-
PDF
- Split View
-
Views
-
Cite
Cite
Robert W Finberg, Riin Lanno, David Anderson, Roman Fleischhackl, Wilbert van Duijnhoven, Robert S Kauffman, Teddy Kosoglou, Johan Vingerhoets, Lorant Leopold, Phase 2b Study of Pimodivir (JNJ-63623872) as Monotherapy or in Combination With Oseltamivir for Treatment of Acute Uncomplicated Seasonal Influenza A: TOPAZ Trial, The Journal of Infectious Diseases, Volume 219, Issue 7, 1 April 2019, Pages 1026–1034, https://doi.org/10.1093/infdis/jiy547
Close - Share Icon Share
Abstract
Pimodivir, a first-in-class inhibitor of influenza virus polymerase basic protein 2, is being developed for hospitalized and high-risk patients with influenza A.
In this double-blinded phase 2b study, adults with acute uncomplicated influenza A were randomized 1:1:1:1 to receive one of the following treatments twice daily for 5 days: placebo, pimodivir 300 mg or 600 mg, or pimodivir 600 mg plus oseltamivir 75 mg. Antiviral activity, safety, and pharmacokinetics of pimodivir alone or in combination were evaluated.
Of 292 patients randomized, 223 were treated and had confirmed influenza A virus infection. The trial was stopped early because the primary end point was met; the area under the curve of the viral load, determined by quantitative reverse transcription–polymerase chain reaction analysis, in nasal secretions from baseline to day 8 significantly decreased in the active treatment groups, compared with the placebo group (300 mg group, −3.6 day*log10 copies/mL [95% confidence interval {CI}, −7.1 to −0.1]; 600 mg group, −4.5 [95%CI −8.0 to −1.0]; and combination group, −8.6 [95% CI, −12.0 to −5.1]). Pimodivir plus oseltamivir yielded a significantly lower viral load titer over time than placebo and a trend for a shorter time to symptom resolution than placebo. Pimodivir plasma concentrations increased in a dose-proportional manner. The most commonly reported adverse event was mild or moderate diarrhea.
Pimodivir (with or without oseltamivir) resulted in significant virologic improvements over placebo, demonstrated trends in clinical improvement, and was well tolerated. Pimodivir 600 mg twice daily is in further development.
NCT02342249, 2014-004068-39, and CR107745.
(See the Editorial Commentary by Lee and Ison on pages 1013–5.)
Influenza remains a global health problem, affecting >10% of the population worldwide, and is a major cause of morbidity and mortality [1–3]. Currently approved antiviral drugs, adamantanes and neuraminidase inhibitors (NAIs), when administered within 48 hours of influenza symptom onset, have been shown to reduce the duration and severity of symptoms in the outpatient setting [4], but they have not demonstrated clinical benefits in complicated or hospitalized patients. There remains a significant need for novel therapeutic options for the treatment of influenza, specifically for antivirals effective against influenza A and B virus strains and known neuraminidase (NA)– and amantadine-resistant strains, that also extend the window from onset of symptoms to treatment initiation. Importantly, these benefits need to be demonstrated in hospitalized and high-risk patient populations.
Pimodivir (JNJ-63623872; formerly VX-787) is a first-in-class, orally bioavailable nonnucleoside inhibitor of the polymerase basic protein 2 (PB2) subunit of the influenza A virus polymerase complex that inhibits viral replication. Pimodivir inhibits viral RNA transcription in influenza A virus infection at nanomolar concentrations by targeting a highly conserved site of the influenza virus PB2 protein [5–7]. In vitro experiments have confirmed the synergy of pimodivir with oseltamivir and the antiviral activity of pimodivir against amantadine or NA-resistant strains [6]. In mouse models, pimodivir was highly efficacious in both prophylaxis and treatment models and was superior to oseltamivir, with 100% survival when administered up to 96 hours after infection [7]. In a proof-of-concept human challenge study evaluating different doses of pimodivir starting 24 hours after viral inoculation (with an influenza A[H3N2] virus challenge strain) in healthy volunteers, pimodivir 600 mg was effective in decreasing viral shedding in patients with active influenza A virus infection, with a concomitant reduction in symptoms [8]. In a pharmacokinetic study, pimodivir 600 mg showed no clinically relevant drug-drug interactions when combined with oseltamivir (clinical trials registration NCT02262715). Pimodivir is currently in late-phase development for influenza A virus infection and has received fast track designation by the Food and Drug Administration for its potential to address an unmet need in the treatment of influenza [9, 10].
Population sequencing data demonstrated the presence of the known PB2 resistance–associated amino acid substitution M431I in 4 subjects receiving pimodivir. This mutation is associated with a 57-fold decrease in the susceptibility to pimodivir but a reduced replication capacity, compared with wild-type strains. The PB2 substitutions S324C, K376R, and M431L/R/V, previously identified in vitro to confer resistance to pimodivir, were observed in 1 subject each. Emergence of these variants was not coincident with viral rebound, and all subjects subsequently cleared virus after treatment [8]. The purpose of the current dose-ranging study was to evaluate the optimal dose of pimodivir in uncomplicated seasonal influenza A virus infection, contribute to a large safety database, and explore potential benefits of combination versus monotherapy with pimodivir.
METHODS
Study Design
This was a phase 2b, randomized, double-blinded, placebo-controlled, parallel-group, dose-response, multicenter study of pimodivir as monotherapy and as combination therapy with oseltamivir conducted in adult patients with influenza A. The study was conducted in 244 sites across the northern (in Belgium, Bulgaria, Canada, Estonia, Latvia, and the United States) and southern (in South Africa) hemispheres. The planned study duration for each patient was 14 days, with an additional 5 days for safety follow-up (Supplementary Figure 1). Patients were randomized (1:1:1:1) to receive a twice-daily oral dose of pimodivir 300 mg, pimodivir 600 mg, a combination of pimodivir 600 mg and oseltamivir 75 mg, or placebo (Supplementary Figure 2).
Randomization was stratified by time from onset of influenza symptoms to time of first study drug intake (≤24 hours or >24 to 48 hours) to ensure a balance between treatment groups. Patients received 10 doses of the oral drugs administered at approximately 12-hour intervals without regard to food intake. An unblinded independent data monitoring committee appointed before study initiation reviewed all unblinded safety data during the study. The study protocol and amendments were reviewed and approved by the institutional review board for each site. The study was conducted in compliance with the Declaration of Helsinki and applicable regulatory requirements (clinical trials registration NCT02342249). Written informed consent was obtained from all patients before study enrollment. A formal interim analysis was performed at the end of the second influenza season (2015–2016) in the northern hemisphere.
Patient Population
Adult patients (aged 18–64 years) were eligible if they presented to the clinic with symptoms suggestive of influenza, had a documented oral temperature of ≥38°C, and had at least 1 respiratory symptom (ie, cough, sore throat, and nasal stuffiness) and at least 1 systemic symptom (eg, headache, muscle or joint pain, feverishness, and fatigue) of moderate or worse severity. The time from onset of influenza-like symptoms to the start of study drug had to be ≤48 hours, and patients had to test positive for influenza A virus by a rapid influenza diagnostic test (ie, the centrally supplied Quickvue Influenza A+B test; Quidel, San Diego, CA).
Patients with major comorbidities and a history of relevant drug allergies, chronic diseases, significant cardiovascular or central nervous system disease, or a history of most cancers were excluded. Patients could not have used a live attenuated intranasal spray influenza vaccine in the 3 weeks before study entry. During the study, patients could use acetaminophen/paracetamol as needed for fever.
Study Assessments
Venous blood samples were collected from all patients, one each for pimodivir and oseltamivir analysis, on days 1 (baseline), 3, 4, 6, and 8, to measure plasma concentrations of the drugs. Influenza virus load and viral titer were measured in nasopharyngeal swab specimens on days 1, 3, 4, 6, and 8, using quantitative reverse transcription–polymerase chain reaction (qRT-PCR) and viral culture, respectively.
Module 1 of the Flu-iiQ questionnaire was used to assess symptom severity at baseline and every 8 hours after the start of treatment through day 8 and once daily on days 9–14 [11]. The time taken for resolution of symptoms was measured as the time from the start of treatment of the first of 3 evaluations over 24 hours in which symptoms scores for each of 3 assessment were 0 (defined as not present) or 1 (defined as mild for all 7 of the following respiratory and systemic influenza symptoms: cough, sore throat, headache, nasal stuffiness, feverishness or chills, muscle or joint pain, and fatigue; Supplementary Materials).
Sanger sequencing of PB2 and NA were performed in all baseline samples and the last postbaseline sample with a sufficient virus load to evaluate the emergence of amino acid substitutions potentially associated with resistance to pimodivir (Supplementary Materials). The following PB2 positions of interest (ie, the positions potentially associated with resistance to pimodivir) were defined on the basis of in vitro and human challenge study data: Q306, F323, S324, F325, S337, H357, F363, K376, T378, F404, Q406, M431, and N510. Pimodivir susceptibility testing was performed using the Influenza ViroSpot assay (Viroclinics Biosciences, Rotterdam, Netherlands). Oseltamivir susceptibility testing was assessed using the influenza NAI reagent kit (NA Star; Applied Biosystems, Foster City, CA) with a chemiluminescent readout of NA enzyme activity. Phenotypic resistance to pimodivir and oseltamivir was defined using a preliminary cutoff of >4.0 in the fold change in the value calculated by dividing the 50% effective concentration (EC50) by the 50% inhibitory concentration. Safety assessments included recording of adverse events (AEs), physical examinations, clinical laboratory tests, electrocardiography, and vital sign assessments.
Study End Points
The primary efficacy end point was the area under the curve (AUC) for the virus load in nasal secretions, as determined by qRT-PCR, from baseline to day 8. The antiviral effect of pimodivir in combination with oseltamivir, as measured by the viral load AUC (determined by qRT-PCR) and the viral titer AUC (determined by viral culture) in nasopharyngeal swab specimens collected from baseline to day 8 and the change in the duration and severity of clinical symptoms, were also evaluated (Supplementary Materials). Secondary end points included evaluation of the time to resolution of 7 respiratory and systemic influenza symptoms. Viral resistance to pimodivir in terms of genotypic and phenotypic changes in influenza A virus variants were explored along with safety and tolerability profiles.
Statistical Analysis
A sample size of 107 evaluable patients per group was sufficient to detect a dose-response relation of the virus load AUC after pimodivir administration. Efficacy analyses were performed in the full analysis set, defined as all randomized patients who received ≥1 dose of study drug and who had a confirmed infection with influenza A virus. Safety analyses were conducted in the safety set, defined as all patients who received ≥1 dose of study drug. The virus load data were analyzed using a mixed model for repeated measures, and dose response was tested by means of a multiple comparisons approach, using a linear contrast and a contrast comparing both monotherapies combined versus placebo, with adjustment for multiple comparisons. For time-to-event data, treatment effects versus placebo effects were reported as acceleration factors, including 95% confidence intervals (CIs), for each treatment group separately. Kaplan-Meier curves were produced to graphically describe the time-to-event data.
RESULTS
Patient Disposition and Baseline Characteristics
Of the 967 patients screened, 293 (30.3%) were randomized and/or treated. The major reason for ineligibility based on screening findings was negativity for influenza A virus by a rapid influenza diagnostic test. One patient was treated but not randomized, and another patient was randomized but not treated (Supplementary Figure 2). Confirmation of influenza A virus infection (by PCR) was not obtained for 69 of 292 treated patients (23.5%), and these patients were not included in the full analysis set. Thirty-three patients (14.8%) in the full analysis set discontinued treatment because of an AE (n = 17), consent withdrawal (n = 5), physician decision (n = 5), or other reasons (n = 6). In the full analysis set, the sex of 113 patients (50.7%) was female, the median age was 41 years, and the majority of patients (118 [84.3%]) were white. The demographic characteristics were balanced across the treatment groups (Table 1). During the study, 61.9% of patients (138) used paracetamol, and 36.8% (82) used ibuprofen, and overall, the use of concomitant therapies was similar across the treatment groups.
| Characteristic . | Pimodivir 300 mg Twice Daily (n = 58) . | Pimodivir 600 mg Twice Daily (n = 57) . | Pimodivir 600 mg + oseltamivir 75 mg Twice Daily (n = 57) . | Placebo (n = 51) . |
|---|---|---|---|---|
| Female sex | 28 (48.3) | 30 (52.6) | 31 (54.4) | 24 (47.1) |
| Race | ||||
| Asian | 6 (10.3) | 3 (5.3) | 4 (7.0) | 1 (2.0) |
| Black or African American | 8 (13.8) | 6 (10.5) | 3 (5.3) | 4 (7.8) |
| White | 44 (75.9) | 48 (84.2) | 50 (87.7) | 46 (90.2) |
| Ethnicity | ||||
| Hispanic or Latino | 11 (19.0) | 12 (21.1) | 12 (21.1) | 10 (19.6) |
| Not Hispanic or Latino | 47 (81.0) | 45 (78.9) | 45 (78.9) | 41 (80.4) |
| Age, y | 41.3 ± 13.32 | 37.1 ± 14.11 | 42.0 ± 12.92 | 40.1 ± 12.23 |
| Body mass indexa | 26.09 ± 4.23 | 26.76 ± 4.59 | 27.08 ± 3.71 | 26.68 ± 3.95 |
| Influenza virus subtypeb | ||||
| A(H3N2) | 29 (52.7) | 28 (50.0) | 30 (54.5) | 23 (47.9) |
| A(H1N1) | 26 (47.3) | 28 (50.0) | 25 (45.5) | 25 (52.1) |
| Characteristic . | Pimodivir 300 mg Twice Daily (n = 58) . | Pimodivir 600 mg Twice Daily (n = 57) . | Pimodivir 600 mg + oseltamivir 75 mg Twice Daily (n = 57) . | Placebo (n = 51) . |
|---|---|---|---|---|
| Female sex | 28 (48.3) | 30 (52.6) | 31 (54.4) | 24 (47.1) |
| Race | ||||
| Asian | 6 (10.3) | 3 (5.3) | 4 (7.0) | 1 (2.0) |
| Black or African American | 8 (13.8) | 6 (10.5) | 3 (5.3) | 4 (7.8) |
| White | 44 (75.9) | 48 (84.2) | 50 (87.7) | 46 (90.2) |
| Ethnicity | ||||
| Hispanic or Latino | 11 (19.0) | 12 (21.1) | 12 (21.1) | 10 (19.6) |
| Not Hispanic or Latino | 47 (81.0) | 45 (78.9) | 45 (78.9) | 41 (80.4) |
| Age, y | 41.3 ± 13.32 | 37.1 ± 14.11 | 42.0 ± 12.92 | 40.1 ± 12.23 |
| Body mass indexa | 26.09 ± 4.23 | 26.76 ± 4.59 | 27.08 ± 3.71 | 26.68 ± 3.95 |
| Influenza virus subtypeb | ||||
| A(H3N2) | 29 (52.7) | 28 (50.0) | 30 (54.5) | 23 (47.9) |
| A(H1N1) | 26 (47.3) | 28 (50.0) | 25 (45.5) | 25 (52.1) |
Data are no. (%) of participants or mean value ± SD.
aCalculated as the weight in kilograms divided by the height in meters squared.
bData are for 55 participants in the pimodivir 300 mg group, 56 in the pimodivir 600 mg group, 55 in the pimodivir 600 mg + oseltamivir 75 mg group, and 48 in the placebo group.
| Characteristic . | Pimodivir 300 mg Twice Daily (n = 58) . | Pimodivir 600 mg Twice Daily (n = 57) . | Pimodivir 600 mg + oseltamivir 75 mg Twice Daily (n = 57) . | Placebo (n = 51) . |
|---|---|---|---|---|
| Female sex | 28 (48.3) | 30 (52.6) | 31 (54.4) | 24 (47.1) |
| Race | ||||
| Asian | 6 (10.3) | 3 (5.3) | 4 (7.0) | 1 (2.0) |
| Black or African American | 8 (13.8) | 6 (10.5) | 3 (5.3) | 4 (7.8) |
| White | 44 (75.9) | 48 (84.2) | 50 (87.7) | 46 (90.2) |
| Ethnicity | ||||
| Hispanic or Latino | 11 (19.0) | 12 (21.1) | 12 (21.1) | 10 (19.6) |
| Not Hispanic or Latino | 47 (81.0) | 45 (78.9) | 45 (78.9) | 41 (80.4) |
| Age, y | 41.3 ± 13.32 | 37.1 ± 14.11 | 42.0 ± 12.92 | 40.1 ± 12.23 |
| Body mass indexa | 26.09 ± 4.23 | 26.76 ± 4.59 | 27.08 ± 3.71 | 26.68 ± 3.95 |
| Influenza virus subtypeb | ||||
| A(H3N2) | 29 (52.7) | 28 (50.0) | 30 (54.5) | 23 (47.9) |
| A(H1N1) | 26 (47.3) | 28 (50.0) | 25 (45.5) | 25 (52.1) |
| Characteristic . | Pimodivir 300 mg Twice Daily (n = 58) . | Pimodivir 600 mg Twice Daily (n = 57) . | Pimodivir 600 mg + oseltamivir 75 mg Twice Daily (n = 57) . | Placebo (n = 51) . |
|---|---|---|---|---|
| Female sex | 28 (48.3) | 30 (52.6) | 31 (54.4) | 24 (47.1) |
| Race | ||||
| Asian | 6 (10.3) | 3 (5.3) | 4 (7.0) | 1 (2.0) |
| Black or African American | 8 (13.8) | 6 (10.5) | 3 (5.3) | 4 (7.8) |
| White | 44 (75.9) | 48 (84.2) | 50 (87.7) | 46 (90.2) |
| Ethnicity | ||||
| Hispanic or Latino | 11 (19.0) | 12 (21.1) | 12 (21.1) | 10 (19.6) |
| Not Hispanic or Latino | 47 (81.0) | 45 (78.9) | 45 (78.9) | 41 (80.4) |
| Age, y | 41.3 ± 13.32 | 37.1 ± 14.11 | 42.0 ± 12.92 | 40.1 ± 12.23 |
| Body mass indexa | 26.09 ± 4.23 | 26.76 ± 4.59 | 27.08 ± 3.71 | 26.68 ± 3.95 |
| Influenza virus subtypeb | ||||
| A(H3N2) | 29 (52.7) | 28 (50.0) | 30 (54.5) | 23 (47.9) |
| A(H1N1) | 26 (47.3) | 28 (50.0) | 25 (45.5) | 25 (52.1) |
Data are no. (%) of participants or mean value ± SD.
aCalculated as the weight in kilograms divided by the height in meters squared.
bData are for 55 participants in the pimodivir 300 mg group, 56 in the pimodivir 600 mg group, 55 in the pimodivir 600 mg + oseltamivir 75 mg group, and 48 in the placebo group.
Efficacy
Viral Load Over Time (Primary End Point)
The mean virus load AUC measured using qRT-PCR from baseline to day 8 was lower for the pimodivir treatment groups than the placebo group, and a significant dose-response relationship for the reduction in the virus load AUC was observed. The average changes in the virus load AUCs from that in the placebo group were −3.6 day*log10 copies/mL (95% CI, −7.1 to −0.1; P = .044), −4.5 day*log10 copies/mL (95% CI, −8.0 to −1.0; P = .012), and −8.6 day*log10 copies/mL (95% CI, −12.0 to −5.1; P < .001) for the pimodivir 300 mg, pimodivir 600 mg, and pimodivir 600 mg plus oseltamivir 75 mg combination groups, respectively. The combination of pimodivir 600 mg plus oseltamivir 75 mg resulted in a significantly lower virus load AUC, compared with pimodivir 600 mg alone (Figure 1A). As the primary end point of virus load AUC was met during a planned interim analysis, the study was discontinued early.
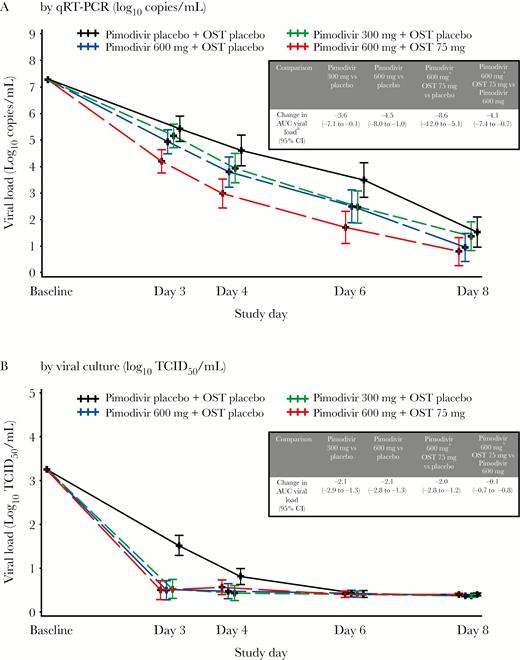
Viral load over time in full analysis set, as determined by quantitative reverse transcription–polymerase chain reaction (qRT-PCR) analysis (A) and viral culture (B). The limit of quantification for the viral load assay was 4.0 log10 copies/mL, with a limit of detection of 3.48 log10 copies/mL. Results less than the limit of quantification and greater than the limit of detection (“target detected”) are imputed with 3.75 log10 copies/mL; results less than the LOD (“target not detected”) are imputed with 0 log10 copies/mL. Error bars are 95% confidence intervals (CIs). AUC, area under the curve; OST, oseltamivir; TCID50, median tissue culture infectious dose.
A statistically significant dose-response relationship was obtained for the 300 mg and 600 mg pimodivir monotherapy groups combined versus the placebo group (P = .008 and P = .010, after adjustment for multiple comparisons), and a linear dose-response trend, respectively, as compared to the 1-sided type 1 error limit of 0.016.
Viral culture data are consistent with qRT-PCR results in demonstrating a greater effect of the treatments as compared to placebo, although the differences observed in qRT-PCR findings with respect to pimodivir dose and combination therapy versus monotherapy were not observed in viral culture data (Figure 1B). In viral culture data, the average changes in the virus load AUCs from that of the placebo group were −2.1 log10 median tissue culture infectious doses (TCID50)/mL (95% CI, −2.9 to −1.3; P < .001), −2.1 log10 TCID50/mL (95% CI, −2.8 to −1.3; P < .001), and −2.0 log10 TCID50/mL (95% CI, −2.8 to −1.2; P < .001) for the pimodivir 300 mg, pimodivir 600 mg, and pimodivir 600 mg plus oseltamivir 75 mg combination groups, respectively. The average change in virus load AUC in the combination group versus the pimodivir 600 mg group was 0.1 log10 TCID50/mL (P = .837).
Duration of Viral Shedding
The time to virus negativity (determined by qRT-PCR) was reduced by 13% in the pimodivir 300 mg group, 18% in the pimodivir 600 mg group, and 31% in the pimodivir 600 mg plus oseltamivir 75 mg group, compared with the placebo group. Combination therapy resulted in a 16% reduction in the time to virus negativity, compared with pimodivir 600 mg alone (Figure 2A). The time to virus negativity (determined by viral culture) was reduced by 29% in the pimodivir 300 mg group, 28% in the pimodivir 600 mg group, and 37% in the pimodivir 600 mg plus oseltamivir 75 mg group, compared with the placebo group. Combination therapy resulted in a 13% reduction in the time to virus negativity, compared with pimodivir 600 mg alone (Figure 2B).
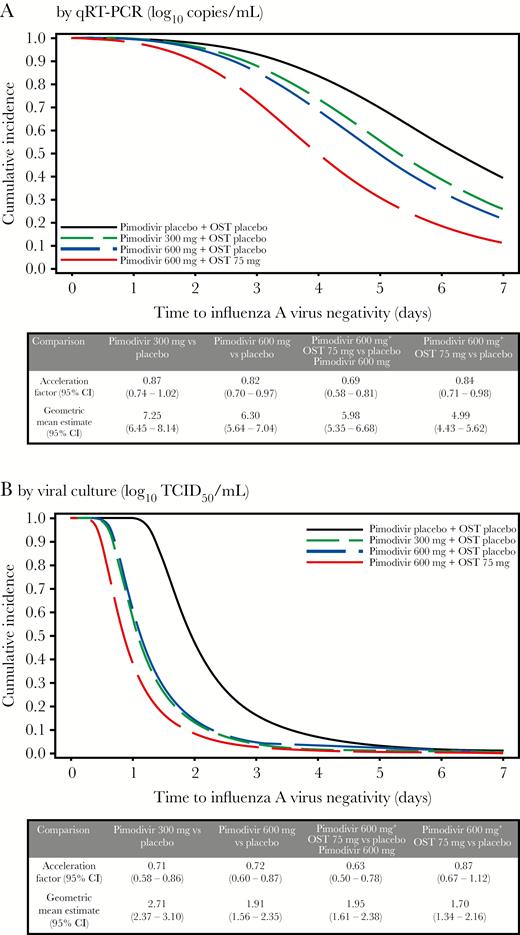
Duration of viral shedding, determined using an accelerated failure time model, in the full analysis set. A, Quantitative revere transcription–polymerase chain reaction (qRT-PCR) analysis findings. B, Viral culture findings. CI, confidence interval; OST, oseltamivir.
Time to Resolution of Influenza Symptoms
The accelerated failure time model (expressed as the ratio of the time to resolution of 7 primary influenza symptoms in the pimodivir treatment groups as compared to the placebo group), adjusted for baseline composite score and stratum, showed trends but no statistically significant differences, with a faster time to resolution of influenza symptoms versus placebo observed with pimodivir 600 mg (13%) and pimodivir 600 mg plus oseltamivir 75 mg (17%). Pimodivir 600 mg and oseltamivir combination therapy led to a similar time to resolution of influenza symptoms versus pimodivir 600 mg alone (Figure 3A and 3B).
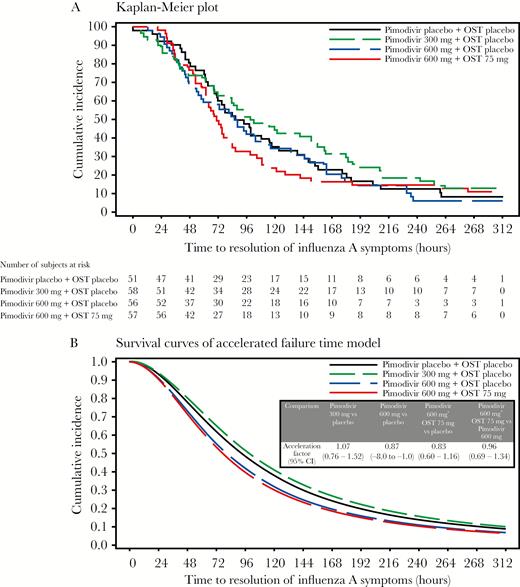
Time to resolution of 7 primary influenza symptoms. A, Kaplan-Meier plot. B, Survival curves of the accelerated failure time model. CI, confidence interval; OST, oseltamivir.
Pharmacokinetics
In all active treatment groups, the mean pimodivir concentration on day 3 was comparable to that on day 4 (ie, 873 ng/mL and 728 ng/mL, respectively, in the pimodivir 300 mg group; 1136 ng/mL and 1638 ng/mL, respectively, in the pimodivir 600 mg group; and 1359 ng/mL and 1034 ng/mL, respectively, in the pimodivir 600 mg plus oseltamivir 75 mg group), indicating that steady state was reached between days 3 and 4. Plasma concentrations of pimodivir 600 mg were similar with and without oseltamivir 75 mg (Supplementary Table 1). Mean oseltamivir and oseltamivir carboxylate plasma concentrations were also comparable on days 3 and 4, indicating that, from day 3 onward, steady state was reached (data not shown). No apparent drug-drug interaction was observed between pimodivir and oseltamivir.
Viral Resistance
Baseline sequences for PB2 and NA were available for a majority (92.4%) of patients. None of the baseline polymorphisms in PB2 were at any of the predefined positions of interest. Two patients receiving oseltamivir were identified with NA substitutions conferring reduced susceptibility, with 1 patient in the 300 mg pimodivir group harboring H275Y and 1 patient in the 600 mg pimodivir group harboring D199N. Baseline fold changes in EC50/IC50 values for pimodivir and oseltamivir were available for 81.7% and 73.5% of patients, respectively. No pimodivir fold change was >4.0 at baseline. Three patients had baseline resistance to oseltamivir (fold change, >4.0). Analysis of samples collected after baseline while undergoing treatment demonstrated emergence of PB2 substitutions or phenotypic resistance to pimodivir in 11 patients (4, 6, and 1 in the pimodivir 300 mg, pimodivir 600 mg, and combination groups, respectively). The mutations included S324K/N/R, F325L, S337P, K376N/R, T378S, and N510K, and their emergence was typically associated with a concomitant increase in the pimodivir fold change (range, 9.4 to >372.0). Further details will be reported elsewhere.
Safety
The most frequently reported AEs (by >5% patients in any group), diarrhea and nausea, were mild or moderate in severity (Table 2). One patient reported a severe diarrhea event in the pimodivir 600 mg group, and one patient had severe nausea in the combination treatment group. Diarrhea was more frequently reported in the higher pimodivir dose groups (27.0% of patients [20] in the pimodivir 600 mg group and 17.8% [13] in the pimodivir 600 mg plus oseltamivir 75 mg group, compared with 5.6% (4) in the placebo group and 6.8% [5] in the pimodivir 300 mg group). The events of diarrhea reported in the pimodivir treatment groups were often considered to be at least possibly related to the study drug and were typically mild in severity and transient and did not require any intervention.
Summary of Treatment-Emergent Adverse Events (AEs), by Treatment Group: Safety Set
| Variable . | Pimodivir 300 mg Twice Daily (n = 74) . | Pimodivir 600 mg Twice Daily (n = 74) . | Pimodivir 600 mg + OST 75 mg Twice Daily (n = 73) . | Placebo (n = 71) . |
|---|---|---|---|---|
| Treatment-emergent AEs, no. (%) | ||||
| Any | 32 (43.2) | 39 (52.7) | 30 (41.1) | 31 (43.7) |
| Serious | 0 | 1 (1.4) | 0 | 1 (1.4) |
| Leading to drug discontinuation | 7 (9.5) | 3 (4.1) | 6 (8.2) | 5 (7.0) |
| AEs in ≥5% of subjects in any treatment group, no. (%) | ||||
| Diarrhea | 5 (6.8) | 20 (27.0) | 13 (17.8) | 4 (5.6) |
| Nausea | 3 (4.1) | 3 (4.1) | 8 (11.0) | 0 |
| Vomiting | 1 (1.4) | 3 (4.1) | 6 (8.2) | 0 |
| Blood creatinine phosphokinase increase | 2 (2.7) | 1 (1.4) | 3 (4.1) | 4 (5.6) |
| Glomerular filtration rate decrease | 4 (5.4) | 1 (1.4) | 1 (1.4) | 2 (2.8) |
| Neutrophil count decrease | 1 (1.4) | 4 (5.4) | 1 (1.4) | 0 |
| Variable . | Pimodivir 300 mg Twice Daily (n = 74) . | Pimodivir 600 mg Twice Daily (n = 74) . | Pimodivir 600 mg + OST 75 mg Twice Daily (n = 73) . | Placebo (n = 71) . |
|---|---|---|---|---|
| Treatment-emergent AEs, no. (%) | ||||
| Any | 32 (43.2) | 39 (52.7) | 30 (41.1) | 31 (43.7) |
| Serious | 0 | 1 (1.4) | 0 | 1 (1.4) |
| Leading to drug discontinuation | 7 (9.5) | 3 (4.1) | 6 (8.2) | 5 (7.0) |
| AEs in ≥5% of subjects in any treatment group, no. (%) | ||||
| Diarrhea | 5 (6.8) | 20 (27.0) | 13 (17.8) | 4 (5.6) |
| Nausea | 3 (4.1) | 3 (4.1) | 8 (11.0) | 0 |
| Vomiting | 1 (1.4) | 3 (4.1) | 6 (8.2) | 0 |
| Blood creatinine phosphokinase increase | 2 (2.7) | 1 (1.4) | 3 (4.1) | 4 (5.6) |
| Glomerular filtration rate decrease | 4 (5.4) | 1 (1.4) | 1 (1.4) | 2 (2.8) |
| Neutrophil count decrease | 1 (1.4) | 4 (5.4) | 1 (1.4) | 0 |
Abbreviation: OST, oseltamivir.
Summary of Treatment-Emergent Adverse Events (AEs), by Treatment Group: Safety Set
| Variable . | Pimodivir 300 mg Twice Daily (n = 74) . | Pimodivir 600 mg Twice Daily (n = 74) . | Pimodivir 600 mg + OST 75 mg Twice Daily (n = 73) . | Placebo (n = 71) . |
|---|---|---|---|---|
| Treatment-emergent AEs, no. (%) | ||||
| Any | 32 (43.2) | 39 (52.7) | 30 (41.1) | 31 (43.7) |
| Serious | 0 | 1 (1.4) | 0 | 1 (1.4) |
| Leading to drug discontinuation | 7 (9.5) | 3 (4.1) | 6 (8.2) | 5 (7.0) |
| AEs in ≥5% of subjects in any treatment group, no. (%) | ||||
| Diarrhea | 5 (6.8) | 20 (27.0) | 13 (17.8) | 4 (5.6) |
| Nausea | 3 (4.1) | 3 (4.1) | 8 (11.0) | 0 |
| Vomiting | 1 (1.4) | 3 (4.1) | 6 (8.2) | 0 |
| Blood creatinine phosphokinase increase | 2 (2.7) | 1 (1.4) | 3 (4.1) | 4 (5.6) |
| Glomerular filtration rate decrease | 4 (5.4) | 1 (1.4) | 1 (1.4) | 2 (2.8) |
| Neutrophil count decrease | 1 (1.4) | 4 (5.4) | 1 (1.4) | 0 |
| Variable . | Pimodivir 300 mg Twice Daily (n = 74) . | Pimodivir 600 mg Twice Daily (n = 74) . | Pimodivir 600 mg + OST 75 mg Twice Daily (n = 73) . | Placebo (n = 71) . |
|---|---|---|---|---|
| Treatment-emergent AEs, no. (%) | ||||
| Any | 32 (43.2) | 39 (52.7) | 30 (41.1) | 31 (43.7) |
| Serious | 0 | 1 (1.4) | 0 | 1 (1.4) |
| Leading to drug discontinuation | 7 (9.5) | 3 (4.1) | 6 (8.2) | 5 (7.0) |
| AEs in ≥5% of subjects in any treatment group, no. (%) | ||||
| Diarrhea | 5 (6.8) | 20 (27.0) | 13 (17.8) | 4 (5.6) |
| Nausea | 3 (4.1) | 3 (4.1) | 8 (11.0) | 0 |
| Vomiting | 1 (1.4) | 3 (4.1) | 6 (8.2) | 0 |
| Blood creatinine phosphokinase increase | 2 (2.7) | 1 (1.4) | 3 (4.1) | 4 (5.6) |
| Glomerular filtration rate decrease | 4 (5.4) | 1 (1.4) | 1 (1.4) | 2 (2.8) |
| Neutrophil count decrease | 1 (1.4) | 4 (5.4) | 1 (1.4) | 0 |
Abbreviation: OST, oseltamivir.
Overall, 22.3% of patients (65) had treatment-emergent AEs. Drug discontinuations due to treatment-emergent AEs were similar in all treatment groups (7 in the pimodivir 300 mg, 3 in the pimodivir 600 mg group, 6 in the pimodivir 600 mg plus oseltamivir 75 mg group, and 5 in the placebo group). All the events leading to discontinuation of the study drug in the placebo group and 6 of 7 events leading to discontinuation in the pimodivir 300 mg group were mild to moderate in severity and not considered related to the study medication. The seventh patient in the pimodivir 300 mg group presented with 2 treatment-emergent AEs: insomnia (mild) and paresthesia (moderate) considered possibly and probably related to the study drug, respectively. The 3 events in the pimodivir 600 mg group were considered probably related to the study drug (elevated liver enzyme levels; moderate severity) and possibly related to the study drug (low absolute neutrophil count and low neutrophils; both moderate severity). In the combination treatment group, 6 patients had treatment-emergent AEs, including elevated alanine aminotransferase level (moderate), increased urine protein level (severe), decreased absolute neutrophil count (severe), decreased white blood cell count (moderate), gastrointestinal events (nausea, vomiting, and abdominal cramps [all mild] and diarrhea [moderate]), and sinus bradycardia (mild).
Complications due to influenza were not observed in the pimodivir 600 mg and combination treatment groups. Patients developed complications in the pimodivir 300 mg (n = 4) and placebo groups (n = 4), which included bronchitis (1 in the pimodivir 300 mg group and 3 in the placebo group), otitis media (1 in the placebo group), and sinusitis (3 in the pimodivir 300 mg group). Two patients in each group took antibacterial medication. No hospitalizations due to influenza or influenza-related complications were noted during the study.
Two serious AEs were reported during the study: 1 patient in the pimodivir 600 mg group had an increase in alanine aminotransferase levels, which was moderately severe and resolved after 21 weeks; and 1 patient in the placebo group reported thrombocytopenia that was considered to be possibly related to the study drug by the investigator. Of the grade 3 laboratory abnormalities, 4 events occurred in ≥3 patients in any treatment group, including elevated levels of cholesterol and urine red blood cell counts and decreases in hemoglobin levels and neutrophil counts (Supplementary Table 2). There were no clinically relevant changes in electrocardiographic parameters and vital signs or on physical examinations. No deaths were reported during this study.
DISCUSSION
This study was designed to assess the safety and efficacy of the novel nonnucleoside PB2 inhibitor pimodivir in patients with naturally acquired influenza A virus infection and to select a dose of the compound for further development. In this study, treatment with pimodivir resulted in a statistically significant and dose-dependent decrease in virus load AUC over 7 days from the start of dosing. Pimodivir combined with oseltamivir resulted in a statistically significant lower virus load AUC versus pimodivir 600 mg alone. A faster time to viral negativity versus placebo was observed with pimodivir 600 mg alone and in combination with oseltamivir.
A formal interim analysis was performed at the end of the second influenza season in the northern hemisphere (2015–2016) in patients who had enrolled up to that time point and were part of the full analysis set, and the primary end point was found to have been met. At the time of the interim analysis, 223 patients with confirmed influenza A virus infection were treated (versus the initially planned 500 patients). As the resulting sample sizes per group were relatively small, overall clinical outcome comparisons had reduced power to show differences in secondary end points.
Subsequently, trends but no statistically significant differences in the time to resolution of 7 influenza symptoms were found by the patient-reported outcome assessment tool Flu-iiQ. By study day 6, all patients experienced improvements in symptoms as assessed by the Flu-iiQ, regardless of treatment received. The greatest improvements were experienced by patients receiving pimodivir 600 mg and pimodivir 600 mg plus oseltamivir 75 mg. Pimodivir concentrations reached steady state between days 3 and 4, with no evidence of a pimodivir drug effect on oseltamivir.
A favorable safety profile for pimodivir was established in this study. Diarrhea, the most common AE reported in the pimodivir groups, was typically mild and transient and was seen more commonly in the pimodivir 600 mg groups versus placebo. Complications typically associated with influenza (ie, sinusitis, bronchitis, and otitis) were not observed in the 600 mg pimodivir groups (monotherapy or combination therapy), whereas the incidence of these complications was 7.8% in the placebo group and 6.9% in the pimodivir 300 mg group.
As the oseltamivir efficacy and safety profiles are well established [12, 13], a separate oseltamivir treatment group as a direct comparator was not included in the study. A placebo-controlled study was considered acceptable in this otherwise healthy outpatient population. Additionally, not including an oseltamivir only treatment group did not allow for a comparison of pimodivir to oseltamivir (different mode of actions). Combining pimodivir with oseltamivir resulted in a lower frequency of pimodivir resistance, although the number of patients developing any resistance overall was small. The pimodivir 600 mg twice-daily dose for 5 days is being further evaluated in ongoing clinical trials in hospitalized patients and high-risk outpatient settings (clinical trials registration NCT02532283, NCT03376321, and NCT03381196).
The overall risk-benefit assessment of pimodivir was assessed as favorable, which is significant especially considering the intent to further study this compound in a population of hospitalized patients and patients at high risk for influenza A for whom no antivirals to date have been able to demonstrate a clinical benefit. While only trends toward a clinical benefit were observed in this study, owing to a low power to detect statistically significant differences, these trends, along with the favorable safety and dosing profiles, provide an overall compelling case for further investigation. Based on this study, the dose of pimodivir 600 mg twice daily for 5 days was selected for future studies. This decision was based on the more favorable virologic and clinical outcomes observed versus the 300 mg twice-daily dose and on the opinion that the safety profile at the 600 mg dose remained reasonable and with an overall positive risk-benefit assessment.
In conclusion, pimodivir resulted in a significant and dose-dependent decrease in viral load, with the largest decrease in viral load observed for pimodivir 600 mg combined with oseltamivir 75 mg in patients with acute uncomplicated influenza A virus infection. Along with a favorable safety profile, pimodivir showed virologic benefit and the potential for demonstrating clinical benefit in influenza A virus–infected patients.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank Ineke Seghers, for her contributions to the analyses; Sabrina Khouri of Vertex, for her contributions to the management of the study; the study participants, without whom this study would not have been accomplished; and the investigators, for their participation in this study.
Janssen Research & Development facilitated the study design, provided writing assistance and editorial support for the manuscript, and reviewed and approved the manuscript prior to submission. Writing assistance was provided by Lakshmi Kasthurirangan, PhD (SIRO Clinpharm), and editorial support was provided by Bradford Challis, PhD (Janssen Research and Development).
All authors participated in the original design of the studies; supervised recruitment and monitoring of data quality; contributed to the data interpretation, development, and review of this manuscript; and confirm that they have read The Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines. The authors independently collected the data, interpreted the results, and had the final decision to submit the manuscript for publication.
Financial support. This work was supported by Janssen Research and Development.
Potential conflicts of interest. D. A., R. F., L. L., T. K., J. V., and W. v. D. are employees of Janssen. R. S. K. was an employee of Vertex. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: 5th International Society for Influenza and Other Respiratory Virus Diseases–Antiviral Group Conference, Shanghai, China, 14–16 June 2017; 6th European Scientific Working Group on Influenza Conference, 10–13 September 2017, Riga, Latvia.




