-
PDF
- Split View
-
Views
-
Cite
Cite
Christopher T Lee, Jose E Hagan, Baigalmaa Jantsansengee, Oyun-Erdene Tumurbaatar, Samdan Altanchimeg, Buyanjargal Yadamsuren, Sodbayar Demberelsuren, Chinbayar Tserendorj, Oyungerel Munkhtogoo, Darmaa Badarch, Nyamaa Gunregjav, Bolortuya Baatarkhuu, Chimedsuren Ochir, LaShondra Berman, Raydel Anderson, Minal K Patel, Christopher J Gregory, James L Goodson, Increase in Infant Measles Deaths During a Nationwide Measles Outbreak—Mongolia, 2015–2016, The Journal of Infectious Diseases, Volume 220, Issue 11, 1 December 2019, Pages 1771–1779, https://doi.org/10.1093/infdis/jiz140
Close - Share Icon Share
Abstract
Surveillance data from a large measles outbreak in Mongolia suggested increased case fatality ratio (CFR) in the second of 2 waves. To confirm the increase in CFR and identify risk factors for measles death, we enhanced mortality ascertainment and conducted a case-control study among infants hospitalized for measles.
We linked national vital records with surveillance data of clinically or laboratory-confirmed infant (aged <12 months) measles cases with rash onset during March–September 2015 (wave 1) and October 2015–June 2016 (wave 2). We abstracted medical charts of 95 fatal cases and 273 nonfatal cases hospitalized for measles, matched by age and sex. We calculated adjusted matched odds ratios (amORs) and 95% confidence intervals (CIs) for risk factors.
Infant measles deaths increased from 3 among 2224 cases (CFR: 0.13%) in wave 1 to 113 among 4884 cases (CFR: 2.31%) in wave 2 (P < .001). Inpatient admission, 7–21 days before measles rash onset, for pneumonia or influenza (amOR: 4.5; CI, 2.6–8.0), but not other diagnoses, was significantly associated with death.
Measles infection among children hospitalized with respiratory infections likely increased deaths due to measles during wave 2. Preventing measles virus nosocomial transmission likely decreases measles mortality.
Measles virus is a paramyxovirus of the genus Morbillivirus transmitted from person to person by droplet or aerosolized respiratory fluids of measles virus-infected persons [1]. The incubation period is 7–21 days; signs and symptoms of measles include fever, generalized maculopapular erythematous rash, cough, coryza, and conjunctivitis. Common measles complications include pneumonia, otitis media, thrombocytopenia, diarrhea, and encephalitis. Measles can lead to death or disability including blindness, deafness, or intellectual disabilities associated with encephalitis [2].
Despite the availability of a safe and highly effective vaccine, measles remains a leading cause of under-5 mortality [3]. Measles mortality can be underreported to routine surveillance systems, as the death often occurs weeks after the initial report of the case or after a case has been discharged from the hospital. Independent risk factors for measles mortality include age <3 years, neurological complications, lack of measles vaccination, lack of receiving a full course of vitamin A for case management, and exposure in the household [1, 4–6].
In Mongolia, measles-containing vaccine (MCV) administered at 9 months of age was introduced into the national Expanded Programme on Immunization (EPI) in 1973, and a second dose given at 2 years of age was introduced in 1989. Following increasing vaccination coverage, no cases of measles were reported during 2011–2014. Mongolia was certified measles free by the World Health Organization (WHO) Western Pacific Regional Verification Commission in July 2014 [7].
Beginning in March 2015, a large nationwide measles outbreak began, with 49 908 suspected cases reported during March 2015–June 2016. The outbreak was the largest documented measles outbreak in any setting after verification of measles elimination. Measles virus transmission occurred during 2 waves: March–September 2015 (wave 1) [8] and October 2015–June 2016 (wave 2). Surveillance data suggested increased infant mortality during the second versus the first wave, although it was unclear whether this difference was due to reporting or detection bias, the population affected, clinical care or delays to clinical care among rural populations, or other factors. Because of the apparent increase in mortality among infants, we sought to verify the increase in mortality and case fatality ratio (CFR) by enhancing death ascertainment of confirmed infant measles cases and to identify independent risk factors for infant measles mortality during the second wave using a matched case-control study.
METHODS
Participants and Data Collection
We analyzed case-based surveillance data reported from health care providers and collected by the national EPI program during March 2015–June 2016. We restricted analyses to confirmed measles cases among persons with laboratory evidence of measles virus infection (ie, presence of measles immunoglobulin-M [IgM] by enzyme-linked immunosorbent assay or detection of measles RNA by reverse transcriptase polymerase chain reaction [RT-PCR]) or clinical evidence of infection (ie, presence of fever and maculopapular rash, and ≥1 of cough, coryza, or conjunctivitis). We also analyzed surveillance data from the Mongolia National Influenza Center (MNIC) on nationwide influenza-like illness hospitalizations among infants to ascertain when the influenza season among infants occurred, and analyzed MNIC influenza subtype data using real-time RT-PCR from sentinel sites during the same period.
We defined an infant measles death as a death that occurred <30 days of rash onset in a confirmed measles patient <12 months of age, without another unrelated cause (eg, trauma) [9]. To enhance ascertainment of measles deaths, we matched case-based surveillance data with national vital statistics data routinely collected by the National Center for Health Development with corresponding International Classification of Diseases, 10th Revision (ICD-10) codes of infant deaths that occurred during 2015–2016, using a probabilistic record linkage algorithm described elsewhere [10]. Databases were matched on name, sex, date of birth, and national registration number. All matches were manually confirmed.
We conducted a matched case-control study among hospitalized measles cases to identify risk factors associated with infant measles death. Cases were defined as fatal measles cases among the population of hospitalized confirmed infant measles cases with rash onset during the second wave (ie, 1 October 2015–27 June 2016) for whom we could locate medical records (Figure 1); controls were nonfatal measles cases drawn from the same population. Target sample size (100 cases matched with 300 controls) was calculated to detect a minimum odds ratio of 2.0, power of 0.80, prespecified alpha 0.05, assuming correlation coefficient (ϕ) between the exposure status of the ith pair of the case and control of 0.2 and prevalence of the risk factor among controls of 0.4. We obtained medical records for 95 (84%) of 106 measles deaths and matched them 3:1 with 273 controls on sex and birth week.
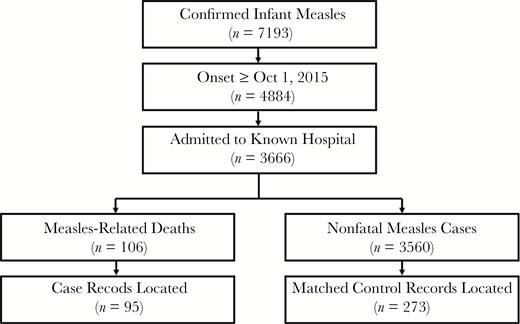
Flow diagram of infant measles mortality case-control study population and sample. The study population was infant measles cases with laboratory evidence of measles infection (positive measles-specific immunoglobulin M antibody or polymerase chain reaction) or clinical evidence of measles infection (fever and rash with 1 of cough, coryza, or conjunctivitis) during the second outbreak phase (rash onset after 1 October 2015), admitted to a hospital from which we could obtain medical records. Fatal measles case patients (measles-related deaths) were matched 3:1 on age and date of birth with nonfatal measles control patients.
This public health response was determined not to be human subjects’ research by the US Centers for Disease Control and Prevention (CDC) and the Mongolia National Center for Communicable Diseases.
Medical Record Abstraction
Abstraction variables included demographic and anthropometric variables (date of birth, sex, Aimag [province] and Soum [district] of residence, weight, length); details on illness history (date of rash onset, signs and symptoms, preceding episodes of inpatient and outpatient care, Aimag and Soum of measles hospitalization); vaccination status as documented in the medical chart (MCV, pentavalent conjugate vaccine); underlying medical conditions before measles hospitalization; and treatment received after rash onset (intubation/mechanical ventilation, antibiotics, vitamin A, steroids). The tool was developed in English, translated to Mongolian, and independently back-translated. When autopsy results from the Mongolia National Pathology Center were available for review, we abstracted findings from diagnostic histopathological examination of formalin-fixed paraffin-embedded lung tissue, which was stained in all cases using hematoxylin and eosin and Lillie-Twort tissue Gram stains. Tissue sample RNA from a subset of 6 fatal laboratory-confirmed infant measles cases was extracted from FTA cards using the QIamp viral RNA mini kit (Qiagen) as described previously [11]. The presence of measles RNA was confirmed in all 6 samples by measles real-time RT-PCR [12]. The samples were tested for influenza A and B using CDC real-time RT-PCR assays. Any influenza A or B positive specimens were further characterized by subtypes and/or genotypes.
Surveillance Database Analysis
Data analysis was performed using Stata v15.0 (College Station, TX). Differences in proportions during wave 1 versus wave 2 were compared by Pearson Chi [2] (χ2) test.
Case-Control Analysis
Low weight-for-age was calculated as a dichotomous variable using the WHO 2006 Growth Standards for child weight-for-age less than −2 Z-scores [13]. We created a malnutrition variable combining patients with low weight-for-age with those with a clinical diagnosis of malnutrition. Health care-associated measles infection (HAI) was defined as a confirmed measles case with history of any hospitalization 7–21 days before measles rash onset (incubation period). We created dummy variables for measles HAI for a pneumonia or influenza hospitalization, measles HAI for other type of hospitalization (ie, diarrheal disease, upper respiratory tract infection, urinary tract infection, meningitis, or other), and non-HAI (ie, no history of hospitalization in the 7–21 days before rash onset). We calculated matched odds ratios using conditional logistic regression to compare discordant pairs of matched cases and controls adjusted for age and sex for bivariate associations. Model selection was performed by comparing nested multivariable models using Akaike’s Information Criterion (AIC), a measure of log likelihood and variable parsimony. Variables collinear with the outcome (ie, intensive care unit admission, intubation/mechanical ventilation) were excluded from the model. Residence in Ulaanbaatar was collinear with first measles hospitalization in Ulaanbaatar (ie, all residents of Ulaanbaatar were hospitalized in Ulaanbaatar; n = 17 patients from outside of Ulaanbaatar were hospitalized in Ulaanbaatar), so the variable was not included in the model. We compared a base model, adjusted for age, sex, underlying medical conditions, and history of hospitalization (AIC 332.4) with a model that additionally adjusted for receipt of medical interventions, including vitamin A, antibiotics, and corticosteroids (AIC 290.2). The second model demonstrated superior fit and was selected as the final model. We found no improvement in model fit by including history of breastfeeding or interaction terms. We used an n-fold cross-validation of the final model to assess goodness of fit and found that the model performed well to classify the full data (86%) and test data (83%). We calculated adjusted matched odds ratios (amOR) to measure multivariable associations and corresponding 95% confidence intervals (CI).
RESULTS
Descriptive Epidemiology
A total of 33 947 confirmed measles cases were reported during the outbreak; 14 010 (41%) during wave 1 and 19 937 (59%) during wave 2 (Figure 2A). The cumulative incidence was 11 581 per million. There were several differences in the epidemiology of the 2 outbreak waves. During the first wave, 9671 (69%; average weekly incidence [AWI] 153 per million) cases occurred among adults ≥15 years and 2224 (16%; AWI 1094 per million) cases occurred among infants. During the second wave, 11 346 (57%; AWI 138 per million) cases occurred among adults and 4884 (25%; AWI 1850 per million) cases occurred among infants. Whereas the majority (12 717, 91%) of cases during wave 1 occurred in Ulaanbaatar, in wave 2, approximately half (9258, 46%) occurred in outlying Aimags (Figure 2B). There was a significant increase in the proportion of HAI cases, from 2% (n = 331) during wave 1 to 5% (n = 924) in wave 2 (χ2P < .001) (Figure 2C). The number of total reported deaths increased from wave 1 (n = 3, CFR 0.02%) to wave 2 (n = 113, CFR 0.57%) (Figure 2D). Of the 116 deaths, 97 (84%) occurred among infants. The infant CFR increased, from 0.04% in wave 1 to 1.97% in wave 2 (χ2P < .001; Table 1). The second wave of the outbreak coincided with the seasonal influenza period (Figure 2E), with influenza B as the predominant circulating strain (Supplementary Figure 1).
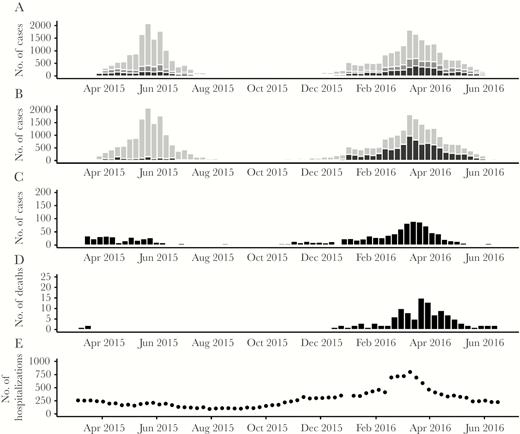
Descriptive epidemiology of Mongolia measles outbreak. A, Epidemiologic curve of measles cases by date of rash onset and age category (infants <12 months, black; children 12 months–14 years, dark grey; adults ≥15 years, light grey). B, Epidemiologic curve of measles cases by date of rash onset and residence: Aimags (provinces), black; Ulaanbaatar (capital city), grey. C, Epidemiologic curve of health care-associated measles infections (ie, hospitalized during 7–21 days before rash onset) by date of rash onset. D, Epidemiologic curve of reported measles deaths by date of rash onset. E, Trend of infant hospitalizations for influenza-like illness by epidemiologic week.
Infant Measles-Related Deaths Reported to Surveillance, Enhanced by Vital Statistics Record Linkage and Case Fatality Ratio, by Outbreak Phase, Mongolia, 2015–2016
| Wave . | Infant Measles Casesa . | Infant Measles-Related Deathsb Reported to Surveillance . | Infant Deaths Reported to Surveillance or Identified by Vital Statistics Linkagec . | Case Fatality Ratio, % . |
|---|---|---|---|---|
| 1, March 2015–September 2015 | 2224 | 1 | 3 | 0.13 |
| 2, October 2015–June 2016 | 4884 | 96 | 113 | 2.31 |
| Wave . | Infant Measles Casesa . | Infant Measles-Related Deathsb Reported to Surveillance . | Infant Deaths Reported to Surveillance or Identified by Vital Statistics Linkagec . | Case Fatality Ratio, % . |
|---|---|---|---|---|
| 1, March 2015–September 2015 | 2224 | 1 | 3 | 0.13 |
| 2, October 2015–June 2016 | 4884 | 96 | 113 | 2.31 |
aInfants with laboratory evidence of measles infection (ie, presence of measles-specific immunoglobulin M antibody or positive measles polymerase chain reaction) or clinical evidence of measles infection (presence of fever and rash and ≥1 of cough, coryza, or conjunctivitis.
bDeath of a measles patient ≤30 days of rash onset due to any related cause.
cProbabilistic record linkage of nationwide infant mortality dataset with measles surveillance database.
Infant Measles-Related Deaths Reported to Surveillance, Enhanced by Vital Statistics Record Linkage and Case Fatality Ratio, by Outbreak Phase, Mongolia, 2015–2016
| Wave . | Infant Measles Casesa . | Infant Measles-Related Deathsb Reported to Surveillance . | Infant Deaths Reported to Surveillance or Identified by Vital Statistics Linkagec . | Case Fatality Ratio, % . |
|---|---|---|---|---|
| 1, March 2015–September 2015 | 2224 | 1 | 3 | 0.13 |
| 2, October 2015–June 2016 | 4884 | 96 | 113 | 2.31 |
| Wave . | Infant Measles Casesa . | Infant Measles-Related Deathsb Reported to Surveillance . | Infant Deaths Reported to Surveillance or Identified by Vital Statistics Linkagec . | Case Fatality Ratio, % . |
|---|---|---|---|---|
| 1, March 2015–September 2015 | 2224 | 1 | 3 | 0.13 |
| 2, October 2015–June 2016 | 4884 | 96 | 113 | 2.31 |
aInfants with laboratory evidence of measles infection (ie, presence of measles-specific immunoglobulin M antibody or positive measles polymerase chain reaction) or clinical evidence of measles infection (presence of fever and rash and ≥1 of cough, coryza, or conjunctivitis.
bDeath of a measles patient ≤30 days of rash onset due to any related cause.
cProbabilistic record linkage of nationwide infant mortality dataset with measles surveillance database.
Enhanced Infant Measles Mortality Ascertainment and Estimation of Case Fatality Ratio
Using linkage of case-based surveillance and vital statistics data, we identified 2 additional infant deaths in wave 1 and 17 additional deaths in wave 2 (Table 1). We calculated CFR using the enhanced mortality data; we identified an increase in CFR from wave 1 (0.13%) to wave 2 (2.31%; P < .001). Of 96 records with ICD-10 codes assigned, 86 (89.5%) of infant measles deaths were assigned an ICD-10 code for measles death, and 9 (9.5%) were assigned pneumonia (unspecified) ICD-10 codes.
Case-Control Sample Characteristics and Bivariate Analyses
Fatal cases and hospitalized controls were well matched; there were no significant differences between cases and controls for age at rash onset or sex (Table 2). A higher proportion of cases (67%) than nonfatal hospitalized controls (46%) resided in outlying Aimags. Most (316/368) of the cases and controls were too young to receive the first routine dose of MCV. Receipt of ≥1 dose of MCV was low overall, and did not significantly differ between cases (5%) and controls (2%). Comorbid medical conditions including premature birth or birth defects, immune deficiencies, rickets, malnutrition, and anemia were all statistically significantly more common among cases than controls (Table 2). Cases were more likely to have been admitted to a hospital during the measles incubation period (ie, measles virus HAI) than controls (53% versus 26%; χ2P < .001) and were more likely to be admitted for pneumonia or influenza diagnoses (86% of hospitalizations) than controls (61%; χ2P < .001). However, the proportion of antecedent hospitalizations for other diagnoses (eg, urinary tract infection, diarrhea, upper respiratory infections) did not differ between groups. The mean duration of hospitalization was 25 days (range 9‒68) and did not differ significantly between cases and controls. The mean duration between hospital admission and rash onset was 14 days (range 8‒21). There was no difference in delay to hospitalization after rash onset; most cases (69%) and controls (63%) were hospitalized <2 days after rash onset. A higher proportion of cases (64%) than controls (43%) were hospitalized for measles in Aimags versus Ulaanbaatar (χ2P < .001). There were no significant differences in receipt of vitamin A among cases (17%) versus controls (22%). Case patients were less likely to receive a dose of antibiotics in the hospital (95%) than controls (99%; χ2P = .02).
Characteristics of Fatal Infant Measles Cases and Matched Nonfatal Measles Controls, Mongolia, 2015–2016
| Characteristics . | Total, n (%) (n = 368) . | Cases, n (%) (n = 95) . | Controls, n (%) (n = 273) . | χ2P . |
|---|---|---|---|---|
| Infant demographic factors | ||||
| Age at rash onset | .88 | |||
| <3 mo | 33 (9.0) | 7 (7.4) | 26 (9.5) | |
| 3 mo to <6 mo | 134 (36.4) | 34 (35.8) | 100 (36.6) | |
| 6 mo to <9 mo | 149 (40.5) | 39 (41.1) | 110 (40.3) | |
| ≥9 mo to <12 mo | 52 (14.1) | 15 (15.8) | 37 (13.6) | |
| Sex | .96 | |||
| Female | 148 (40.2) | 38 (40.0) | 110 (40.3) | |
| Male | 220 (59.8) | 57 (60.0) | 163 (59.7) | |
| Residence | <.001 | |||
| Ulaanbaatar City | 178 (48.4) | 31 (32.6) | 147 (53.9) | |
| Aimags (provinces) | 190 (51.6) | 64 (67.4) | 126 (46.2) | |
| Infant medical and vaccination history | ||||
| Received ≥1 dose of vaccine | ||||
| MCVa | 10 (2.7) | 5 (5.3) | 5 (1.8) | .21 |
| Hib/pentavalentb | 283 (77.5) | 64 (67.4) | 219 (81.1) | .02 |
| Influenza | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Underlying medical conditions | ||||
| Premature birth or birth defect | 27 (7.3) | 12 (12.6) | 15 (5.5) | .02 |
| Immune deficiency | 38 (10.3) | 16 (16.8) | 22 (8.1) | .015 |
| Rickets | 34 (9.2) | 24 (25.3) | 10 (3.7) | <.001 |
| Malnutritionc | 21 (5.7) | 11 (11.6) | 10 (3.7) | .004 |
| Anemia | 67 (18.2) | 49 (51.6) | 18 (6.6) | <.001 |
| Breastfeeding history | ||||
| Breastfed ever | 303 (82.3) | 73 (76.8) | 230 (84.3) | .10 |
| Health care exposures before rash onset | ||||
| Admitted to hospital 7–21 days before rash onset | 122 (33.2) | 50 (52.6) | 72 (26.4) | <.001 |
| Diagnoses during hospitalization | ||||
| Pneumonia or influenza | 87 (23.6) | 43 (45.3) | 44 (16.1) | <.001 |
| Other diagnosisd | 35 (9.5) | 7 (7.4) | 28 (10.3) | .41 |
| Health care exposures after rash onset | ||||
| Days to hospitalization after rash onset | ||||
| 0 days | 103 (28.0) | 34 (35.8) | 69 (25.3) | .14 |
| 1 day | 134 (36.4) | 32 (33.7) | 102 (37.4) | |
| >1 day | 131 (35.6) | 29 (30.5) | 102 (37.4) | |
| Location of first hospitalization after rash onset | ||||
| Ulaanbaatar | 191 (52.0) | 34 (36.2) | 157 (57.5) | <.001 |
| Outside of Ulaanbaatar | 176 (48.0) | 60 (63.8) | 116 (42.5) | |
| Treatment received | ||||
| Received vitamin A (any) | 75 (20.4) | 16 (16.8) | 59 (21.6) | .32 |
| Received antibiotics (any) | 360 (97.8) | 90 (94.8) | 270 (98.9) | .02 |
| Characteristics . | Total, n (%) (n = 368) . | Cases, n (%) (n = 95) . | Controls, n (%) (n = 273) . | χ2P . |
|---|---|---|---|---|
| Infant demographic factors | ||||
| Age at rash onset | .88 | |||
| <3 mo | 33 (9.0) | 7 (7.4) | 26 (9.5) | |
| 3 mo to <6 mo | 134 (36.4) | 34 (35.8) | 100 (36.6) | |
| 6 mo to <9 mo | 149 (40.5) | 39 (41.1) | 110 (40.3) | |
| ≥9 mo to <12 mo | 52 (14.1) | 15 (15.8) | 37 (13.6) | |
| Sex | .96 | |||
| Female | 148 (40.2) | 38 (40.0) | 110 (40.3) | |
| Male | 220 (59.8) | 57 (60.0) | 163 (59.7) | |
| Residence | <.001 | |||
| Ulaanbaatar City | 178 (48.4) | 31 (32.6) | 147 (53.9) | |
| Aimags (provinces) | 190 (51.6) | 64 (67.4) | 126 (46.2) | |
| Infant medical and vaccination history | ||||
| Received ≥1 dose of vaccine | ||||
| MCVa | 10 (2.7) | 5 (5.3) | 5 (1.8) | .21 |
| Hib/pentavalentb | 283 (77.5) | 64 (67.4) | 219 (81.1) | .02 |
| Influenza | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Underlying medical conditions | ||||
| Premature birth or birth defect | 27 (7.3) | 12 (12.6) | 15 (5.5) | .02 |
| Immune deficiency | 38 (10.3) | 16 (16.8) | 22 (8.1) | .015 |
| Rickets | 34 (9.2) | 24 (25.3) | 10 (3.7) | <.001 |
| Malnutritionc | 21 (5.7) | 11 (11.6) | 10 (3.7) | .004 |
| Anemia | 67 (18.2) | 49 (51.6) | 18 (6.6) | <.001 |
| Breastfeeding history | ||||
| Breastfed ever | 303 (82.3) | 73 (76.8) | 230 (84.3) | .10 |
| Health care exposures before rash onset | ||||
| Admitted to hospital 7–21 days before rash onset | 122 (33.2) | 50 (52.6) | 72 (26.4) | <.001 |
| Diagnoses during hospitalization | ||||
| Pneumonia or influenza | 87 (23.6) | 43 (45.3) | 44 (16.1) | <.001 |
| Other diagnosisd | 35 (9.5) | 7 (7.4) | 28 (10.3) | .41 |
| Health care exposures after rash onset | ||||
| Days to hospitalization after rash onset | ||||
| 0 days | 103 (28.0) | 34 (35.8) | 69 (25.3) | .14 |
| 1 day | 134 (36.4) | 32 (33.7) | 102 (37.4) | |
| >1 day | 131 (35.6) | 29 (30.5) | 102 (37.4) | |
| Location of first hospitalization after rash onset | ||||
| Ulaanbaatar | 191 (52.0) | 34 (36.2) | 157 (57.5) | <.001 |
| Outside of Ulaanbaatar | 176 (48.0) | 60 (63.8) | 116 (42.5) | |
| Treatment received | ||||
| Received vitamin A (any) | 75 (20.4) | 16 (16.8) | 59 (21.6) | .32 |
| Received antibiotics (any) | 360 (97.8) | 90 (94.8) | 270 (98.9) | .02 |
aFirst dose of measles-containing vaccine (MCV) at 9 months of age.
bFirst dose at 2 months of age.
c2006 WHO Growth Curve Z-score for weight for age less than −2 at the time of first hospital admission after rash onset or chart diagnosis of malnutrition.
dDiarrhea, bronchitis, laryngitis, seizure, meningitis, urinary infection, other.
Characteristics of Fatal Infant Measles Cases and Matched Nonfatal Measles Controls, Mongolia, 2015–2016
| Characteristics . | Total, n (%) (n = 368) . | Cases, n (%) (n = 95) . | Controls, n (%) (n = 273) . | χ2P . |
|---|---|---|---|---|
| Infant demographic factors | ||||
| Age at rash onset | .88 | |||
| <3 mo | 33 (9.0) | 7 (7.4) | 26 (9.5) | |
| 3 mo to <6 mo | 134 (36.4) | 34 (35.8) | 100 (36.6) | |
| 6 mo to <9 mo | 149 (40.5) | 39 (41.1) | 110 (40.3) | |
| ≥9 mo to <12 mo | 52 (14.1) | 15 (15.8) | 37 (13.6) | |
| Sex | .96 | |||
| Female | 148 (40.2) | 38 (40.0) | 110 (40.3) | |
| Male | 220 (59.8) | 57 (60.0) | 163 (59.7) | |
| Residence | <.001 | |||
| Ulaanbaatar City | 178 (48.4) | 31 (32.6) | 147 (53.9) | |
| Aimags (provinces) | 190 (51.6) | 64 (67.4) | 126 (46.2) | |
| Infant medical and vaccination history | ||||
| Received ≥1 dose of vaccine | ||||
| MCVa | 10 (2.7) | 5 (5.3) | 5 (1.8) | .21 |
| Hib/pentavalentb | 283 (77.5) | 64 (67.4) | 219 (81.1) | .02 |
| Influenza | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Underlying medical conditions | ||||
| Premature birth or birth defect | 27 (7.3) | 12 (12.6) | 15 (5.5) | .02 |
| Immune deficiency | 38 (10.3) | 16 (16.8) | 22 (8.1) | .015 |
| Rickets | 34 (9.2) | 24 (25.3) | 10 (3.7) | <.001 |
| Malnutritionc | 21 (5.7) | 11 (11.6) | 10 (3.7) | .004 |
| Anemia | 67 (18.2) | 49 (51.6) | 18 (6.6) | <.001 |
| Breastfeeding history | ||||
| Breastfed ever | 303 (82.3) | 73 (76.8) | 230 (84.3) | .10 |
| Health care exposures before rash onset | ||||
| Admitted to hospital 7–21 days before rash onset | 122 (33.2) | 50 (52.6) | 72 (26.4) | <.001 |
| Diagnoses during hospitalization | ||||
| Pneumonia or influenza | 87 (23.6) | 43 (45.3) | 44 (16.1) | <.001 |
| Other diagnosisd | 35 (9.5) | 7 (7.4) | 28 (10.3) | .41 |
| Health care exposures after rash onset | ||||
| Days to hospitalization after rash onset | ||||
| 0 days | 103 (28.0) | 34 (35.8) | 69 (25.3) | .14 |
| 1 day | 134 (36.4) | 32 (33.7) | 102 (37.4) | |
| >1 day | 131 (35.6) | 29 (30.5) | 102 (37.4) | |
| Location of first hospitalization after rash onset | ||||
| Ulaanbaatar | 191 (52.0) | 34 (36.2) | 157 (57.5) | <.001 |
| Outside of Ulaanbaatar | 176 (48.0) | 60 (63.8) | 116 (42.5) | |
| Treatment received | ||||
| Received vitamin A (any) | 75 (20.4) | 16 (16.8) | 59 (21.6) | .32 |
| Received antibiotics (any) | 360 (97.8) | 90 (94.8) | 270 (98.9) | .02 |
| Characteristics . | Total, n (%) (n = 368) . | Cases, n (%) (n = 95) . | Controls, n (%) (n = 273) . | χ2P . |
|---|---|---|---|---|
| Infant demographic factors | ||||
| Age at rash onset | .88 | |||
| <3 mo | 33 (9.0) | 7 (7.4) | 26 (9.5) | |
| 3 mo to <6 mo | 134 (36.4) | 34 (35.8) | 100 (36.6) | |
| 6 mo to <9 mo | 149 (40.5) | 39 (41.1) | 110 (40.3) | |
| ≥9 mo to <12 mo | 52 (14.1) | 15 (15.8) | 37 (13.6) | |
| Sex | .96 | |||
| Female | 148 (40.2) | 38 (40.0) | 110 (40.3) | |
| Male | 220 (59.8) | 57 (60.0) | 163 (59.7) | |
| Residence | <.001 | |||
| Ulaanbaatar City | 178 (48.4) | 31 (32.6) | 147 (53.9) | |
| Aimags (provinces) | 190 (51.6) | 64 (67.4) | 126 (46.2) | |
| Infant medical and vaccination history | ||||
| Received ≥1 dose of vaccine | ||||
| MCVa | 10 (2.7) | 5 (5.3) | 5 (1.8) | .21 |
| Hib/pentavalentb | 283 (77.5) | 64 (67.4) | 219 (81.1) | .02 |
| Influenza | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Underlying medical conditions | ||||
| Premature birth or birth defect | 27 (7.3) | 12 (12.6) | 15 (5.5) | .02 |
| Immune deficiency | 38 (10.3) | 16 (16.8) | 22 (8.1) | .015 |
| Rickets | 34 (9.2) | 24 (25.3) | 10 (3.7) | <.001 |
| Malnutritionc | 21 (5.7) | 11 (11.6) | 10 (3.7) | .004 |
| Anemia | 67 (18.2) | 49 (51.6) | 18 (6.6) | <.001 |
| Breastfeeding history | ||||
| Breastfed ever | 303 (82.3) | 73 (76.8) | 230 (84.3) | .10 |
| Health care exposures before rash onset | ||||
| Admitted to hospital 7–21 days before rash onset | 122 (33.2) | 50 (52.6) | 72 (26.4) | <.001 |
| Diagnoses during hospitalization | ||||
| Pneumonia or influenza | 87 (23.6) | 43 (45.3) | 44 (16.1) | <.001 |
| Other diagnosisd | 35 (9.5) | 7 (7.4) | 28 (10.3) | .41 |
| Health care exposures after rash onset | ||||
| Days to hospitalization after rash onset | ||||
| 0 days | 103 (28.0) | 34 (35.8) | 69 (25.3) | .14 |
| 1 day | 134 (36.4) | 32 (33.7) | 102 (37.4) | |
| >1 day | 131 (35.6) | 29 (30.5) | 102 (37.4) | |
| Location of first hospitalization after rash onset | ||||
| Ulaanbaatar | 191 (52.0) | 34 (36.2) | 157 (57.5) | <.001 |
| Outside of Ulaanbaatar | 176 (48.0) | 60 (63.8) | 116 (42.5) | |
| Treatment received | ||||
| Received vitamin A (any) | 75 (20.4) | 16 (16.8) | 59 (21.6) | .32 |
| Received antibiotics (any) | 360 (97.8) | 90 (94.8) | 270 (98.9) | .02 |
aFirst dose of measles-containing vaccine (MCV) at 9 months of age.
bFirst dose at 2 months of age.
c2006 WHO Growth Curve Z-score for weight for age less than −2 at the time of first hospital admission after rash onset or chart diagnosis of malnutrition.
dDiarrhea, bronchitis, laryngitis, seizure, meningitis, urinary infection, other.
Multivariable Conditional Logistic Regression
After adjustment for covariates, underlying medical conditions, including premature birth or birth defects, immune deficiencies, rickets, and anemia were significantly associated with measles death (Table 3). First hospitalization after rash onset outside of Ulaanbaatar was significantly associated with measles death (amOR 2.5; CI, 1.1–5.8). In a sensitivity analysis conducted by including residence within or outside of Ulaanbaatar as a predictor variable, hospitalization outside of Ulaanbaatar and not residence outside of Ulaanbaatar was significantly associated with measles death (data not shown). Compared with infants who were not hospitalized during the measles incubation period, infants admitted during the incubation period with pneumonia or influenza diagnosis (amOR 4.5; CI, 1.9–10.8) were more likely to die whereas those admitted for other diagnoses (amOR 0.7; CI, 0.2–2.7) were not.
Multivariable Conditional Logistic Regression for Measles Mortality Among Fatal Measles Cases and Matched Nonfatal Measles Controls, Mongolia, 2015–2016
| Characteristics . | Bivariatea OR (95% CI) . | Adjustedb OR (95% CI) . |
|---|---|---|
| Infant medical and vaccination history | ||
| Received ≥1 dose of vaccine: | ||
| MCVc | 2.9 (.8–10.4) | 7.1 (.9–58.2) |
| Hib/pentavalentd | 0.4 (.2–.7) | 0.9 (.4–2.0) |
| Underlying medical conditions | ||
| Premature birth or birth defect | 2.3 (1.0–5.0) | 4.8 (1.5–15.2) |
| Immune deficiency | 2.3 (1.1–4.8) | 3.6 (1.1–11.3) |
| Rickets | 9.2 (3.9–21.7) | 5.5 (1.7–17.1) |
| Malnutritione | 3.1 (1.3–7.4) | 0.7 (.2–3.5) |
| Anemia | 15.1 (7.2–31.7) | 12.3 (4.9–31.1) |
| Health care-associated infection | ||
| Admitted to hospital 7–21 days before rash onset | ||
| No (non-HAI) | REF | REF |
| HAI: pneumonia or influenza diagnosis | 4.5 (2.6–8.0) | 4.5 (1.9–10.8) |
| HAI: other diagnosisf | 1.1 (.4–2.7) | 0.7 (.2–2.7) |
| Health care exposures after rash onset | ||
| Location of first hospitalization after rash onset | ||
| Ulaanbaatar | REF | REF |
| Outside of Ulaanbaatar | 2.7 (1.6–4.6) | 2.4 (1.1–5.8) |
| Treatment received | ||
| Received vitamin A (any) | 0.6 (.3–1.2) | 0.5 (.2–1.3) |
| Received antibiotics (any) | 0.2 (.0–.8) | 0.2 (.0–1.7) |
| Characteristics . | Bivariatea OR (95% CI) . | Adjustedb OR (95% CI) . |
|---|---|---|
| Infant medical and vaccination history | ||
| Received ≥1 dose of vaccine: | ||
| MCVc | 2.9 (.8–10.4) | 7.1 (.9–58.2) |
| Hib/pentavalentd | 0.4 (.2–.7) | 0.9 (.4–2.0) |
| Underlying medical conditions | ||
| Premature birth or birth defect | 2.3 (1.0–5.0) | 4.8 (1.5–15.2) |
| Immune deficiency | 2.3 (1.1–4.8) | 3.6 (1.1–11.3) |
| Rickets | 9.2 (3.9–21.7) | 5.5 (1.7–17.1) |
| Malnutritione | 3.1 (1.3–7.4) | 0.7 (.2–3.5) |
| Anemia | 15.1 (7.2–31.7) | 12.3 (4.9–31.1) |
| Health care-associated infection | ||
| Admitted to hospital 7–21 days before rash onset | ||
| No (non-HAI) | REF | REF |
| HAI: pneumonia or influenza diagnosis | 4.5 (2.6–8.0) | 4.5 (1.9–10.8) |
| HAI: other diagnosisf | 1.1 (.4–2.7) | 0.7 (.2–2.7) |
| Health care exposures after rash onset | ||
| Location of first hospitalization after rash onset | ||
| Ulaanbaatar | REF | REF |
| Outside of Ulaanbaatar | 2.7 (1.6–4.6) | 2.4 (1.1–5.8) |
| Treatment received | ||
| Received vitamin A (any) | 0.6 (.3–1.2) | 0.5 (.2–1.3) |
| Received antibiotics (any) | 0.2 (.0–.8) | 0.2 (.0–1.7) |
Abbreviations: CI, confidence interval; HAI, health care-associated infection; MCV, measles-containing vaccine; OR, odds ratio; REF, reference.
Bold indicates P value <0.05 in the conditional logistic regression model.
aConditional logistic regression OR accounting for matching on age and sex.
bConditional logistic regression OR accounting for matching on age, sex, and controlling for all other variables in Table 3.
cFirst dose of MCV at 9 months of age.
dFirst dose at 2 months of age.
e2006 WHO Growth Curve Z-score for weight for age less than −2 at the time of first hospital admission after rash onset or chart diagnosis of malnutrition.
fDiarrhea, bronchitis, laryngitis, seizure, meningitis, urinary infection, other.
Multivariable Conditional Logistic Regression for Measles Mortality Among Fatal Measles Cases and Matched Nonfatal Measles Controls, Mongolia, 2015–2016
| Characteristics . | Bivariatea OR (95% CI) . | Adjustedb OR (95% CI) . |
|---|---|---|
| Infant medical and vaccination history | ||
| Received ≥1 dose of vaccine: | ||
| MCVc | 2.9 (.8–10.4) | 7.1 (.9–58.2) |
| Hib/pentavalentd | 0.4 (.2–.7) | 0.9 (.4–2.0) |
| Underlying medical conditions | ||
| Premature birth or birth defect | 2.3 (1.0–5.0) | 4.8 (1.5–15.2) |
| Immune deficiency | 2.3 (1.1–4.8) | 3.6 (1.1–11.3) |
| Rickets | 9.2 (3.9–21.7) | 5.5 (1.7–17.1) |
| Malnutritione | 3.1 (1.3–7.4) | 0.7 (.2–3.5) |
| Anemia | 15.1 (7.2–31.7) | 12.3 (4.9–31.1) |
| Health care-associated infection | ||
| Admitted to hospital 7–21 days before rash onset | ||
| No (non-HAI) | REF | REF |
| HAI: pneumonia or influenza diagnosis | 4.5 (2.6–8.0) | 4.5 (1.9–10.8) |
| HAI: other diagnosisf | 1.1 (.4–2.7) | 0.7 (.2–2.7) |
| Health care exposures after rash onset | ||
| Location of first hospitalization after rash onset | ||
| Ulaanbaatar | REF | REF |
| Outside of Ulaanbaatar | 2.7 (1.6–4.6) | 2.4 (1.1–5.8) |
| Treatment received | ||
| Received vitamin A (any) | 0.6 (.3–1.2) | 0.5 (.2–1.3) |
| Received antibiotics (any) | 0.2 (.0–.8) | 0.2 (.0–1.7) |
| Characteristics . | Bivariatea OR (95% CI) . | Adjustedb OR (95% CI) . |
|---|---|---|
| Infant medical and vaccination history | ||
| Received ≥1 dose of vaccine: | ||
| MCVc | 2.9 (.8–10.4) | 7.1 (.9–58.2) |
| Hib/pentavalentd | 0.4 (.2–.7) | 0.9 (.4–2.0) |
| Underlying medical conditions | ||
| Premature birth or birth defect | 2.3 (1.0–5.0) | 4.8 (1.5–15.2) |
| Immune deficiency | 2.3 (1.1–4.8) | 3.6 (1.1–11.3) |
| Rickets | 9.2 (3.9–21.7) | 5.5 (1.7–17.1) |
| Malnutritione | 3.1 (1.3–7.4) | 0.7 (.2–3.5) |
| Anemia | 15.1 (7.2–31.7) | 12.3 (4.9–31.1) |
| Health care-associated infection | ||
| Admitted to hospital 7–21 days before rash onset | ||
| No (non-HAI) | REF | REF |
| HAI: pneumonia or influenza diagnosis | 4.5 (2.6–8.0) | 4.5 (1.9–10.8) |
| HAI: other diagnosisf | 1.1 (.4–2.7) | 0.7 (.2–2.7) |
| Health care exposures after rash onset | ||
| Location of first hospitalization after rash onset | ||
| Ulaanbaatar | REF | REF |
| Outside of Ulaanbaatar | 2.7 (1.6–4.6) | 2.4 (1.1–5.8) |
| Treatment received | ||
| Received vitamin A (any) | 0.6 (.3–1.2) | 0.5 (.2–1.3) |
| Received antibiotics (any) | 0.2 (.0–.8) | 0.2 (.0–1.7) |
Abbreviations: CI, confidence interval; HAI, health care-associated infection; MCV, measles-containing vaccine; OR, odds ratio; REF, reference.
Bold indicates P value <0.05 in the conditional logistic regression model.
aConditional logistic regression OR accounting for matching on age and sex.
bConditional logistic regression OR accounting for matching on age, sex, and controlling for all other variables in Table 3.
cFirst dose of MCV at 9 months of age.
dFirst dose at 2 months of age.
e2006 WHO Growth Curve Z-score for weight for age less than −2 at the time of first hospital admission after rash onset or chart diagnosis of malnutrition.
fDiarrhea, bronchitis, laryngitis, seizure, meningitis, urinary infection, other.
Clinical and Diagnostic Findings
A higher proportion of fatal cases (91/95, 96%) than hospitalized controls (182/273, 67%) had clinical pneumonia diagnoses in the chart, and a higher proportion of fatal cases (19/95, 20%) than hospitalized controls (1/273, 0.4%) were diagnosed with acute respiratory distress syndrome (Table 4). Influenza A or B testing results were not identified in any charts. Fatal cases were more likely to have neurologic complications than hospitalized controls, including encephalitis, meningitis, and seizure. Autopsy was performed on a subset (n = 10) of fatal cases (Supplementary Table 1); none had evidence of bacterial pneumonia, whereas 6 (60%) had an interstitial pneumonia pattern, of which 5 had a pathological finding of giant cell viral pneumonitis consistent with primary measles pneumonia (Figure 3). Two of 6 postmortem lung tissue specimens that were positive for measles RNA were positive for influenza B virus by RT-PCR.
Clinical and Diagnostic Findings Among Fatal Measles Cases and Nonfatal Measles Controls, Mongolia, 2015–2016
| Findings . | Cases, n (%) (n = 95) . | Controls, n (%) (n = 273) . |
|---|---|---|
| Pneumonia | 91 (95.8) | 182 (66.7) |
| Acute respiratory distress syndrome | 19 (20.0) | 1 (0.4) |
| Chest radiograph findings | ||
| Bilateral consolidation | 53 (55.8) | 101 (37.0) |
| Interstitial pattern | 13 (13.7) | 61 (22.3) |
| None performed | 36 (37.9) | 105 (38.5) |
| Neurologic complications | 52 (54.7) | 52 (19.1) |
| Encephalitis | 8 (8.4) | 0 (0.0) |
| Meningitis | 4 (4.2) | 1 (0.4) |
| Seizure | 52 (54.7) | 52 (19.1) |
| Findings . | Cases, n (%) (n = 95) . | Controls, n (%) (n = 273) . |
|---|---|---|
| Pneumonia | 91 (95.8) | 182 (66.7) |
| Acute respiratory distress syndrome | 19 (20.0) | 1 (0.4) |
| Chest radiograph findings | ||
| Bilateral consolidation | 53 (55.8) | 101 (37.0) |
| Interstitial pattern | 13 (13.7) | 61 (22.3) |
| None performed | 36 (37.9) | 105 (38.5) |
| Neurologic complications | 52 (54.7) | 52 (19.1) |
| Encephalitis | 8 (8.4) | 0 (0.0) |
| Meningitis | 4 (4.2) | 1 (0.4) |
| Seizure | 52 (54.7) | 52 (19.1) |
Clinical and Diagnostic Findings Among Fatal Measles Cases and Nonfatal Measles Controls, Mongolia, 2015–2016
| Findings . | Cases, n (%) (n = 95) . | Controls, n (%) (n = 273) . |
|---|---|---|
| Pneumonia | 91 (95.8) | 182 (66.7) |
| Acute respiratory distress syndrome | 19 (20.0) | 1 (0.4) |
| Chest radiograph findings | ||
| Bilateral consolidation | 53 (55.8) | 101 (37.0) |
| Interstitial pattern | 13 (13.7) | 61 (22.3) |
| None performed | 36 (37.9) | 105 (38.5) |
| Neurologic complications | 52 (54.7) | 52 (19.1) |
| Encephalitis | 8 (8.4) | 0 (0.0) |
| Meningitis | 4 (4.2) | 1 (0.4) |
| Seizure | 52 (54.7) | 52 (19.1) |
| Findings . | Cases, n (%) (n = 95) . | Controls, n (%) (n = 273) . |
|---|---|---|
| Pneumonia | 91 (95.8) | 182 (66.7) |
| Acute respiratory distress syndrome | 19 (20.0) | 1 (0.4) |
| Chest radiograph findings | ||
| Bilateral consolidation | 53 (55.8) | 101 (37.0) |
| Interstitial pattern | 13 (13.7) | 61 (22.3) |
| None performed | 36 (37.9) | 105 (38.5) |
| Neurologic complications | 52 (54.7) | 52 (19.1) |
| Encephalitis | 8 (8.4) | 0 (0.0) |
| Meningitis | 4 (4.2) | 1 (0.4) |
| Seizure | 52 (54.7) | 52 (19.1) |
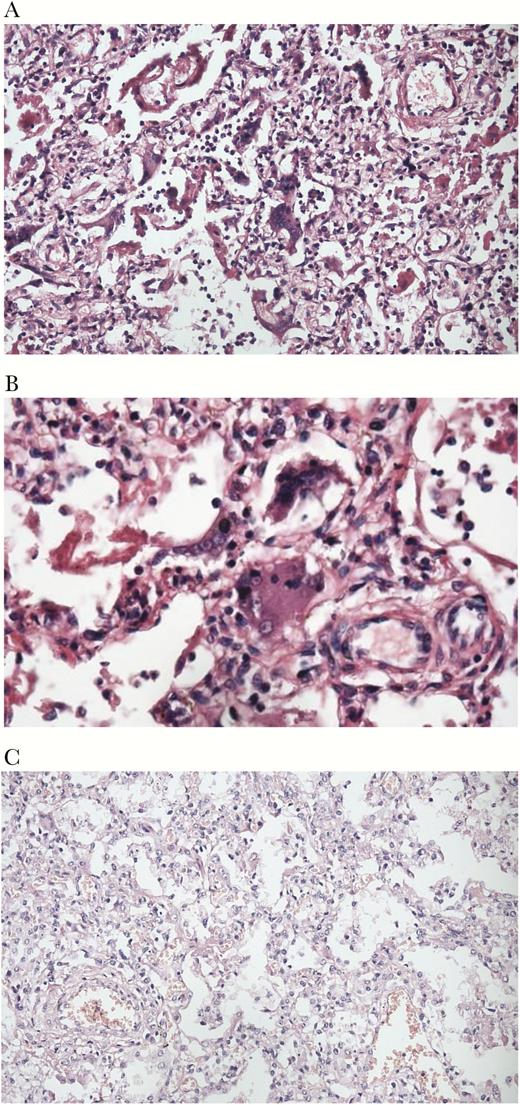
Hematoxylin and eosin stained specimens of lung tissue from 2 fatal measles cases. A, Interstitial pneumonia pattern with Hecht’s giant cells in a fatal measles patient that was hospitalized during the measles incubation period, but for a diagnosis other than pneumonia or influenza (×20). B, Specimen from the same patient as in (A), at ×40, with Hecht’s giant cells. C, interstitial pneumonia pattern without Hecht’s giant cells in a fatal measles patient that was hospitalized during the measles incubation period for influenza.
DISCUSSION
During a response to the largest measles outbreak in any postelimination setting, we confirmed a significant increase in CFR during outbreak and identified nosocomial measles infections among infants diagnosed with influenza or pneumonia as a risk factor for measles death among infants hospitalized for measles. Incidence among infants was high during both waves of the outbreak despite a high coverage supplemental immunization activity conducted during the first wave [14], as infants infected during the second wave of the outbreak were too young to be vaccinated during the campaign.
Linkage of national surveillance and vital statistics data allowed for rapid confirmation of increased CFR during the outbreak rather than a reporting artifact. Record linkage has been implemented during outbreaks to better understand frequencies of complications and deaths [15], hospitalizations [16], and the sensitivity of surveillance systems [10]. In addition to the use of probabilistic record linkage to verify CFR, our study provides clinical data from medical charts for both cases and controls, which supplement epidemiologic descriptions of the outbreak [17]. Although the majority of measles deaths were assigned the ICD-10 code for measles, approximately 10% had a nonspecific pneumonia ICD-10 code, suggesting that analysis of vital statistics data to determine under-5 mortality alone might result in underascertainment of the measles mortality burden [3].
Epidemiologic evidence from our study suggested mortality was associated with nosocomial measles virus transmission among infants with preexisting pneumonia or influenza diagnoses, and hospitalization outside of the capital city. Nosocomial transmission has been increasingly recognized as a barrier to measles elimination in low-incidence settings and has been attributed to the high basic reproduction number of the disease and the ability of measles virus to remain viable in aerosol suspension for up to 2 hours [18, 19]. HAI was found to be a risk factor for infection during the first wave of the outbreak [20] and might be more severe than community-acquired infections, as patients have underlying illnesses and might be subject to intense virus exposure in a closed setting [18, 21]. Health care workers might be at higher risk for measles infection than persons in the community, and transmission in health care facilities poses substantial risks to infants and immunocompromised persons [22, 23]. WHO recommends that all health care workers be measles immune, and proof of vaccination or immunity should be a condition of enrollment into training or employment [23]. Infection control measures should be implemented to prevent nosocomial measles virus transmission, which include rapid evaluation and respiratory isolation of patients with suspected or confirmed measles infection and use of respiratory protection, eye protection, gloves, and appropriate hand washing by health care workers [19]. An investigation of nosocomial infections during the outbreak identified at least 55 measles infections among health care workers during the outbreak [24]. Although policies for infection control were available in Mongolia, infrastructure limitations and the high number of cases did not allow for their full implementation [24].
Hospitalization for pneumonia or influenza diagnoses during the measles incubation period was associated with measles death, suggesting that infants were hospitalized for respiratory illnesses prior to measles infection. Although influenza testing was not routinely performed, epidemiologic evidence of the circulation of influenza B during the second wave of the outbreak and laboratory data suggest a possible mechanism of sequential influenza B and measles virus infections increasing measles mortality among infants. Among 10 infants with pathologic specimens available, none had evidence of bacterial pneumonia, whereas influenza B viral RNA was detected in postmortem specimens from 2 of 6 fatal measles cases that were also positive for measles virus RNA. Coinfection with other respiratory pathogens has been associated with increased severity of measles [25]. Although influenza and measles coinfections have been previously observed, to our knowledge, this is the first study to suggest that previous influenza infection might increase measles fatality. Because measles deaths are defined as deaths due to any related cause (ie, including due to influenza or pneumonia) [9], and measles is known to produce long-term immune modulation and increase mortality from other infectious causes [26], it is possible that some of these measles infections were a “second hit” for previously ill infants with preexisting severe respiratory infections. Although we cannot conclusively determine whether measles-related immunodeficiency increased the risk of death among patients with preexisting lung infection or whether preexisting lung infection increased the risk of measles mortality, pathologic evidence from 5 fatal measles cases consistent with primary measles pneumonia is suggestive of measles being the proximal cause of deaths among these patients. Prevention of other respiratory infections, including vaccine-preventable bacterial and viral causes, might reduce the morbidity and mortality observed during measles outbreaks.
Only a minority of infants received appropriate vitamin A supplementation. Vitamin A is an essential nutrient that regulates human immune function [27]. Vitamin A supplementation among children <5 years of age significantly reduces child measles morbidity and mortality [28], and is recommended by WHO on 2 consecutive days (at the time of diagnosis and on the following day) for all children <5 years of age when measles CFR >1%, in areas of known vitamin A deficiency, and as part of case management for suspected measles cases [29, 30]. Our finding of an association between anemia and rickets diagnosis with measles mortality, conditions not previously known to be associated with measles death, might have represented a proxy for vitamin A or other micronutrient deficiencies, as these conditions are known to be associated with underlying micronutrient deficiencies [31, 32].
The interpretation of these results is subject to several limitations. First, we were unable to directly test the hypothesis that influenza coinfection was more prevalent in fatal cases because influenza testing results were not documented; the lack of documentation of influenza testing results also limited our ability to distinguish between influenza and viral or bacterial pneumonias. Second, interpreting pneumonia etiology (ie, viral coinfection, measles pneumonia, or bacterial pneumonia) was limited by the small number of pathologic specimens available among fatal cases. Because of the potentially long interval between influenza B infection and measles death, it is possible that the pathologic specimens we tested underestimated the number of infant measles deaths with influenza B coinfection. We were unable to directly measure micronutrient deficiencies, including vitamin A, which might be associated with measles mortality, but are associated with 2 covariates in the model (rickets and anemia), which resulted in potentially spurious associations between these conditions and measles mortality. Last, because we restricted the case control sample to infants hospitalized with measles infection during the second wave, we were unable to make inferences about other age groups, or other seasonal factors including the severe winter in Mongolia (known as Dzud) that occurred during the period of study. Similarly, as medical record abstraction was used to obtain data for the study, other social determinants of health could not be measured as potential risk factors for mortality.
This investigation, which took place during a large outbreak, highlighted factors that might aid in future outbreak response in postelimination settings. Strong vital registration systems can aid in verifying mortality. Prevention of measles HAI might decrease mortality. Implementation of national strategies for health care facilities, including appropriate infection prevention and control measures, vitamin A supplementation, and adequate respiratory support, are critical to reduce mortality from this preventable illness.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Presented in part: 66th Annual Epidemic Intelligence Service Conference, Atlanta, Georgia, April 2017.
Notes
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention. The World Health Organization contributed to study conception.
Financial support. This work was supported by the World Health Organization.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.




