8–15, and 100% of the positive samples for day 16 PSO onward. The EUROIMMUN IgG assay detected 0%, 0%, 80%, 92%, and 90% of the positive samples from days 1–7, 8–15, 16–26, 27–59, and over 60 PSO, respectively (Figure 3B). When the equivocal results are combined with the positive results, the sensitivity of the EUROIMMUN IgG assay improves to 0%, 40%, 100%, 92%, and 100%, respectively, for each group.
Time-Dependent Measurements
A time course of IgM and IgG levels of 3 Zika-positive patients (patients nos. 7, 9, and 10; Table 1) is presented in Figure 4. For each patient, measurements were taken at 3 different time points over 2 months PSO. For all 3 patients, IgM levels decreased over time (Figure 4A), whereas the IgG levels gradually increased (Figure 4B).
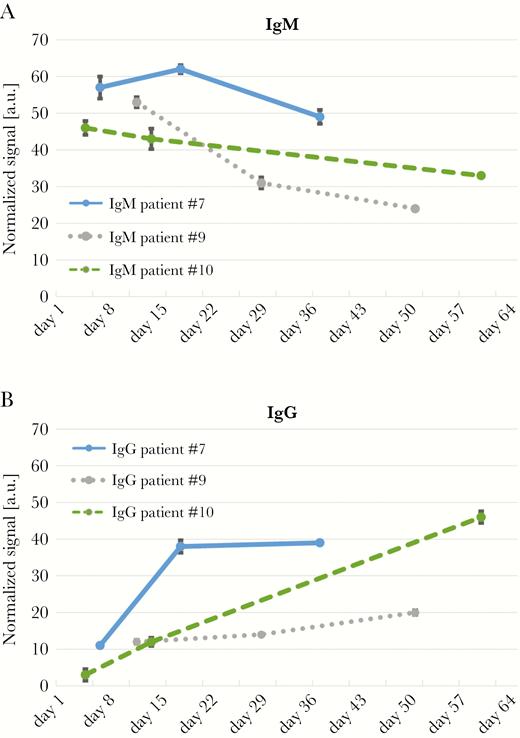
Time course of Zika immunoglobulin (Ig)M and IgG levels. (a) Zika IgM and (b) IgG levels of 3 Zika-positive patients. The blood samples were taken at 3 different time points over 2 months. Error bars represent the standard deviation of 3 measurements (n = 3).
Magnetic Modulation Biosensing and EUROIMMUN False-Positive Results
Table 2 lists 11 samples that were obtained from 10 patients, who tested negative for ZIKV using qRT-PCR and the neutralization test but tested positive in EUROIMMUN IgM or IgG assays. The MMB IgG assay was negative for all 11 samples, and the MMB IgM assay was negative for 10 of the 11 samples. The MMB IgM assay was positive for 1 of the samples.
Characteristics of Ten Israeli Patients With Zika-Positive NS1-Based ELISA Results, but Negative in qRT-PCR/Neutralization
Tests (Total of 11 Samples, n = 11)a
| . | IgM . | IgG . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patient No. . | Country of Possible Exposure . | Gender/Age (Year) . | RT- PCR Result . | Neutrali zation Result . | Sample Number . | Time From Onset (Days) . | ELISA . | MMB . | ELISA . | MMB . |
| 21 | Colombia | M/31 | Neg | Neg | 1st | 5 | Pos | Neg | Neg | Neg |
| 22 | Mexico | F/28 | Neg | Neg | 1st | ~20 | Pos | Neg | Neg | Neg |
| 2nd | ~37 | Pos | Neg | Neg | Neg | |||||
| 23 | Thailand | F/33 | Neg | Neg | 1st | ~90 | Pos | Pos | Neg | Neg |
| 24 | Thailand | F/33 | Neg | Neg | 1st | ~45 | Pos | Neg | Neg | Neg |
| 25 | Philippines | F/28 | Neg | Neg | 1st | ~50 | Neg | Neg | Pos | Neg |
| 26 | Thailand | F/34 | Neg | Neg | 1st | 8 | Equ | Neg | Neg | Neg |
| 27 | Central America | M/25 | Neg | Neg | 1st | N.A. | Pos | Neg | Neg | Neg |
| 28 | Thailand | F/25 | Neg | Neg | 1st | ~50 | Pos | Neg | Neg | Neg |
| 29 | Viatnam/ Thailand/ Singapore | M/34 | Neg | Neg | 1st | ~150 | Equ | Neg | Pos | Neg |
| 30 | Thailand | M/25 | Neg | Neg | 1st | 15 | Pos | Neg | Neg | Neg |
| . | IgM . | IgG . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patient No. . | Country of Possible Exposure . | Gender/Age (Year) . | RT- PCR Result . | Neutrali zation Result . | Sample Number . | Time From Onset (Days) . | ELISA . | MMB . | ELISA . | MMB . |
| 21 | Colombia | M/31 | Neg | Neg | 1st | 5 | Pos | Neg | Neg | Neg |
| 22 | Mexico | F/28 | Neg | Neg | 1st | ~20 | Pos | Neg | Neg | Neg |
| 2nd | ~37 | Pos | Neg | Neg | Neg | |||||
| 23 | Thailand | F/33 | Neg | Neg | 1st | ~90 | Pos | Pos | Neg | Neg |
| 24 | Thailand | F/33 | Neg | Neg | 1st | ~45 | Pos | Neg | Neg | Neg |
| 25 | Philippines | F/28 | Neg | Neg | 1st | ~50 | Neg | Neg | Pos | Neg |
| 26 | Thailand | F/34 | Neg | Neg | 1st | 8 | Equ | Neg | Neg | Neg |
| 27 | Central America | M/25 | Neg | Neg | 1st | N.A. | Pos | Neg | Neg | Neg |
| 28 | Thailand | F/25 | Neg | Neg | 1st | ~50 | Pos | Neg | Neg | Neg |
| 29 | Viatnam/ Thailand/ Singapore | M/34 | Neg | Neg | 1st | ~150 | Equ | Neg | Pos | Neg |
| 30 | Thailand | M/25 | Neg | Neg | 1st | 15 | Pos | Neg | Neg | Neg |
Abbreviations: ELISA, enzyme-linked immunosorbent assay; Equ, equivocal; F, female; Ig, immunoglobulin; M, male; MMB, magnetic modulation biosensing; N.A., not applicable; Neg, negative; NS1, nonstructural 1; Pos, positive; qRT-PCR, quantitative reverse-transcription polymerase chain reaction; WNV, West Nile virus.
aAll of the samples were negative to dengue IgM and IgG. The first sample of patient no. 22 was positive to WNV IgG. The sample of patient no. 27 was equivocal to WNV IgG.
Characteristics of Ten Israeli Patients With Zika-Positive NS1-Based ELISA Results, but Negative in qRT-PCR/Neutralization
Tests (Total of 11 Samples, n = 11)a
| . | IgM . | IgG . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patient No. . | Country of Possible Exposure . | Gender/Age (Year) . | RT- PCR Result . | Neutrali zation Result . | Sample Number . | Time From Onset (Days) . | ELISA . | MMB . | ELISA . | MMB . |
| 21 | Colombia | M/31 | Neg | Neg | 1st | 5 | Pos | Neg | Neg | Neg |
| 22 | Mexico | F/28 | Neg | Neg | 1st | ~20 | Pos | Neg | Neg | Neg |
| 2nd | ~37 | Pos | Neg | Neg | Neg | |||||
| 23 | Thailand | F/33 | Neg | Neg | 1st | ~90 | Pos | Pos | Neg | Neg |
| 24 | Thailand | F/33 | Neg | Neg | 1st | ~45 | Pos | Neg | Neg | Neg |
| 25 | Philippines | F/28 | Neg | Neg | 1st | ~50 | Neg | Neg | Pos | Neg |
| 26 | Thailand | F/34 | Neg | Neg | 1st | 8 | Equ | Neg | Neg | Neg |
| 27 | Central America | M/25 | Neg | Neg | 1st | N.A. | Pos | Neg | Neg | Neg |
| 28 | Thailand | F/25 | Neg | Neg | 1st | ~50 | Pos | Neg | Neg | Neg |
| 29 | Viatnam/ Thailand/ Singapore | M/34 | Neg | Neg | 1st | ~150 | Equ | Neg | Pos | Neg |
| 30 | Thailand | M/25 | Neg | Neg | 1st | 15 | Pos | Neg | Neg | Neg |
| . | IgM . | IgG . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patient No. . | Country of Possible Exposure . | Gender/Age (Year) . | RT- PCR Result . | Neutrali zation Result . | Sample Number . | Time From Onset (Days) . | ELISA . | MMB . | ELISA . | MMB . |
| 21 | Colombia | M/31 | Neg | Neg | 1st | 5 | Pos | Neg | Neg | Neg |
| 22 | Mexico | F/28 | Neg | Neg | 1st | ~20 | Pos | Neg | Neg | Neg |
| 2nd | ~37 | Pos | Neg | Neg | Neg | |||||
| 23 | Thailand | F/33 | Neg | Neg | 1st | ~90 | Pos | Pos | Neg | Neg |
| 24 | Thailand | F/33 | Neg | Neg | 1st | ~45 | Pos | Neg | Neg | Neg |
| 25 | Philippines | F/28 | Neg | Neg | 1st | ~50 | Neg | Neg | Pos | Neg |
| 26 | Thailand | F/34 | Neg | Neg | 1st | 8 | Equ | Neg | Neg | Neg |
| 27 | Central America | M/25 | Neg | Neg | 1st | N.A. | Pos | Neg | Neg | Neg |
| 28 | Thailand | F/25 | Neg | Neg | 1st | ~50 | Pos | Neg | Neg | Neg |
| 29 | Viatnam/ Thailand/ Singapore | M/34 | Neg | Neg | 1st | ~150 | Equ | Neg | Pos | Neg |
| 30 | Thailand | M/25 | Neg | Neg | 1st | 15 | Pos | Neg | Neg | Neg |
Abbreviations: ELISA, enzyme-linked immunosorbent assay; Equ, equivocal; F, female; Ig, immunoglobulin; M, male; MMB, magnetic modulation biosensing; N.A., not applicable; Neg, negative; NS1, nonstructural 1; Pos, positive; qRT-PCR, quantitative reverse-transcription polymerase chain reaction; WNV, West Nile virus.
aAll of the samples were negative to dengue IgM and IgG. The first sample of patient no. 22 was positive to WNV IgG. The sample of patient no. 27 was equivocal to WNV IgG.
DISCUSSION
Current ZIKV serological diagnosis is designed to detect IgM and IgG antibodies for either envelope or NS1 proteins. The envelope-based ELISA tests are sensitive but lack specificity and have high cross-reactivity [16]. The NS1-based ELISA tests are considered to be highly specific [11] but less sensitive [5, 28]. In this study, using neutralization and qRT-PCR ZIKV-positive samples, we show that the sensitivity of the EUROIMMUN ZIKV ELISA is only 38% for IgM and 74% for IgG. These results are consistent with previous EUROIMMUN ZIKV ELISA studies, which reported sensitivities of 37%–68% for IgM and 79% for IgG [5, 28].
The MMB technology enables highly sensitive detection of fluorescently labeled target molecules. Here, we demonstrate that MMB-based assays provide a 5- to 85-fold improvement over EUROIMMUN ELISA assays in detecting recombinant ZIKV IgM and IgG anti-NS1 antibodies. Moreover, using qRT-PCR ZIKV-positive samples, we show that the sensitivities of the MMB assays are 88% for IgM and 97% for IgG. In addition, using healthy patients’ serum samples, we show that the specificity of the MMB assay is 100% for both IgM and IgG.
The cross-reactivity of the Zika MMB IgM assay was 0% for both DENV (n = 24) and WNV (n = 24). The cross-reactivity of the Zika MMB IgG assay was 4% for DENV and 0% for WNV. These results are equivalent or superior to results from recent studies with the EUROIMMUN Zika IgM and IgG ELISA assays that use NS1 protein as a capture antigen [9, 29]. For example, L’Huillier et al [9] evaluated the EUROIMMUN Zika IgM and IgG ELISA assays and reported a cross-reactivity of 66% for both assays when tested with DENV neutralization-positive samples (n = 21). In 2016, Granger et al [29] evaluated the EUROIMMUN Zika IgM assay and reported cross-reactivities of 6.2% and 0% for DENV (n = 16) and WNV (n = 10), respectively. In addition, with the EUROIMMUN Zika IgG assay, the cross-reactivities were 30% and 0% for DENV (n = 20) and WNV (n = 13), respectively [29].
In addition to increased sensitivity, the MMB assays significantly extend the number of days PSO in which the IgM and IgG antibodies are detectable. Although the EUROIMMUN IgM assay was able to detect IgM antibodies from days 7 to 29 PSO only, the MMB assay detected IgM antibodies from days 5 to 180 PSO, using the same samples. In addition, the EUROIMMUN IgG assay was able to detect IgG antibodies from day 16 PSO only, whereas the MMB IgG assay detected IgG antibodies from as early as day 7 PSO. In particular, 4 of 5 serum samples that were taken on days 8–15 PSO tested positive with the MMB IgG assay and negative with the EUROIMMUN IgG assay. Even considering the combined positive and equivocal results of the EUROIMMUN ELISA assays, the MMB assays have a substantially broader detection window and offer significantly higher sensitivity.
To determine acute or convalescent stages of infection, IgM and IgG levels are measured over time, usually at relatively long time intervals (eg, 14 days apart). Here, time-dependent measurements of samples from 3 patients showed the expected trends in IgM and IgG levels. The combination of the high dynamic range (3 and 2 logs dynamic range for IgM and IgG, respectively) and high sensitivity of the MMB assays allows quantitative monitoring of patients’ IgM and IgG levels. To determine the minimal time interval between measurements needed to see the trend in the IgM or IgG levels and to define the clinical status of patients, a large-scale clinical trial is required.
The EUROIMMUN ELISA assays exhibited a higher incidence of false-positive results than the MMB assays. Eleven serum samples that were negative for qRT-PCR and neutralization tests, but positive in the EUROIMMUN IgM or IgG assays, were tested by the MMB assays. Ten of the eleven samples were negative, and 1 sample was falsely defined as positive by the MMB assays. The greater number of false-positive results of the EUROIMMUN assays can be attributed to higher nonspecific binding. Although both the EUROIMMUN ELISA and the MMB assays utilize the same ZIKV NS1 capture antigen, they have different detection antibodies. Therefore, one reason for the higher nonspecific binding in the ELISA could be the difference in the specificity of the detection antibodies. Other causes could be the type of capture surface, functionality, and blocking treatment used in each assay [30].
The limitation of the study is its restriction to returning travelers only. Future research should evaluate the sensitivity of the MMB-based assays on Zika-positive samples that were collected from patients living in Zika-endemic areas.
CONCLUSIONS
In conclusion, the MMB ZIKV assays provide high sensitivity and specificity and low cross-reactivity. In this study, to evaluate their performance, we carried out a small-scale clinical trial and compared the results to EUROIMMUN ZIKV assays, which are commonly used as the primary tool to detect ZIKV-infected patients. The MMB system proved to be a rapid, highly sensitive, and quantitative tool that shortens the detection time and enables simple operation in low-resource settings. Combining the MMB system with an NS1-based serological assay for the detection of ZIKV has the potential to improve the currently available ZIKV serological tests and significantly advance the field of serology.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank Tal Iluz and Michael Margulis for technical assistance and Jonas Schmidt-Chanasit (Bernhard Nocht Institute for Tropical Medicine, Hamburg) for the dengue-positive samples from Germany. James Ballard provided an editorial review of the manuscript.
Financial support. This work was funded by Israel Science Foundation Grants 2152/15 and 1142/15.
Potential conflicts of interest. A. D. has a financial interest in MagBiosense, Inc., which, however, did not financially support this work. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
Author notes
Y. M. and Y. L. contributed equally to this work.




