-
PDF
- Split View
-
Views
-
Cite
Cite
José E Hagan, Yoshihiro Takashima, Amarzaya Sarankhuu, Otgonbayar Dashpagma, Baigalmaa Jantsansengee, Roberta Pastore, Gunregjav Nyamaa, Buyanjargal Yadamsuren, Mick N Mulders, Kathleen A Wannemuehler, Raydel Anderson, Bettina Bankamp, Paul Rota, James L Goodson, Risk Factors for Measles Virus Infection Among Adults During a Large Outbreak in Postelimination Era in Mongolia, 2015, The Journal of Infectious Diseases, Volume 216, Issue 10, 15 November 2017, Pages 1187–1195, https://doi.org/10.1093/infdis/jix449
Close - Share Icon Share
Abstract
In 2015, a large nationwide measles outbreak occurred in Mongolia, with very high incidence in the capital city of Ulaanbaatar and among young adults.
We conducted an outbreak investigation including a matched case-control study of risk factors for laboratory-confirmed measles among young adults living in Ulaanbaatar. Young adults with laboratory-confirmed measles, living in the capital city of Ulaanbaatar, were matched with 2–3 neighborhood controls. Conditional logistic regression was used to estimate adjusted matched odds ratios (aMORs) for risk factors, with 95% confidence intervals.
During March 1–September 30, 2015, 20 077 suspected measles cases were reported; 14 010 cases were confirmed. Independent risk factors for measles included being unvaccinated (adjusted matched odds ratio [aMOR] 2.0, P < .01), being a high school graduate without college education (aMOR 2.6, P < .01), remaining in Ulaanbaatar during the outbreak (aMOR 2.5, P < .01), exposure to an inpatient healthcare facility (aMOR 4.5 P < .01), and being born outside of Ulaanbaatar (aMOR 1.8, P = .02).
This large, nationwide outbreak shortly after verification of elimination had high incidence among young adults, particularly those born outside the national capital. In addition, findings indicated that nosocomial transmission within health facilities helped amplify the outbreak.
All 6 World Health Organization (WHO) regions have established goals for measles elimination [1]. In the WHO Western Pacific Region (WPR), the Regional Committee noted in 2003 that measles elimination should be a goal, through implementation of nationwide, case-based surveillance and providing 2 doses of measles-containing vaccine (MCV) to achieve ≥95% population immunity. In 2005, the WPR countries established a goal to achieve measles elimination [2].
Mongolia is an independent country in WPR with an estimated population of 2.96 million, bordering Russia to the north and sharing a long southern border with China, where a large measles resurgence occurred during 2013–2014, after a historic low measles incidence of 4.6 cases per million population in 2012 [3, 4]. The Mongolian terrain of arid steppes is sparsely populated with some of the lowest population densities in the world; 46% of the population lives in the capital city of Ulaanbaatar (UB), which has experienced recent urbanization, growing 79% from a population of 760 000 in 2000 to 1.36 million in 2015 [5]. The winter season in Mongolia is long, with average daily temperature below 0°C from November to March, and the average yearly low temperature is −33°C [6]. Despite these logistical challenges for delivery of health and immunization services, estimated coverage of the first MCV dose (MCV1) has been >95% in Mongolia since 2001 based on WHO/UNICEF Estimates of National Immunization Coverage (WUENIC) [7]. The routine second MCV dose (MCV2) was introduced in 1985 with 95% estimated MCV2 coverage among children 2 years of age since 2001. Routine MCV is currently administered at age 9 months, with a second dose at age 24 months. In addition, beginning in 1994, periodic nationwide measles supplemental immunization activities (SIAs) were implemented, with target age groups that included, at least once, each birth cohort born since 1986. The National Verification Committee for measles elimination provided documentation that endemic measles virus transmission had been interrupted for a period >36 months [8], and the achievement of measles elimination in Mongolia was verified by the WPR Verification Commission in March 2014 [9]. However, after verification of elimination, a large nationwide measles outbreak occurred starting in March 2015 (Figure 1), continuing until July 2016 in 2 distinct waves with different epidemiologic characteristics. This outbreak became the largest recorded in a postelimination setting since a measles outbreak in Sao Paulo, Brazil in 1997, and it had the largest proportion of adult cases in any outbreak of this size. To inform elimination efforts, we conducted an outbreak investigation to describe the epidemiology of the first wave of epidemic transmission, determined the likely causes of the outbreak, and implemented a case-control study to identify risk factors for measles among young adults who had the highest attack rate during the outbreak.
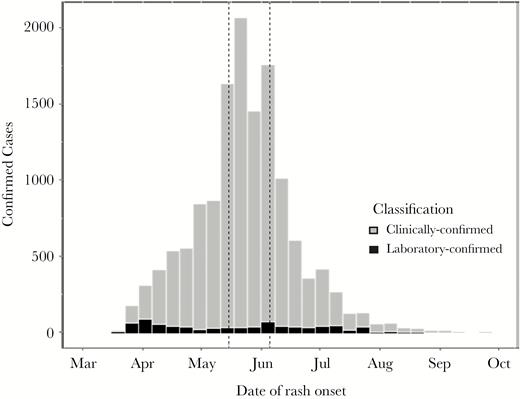
Clinically confirmed (gray, n = 13 064) and laboratory-confirmed (black, n = 946) measles cases by week of rash onset—Mongolia, March 1–September 30, 2015 (N = 14 010). Note: During May 15–June 5, 2015 (indicated by dotted lines), a nationwide outbreak response immunization (ORI) campaign was implemented using measles vaccine with a target age group of 6–71 months, 371 971 doses were delivered, and the ORI administrative coverage was 94%.
METHODS
Analysis of Measles Surveillance and Vaccination Coverage Data
Clinically diagnosed measles cases reported to the Mongolia Ministry of Health and Sports (MOH) were investigated by the surveillance team, Expanded Programme on Immunizations, National Center for Communicable Diseases (NCCD). Surveillance officers used an individual case investigation form (CIF) to collect information on suspected cases on demographics, occupation, address and contact information, date of rash onset, illness signs and symptoms, documented number of MCV doses received, and date of last measles vaccination. In addition, because the initial detected cases were hospital-associated, in 2015, the CIF included dates of recent visit to a healthcare facility before onset of rash. Laboratory testing for measles and rubella confirmation was performed by NCCD using a commercial enzyme-linked immunosorbent assay ([ELISA], Siemens, Munich, Germany) for immunoglobulin (Ig)M antibody [10]. Measles genotype testing was performed at NCCD and the US Centers for Disease Control and Prevention, using real-time polymerase chain reaction [11] on oral fluid specimens. We reviewed measles case-based surveillance data from March 1, 2015 to September 30, 2015, and we defined “confirmed measles” as laboratory-confirmed or clinically confirmed with rash and fever and one of the following: cough, coryza, or conjunctivitis.
We reviewed routine MCV coverage data from WUENIC, MOH reported administrative coverage for SIAs, and nationally representative MCV coverage surveys. Using these coverage estimates and population estimates from the 2014 national census, we calculated a susceptibility profile for Mongolia to estimate the total number of measles-susceptible individuals in each birth cohort [12], assuming vaccine effectiveness of 85% for MCV1 and 95% for MCV2 and supplemental doses, and that supplemental immunization campaigns successfully reached previously unimmunized individuals. As a sensitivity analysis to estimate an upper bound for the possible number of susceptible individuals, the susceptibility profile was constructed using the most conservative assumption that all individuals missed by the routine immunization system were also missed by supplemental campaigns, so SIA doses served as a booster dose but did not immunize new individuals.
Case-Control Study
A matched, case-control study was conducted during September 25–October 6, 2015. A case was defined as laboratory-confirmed measles in an adult born during October 1, 1986–December 31, 1999 (aged 15–28 years in 2015) who lived in UB ≥3 weeks during March 1–August 31, 2015. The target sample size was calculated to be 140 cases and 2 controls per case [13]. During data collection, we increased the target number of controls to 3 per case to account for nonresponse among cases who could not be reached because of summer season travel outside the city, common among UB residents.
Controls aged 15–28 years were matched to cases within the immediate neighborhood of the case. After selecting a household near a case household using a random walk technique [14], a control was selected using simple random sampling from an enumerated list of age-eligible household members. Potential controls were excluded if they had history of fever and rash or did not live in UB for ≥3 weeks during March 1–August 31, 2015. Data on signs and symptoms, vaccination status, and exposures were obtained through face-to-face interviews.
Data were collected by teams composed of trained local epidemiologists, residents of the Mongolia Field Epidemiology and Laboratory Training Program, and students from the Mongolia School of Public Health. Verbal consent was obtained from case-patients and control-volunteers before data collection; data were collected using deidentified study numbers.
Data Management and Statistical Analysis of Risk Factors
Field study data were collected on handheld cellular smartphone devices and uploaded via cellular data to a database managed using REDCap version 6.4.4 [15]. Univariate and multivariate analyses were performed using R version 3.2 [16]. Age and sex were included in a multivariate conditional logistic regression model regardless of significance in univariate analysis; other variables were included in the regression model if P value on the univariate analysis was <.05. All P values were 2-sided. Risk factors were expressed using odds ratio (OR) and 95% confidence interval (CI), calculated using maximum likelihood.
RESULTS
Epidemiology of the Outbreak
The first 2 detected cases were reported in March 2015, with rash onset during epidemiological week (epi-week) 11, 1 from UB and 1 from Umnugobi Aimag, bordering China; they were not epidemiologically linked to each other or to another case and had no known travel or source exposure history. The earliest confirmed case had rash onset on March 7, 2015 (epi-week 9), but it was not detected and reported until epi-week 12. During March 1–September 30, 2015, a total of 20 077 suspected measles cases were reported. Of these, 14 010 cases were confirmed, including 946 (7%) laboratory-confirmed and 13 064 (93%) clinically confirmed (Figure 1, Supplemental Table 1). Suspected measles cases were reported from all 21 provinces (aimags) in the country within 10 weeks of initial case detection (Supplemental Figure 1). Of the confirmed cases, 12 717 (91%) were reported from UB, but all 21 aimags reported at least 1 laboratory-confirmed case (Supplemental Table 1). During March 1–September 30, 2015, the cumulative confirmed measles incidence per million population was 4779 nationwide and 9670 in UB. The highest age-specific, confirmed measles attack rates occurred among infants and among adults aged 15–25 years (born during 1990–2000) (Figure 3). Of confirmed measles cases, 1840 (13%) were among infants aged <9 months (before the age of eligibility for MCV1), and 9671 (69%) were among adults aged ≥15 years (Supplemental Table 1). The age distribution of confirmed cases by epi-week varied during the progression of the outbreak (Figure 2), occurring predominantly among infants in the early weeks of the outbreak, and among young adults at the peak of transmission intensity. The majority (95%) of cases did not have documentation of vaccination status (Table 1). Among case-patients aged 9 months–4 years, 25% had documentation of one dose of MCV, and 4% had 2 or more MCV doses. Estimated coverage with 2 doses of MCV was ≥94% from 2001 to 2015 [7]; however, in 5 coverage surveys conducted during 1995–2013 [17–21] estimated MCV1 coverage ranged from 76.1% to 86.9% (Figure 3), and coverage varied significantly by region. The adult age cohorts that were born during 1990–2000 and had the highest confirmed incidence were all targeted by at least 1 SIA. The population susceptibility profile indicated that 2246 total individuals in the 1990–2000 birth cohorts in Mongolia were measles-susceptible at the beginning of 2015, assuming that SIAs targeting these birth cohorts were effective in reaching unvaccinated children. However, approximately three times as many (7006) confirmed measles cases in these birth cohorts (adults aged 15–25 years) were detected by case-based surveillance during March 1–September 30, 2015. Using the conservative assumption that SIAs were only effective as a booster dose for those who had been reached at least once by the routine immunization progamme, we estimated 26 378 measles-susceptible adults in this age range nationwide.
Measles Vaccination Status and Healthcare Facility Exposure Within 60 Days Before Rash Onset Among Confirmed Measles Cases, by Age Group—Mongolia, March 1–September 30, 2015
| Age Group . | Total Number of Confirmed Cases . | Measles Vaccination Status n (%) . | Healthcare Facility Exposure Within 60 Days Before Rash Onset n (%) . | |||||
|---|---|---|---|---|---|---|---|---|
| Zero Doses or No Documentation . | One Dose . | ≥Two Doses . | No . | Yes . | Timing of Healthcare Exposure Before Rash Onset . | |||
| 7–21 Days . | <7 or >21 Days . | |||||||
| <9 months | 1840 | 1753 (95%) | 87 (5%) | 0 (0%) | 1598 (87%) | 242 (13%) | 167 (69%) | 75 (31%) |
| 9 months–4 years | 1567 | 1116 (71%) | 1116 (71%) | 61 (4%) | 1435 (92%) | 132 (8%) | 82 (62%) | 50 (38%) |
| 5 years–14 years | 932 | 827 (89%) | 827 (89%) | 21 (2%) | 923 (99%) | 9 (1%) | 6 (67%) | 3 (33%) |
| ≥15 years | 9671 | 9422 (97%) | 9422 (97%) | 8 (0%) | 9558 (99%) | 113 (1%) | 76 (67%) | 37 (33%) |
| All ages | 14010 | 13118 (94%) | 13118 (94%) | 90 (1%) | 13514 (96%) | 496 (4%) | 331 (67%) | 165 (33%) |
| Age Group . | Total Number of Confirmed Cases . | Measles Vaccination Status n (%) . | Healthcare Facility Exposure Within 60 Days Before Rash Onset n (%) . | |||||
|---|---|---|---|---|---|---|---|---|
| Zero Doses or No Documentation . | One Dose . | ≥Two Doses . | No . | Yes . | Timing of Healthcare Exposure Before Rash Onset . | |||
| 7–21 Days . | <7 or >21 Days . | |||||||
| <9 months | 1840 | 1753 (95%) | 87 (5%) | 0 (0%) | 1598 (87%) | 242 (13%) | 167 (69%) | 75 (31%) |
| 9 months–4 years | 1567 | 1116 (71%) | 1116 (71%) | 61 (4%) | 1435 (92%) | 132 (8%) | 82 (62%) | 50 (38%) |
| 5 years–14 years | 932 | 827 (89%) | 827 (89%) | 21 (2%) | 923 (99%) | 9 (1%) | 6 (67%) | 3 (33%) |
| ≥15 years | 9671 | 9422 (97%) | 9422 (97%) | 8 (0%) | 9558 (99%) | 113 (1%) | 76 (67%) | 37 (33%) |
| All ages | 14010 | 13118 (94%) | 13118 (94%) | 90 (1%) | 13514 (96%) | 496 (4%) | 331 (67%) | 165 (33%) |
Measles Vaccination Status and Healthcare Facility Exposure Within 60 Days Before Rash Onset Among Confirmed Measles Cases, by Age Group—Mongolia, March 1–September 30, 2015
| Age Group . | Total Number of Confirmed Cases . | Measles Vaccination Status n (%) . | Healthcare Facility Exposure Within 60 Days Before Rash Onset n (%) . | |||||
|---|---|---|---|---|---|---|---|---|
| Zero Doses or No Documentation . | One Dose . | ≥Two Doses . | No . | Yes . | Timing of Healthcare Exposure Before Rash Onset . | |||
| 7–21 Days . | <7 or >21 Days . | |||||||
| <9 months | 1840 | 1753 (95%) | 87 (5%) | 0 (0%) | 1598 (87%) | 242 (13%) | 167 (69%) | 75 (31%) |
| 9 months–4 years | 1567 | 1116 (71%) | 1116 (71%) | 61 (4%) | 1435 (92%) | 132 (8%) | 82 (62%) | 50 (38%) |
| 5 years–14 years | 932 | 827 (89%) | 827 (89%) | 21 (2%) | 923 (99%) | 9 (1%) | 6 (67%) | 3 (33%) |
| ≥15 years | 9671 | 9422 (97%) | 9422 (97%) | 8 (0%) | 9558 (99%) | 113 (1%) | 76 (67%) | 37 (33%) |
| All ages | 14010 | 13118 (94%) | 13118 (94%) | 90 (1%) | 13514 (96%) | 496 (4%) | 331 (67%) | 165 (33%) |
| Age Group . | Total Number of Confirmed Cases . | Measles Vaccination Status n (%) . | Healthcare Facility Exposure Within 60 Days Before Rash Onset n (%) . | |||||
|---|---|---|---|---|---|---|---|---|
| Zero Doses or No Documentation . | One Dose . | ≥Two Doses . | No . | Yes . | Timing of Healthcare Exposure Before Rash Onset . | |||
| 7–21 Days . | <7 or >21 Days . | |||||||
| <9 months | 1840 | 1753 (95%) | 87 (5%) | 0 (0%) | 1598 (87%) | 242 (13%) | 167 (69%) | 75 (31%) |
| 9 months–4 years | 1567 | 1116 (71%) | 1116 (71%) | 61 (4%) | 1435 (92%) | 132 (8%) | 82 (62%) | 50 (38%) |
| 5 years–14 years | 932 | 827 (89%) | 827 (89%) | 21 (2%) | 923 (99%) | 9 (1%) | 6 (67%) | 3 (33%) |
| ≥15 years | 9671 | 9422 (97%) | 9422 (97%) | 8 (0%) | 9558 (99%) | 113 (1%) | 76 (67%) | 37 (33%) |
| All ages | 14010 | 13118 (94%) | 13118 (94%) | 90 (1%) | 13514 (96%) | 496 (4%) | 331 (67%) | 165 (33%) |
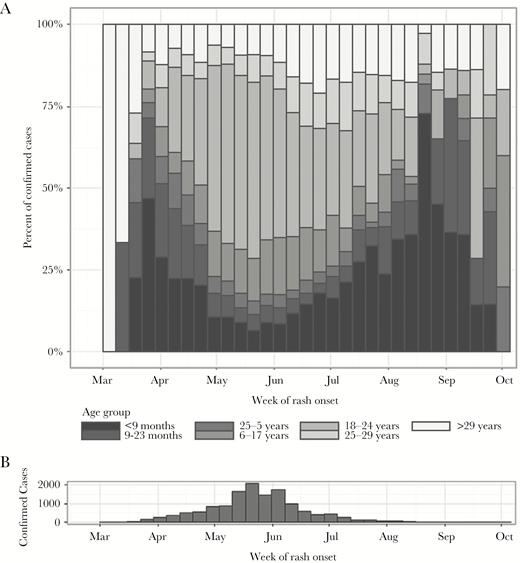
(A) Age-specific proportion of confirmed measles cases by epidemiological week, and (B) histogram of confirmed cases (N = 14 010) by epidemiological week—Mongolia, March 1–September 30, 2015. Note: During May 15–June 5, 2015, a nationwide outbreak response immunization (ORI) campaign was implemented using measles-containing vaccine with a target age group of 6–71 months, 371 971 doses were delivered, and the ORI administrative coverage was 94%.
![Vaccination coverage (right axis) of first dose (measles-containing vaccine [MCV]1, solid line) and second dose (MCV2, dashed line) of measles-containing vaccine (World Health Organization-UNICEF estimates [16]), supplemental immunization activities ([SIA] dotted lines), and MCV1 by 1 year of age according to Multiple Indicator Cluster Survey [17–21] (diamonds; details of each SIA are provided in the inset table); and age-specific attack rate (black bars, left axis) of confirmed measles (N = 14 010 cases) during the outbreak—Mongolia, March 1–September 30, 2015. Note: MCV2 was introduced in 1985, but coverage estimates were available only from 2000.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jid/216/10/10.1093_infdis_jix449/1/m_jix44903.jpeg?Expires=1750217465&Signature=aE67jZ~SHEMRAXYcxpNZA60EFegshY9epLTPHraKBJC0L1gpXBNdpaVBYoeGufjMhxHZPayI~YT3yQoV-uhD4lDm2ZYpnYv1fOI6-sR10eYo7Wwvw48sL2y0jRFPBJhULAFawYoHcBcbkBuylAqWUoJJGNSqyB3QGs8n7GjDDFLn0hOR5j9hf158dqnUPprPUBgY6VuXlYBaWf59xgx-h1WfbpAH6xPWbRgRxQH-jHCGHhLuX~ToGkqi2psIGWQiaNGZTYyuEkvurOJp7iq~AGu~tn3aACAGF7VwpZastKYfaiTr0gH3INbuBVm5pNQ~HHoyfSW-6qhvbiBjTDHCxA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Vaccination coverage (right axis) of first dose (measles-containing vaccine [MCV]1, solid line) and second dose (MCV2, dashed line) of measles-containing vaccine (World Health Organization-UNICEF estimates [16]), supplemental immunization activities ([SIA] dotted lines), and MCV1 by 1 year of age according to Multiple Indicator Cluster Survey [17–21] (diamonds; details of each SIA are provided in the inset table); and age-specific attack rate (black bars, left axis) of confirmed measles (N = 14 010 cases) during the outbreak—Mongolia, March 1–September 30, 2015. Note: MCV2 was introduced in 1985, but coverage estimates were available only from 2000.
Exposure to a healthcare facility within 60 days before rash onset was reported for 496 (4%) of the confirmed case-patients (13% of those aged 0–8 months, and 8% of those aged 9 months–4 years) (Table 1). Of the 496 cases, 331 (67%) had reported exposure during the measles incubation period (7–21 days before rash onset) (Figure 4). During the first 4 weeks of the outbreak, 48 (17%) of 278 confirmed case-patients visited a healthcare facility before rash onset, during the measles incubation period.
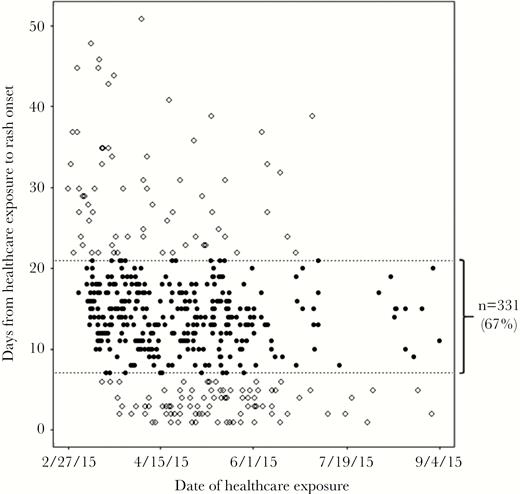
Scatterplot of confirmed measles cases that reported exposure to a healthcare facility 1–60 days before rash onset—Mongolia, March 1–September 30, 2015 (N = 496). Each filled circle represents 1 case with healthcare exposure that occurred 7–21 days before rash (measles incubation period) onset (67% of total). Open circles represent cases with healthcare exposure 1–6 or 22–60 days before rash onset (33% of total).
During March 1–September 30, 2015, 4879 (35%) of 14 010 confirmed case-patients were hospitalized. Hospitalization was most common among infants less than 1 year of age (1390 (63%)); 831 (39%) children aged 1–15 years and 2658 (27%) adults aged >15 years were hospitalized. Three reported deaths during this period were officially attributed to measles. The NCCD reported a total of 38 312 visits and 8953 admissions for suspected measles, including 425 admissions to the intensive care unit, as well as 19 confirmed measles cases among NCCD healthcare workers during March 1–August 31, 2015. Measles complications included pneumonia, severe diarrhea, seizure, and encephalitis.
During March 1–September 30, 2015, a total of 1827 cases had sera tested for measles-specific IgM antibody: 904 (49%) were positive, 143 (8%) were equivocal, and 780 (43%) were negative. Laboratory testing for specific IgM antibody was conducted on 715 (92%) sera that were measles IgM negative; 6 (1%) were rubella antibody positive, and, of these, 4 (67%) were collected from infants 10–11 months of age who had recently received rubella-containing vaccine.
Genotype results from 46 cases from UB and 9 other provinces during March–September 2015, were all genotype H1, and the majority (42, 91%) exactly matched the named strain MVs/Hong Kong.CHN/49.12/ represented by 3739 sequences in the WHO Measles Nucleotide Surveillance database [22, 23], and circulating since 2012, primarily in China. Only 1 nucleotide difference was found between any given pair of sequences, consistent with a single importation during the outbreak.
Risk Factors for Measles Among Young Adults
A total of 124 case-patients were identified in the national, case-based surveillance database and enrolled in the case-control study. An additional 16 case-patients met study criteria but could not be reached because of missing or incorrect contact information or because they were traveling during the data collection period. A total of 302 matched controls (2–3 per case) were included in the study. The most common complications were diarrhea (14%), pneumonia (4%), and eye problems (3%) (Table 2). Eighty-nine percent of case-patients were hospitalized, almost all (98%) at NCCD. There were no significant differences in measles signs, symptoms, or complications between vaccinated and unvaccinated case-patients.
Clinical Characteristics of Laboratory-Confirmed Measles, Matched Case-Control Study—Ulaanbaatar, Mongolia, March 1–September 25, 2015
| Case characteristic Symptom . | All Cases (N = 124) . | Unvaccinateda Cases (N = 95) . | Vaccinated Cases (N = 29) . |
|---|---|---|---|
| n (%) or Median (IQR) . | |||
| Fever | 122 (98%) | 94 (99%) | 28 (97%) |
| Rash | 120 (97%) | 92 (97%) | 28 (97%) |
| Cough | 87 (70%) | 69 (73%) | 18 (62%) |
| Conjunctivitis | 60 (48%) | 51 (54%) | 9 (31%) |
| Coryza | 56 (45%) | 43 (45%) | 13 (45%) |
| Headache | 103 (83%) | 80 (84%) | 23 (79%) |
| Confusion | 15 (12%) | 9 (9%) | 6 (21%) |
| Other | 48 (39%) | 32 (34%) | 16 (55%) |
| Complication | |||
| None | 94 (76%) | 72 (76%) | 22 (75%) |
| Pneumonia | 5 (4%) | 3 (3%) | 2 (7%) |
| Diarrhea | 17 (14%) | 13 (14%) | 4 (14%) |
| Otitis | 2 (2%) | 2 (2%) | 0 (0%) |
| Eye problems | 4 (3%) | 4 (4%) | 0 (0%) |
| Other | 7 (6%) | 6 (6%) | 1 (3%) |
| Death | 0 (0%) | 0 (0%) | 0 (0%) |
| Hospitalized | 110 (89%) | 86 (90%) | 24 (83%) |
| NCCD | 108 (98%) | 84 (98%) | 24 (100%) |
| Other national specialty hospital | 2 (2%) | 2 (2%) | 0 (0%) |
| Length of hospitalization | 7 days (5–8) | 7 days (5–7) | 6 days (5–8) |
| Number of secondary cases | |||
| 0 | 74 (60%) | 53 (56%) | 21 (72%) |
| 1 | 24 (19%) | 20 (21%) | 4 (14%) |
| 2 | 15 (12%) | 12 (13%) | 3 (10%) |
| 3 | 8 (6%) | 7 (7%) | 1 (3%) |
| 4 | 2 (2%) | 2 (2%) | 0 (0%) |
| 5 | 1 (1%) | 1 (1%) | 0 (0%) |
| Any secondary casesb | 50 (40%) | 42 (44%) | 8 (28%) |
| Case characteristic Symptom . | All Cases (N = 124) . | Unvaccinateda Cases (N = 95) . | Vaccinated Cases (N = 29) . |
|---|---|---|---|
| n (%) or Median (IQR) . | |||
| Fever | 122 (98%) | 94 (99%) | 28 (97%) |
| Rash | 120 (97%) | 92 (97%) | 28 (97%) |
| Cough | 87 (70%) | 69 (73%) | 18 (62%) |
| Conjunctivitis | 60 (48%) | 51 (54%) | 9 (31%) |
| Coryza | 56 (45%) | 43 (45%) | 13 (45%) |
| Headache | 103 (83%) | 80 (84%) | 23 (79%) |
| Confusion | 15 (12%) | 9 (9%) | 6 (21%) |
| Other | 48 (39%) | 32 (34%) | 16 (55%) |
| Complication | |||
| None | 94 (76%) | 72 (76%) | 22 (75%) |
| Pneumonia | 5 (4%) | 3 (3%) | 2 (7%) |
| Diarrhea | 17 (14%) | 13 (14%) | 4 (14%) |
| Otitis | 2 (2%) | 2 (2%) | 0 (0%) |
| Eye problems | 4 (3%) | 4 (4%) | 0 (0%) |
| Other | 7 (6%) | 6 (6%) | 1 (3%) |
| Death | 0 (0%) | 0 (0%) | 0 (0%) |
| Hospitalized | 110 (89%) | 86 (90%) | 24 (83%) |
| NCCD | 108 (98%) | 84 (98%) | 24 (100%) |
| Other national specialty hospital | 2 (2%) | 2 (2%) | 0 (0%) |
| Length of hospitalization | 7 days (5–8) | 7 days (5–7) | 6 days (5–8) |
| Number of secondary cases | |||
| 0 | 74 (60%) | 53 (56%) | 21 (72%) |
| 1 | 24 (19%) | 20 (21%) | 4 (14%) |
| 2 | 15 (12%) | 12 (13%) | 3 (10%) |
| 3 | 8 (6%) | 7 (7%) | 1 (3%) |
| 4 | 2 (2%) | 2 (2%) | 0 (0%) |
| 5 | 1 (1%) | 1 (1%) | 0 (0%) |
| Any secondary casesb | 50 (40%) | 42 (44%) | 8 (28%) |
Abbreviations: IQR, interquartile range; NCCD, National Center for Communicable Diseases.
aIncludes 66 (69%) cases with unknown vaccination status.
bP = .11 by χ2.
Clinical Characteristics of Laboratory-Confirmed Measles, Matched Case-Control Study—Ulaanbaatar, Mongolia, March 1–September 25, 2015
| Case characteristic Symptom . | All Cases (N = 124) . | Unvaccinateda Cases (N = 95) . | Vaccinated Cases (N = 29) . |
|---|---|---|---|
| n (%) or Median (IQR) . | |||
| Fever | 122 (98%) | 94 (99%) | 28 (97%) |
| Rash | 120 (97%) | 92 (97%) | 28 (97%) |
| Cough | 87 (70%) | 69 (73%) | 18 (62%) |
| Conjunctivitis | 60 (48%) | 51 (54%) | 9 (31%) |
| Coryza | 56 (45%) | 43 (45%) | 13 (45%) |
| Headache | 103 (83%) | 80 (84%) | 23 (79%) |
| Confusion | 15 (12%) | 9 (9%) | 6 (21%) |
| Other | 48 (39%) | 32 (34%) | 16 (55%) |
| Complication | |||
| None | 94 (76%) | 72 (76%) | 22 (75%) |
| Pneumonia | 5 (4%) | 3 (3%) | 2 (7%) |
| Diarrhea | 17 (14%) | 13 (14%) | 4 (14%) |
| Otitis | 2 (2%) | 2 (2%) | 0 (0%) |
| Eye problems | 4 (3%) | 4 (4%) | 0 (0%) |
| Other | 7 (6%) | 6 (6%) | 1 (3%) |
| Death | 0 (0%) | 0 (0%) | 0 (0%) |
| Hospitalized | 110 (89%) | 86 (90%) | 24 (83%) |
| NCCD | 108 (98%) | 84 (98%) | 24 (100%) |
| Other national specialty hospital | 2 (2%) | 2 (2%) | 0 (0%) |
| Length of hospitalization | 7 days (5–8) | 7 days (5–7) | 6 days (5–8) |
| Number of secondary cases | |||
| 0 | 74 (60%) | 53 (56%) | 21 (72%) |
| 1 | 24 (19%) | 20 (21%) | 4 (14%) |
| 2 | 15 (12%) | 12 (13%) | 3 (10%) |
| 3 | 8 (6%) | 7 (7%) | 1 (3%) |
| 4 | 2 (2%) | 2 (2%) | 0 (0%) |
| 5 | 1 (1%) | 1 (1%) | 0 (0%) |
| Any secondary casesb | 50 (40%) | 42 (44%) | 8 (28%) |
| Case characteristic Symptom . | All Cases (N = 124) . | Unvaccinateda Cases (N = 95) . | Vaccinated Cases (N = 29) . |
|---|---|---|---|
| n (%) or Median (IQR) . | |||
| Fever | 122 (98%) | 94 (99%) | 28 (97%) |
| Rash | 120 (97%) | 92 (97%) | 28 (97%) |
| Cough | 87 (70%) | 69 (73%) | 18 (62%) |
| Conjunctivitis | 60 (48%) | 51 (54%) | 9 (31%) |
| Coryza | 56 (45%) | 43 (45%) | 13 (45%) |
| Headache | 103 (83%) | 80 (84%) | 23 (79%) |
| Confusion | 15 (12%) | 9 (9%) | 6 (21%) |
| Other | 48 (39%) | 32 (34%) | 16 (55%) |
| Complication | |||
| None | 94 (76%) | 72 (76%) | 22 (75%) |
| Pneumonia | 5 (4%) | 3 (3%) | 2 (7%) |
| Diarrhea | 17 (14%) | 13 (14%) | 4 (14%) |
| Otitis | 2 (2%) | 2 (2%) | 0 (0%) |
| Eye problems | 4 (3%) | 4 (4%) | 0 (0%) |
| Other | 7 (6%) | 6 (6%) | 1 (3%) |
| Death | 0 (0%) | 0 (0%) | 0 (0%) |
| Hospitalized | 110 (89%) | 86 (90%) | 24 (83%) |
| NCCD | 108 (98%) | 84 (98%) | 24 (100%) |
| Other national specialty hospital | 2 (2%) | 2 (2%) | 0 (0%) |
| Length of hospitalization | 7 days (5–8) | 7 days (5–7) | 6 days (5–8) |
| Number of secondary cases | |||
| 0 | 74 (60%) | 53 (56%) | 21 (72%) |
| 1 | 24 (19%) | 20 (21%) | 4 (14%) |
| 2 | 15 (12%) | 12 (13%) | 3 (10%) |
| 3 | 8 (6%) | 7 (7%) | 1 (3%) |
| 4 | 2 (2%) | 2 (2%) | 0 (0%) |
| 5 | 1 (1%) | 1 (1%) | 0 (0%) |
| Any secondary casesb | 50 (40%) | 42 (44%) | 8 (28%) |
Abbreviations: IQR, interquartile range; NCCD, National Center for Communicable Diseases.
aIncludes 66 (69%) cases with unknown vaccination status.
bP = .11 by χ2.
There were no significant differences in age or sex among cases and controls (Table 3). Cases were less likely to have a reported history of ≥1 MCV dose (adjusted matched OR [aMOR], 0.47; 95% CI, 0.28–0.79). Case-patients were more likely to be high school graduates without college education (aMOR, 2.57; 95% CI, 1.32–5.01) and more likely born outside of UB (aMOR, 1.79; 95% CI, 1.10–2.93). Travel outside of UB during the outbreak period was protective (aMOR, 0.40; 95% CI, 0.20–0.80). Finally, exposure to an inpatient healthcare facility during the month before rash onset was an independent risk factor for measles (aMOR, 4.48; 95% CI, 1.86–10.82).
Description of Cases and Controls and Risk Factors for Measles, Matched Case-Control Study—Ulaanbaatar, Mongolia, March 1–September 25, 2015
| Risk Factor . | Case (N = 124) . | Control (N = 302) . | Univariate Model . | Multivariate Model . | ||
|---|---|---|---|---|---|---|
| n (%) or Median (IQR) . | MOR (P Value) . | 95% CI . | aMOR (P Value) . | 95% CI . | ||
| Age in years | 20.6 (19.3–23.7) | 21.1 (18.7–24.5) | 1.0 (P = .82) | 0.9–1.1 | 1.0 (P = .48) | 0.9–1.0 |
| Female sex | 82 (66%) | 170 (56%) | 1.5 (P = .09) | 1.0–2.4 | 0.7 (P = .21) | 0.5–1.2 |
| Vaccinateda | 29 (23%) | 111 (37%) | 0.5 (P < .01) | 0.3–0.8 | 0.5 (P < .01) | 0.3–0.8 |
| Education | ||||||
| <12th grade | 15 (12%) | 69 (23%) | Ref | Ref | ||
| 12th grade completed | 62 (50%) | 98 (32%) | 3.1 (P < .01) | 1.6–6.1 | 2.6 (P < .01) | 1.3–5.0 |
| At least some college | 47 (38%) | 135 (45%) | 1.6 (P = .15) | 0.8–3.1 | 1.2 (P = .60) | 0.6–2.5 |
| Travel outside of UB during outbreak | 13 (10%) | 19 (58%) | 0.5 (P=.03) | 0.3–0.9 | 0.4 (P < .01) | 0.2–0.8 |
| Healthcare facility exposure <30 days before case rash onset | ||||||
| None | 28 (23%) | 63 (21%) | Ref | Ref | ||
| Outpatient | 79 (64%) | 229 (76%) | 1.4 (P = .26) | 0.8–2.3 | 1.5 (P = .15) | 0.9–2.6 |
| Inpatient | 17 (14%) | 10 (3%) | 5.1 (P < .01) | 2.1–12.2 | 4.5 (P < .01) | 1.9–10.8 |
| Birth outside of UB | 87 (70%) | 169 (56%) | 1.9 (P < .01) | 1.2–3.0 | 1.8 (P=.02) | 1.1–2.9 |
| Risk Factor . | Case (N = 124) . | Control (N = 302) . | Univariate Model . | Multivariate Model . | ||
|---|---|---|---|---|---|---|
| n (%) or Median (IQR) . | MOR (P Value) . | 95% CI . | aMOR (P Value) . | 95% CI . | ||
| Age in years | 20.6 (19.3–23.7) | 21.1 (18.7–24.5) | 1.0 (P = .82) | 0.9–1.1 | 1.0 (P = .48) | 0.9–1.0 |
| Female sex | 82 (66%) | 170 (56%) | 1.5 (P = .09) | 1.0–2.4 | 0.7 (P = .21) | 0.5–1.2 |
| Vaccinateda | 29 (23%) | 111 (37%) | 0.5 (P < .01) | 0.3–0.8 | 0.5 (P < .01) | 0.3–0.8 |
| Education | ||||||
| <12th grade | 15 (12%) | 69 (23%) | Ref | Ref | ||
| 12th grade completed | 62 (50%) | 98 (32%) | 3.1 (P < .01) | 1.6–6.1 | 2.6 (P < .01) | 1.3–5.0 |
| At least some college | 47 (38%) | 135 (45%) | 1.6 (P = .15) | 0.8–3.1 | 1.2 (P = .60) | 0.6–2.5 |
| Travel outside of UB during outbreak | 13 (10%) | 19 (58%) | 0.5 (P=.03) | 0.3–0.9 | 0.4 (P < .01) | 0.2–0.8 |
| Healthcare facility exposure <30 days before case rash onset | ||||||
| None | 28 (23%) | 63 (21%) | Ref | Ref | ||
| Outpatient | 79 (64%) | 229 (76%) | 1.4 (P = .26) | 0.8–2.3 | 1.5 (P = .15) | 0.9–2.6 |
| Inpatient | 17 (14%) | 10 (3%) | 5.1 (P < .01) | 2.1–12.2 | 4.5 (P < .01) | 1.9–10.8 |
| Birth outside of UB | 87 (70%) | 169 (56%) | 1.9 (P < .01) | 1.2–3.0 | 1.8 (P=.02) | 1.1–2.9 |
Abbreviations: aMOR, adjusted matched odds ratio; CI, confidence interval; IQR, interquartile range; MOR, matched odds ratio; NA, not applicable; Ref, reference category; UB, Ulaanbaatar.
aVaccination status by recall if documentation unavailable.
Bold text indicates statistically significant risk factors in multivariate model.
Description of Cases and Controls and Risk Factors for Measles, Matched Case-Control Study—Ulaanbaatar, Mongolia, March 1–September 25, 2015
| Risk Factor . | Case (N = 124) . | Control (N = 302) . | Univariate Model . | Multivariate Model . | ||
|---|---|---|---|---|---|---|
| n (%) or Median (IQR) . | MOR (P Value) . | 95% CI . | aMOR (P Value) . | 95% CI . | ||
| Age in years | 20.6 (19.3–23.7) | 21.1 (18.7–24.5) | 1.0 (P = .82) | 0.9–1.1 | 1.0 (P = .48) | 0.9–1.0 |
| Female sex | 82 (66%) | 170 (56%) | 1.5 (P = .09) | 1.0–2.4 | 0.7 (P = .21) | 0.5–1.2 |
| Vaccinateda | 29 (23%) | 111 (37%) | 0.5 (P < .01) | 0.3–0.8 | 0.5 (P < .01) | 0.3–0.8 |
| Education | ||||||
| <12th grade | 15 (12%) | 69 (23%) | Ref | Ref | ||
| 12th grade completed | 62 (50%) | 98 (32%) | 3.1 (P < .01) | 1.6–6.1 | 2.6 (P < .01) | 1.3–5.0 |
| At least some college | 47 (38%) | 135 (45%) | 1.6 (P = .15) | 0.8–3.1 | 1.2 (P = .60) | 0.6–2.5 |
| Travel outside of UB during outbreak | 13 (10%) | 19 (58%) | 0.5 (P=.03) | 0.3–0.9 | 0.4 (P < .01) | 0.2–0.8 |
| Healthcare facility exposure <30 days before case rash onset | ||||||
| None | 28 (23%) | 63 (21%) | Ref | Ref | ||
| Outpatient | 79 (64%) | 229 (76%) | 1.4 (P = .26) | 0.8–2.3 | 1.5 (P = .15) | 0.9–2.6 |
| Inpatient | 17 (14%) | 10 (3%) | 5.1 (P < .01) | 2.1–12.2 | 4.5 (P < .01) | 1.9–10.8 |
| Birth outside of UB | 87 (70%) | 169 (56%) | 1.9 (P < .01) | 1.2–3.0 | 1.8 (P=.02) | 1.1–2.9 |
| Risk Factor . | Case (N = 124) . | Control (N = 302) . | Univariate Model . | Multivariate Model . | ||
|---|---|---|---|---|---|---|
| n (%) or Median (IQR) . | MOR (P Value) . | 95% CI . | aMOR (P Value) . | 95% CI . | ||
| Age in years | 20.6 (19.3–23.7) | 21.1 (18.7–24.5) | 1.0 (P = .82) | 0.9–1.1 | 1.0 (P = .48) | 0.9–1.0 |
| Female sex | 82 (66%) | 170 (56%) | 1.5 (P = .09) | 1.0–2.4 | 0.7 (P = .21) | 0.5–1.2 |
| Vaccinateda | 29 (23%) | 111 (37%) | 0.5 (P < .01) | 0.3–0.8 | 0.5 (P < .01) | 0.3–0.8 |
| Education | ||||||
| <12th grade | 15 (12%) | 69 (23%) | Ref | Ref | ||
| 12th grade completed | 62 (50%) | 98 (32%) | 3.1 (P < .01) | 1.6–6.1 | 2.6 (P < .01) | 1.3–5.0 |
| At least some college | 47 (38%) | 135 (45%) | 1.6 (P = .15) | 0.8–3.1 | 1.2 (P = .60) | 0.6–2.5 |
| Travel outside of UB during outbreak | 13 (10%) | 19 (58%) | 0.5 (P=.03) | 0.3–0.9 | 0.4 (P < .01) | 0.2–0.8 |
| Healthcare facility exposure <30 days before case rash onset | ||||||
| None | 28 (23%) | 63 (21%) | Ref | Ref | ||
| Outpatient | 79 (64%) | 229 (76%) | 1.4 (P = .26) | 0.8–2.3 | 1.5 (P = .15) | 0.9–2.6 |
| Inpatient | 17 (14%) | 10 (3%) | 5.1 (P < .01) | 2.1–12.2 | 4.5 (P < .01) | 1.9–10.8 |
| Birth outside of UB | 87 (70%) | 169 (56%) | 1.9 (P < .01) | 1.2–3.0 | 1.8 (P=.02) | 1.1–2.9 |
Abbreviations: aMOR, adjusted matched odds ratio; CI, confidence interval; IQR, interquartile range; MOR, matched odds ratio; NA, not applicable; Ref, reference category; UB, Ulaanbaatar.
aVaccination status by recall if documentation unavailable.
Bold text indicates statistically significant risk factors in multivariate model.
DISCUSSION
In 2015, the largest measles outbreak in Mongolia in >25 years occurred, less than 1 year after verification of measles elimination. The outbreak was first detected when 2 nonepidemiologically linked individuals with rash onset on March 14 tested positive for measles on March 18 (epi-week 11). Measles rapidly spread to all aimags and the capital city, suggesting that the first chains of transmission were likely already established at the time of initial case detection. Measles virus importation might have occurred during the annual Tsagaan Sar lunar new year celebration (February 19–21, 2015), when mass population movement is common, including cross-border travel to and from neighboring China, where genotype H1 is endemic. The likely cause of the outbreak was a large accumulation of measles-susceptible persons due to nonvaccination and migration from sparsely populated rural settings (aimags) that had low vaccination coverage to high population density urban centers, during a prolonged period (≥15 years) of very low measles incidence. During the outbreak, measles virus transmission was sustained by mixing patterns with high contact rates among susceptible infants and young adults and within healthcare facilities. In addition, the initial outbreak response included a narrow target age group for outbreak response immunization (ORI) that failed to interrupt transmission.
Nosocomial transmission played an important role in the early amplification of this epidemic and was an important risk factor for measles virus infection. Nosocomial transmission preceding community transmission has previously been observed during measles outbreaks in other settings [24] with high vaccination coverage and low measles incidence [25]. When nosocomial transmission occurs, it tends to primarily affect infants and children [26], as was observed in this outbreak. Mongolia followed a policy of referring all suspected measles cases to NCCD, and the hospitalization rate for uncomplicated measles was high, which led to an extremely large inpatient burden on that institution in a short time, including approximately 9000 measles admissions within 5 months. This outbreak highlighted the important need for appropriate case referral, triage procedures, and hospital infection prevention and control practices, particularly during outbreaks, to reduce the risk of measles virus transmission through nosocomial exposures. Strategies to limit nosocomial transmission of measles virus should emphasize home-based and primary care case management for uncomplicated measles, designated triage areas for suspected cases waiting to see a clinician, documented measles immunity among all healthcare personnel [26, 27], and during measles outbreaks check the vaccination status of children attending hospitals and offer measles immunization [28, 29].
The outbreak had a wide age distribution, but the highest age-specific incidence occurred among young adults and infants. This combination has been observed in other outbreaks in near- and post-elimination settings [30–32]. When measles susceptibility exists among women of reproductive age, their newborns have no maternal antibodies and are susceptible to measles virus infection until they become age-eligible for vaccination and receive vaccination. The high attack rate among young infants suggested low prevalence of maternal measles antibody due to a large number of measles-susceptible mothers.
The high measles incidence observed among young adults was inconsistent with the expected population immunity in a country with a history of consistently high routine and supplemental immunization coverage. The number of susceptible young adults estimated by the population profile was exceeded by the number of confirmed cases in this age group within the first 6 months of a multiyear outbreak. A nationwide serosurvey completed in 2004 in Mongolia also estimated a higher than expected level of measles-susceptibility of 12%–17% among those aged 13–21 years in 2015 [33]. Despite this apparent emerging measles susceptibility, reported measles incidence remained low in Mongolia after the last major outbreak in 2001. During that outbreak, it appeared that the 1991–1998 birth cohorts might have been protected from measles, because this age group accounted for a small proportion of outbreak cases [34]. However, surprisingly, this age group had a very high age-specific attack-rate during the outbreak in 2015. This unexpected finding of a population with high estimated immunization coverage, which appeared to be protected during a nationwide outbreak in 2001, but highly measles-susceptible during an outbreak 15 years later, could be explained by several factors. For example, an influx of unimmunized persons due to urbanization and migration from low coverage to high coverage areas might have occurred since 2001. However, waning immunity due to reduced measles vaccine field effectiveness in this age group might have also played a role. Estimated vaccine effectiveness between 20% and 70% can be inferred from the results of this case-control study (calculated by 1-aMOR) [35], although this was not an objective of the study because of widespread lack of vaccination records among adults. Laboratory testing of sera, including the detection of high-avidity IgG, might help determine whether case-patients had been previously exposed to measles virus through vaccination and/or natural infection, potentially indicating emerging gaps in protective immunity among the population [36], and could provide evidence supporting the need for vaccination strategies to reach adults.
After gaining independence from China in 1924, Mongolia became closely associated with the Soviet Union as a satellite state. After the collapse of the Soviet Union, the Mongolian public health infrastructure suffered a period of instability during 1990–2000 while transitioning away from highly centralized planning. During this transition period, the delivery of public health services including immunization services was severely disrupted, challenging efforts to achieve high measles immunity through routine immunization services and SIAs, accurately estimate coverage, and properly manage and store vaccines. Breakdowns or gaps in cold-chain quality, where MCV might have been exposed to elevated temperatures, might have adversely affected vaccine potency and vaccine effectiveness [37]. Several former Soviet republics that also reported high coverage with 2 doses of measles vaccine during this transition period have experienced measles outbreaks among young adults born in the same years as those with highest incidence during the Mongolia outbreak [38–42].
The investigation results should be considered in light of limitations. Surveillance data, particularly in the setting of a large outbreak, suffers from limitations in data quality. In addition, the majority of cases in the analysis were confirmed clinically, rather than by laboratory testing, and epidemiological link case classification was not used during the outbreak. However, the available serological evidence was compelling that the outbreak was caused by measles virus and that there were no significant concurrent outbreaks of other febrile rash illnesses. Although our investigation was limited by the low number of tests performed for differential diagnosis to rule out concurrent circulation of other pathogens that cause febrile rash illness, 4 of 6 suspected measles cases that tested positive for rubella were highly likely to be false positive because of recent vaccination. To minimize misclassification bias in determining measles risk factors, only laboratory-confirmed measles cases were selected for the case-control study. In addition, the study assessed the risk factors for measles transmitted within UB; risk factors for measles transmitted in outlying areas may be different. Sixteen cases who were traveling outside UB during the summer months (11% of eligible cases) were not included, which may have been a source of bias.
CONCLUSIONS
Although a nationwide ORI campaign during May 15–June 5, 2015 vaccinated 94% of children aged 6–71 months, a second large wave of measles virus transmission occurred during January through July 2016 that was also predominantly driven by transmission among adults and young infants, but with a much larger proportion of cases occurring in outlying provinces compared with the 2015 epidemic wave. Because the ORI campaign targeting children aged under 6 years was ineffective in stopping the outbreak, a second nationwide ORI campaign was implemented, targeting adults aged 18–30 years (549 846 doses, 88% coverage). In October, 2016, the Regional Verification Committee for the Western Pacific determined that endemic measles virus transmission had been reestablished in Mongolia [9]. This outbreak highlighted the potential latent consequences of residual measles-susceptibility that can occur when immunization services experienced past disruptions, and it demonstrated the need to maintain heightened vigilance for early detection of measles importation events and outbreaks to ensure a rapid response. High-quality surveillance for measles combined with epidemiological capacity for outbreak preparedness and response in all districts are critical for rapid case detection, laboratory confirmation, and effective well-targeted outbreak response to achieve and maintain measles elimination.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We gratefully acknowledge health workers, immunization officers, surveillance officers, medical officers, and laboratory personnel in Mongolia for their dedication during this outbreak. We thank the outbreak investigation team for the field work conducted during the case-control study, Dr. Sodbayar Dembelsuren, and the WHO Western Pacific Regional office, in particular Dr. W. William Schulter, for their guidance and support throughout.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention.
Financial support. Funding for this study was provided by the US Centers for Disease Control and Prevention and the World Health Organization.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: Centers for Disease Control and Prevention, Epidemic Intelligence Service Conference, May 2, 2016, Atlanta, Georgia, and at the United States Public Health Service Symposium, May 9, 2017, Chatanooga, Tennessee.
References




