-
PDF
- Split View
-
Views
-
Cite
Cite
Feng Liu, Wen-Pin Tzeng, Lauren Horner, Ram P Kamal, Heather R Tatum, Elisabeth G Blanchard, Xiyan Xu, Ian York, Terrence M Tumpey, Jacqueline M Katz, Xiuhua Lu, Min Z Levine, Influence of Immune Priming and Egg Adaptation in the Vaccine on Antibody Responses to Circulating A(H1N1)pdm09 Viruses After Influenza Vaccination in Adults, The Journal of Infectious Diseases, Volume 218, Issue 10, 15 November 2018, Pages 1571–1581, https://doi.org/10.1093/infdis/jiy376
Close - Share Icon Share
Abstract
Although ferret antisera used in influenza surveillance did not detect antigenic drift of A(H1N1)pdm09 viruses during the 2015–2016 season, low vaccine effectiveness was reported in adults. We investigated the immune basis of low responses to circulating A(H1N1)pdm09 viruses after vaccination.
Prevaccination and postvaccination serum samples collected from >300 adults (aged 18–49 years) in 6 seasons (2010–2011 to 2015–2016) were analyzed using hemagglutination inhibition assays to evaluate the antibody responses to 13 A(H1N1) viruses circulated from 1977 to 2016. Microneutralization and serum adsorption assays were used to verify the 163K and 223R specificity of antibodies.
Individual antibody profiles to A(H1N1) viruses revealed 3 priming patterns: USSR/77, TW/86, or NC/99 priming. More than 20% of adults had reduced titers to cell-propagated circulating 6B.1 and 6B.2 A(H1N1)pdm09 viruses compared with the A/California/07/2009 vaccine virus X-179A. Significantly reduced antibody reactivity to circulating viruses bearing K163Q was observed only in the USSR/77-primed cohort, whereas significantly lower reactivity caused by egg-adapted Q223R change was detected across all 3 cohorts.
Both 163K specificity driven by immune priming and 223R specificity from egg-adapted changes in the vaccine contributed to low responses to circulating A(H1N1)pdm09 viruses after vaccination. Our study highlights the need to incorporate human serology in influenza surveillance and vaccine strain selection.
Seasonal influenza viruses continue to circulate in human populations and accumulate mutations through antigenic drift requiring annual updating of influenza vaccine compositions. Population immunity, acquired primarily through natural infection but also through influenza vaccination, is thought to be a selective force driving the evolution of human influenza viruses [1]. A(H1N1)pdm09 viruses first emerged in 2009, caused a pandemic, and continued to circulate as seasonal viruses. An A/California/07/09-like strain (CA/09) was originally chosen as the A(H1N1)pdm09 component of multivalent seasonal vaccines and has remained a recommended component through the 2016–2017 season [2]. In 2013–2014, the genetic clade 6B emerged with a featured amino acid substitution from lysine (K) to glutamine (Q) at position 163 (K163Q; H1 numbering) in the hemagglutinin (HA), located in antigenic site Sa [3–5]. Since then, 6B viruses have diverged further into 6B.1 and 6B.2 subclades, still bearing K163Q; the 6B.1 subclade has predominated globally since 2015–2016 [6].
Although 6B, 6B.1, and 6B.2 viruses are considered antigenically similar to the CA/09 vaccine virus as characterized by postinfection ferret antisera [3, 6], A(H1N1)pdm09-specific vaccine effectiveness (VE) for the 2015–2016 subclade 6B.1 predominant season was lower among adults born between 1958 and 1979 (22%) than other age groups (61%) [7]. Similar birth cohort effects were also reported from Canada during the same season [8]. Others have hypothesized that prior exposures to older seasonal A(H1N1) viruses may have influenced antibody responses to contemporary A(H1N1)pdm09 viruses bearing K163Q change after influenza vaccination in middle-aged adults [1, 4].
Compared with the wild-type and cell-propagated circulating A(H1N1)pdm09 viruses, egg-propagated CA/09 vaccine viruses (eg, X-179A) had an amino acid change from Q to arginine (R) at HA position 223 (Q223R; H1 numbering), located within the receptor binding site (RBS) [9, 10]. Most circulating A(H1N1)pdm09 wild-type viruses, including 6B, 6B.1, and 6B.2, have 223Q in the HA, whereas egg-adapted viruses frequently have 223R, which confers α-2,3 avian like receptor specificity and hence promotes virus replication in eggs [10]. Findings of several studies have suggested that such egg-adapted changes can alter antigenicity and thus may influence immune responses to influenza vaccines in humans [10–13]. Egg-adapted changes in the HAs of vaccine viruses have been proposed as a factor contributing to low VE against A(H3N2) [14, 15].
In the current study, we used adult prevaccination and postvaccination serum samples collected from 6 seasons after 2009, and evaluated antibody responses to the CA/09 vaccine component and contemporary 6B and 6B.1 viruses to investigate the impact of immune priming on the reduced antibodies to circulating A(H1N1)pdm09 viruses observed in some populations of adults after vaccination [7]. We used a systematic approach to define A(H1N1) immune priming status based on antibody profiles to historic seasonal A(H1N1) viruses to which individuals were probably exposed earlier in life. Then, within the context of defined A(H1N1) priming of each individual, we evaluated the impact of K163Q and egg-adapted Q223R HA changes on antibody responses to circulating A(H1N1)pdm09 viruses after influenza vaccination. Finally, we explored the postvaccination serum antibody repertoire, using antibody adsorption with priming and circulating viruses.
MATERIAL AND METHODS
Viruses
Influenza viruses were propagated either in eggs or in Madin-Darby canine kidney (MDCK) cells. The A(H1N1)pdm09 viruses used included A/California/07/2009-PR8 (X-179A; vaccine virus) and egg- and cell-propagated 6B.1 and 6B.2 viruses, A/Michigan/45/2015 (MI/15e and MI/15c) and A/Iowa/53/2015 (IW/15e and IW/15c), respectively. Ten pre-2009 seasonal A(H1N1) (sH1N1) viruses representing major antigenic clusters circulated during 1977–2009 were also used: A/USSR/90/1977 (USSR/77), A/England/333/1980 (ENG/80), A/Chile/1/1983 (CHI/83), A/Taiwan/1/1986 (TW/86), A/Texas/ 36/1991(TX/91), A/Bayern/7/1995(BAY/95), A/Beijing/262/ 1995 (BJ/95), A/New Caledonia/20/1999 (NC/99), A/Solomon Islands/3/2006 (SI/06), and A/Brisbane/59/2007 (BR/07). In addition, 3 reverse genetically (RG) engineered egg-propagated viruses were generated, containing the HA and neuraminidase genes from CA/09 and 6 internal genes from A/Puerto Rico/8/1934, including A/California/7/2009-PR8 (RG-163K), A/California/7/2009-PR8-K163Q (RG-K163Q), and A/Calif ornia/7/2009-PR8-D127T (RG-D127T) with a putative glycosylation motif at position 125. Details of RG virus generation are included in the Supplementary Material.
Serum Samples
Prevaccination and 21–28-day postvaccination serum samples from US adults (n = 336) who received inactivated influenza vaccines (IIVs) containing CA/09-like H1 component were collected from 6 seasons after the 2009 A(H1N1) pandemic. These anonymized serum samples were provided by a contract organization, and their use was exempt from the Centers for Disease Control and Prevention (CDC) human subjects review. Postinfection ferret antisera were generated by intranasal inoculation of a single dose of wild-type viruses [16].
Hemagglutination Inhibition, Microneutralization, and Antibody Adsorption Assays
Hemagglutination inhibition (HI) assays were conducted with 4 HA units per 25 μL of virus, using 0.5% turkey erythrocytes, as described elsewhere [17]. Serum samples from low responders were further tested using microneutralization assays [17] to measure neutralizing antibody responses. In addition, a small subset of postvaccination serum samples also underwent antibody adsorption with purified whole viruses to confirm the specificity of antibodies [18, 19]. Details of the assays are described in the Supplementary Material.
Statistical Analyses
Wilcoxon matched paired t tests were used to analyze geometric mean titers (GMTs). Fisher exact test was used to compare the numbers of low responders to A(H1N1)pdm09 viruses. Differences were considered significant at P < .05. GraphPad Prism V5 software (GraphPad Software) was used for statistical analyses.
RESULTS
Reduced Antibody Responses to Circulating A(H1N1) Viruses in a Proportion of Vaccinees After Vaccination
Paired serum samples collected from vaccinated adults (aged 18–49 years; n = 336) in 6 seasons since the 2009 pandemic were first tested using HI assays against the X-179A virus. Samples from 300 individuals (aged 18–49 years; birth year [BY], 1961–1998; median age, 33 years) with postvaccination HI titer ≥40 to vaccine like viruses were further analyzed (Table 1 and Supplementary Table 1). The majority of vaccinees mounted robust antibody responses to X-179A (GMT, 272); 87%–98% had postvaccination HI titers ≥40 to other A(H1N1)pdm09 viruses (Table 1). However, compared with HI titers to egg-propagated X-179A across the 6 seasons (n = 300), 9% and 12% of vaccinees had ≥4 fold reductions in HI titers to egg-propagated circulating 6B.1 and 6B.2 viruses respectively; 22% and 25% of these individual also had ≥4 fold reductions in HI titers to cell-propagated 6B.1 and 6B.2 viruses, respectively (Table 1). Furthermore, there was a significant reduction in HI titers to RG-163Q (GMT, 136) compared with titers to RG-163K (GMT, 202) (P < .05) across 6 seasons and in every individual season (P < .05).
HI Antibody Responses Against Influenza A(H1N1)pdm09 Viruses in Adults After Vaccination
| Season . | Vaccinees, No. . | Virus . | HA Group . | Amino Acid . | GMT . | HI Titer ≥40, % . | Seroconversion, %a . | LRs, No. (%)b . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 163 . | 223 . | Pre . | Post (Range) . | Pre . | Post . | . | . | ||||
| 2010–2011 | 50 | X-179A | NA | K | R | 17 | 394 (40–1280) | 36 | 100 | 90 | NA |
| RG-K163 | NA | K | Q | 17 | 260c (20–1280) | 40 | 98 | 86 | 7 (14) | ||
| RG-K163Q | NA | Q | Q | 13 | 143c,d (5–1280) | 24 | 86 | 80 | 18 (36)d | ||
| MI/15e | 6B.1 | Q | R | 12 | 229c (5–1280) | 22 | 94 | 82 | 9 (18) | ||
| MI/15c | 6B.1 | Q | Q | 11 | 125c,e (5–1280) | 20 | 84 | 82 | 20 (40)e | ||
| IW/15e | 6B.2 | Q | R | 11 | 181c (5–1280) | 14 | 92 | 88 | 10 (20) | ||
| IW/15c | 6B.2 | Q | Q | 10 | 112c,e (5–1280) | 12 | 80 | 84 | 19 (38) | ||
| 2011–2012 | 54 | X-179A | NA | K | R | 27 | 281 (40–1280) | 46 | 100 | 72 | NA |
| RG-K163 | NA | K | Q | 20 | 156c (40–640) | 35 | 100 | 69 | 9 (17) | ||
| RG-K163Q | NA | Q | Q | 16 | 106c,d (5–640) | 31 | 87 | 69 | 19 (35)d | ||
| MI/15e | 6B.1 | Q | R | 21 | 207c (5–1280) | 35 | 98 | 72 | 7 (13) | ||
| MI/15c | 6B.1 | Q | Q | 22 | 166c (5–1280) | 35 | 89 | 69 | 15 (28) | ||
| IW/15e | 6B.2 | Q | R | 19 | 139c (5–1280) | 37 | 94 | 72 | 8 (15) | ||
| IW/15c | 6B.2 | Q | Q | 19 | 110c (5–640) | 33 | 80 | 72 | 20 (37)e | ||
| 2013–2014 | 42 | X-179A | NA | K | R | 17 | 242 (40–1280) | 29 | 100 | 83 | NA |
| RG-K163 | NA | K | Q | 16 | 189c (20–1280) | 31 | 98 | 79 | 7 (17) | ||
| RG-K163Q | NA | Q | Q | 12 | 108c,d (10–640) | 21 | 86 | 74 | 12 (29) | ||
| MI/15e | 6B.1 | Q | R | 20 | 242 (10–1280) | 43 | 98 | 76 | 4 (10) | ||
| MI/15c | 6B.1 | Q | Q | 14 | 119c,e (5–1280) | 24 | 83 | 69 | 12 (29)e | ||
| IW/15e | 6B.2 | Q | R | 13 | 125c (5–640) | 24 | 90 | 74 | 5 (12) | ||
| IW/15c | 6B.2 | Q | Q | 11 | 72c,e (5–640) | 17 | 76 | 62 | 17 (40)e | ||
| 2014–2015 | 61 | X-179A | NA | K | R | 30 | 286 (40–1280) | 49 | 100 | 77 | NA |
| RG-K163 | NA | K | Q | 30 | 220c (5–1280) | 57 | 98 | 74 | 4 (7) | ||
| RG-K163Q | NA | Q | Q | 25 | 164c,d (5–1280) | 49 | 93 | 70 | 9 (15) | ||
| MI/15e | 6B.1 | Q | R | 31 | 264 (10–1280) | 51 | 98 | 75 | 2 (3) | ||
| MI/15c | 6B.1 | Q | Q | 24 | 188c,e (5–1280) | 46 | 92 | 74 | 10 (16)e | ||
| IW/15e | 6B.2 | Q | R | 20 | 173c (5–1280) | 39 | 95 | 72 | 4 (7) | ||
| IW/15c | 6B.2 | Q | Q | 20 | 140c (5–1280) | 41 | 89 | 67 | 16 (25)e | ||
| 2015–2016 | 60 | X-179A | NA | K | R | 50 | 214 (40–1280) | 68 | 100 | 42 | NA |
| RG-K163 | NA | K | Q | 53 | 188c (40–1280) | 72 | 100 | 40 | 1 (2) | ||
| RG-K163Q | NA | Q | Q | 46 | 156c,d (5–1280) | 70 | 97 | 38 | 2 (3) | ||
| MI/15e | 6B.1 | Q | R | 55 | 221 (10–1280) | 68 | 98 | 43 | 1 (2) | ||
| MI/15c | 6B.1 | Q | Q | 50 | 192 (5–1280) | 68 | 97 | 42 | 2 (3) | ||
| IW/15e | 6B.2 | Q | R | 33 | 117c (5–1280) | 60 | 98 | 38 | 4 (7) | ||
| IW/15c | 6B.2 | Q | Q | 34 | 117c (5–1280) | 57 | 97 | 40 | 9 (15) | ||
| 2016–2017 | 33 | X-179A | NA | K | R | 77 | 244 (40–1280) | 85 | 100 | 30 | NA |
| RG-K163 | NA | K | Q | 58 | 224 (40–1280) | 73 | 100 | 36 | 0 | ||
| RG-K163Q | NA | Q | Q | 46 | 144c,d (5–1280) | 70 | 97 | 33 | 4 (12) | ||
| MI/15e | 6B.1 | Q | R | 56 | 181c (5–1280) | 76 | 97 | 39 | 4 (12) | ||
| MI/15c | 6B.1 | Q | Q | 43 | 138c,e (5–1280) | 64 | 82 | 36 | 6 (18) | ||
| IW/15e | 6B.2 | Q | R | 48 | 160c (5–1280) | 73 | 94 | 33 | 4 (13) | ||
| IW/15c | 6B.2 | Q | Q | 47 | 160c (5–1280) | 67 | 97 | 36 | 5 (15) | ||
| Total | 300 | X-179A | NA | K | R | 30 | 272 (40–1280) | 51 | 100 | 67 | NA |
| RG-K163 | NA | K | Q | 28 | 202c (5–1280) | 51 | 99 | 64 | 28 (9) | ||
| RG-K163Q | NA | Q | Q | 23 | 136c,d (5–1280) | 44 | 91 | 62 | 64 (21)d | ||
| MI/15e | 6B.1 | Q | R | 28 | 226c (5–1280) | 48 | 98 | 66 | 27 (9) | ||
| MI/15c | 6B.1 | Q | Q | 24 | 156c,e (5–1280) | 43 | 89 | 63 | 65 (22)e | ||
| IW/15e | 6B.2 | Q | R | 20 | 147c (5–1280) | 40 | 94 | 64 | 35 (12) | ||
| IW/15c | 6B.2 | Q | Q | 20 | 115c,e (5–1280) | 37 | 87 | 61 | 76 (25%)e | ||
| Season . | Vaccinees, No. . | Virus . | HA Group . | Amino Acid . | GMT . | HI Titer ≥40, % . | Seroconversion, %a . | LRs, No. (%)b . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 163 . | 223 . | Pre . | Post (Range) . | Pre . | Post . | . | . | ||||
| 2010–2011 | 50 | X-179A | NA | K | R | 17 | 394 (40–1280) | 36 | 100 | 90 | NA |
| RG-K163 | NA | K | Q | 17 | 260c (20–1280) | 40 | 98 | 86 | 7 (14) | ||
| RG-K163Q | NA | Q | Q | 13 | 143c,d (5–1280) | 24 | 86 | 80 | 18 (36)d | ||
| MI/15e | 6B.1 | Q | R | 12 | 229c (5–1280) | 22 | 94 | 82 | 9 (18) | ||
| MI/15c | 6B.1 | Q | Q | 11 | 125c,e (5–1280) | 20 | 84 | 82 | 20 (40)e | ||
| IW/15e | 6B.2 | Q | R | 11 | 181c (5–1280) | 14 | 92 | 88 | 10 (20) | ||
| IW/15c | 6B.2 | Q | Q | 10 | 112c,e (5–1280) | 12 | 80 | 84 | 19 (38) | ||
| 2011–2012 | 54 | X-179A | NA | K | R | 27 | 281 (40–1280) | 46 | 100 | 72 | NA |
| RG-K163 | NA | K | Q | 20 | 156c (40–640) | 35 | 100 | 69 | 9 (17) | ||
| RG-K163Q | NA | Q | Q | 16 | 106c,d (5–640) | 31 | 87 | 69 | 19 (35)d | ||
| MI/15e | 6B.1 | Q | R | 21 | 207c (5–1280) | 35 | 98 | 72 | 7 (13) | ||
| MI/15c | 6B.1 | Q | Q | 22 | 166c (5–1280) | 35 | 89 | 69 | 15 (28) | ||
| IW/15e | 6B.2 | Q | R | 19 | 139c (5–1280) | 37 | 94 | 72 | 8 (15) | ||
| IW/15c | 6B.2 | Q | Q | 19 | 110c (5–640) | 33 | 80 | 72 | 20 (37)e | ||
| 2013–2014 | 42 | X-179A | NA | K | R | 17 | 242 (40–1280) | 29 | 100 | 83 | NA |
| RG-K163 | NA | K | Q | 16 | 189c (20–1280) | 31 | 98 | 79 | 7 (17) | ||
| RG-K163Q | NA | Q | Q | 12 | 108c,d (10–640) | 21 | 86 | 74 | 12 (29) | ||
| MI/15e | 6B.1 | Q | R | 20 | 242 (10–1280) | 43 | 98 | 76 | 4 (10) | ||
| MI/15c | 6B.1 | Q | Q | 14 | 119c,e (5–1280) | 24 | 83 | 69 | 12 (29)e | ||
| IW/15e | 6B.2 | Q | R | 13 | 125c (5–640) | 24 | 90 | 74 | 5 (12) | ||
| IW/15c | 6B.2 | Q | Q | 11 | 72c,e (5–640) | 17 | 76 | 62 | 17 (40)e | ||
| 2014–2015 | 61 | X-179A | NA | K | R | 30 | 286 (40–1280) | 49 | 100 | 77 | NA |
| RG-K163 | NA | K | Q | 30 | 220c (5–1280) | 57 | 98 | 74 | 4 (7) | ||
| RG-K163Q | NA | Q | Q | 25 | 164c,d (5–1280) | 49 | 93 | 70 | 9 (15) | ||
| MI/15e | 6B.1 | Q | R | 31 | 264 (10–1280) | 51 | 98 | 75 | 2 (3) | ||
| MI/15c | 6B.1 | Q | Q | 24 | 188c,e (5–1280) | 46 | 92 | 74 | 10 (16)e | ||
| IW/15e | 6B.2 | Q | R | 20 | 173c (5–1280) | 39 | 95 | 72 | 4 (7) | ||
| IW/15c | 6B.2 | Q | Q | 20 | 140c (5–1280) | 41 | 89 | 67 | 16 (25)e | ||
| 2015–2016 | 60 | X-179A | NA | K | R | 50 | 214 (40–1280) | 68 | 100 | 42 | NA |
| RG-K163 | NA | K | Q | 53 | 188c (40–1280) | 72 | 100 | 40 | 1 (2) | ||
| RG-K163Q | NA | Q | Q | 46 | 156c,d (5–1280) | 70 | 97 | 38 | 2 (3) | ||
| MI/15e | 6B.1 | Q | R | 55 | 221 (10–1280) | 68 | 98 | 43 | 1 (2) | ||
| MI/15c | 6B.1 | Q | Q | 50 | 192 (5–1280) | 68 | 97 | 42 | 2 (3) | ||
| IW/15e | 6B.2 | Q | R | 33 | 117c (5–1280) | 60 | 98 | 38 | 4 (7) | ||
| IW/15c | 6B.2 | Q | Q | 34 | 117c (5–1280) | 57 | 97 | 40 | 9 (15) | ||
| 2016–2017 | 33 | X-179A | NA | K | R | 77 | 244 (40–1280) | 85 | 100 | 30 | NA |
| RG-K163 | NA | K | Q | 58 | 224 (40–1280) | 73 | 100 | 36 | 0 | ||
| RG-K163Q | NA | Q | Q | 46 | 144c,d (5–1280) | 70 | 97 | 33 | 4 (12) | ||
| MI/15e | 6B.1 | Q | R | 56 | 181c (5–1280) | 76 | 97 | 39 | 4 (12) | ||
| MI/15c | 6B.1 | Q | Q | 43 | 138c,e (5–1280) | 64 | 82 | 36 | 6 (18) | ||
| IW/15e | 6B.2 | Q | R | 48 | 160c (5–1280) | 73 | 94 | 33 | 4 (13) | ||
| IW/15c | 6B.2 | Q | Q | 47 | 160c (5–1280) | 67 | 97 | 36 | 5 (15) | ||
| Total | 300 | X-179A | NA | K | R | 30 | 272 (40–1280) | 51 | 100 | 67 | NA |
| RG-K163 | NA | K | Q | 28 | 202c (5–1280) | 51 | 99 | 64 | 28 (9) | ||
| RG-K163Q | NA | Q | Q | 23 | 136c,d (5–1280) | 44 | 91 | 62 | 64 (21)d | ||
| MI/15e | 6B.1 | Q | R | 28 | 226c (5–1280) | 48 | 98 | 66 | 27 (9) | ||
| MI/15c | 6B.1 | Q | Q | 24 | 156c,e (5–1280) | 43 | 89 | 63 | 65 (22)e | ||
| IW/15e | 6B.2 | Q | R | 20 | 147c (5–1280) | 40 | 94 | 64 | 35 (12) | ||
| IW/15c | 6B.2 | Q | Q | 20 | 115c,e (5–1280) | 37 | 87 | 61 | 76 (25%)e | ||
Abbreviations: GMTs, geometric mean titers; HA, hemagglutinin; HI, hemagglutination inhibition; IW/15, A/Iowa/53/2015 field strain grown in egg (IW/15e) or Madin-Darby canine kidney cells (IW/15c); LRs, low responders; MI/15, A/Michigan/45/2015 field strain grown in egg (MI/15e) or Madin-Darby canine kidney cells (MI/15c); NA, not applicable; Post, postvaccination; Pre, prevaccination; RG-K163, A/California/8/2009-PR8 egg-grown virus possessing HA-K163; RG-K163Q, RG-CA egg-grown virus engineered to possess HA-K163Q mutation; X-179A, A/California/7/2009-PR8 egg-grown vaccine virus. Response to vaccine virus X-179A are in bold.
aPercentage of seroconversion, defined as ≥4-fold increase from prevaccination to postvaccination titers, where the postvaccination titer is ≥40.
bLow responders defined as those with ≥4-fold reduced postvaccination HI antibody titers to the corresponding virus compared with X-179A.
cP < .05 for RG-CA viruses and 2015 field strains vs X-179A vaccine strain.
dP < .05 for RG-K163Q vs RG-K163 virus.
eP < .05 for MI/15 and IW/15 cell-grown viruses vs their paired egg-grown viruses with an egg-adapted mutation (Q223R).
HI Antibody Responses Against Influenza A(H1N1)pdm09 Viruses in Adults After Vaccination
| Season . | Vaccinees, No. . | Virus . | HA Group . | Amino Acid . | GMT . | HI Titer ≥40, % . | Seroconversion, %a . | LRs, No. (%)b . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 163 . | 223 . | Pre . | Post (Range) . | Pre . | Post . | . | . | ||||
| 2010–2011 | 50 | X-179A | NA | K | R | 17 | 394 (40–1280) | 36 | 100 | 90 | NA |
| RG-K163 | NA | K | Q | 17 | 260c (20–1280) | 40 | 98 | 86 | 7 (14) | ||
| RG-K163Q | NA | Q | Q | 13 | 143c,d (5–1280) | 24 | 86 | 80 | 18 (36)d | ||
| MI/15e | 6B.1 | Q | R | 12 | 229c (5–1280) | 22 | 94 | 82 | 9 (18) | ||
| MI/15c | 6B.1 | Q | Q | 11 | 125c,e (5–1280) | 20 | 84 | 82 | 20 (40)e | ||
| IW/15e | 6B.2 | Q | R | 11 | 181c (5–1280) | 14 | 92 | 88 | 10 (20) | ||
| IW/15c | 6B.2 | Q | Q | 10 | 112c,e (5–1280) | 12 | 80 | 84 | 19 (38) | ||
| 2011–2012 | 54 | X-179A | NA | K | R | 27 | 281 (40–1280) | 46 | 100 | 72 | NA |
| RG-K163 | NA | K | Q | 20 | 156c (40–640) | 35 | 100 | 69 | 9 (17) | ||
| RG-K163Q | NA | Q | Q | 16 | 106c,d (5–640) | 31 | 87 | 69 | 19 (35)d | ||
| MI/15e | 6B.1 | Q | R | 21 | 207c (5–1280) | 35 | 98 | 72 | 7 (13) | ||
| MI/15c | 6B.1 | Q | Q | 22 | 166c (5–1280) | 35 | 89 | 69 | 15 (28) | ||
| IW/15e | 6B.2 | Q | R | 19 | 139c (5–1280) | 37 | 94 | 72 | 8 (15) | ||
| IW/15c | 6B.2 | Q | Q | 19 | 110c (5–640) | 33 | 80 | 72 | 20 (37)e | ||
| 2013–2014 | 42 | X-179A | NA | K | R | 17 | 242 (40–1280) | 29 | 100 | 83 | NA |
| RG-K163 | NA | K | Q | 16 | 189c (20–1280) | 31 | 98 | 79 | 7 (17) | ||
| RG-K163Q | NA | Q | Q | 12 | 108c,d (10–640) | 21 | 86 | 74 | 12 (29) | ||
| MI/15e | 6B.1 | Q | R | 20 | 242 (10–1280) | 43 | 98 | 76 | 4 (10) | ||
| MI/15c | 6B.1 | Q | Q | 14 | 119c,e (5–1280) | 24 | 83 | 69 | 12 (29)e | ||
| IW/15e | 6B.2 | Q | R | 13 | 125c (5–640) | 24 | 90 | 74 | 5 (12) | ||
| IW/15c | 6B.2 | Q | Q | 11 | 72c,e (5–640) | 17 | 76 | 62 | 17 (40)e | ||
| 2014–2015 | 61 | X-179A | NA | K | R | 30 | 286 (40–1280) | 49 | 100 | 77 | NA |
| RG-K163 | NA | K | Q | 30 | 220c (5–1280) | 57 | 98 | 74 | 4 (7) | ||
| RG-K163Q | NA | Q | Q | 25 | 164c,d (5–1280) | 49 | 93 | 70 | 9 (15) | ||
| MI/15e | 6B.1 | Q | R | 31 | 264 (10–1280) | 51 | 98 | 75 | 2 (3) | ||
| MI/15c | 6B.1 | Q | Q | 24 | 188c,e (5–1280) | 46 | 92 | 74 | 10 (16)e | ||
| IW/15e | 6B.2 | Q | R | 20 | 173c (5–1280) | 39 | 95 | 72 | 4 (7) | ||
| IW/15c | 6B.2 | Q | Q | 20 | 140c (5–1280) | 41 | 89 | 67 | 16 (25)e | ||
| 2015–2016 | 60 | X-179A | NA | K | R | 50 | 214 (40–1280) | 68 | 100 | 42 | NA |
| RG-K163 | NA | K | Q | 53 | 188c (40–1280) | 72 | 100 | 40 | 1 (2) | ||
| RG-K163Q | NA | Q | Q | 46 | 156c,d (5–1280) | 70 | 97 | 38 | 2 (3) | ||
| MI/15e | 6B.1 | Q | R | 55 | 221 (10–1280) | 68 | 98 | 43 | 1 (2) | ||
| MI/15c | 6B.1 | Q | Q | 50 | 192 (5–1280) | 68 | 97 | 42 | 2 (3) | ||
| IW/15e | 6B.2 | Q | R | 33 | 117c (5–1280) | 60 | 98 | 38 | 4 (7) | ||
| IW/15c | 6B.2 | Q | Q | 34 | 117c (5–1280) | 57 | 97 | 40 | 9 (15) | ||
| 2016–2017 | 33 | X-179A | NA | K | R | 77 | 244 (40–1280) | 85 | 100 | 30 | NA |
| RG-K163 | NA | K | Q | 58 | 224 (40–1280) | 73 | 100 | 36 | 0 | ||
| RG-K163Q | NA | Q | Q | 46 | 144c,d (5–1280) | 70 | 97 | 33 | 4 (12) | ||
| MI/15e | 6B.1 | Q | R | 56 | 181c (5–1280) | 76 | 97 | 39 | 4 (12) | ||
| MI/15c | 6B.1 | Q | Q | 43 | 138c,e (5–1280) | 64 | 82 | 36 | 6 (18) | ||
| IW/15e | 6B.2 | Q | R | 48 | 160c (5–1280) | 73 | 94 | 33 | 4 (13) | ||
| IW/15c | 6B.2 | Q | Q | 47 | 160c (5–1280) | 67 | 97 | 36 | 5 (15) | ||
| Total | 300 | X-179A | NA | K | R | 30 | 272 (40–1280) | 51 | 100 | 67 | NA |
| RG-K163 | NA | K | Q | 28 | 202c (5–1280) | 51 | 99 | 64 | 28 (9) | ||
| RG-K163Q | NA | Q | Q | 23 | 136c,d (5–1280) | 44 | 91 | 62 | 64 (21)d | ||
| MI/15e | 6B.1 | Q | R | 28 | 226c (5–1280) | 48 | 98 | 66 | 27 (9) | ||
| MI/15c | 6B.1 | Q | Q | 24 | 156c,e (5–1280) | 43 | 89 | 63 | 65 (22)e | ||
| IW/15e | 6B.2 | Q | R | 20 | 147c (5–1280) | 40 | 94 | 64 | 35 (12) | ||
| IW/15c | 6B.2 | Q | Q | 20 | 115c,e (5–1280) | 37 | 87 | 61 | 76 (25%)e | ||
| Season . | Vaccinees, No. . | Virus . | HA Group . | Amino Acid . | GMT . | HI Titer ≥40, % . | Seroconversion, %a . | LRs, No. (%)b . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 163 . | 223 . | Pre . | Post (Range) . | Pre . | Post . | . | . | ||||
| 2010–2011 | 50 | X-179A | NA | K | R | 17 | 394 (40–1280) | 36 | 100 | 90 | NA |
| RG-K163 | NA | K | Q | 17 | 260c (20–1280) | 40 | 98 | 86 | 7 (14) | ||
| RG-K163Q | NA | Q | Q | 13 | 143c,d (5–1280) | 24 | 86 | 80 | 18 (36)d | ||
| MI/15e | 6B.1 | Q | R | 12 | 229c (5–1280) | 22 | 94 | 82 | 9 (18) | ||
| MI/15c | 6B.1 | Q | Q | 11 | 125c,e (5–1280) | 20 | 84 | 82 | 20 (40)e | ||
| IW/15e | 6B.2 | Q | R | 11 | 181c (5–1280) | 14 | 92 | 88 | 10 (20) | ||
| IW/15c | 6B.2 | Q | Q | 10 | 112c,e (5–1280) | 12 | 80 | 84 | 19 (38) | ||
| 2011–2012 | 54 | X-179A | NA | K | R | 27 | 281 (40–1280) | 46 | 100 | 72 | NA |
| RG-K163 | NA | K | Q | 20 | 156c (40–640) | 35 | 100 | 69 | 9 (17) | ||
| RG-K163Q | NA | Q | Q | 16 | 106c,d (5–640) | 31 | 87 | 69 | 19 (35)d | ||
| MI/15e | 6B.1 | Q | R | 21 | 207c (5–1280) | 35 | 98 | 72 | 7 (13) | ||
| MI/15c | 6B.1 | Q | Q | 22 | 166c (5–1280) | 35 | 89 | 69 | 15 (28) | ||
| IW/15e | 6B.2 | Q | R | 19 | 139c (5–1280) | 37 | 94 | 72 | 8 (15) | ||
| IW/15c | 6B.2 | Q | Q | 19 | 110c (5–640) | 33 | 80 | 72 | 20 (37)e | ||
| 2013–2014 | 42 | X-179A | NA | K | R | 17 | 242 (40–1280) | 29 | 100 | 83 | NA |
| RG-K163 | NA | K | Q | 16 | 189c (20–1280) | 31 | 98 | 79 | 7 (17) | ||
| RG-K163Q | NA | Q | Q | 12 | 108c,d (10–640) | 21 | 86 | 74 | 12 (29) | ||
| MI/15e | 6B.1 | Q | R | 20 | 242 (10–1280) | 43 | 98 | 76 | 4 (10) | ||
| MI/15c | 6B.1 | Q | Q | 14 | 119c,e (5–1280) | 24 | 83 | 69 | 12 (29)e | ||
| IW/15e | 6B.2 | Q | R | 13 | 125c (5–640) | 24 | 90 | 74 | 5 (12) | ||
| IW/15c | 6B.2 | Q | Q | 11 | 72c,e (5–640) | 17 | 76 | 62 | 17 (40)e | ||
| 2014–2015 | 61 | X-179A | NA | K | R | 30 | 286 (40–1280) | 49 | 100 | 77 | NA |
| RG-K163 | NA | K | Q | 30 | 220c (5–1280) | 57 | 98 | 74 | 4 (7) | ||
| RG-K163Q | NA | Q | Q | 25 | 164c,d (5–1280) | 49 | 93 | 70 | 9 (15) | ||
| MI/15e | 6B.1 | Q | R | 31 | 264 (10–1280) | 51 | 98 | 75 | 2 (3) | ||
| MI/15c | 6B.1 | Q | Q | 24 | 188c,e (5–1280) | 46 | 92 | 74 | 10 (16)e | ||
| IW/15e | 6B.2 | Q | R | 20 | 173c (5–1280) | 39 | 95 | 72 | 4 (7) | ||
| IW/15c | 6B.2 | Q | Q | 20 | 140c (5–1280) | 41 | 89 | 67 | 16 (25)e | ||
| 2015–2016 | 60 | X-179A | NA | K | R | 50 | 214 (40–1280) | 68 | 100 | 42 | NA |
| RG-K163 | NA | K | Q | 53 | 188c (40–1280) | 72 | 100 | 40 | 1 (2) | ||
| RG-K163Q | NA | Q | Q | 46 | 156c,d (5–1280) | 70 | 97 | 38 | 2 (3) | ||
| MI/15e | 6B.1 | Q | R | 55 | 221 (10–1280) | 68 | 98 | 43 | 1 (2) | ||
| MI/15c | 6B.1 | Q | Q | 50 | 192 (5–1280) | 68 | 97 | 42 | 2 (3) | ||
| IW/15e | 6B.2 | Q | R | 33 | 117c (5–1280) | 60 | 98 | 38 | 4 (7) | ||
| IW/15c | 6B.2 | Q | Q | 34 | 117c (5–1280) | 57 | 97 | 40 | 9 (15) | ||
| 2016–2017 | 33 | X-179A | NA | K | R | 77 | 244 (40–1280) | 85 | 100 | 30 | NA |
| RG-K163 | NA | K | Q | 58 | 224 (40–1280) | 73 | 100 | 36 | 0 | ||
| RG-K163Q | NA | Q | Q | 46 | 144c,d (5–1280) | 70 | 97 | 33 | 4 (12) | ||
| MI/15e | 6B.1 | Q | R | 56 | 181c (5–1280) | 76 | 97 | 39 | 4 (12) | ||
| MI/15c | 6B.1 | Q | Q | 43 | 138c,e (5–1280) | 64 | 82 | 36 | 6 (18) | ||
| IW/15e | 6B.2 | Q | R | 48 | 160c (5–1280) | 73 | 94 | 33 | 4 (13) | ||
| IW/15c | 6B.2 | Q | Q | 47 | 160c (5–1280) | 67 | 97 | 36 | 5 (15) | ||
| Total | 300 | X-179A | NA | K | R | 30 | 272 (40–1280) | 51 | 100 | 67 | NA |
| RG-K163 | NA | K | Q | 28 | 202c (5–1280) | 51 | 99 | 64 | 28 (9) | ||
| RG-K163Q | NA | Q | Q | 23 | 136c,d (5–1280) | 44 | 91 | 62 | 64 (21)d | ||
| MI/15e | 6B.1 | Q | R | 28 | 226c (5–1280) | 48 | 98 | 66 | 27 (9) | ||
| MI/15c | 6B.1 | Q | Q | 24 | 156c,e (5–1280) | 43 | 89 | 63 | 65 (22)e | ||
| IW/15e | 6B.2 | Q | R | 20 | 147c (5–1280) | 40 | 94 | 64 | 35 (12) | ||
| IW/15c | 6B.2 | Q | Q | 20 | 115c,e (5–1280) | 37 | 87 | 61 | 76 (25%)e | ||
Abbreviations: GMTs, geometric mean titers; HA, hemagglutinin; HI, hemagglutination inhibition; IW/15, A/Iowa/53/2015 field strain grown in egg (IW/15e) or Madin-Darby canine kidney cells (IW/15c); LRs, low responders; MI/15, A/Michigan/45/2015 field strain grown in egg (MI/15e) or Madin-Darby canine kidney cells (MI/15c); NA, not applicable; Post, postvaccination; Pre, prevaccination; RG-K163, A/California/8/2009-PR8 egg-grown virus possessing HA-K163; RG-K163Q, RG-CA egg-grown virus engineered to possess HA-K163Q mutation; X-179A, A/California/7/2009-PR8 egg-grown vaccine virus. Response to vaccine virus X-179A are in bold.
aPercentage of seroconversion, defined as ≥4-fold increase from prevaccination to postvaccination titers, where the postvaccination titer is ≥40.
bLow responders defined as those with ≥4-fold reduced postvaccination HI antibody titers to the corresponding virus compared with X-179A.
cP < .05 for RG-CA viruses and 2015 field strains vs X-179A vaccine strain.
dP < .05 for RG-K163Q vs RG-K163 virus.
eP < .05 for MI/15 and IW/15 cell-grown viruses vs their paired egg-grown viruses with an egg-adapted mutation (Q223R).
The HI GMTs to cell-propagated 6B.1 (GMT, 156) and 6B.2 (GMT, 115) viruses bearing 223Q were significantly lower (P < .05) than those to the corresponding egg-propagated 6B.1 (GMT, 226) and 6B.2 (GMT, 147) viruses bearing the egg-adapted Q223R change (Table 1). The only difference between the egg- and corresponding cell-propagated 6B.1 and 6B.2 viruses is the Q223R change (Supplementary Table 2). These results suggest that although IIV induced robust HI antibodies to the vaccine, 163K and 223R epitopes in the HA of the vaccine virus contributed to reduced antibody responses to circulating viruses (163Q, 223Q) in some adults.
Defining H1N1 Immune Priming by Individual’s Antibody Profiles to Pre-2009 sH1N1 Viruses
Antibody responses to A(H1N1)pdm09 viruses can be shaped by prior exposure to sH1N1 viruses [4, 20–22]. To systemically identify the likely priming sH1N1 virus for each individual, paired serum samples were tested against 10 sH1N1 viruses representative of antigenic clusters circulated during 1977–2009 (Supplementary Figure 1) to which individuals could have been exposed since birth. Antigenic characterization using ferret antisera illustrated the antigenic drift of these pre-2009 viruses over time. Ferret antisera generated through A(H1N1)pdm09 primary infection do not inhibit any of the pre-2009 sH1N1 viruses (Table 2). In contrast, vaccination with CA/09-like virus in adults not only induced antibodies to the vaccine and circulating A(H1N1)pdm09 viruses; it also elicited recalled antibody responses to multiple pre-2009 sH1N1 viruses (Figure 1 and Supplementary Figure 2). Individuals with postvaccination HI titers ≥40 to ≥2 of the 10 sH1N1 viruses (n = 281) after vaccination were included in further analysis to define immune priming. Using each person’s antibody profile to pre-2009 historic sH1N1 viruses and BY, along with the epidemic periods of the sH1N1 viruses (Supplementary Figure 1B), and also taking into consideration the possible period of delay from birth to the first influenza priming exposure [8], we were able to construct 3 distinct age-related priming patterns to 1977–2009 sH1N1 viruses among study individuals.
| Virusb . | HI Titer with Reference Ferret Antiseraa . | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| USSR/77 . | ENG/80 . | CHI/83 . | TW/86 . | TX/91 . | NC/99 . | SI/06 . | BR/07 . | X-179A . | CA/09 . | MI/15e . | MI/15c . | IW/15e . | IW/15c . | |
| USSR/77 | 640 | 320 | 160 | < | < | < | < | < | < | < | < | < | < | < |
| ENG/80 | 320 | 640 | 160 | < | < | < | < | < | < | < | < | < | < | < |
| CHI/83 | 40 | 160 | 80 | < | < | < | < | < | < | < | < | < | < | < |
| TW/86 | < | 10 | 40 | 320 | 320 | 20 | < | < | < | < | < | < | < | < |
| TX/91 | < | < | 10 | 320 | 160 | 40 | 20 | < | < | < | < | < | < | < |
| BAY/95 | < | < | < | 320 | 320 | 40 | 20 | < | < | < | < | < | < | < |
| NC/99 | < | < | < | < | < | 1280 | 80 | 160 | < | < | < | < | < | < |
| SI/06 | < | < | < | < | < | 160 | 640 | 320 | < | < | < | < | < | < |
| BR/07 | < | < | < | < | < | 80 | 320 | 640 | < | < | < | < | < | < |
| X-179A | < | < | < | < | < | < | < | < | 2560 | 2560 | 1280 | 2560 | 2560 | 2560 |
| CA/09 | < | < | < | < | < | < | < | < | 1280 | 1280 | 640 | 1280 | 640 | 640 |
| MI/15e | < | < | < | < | < | < | < | < | 2560 | 2560 | 1280 | 2560 | 2560 | 2560 |
| MI/15c | < | < | < | < | < | < | < | < | 2560 | 2560 | 1280 | 2560 | 2560 | 2560 |
| IW/15e | < | < | < | < | < | < | < | < | 1280 | 1280 | 1280 | 2560 | 1280 | 1280 |
| IW/15c | < | < | < | < | < | < | < | < | 1280 | 1280 | 1280 | 2560 | 1280 | 1280 |
| RG-K163 | < | < | < | < | < | < | < | < | 5120 | 2560 | 2560 | 2560 | 2560 | 2560 |
| RG-K163Q | < | < | < | < | < | < | < | < | 2560 | 2560 | 1280 | 2560 | 2560 | 2560 |
| RG-D127T | < | < | < | < | < | < | < | < | 1280 | 640 | 640 | 1280 | 640 | 640 |
| Virusb . | HI Titer with Reference Ferret Antiseraa . | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| USSR/77 . | ENG/80 . | CHI/83 . | TW/86 . | TX/91 . | NC/99 . | SI/06 . | BR/07 . | X-179A . | CA/09 . | MI/15e . | MI/15c . | IW/15e . | IW/15c . | |
| USSR/77 | 640 | 320 | 160 | < | < | < | < | < | < | < | < | < | < | < |
| ENG/80 | 320 | 640 | 160 | < | < | < | < | < | < | < | < | < | < | < |
| CHI/83 | 40 | 160 | 80 | < | < | < | < | < | < | < | < | < | < | < |
| TW/86 | < | 10 | 40 | 320 | 320 | 20 | < | < | < | < | < | < | < | < |
| TX/91 | < | < | 10 | 320 | 160 | 40 | 20 | < | < | < | < | < | < | < |
| BAY/95 | < | < | < | 320 | 320 | 40 | 20 | < | < | < | < | < | < | < |
| NC/99 | < | < | < | < | < | 1280 | 80 | 160 | < | < | < | < | < | < |
| SI/06 | < | < | < | < | < | 160 | 640 | 320 | < | < | < | < | < | < |
| BR/07 | < | < | < | < | < | 80 | 320 | 640 | < | < | < | < | < | < |
| X-179A | < | < | < | < | < | < | < | < | 2560 | 2560 | 1280 | 2560 | 2560 | 2560 |
| CA/09 | < | < | < | < | < | < | < | < | 1280 | 1280 | 640 | 1280 | 640 | 640 |
| MI/15e | < | < | < | < | < | < | < | < | 2560 | 2560 | 1280 | 2560 | 2560 | 2560 |
| MI/15c | < | < | < | < | < | < | < | < | 2560 | 2560 | 1280 | 2560 | 2560 | 2560 |
| IW/15e | < | < | < | < | < | < | < | < | 1280 | 1280 | 1280 | 2560 | 1280 | 1280 |
| IW/15c | < | < | < | < | < | < | < | < | 1280 | 1280 | 1280 | 2560 | 1280 | 1280 |
| RG-K163 | < | < | < | < | < | < | < | < | 5120 | 2560 | 2560 | 2560 | 2560 | 2560 |
| RG-K163Q | < | < | < | < | < | < | < | < | 2560 | 2560 | 1280 | 2560 | 2560 | 2560 |
| RG-D127T | < | < | < | < | < | < | < | < | 1280 | 640 | 640 | 1280 | 640 | 640 |
Abbreviations: BAY/95, A/Bayern/7/1995; BR/07, A/Brisbane/59/2007; CA/09, A/California/07/2009; CHI/83, A/Chile/1/1983; ENG/80, A/England/333/1980; HI, hemagglutination inhibition; IW/15c and IW/15e, cell- and egg-propagated A/Iowa/53/2015, respectively; MI/15c and MI/15e, cell- and egg-propagated A/Michigan/45/2015, respectively; NC/99, A/New Caledonia/20/1999; RG-D127T, A/California/7/2009-PR8-D127T; RG-K163K, A/California/7/2009-PR8; RG-K163Q, A/California/7/2009-PR8-K163Q; SI/06, A/Solomon Islands/3/2006; TW/86, A/Taiwan/1/1986; TX/91, A/Texas/36/1991; USSR/77, A/USSR/90/1977; X-179A, A/California/07/2009-PR8.
aAntisera were serum samples from ferrets infected with a single dose of A(H1N1) virus. Less-than symbols (<) denote HI titers <10. Titers in bold represent reactions to the homologous virus.
bDashed line separates H1N1pdm09 viruses from prior-2009 sH1N1 viruses. e denotes viruses propagated in eggs; c denotes viruses propagated in cells. Viruses were egg-propagated unless otherwise specified.
| Virusb . | HI Titer with Reference Ferret Antiseraa . | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| USSR/77 . | ENG/80 . | CHI/83 . | TW/86 . | TX/91 . | NC/99 . | SI/06 . | BR/07 . | X-179A . | CA/09 . | MI/15e . | MI/15c . | IW/15e . | IW/15c . | |
| USSR/77 | 640 | 320 | 160 | < | < | < | < | < | < | < | < | < | < | < |
| ENG/80 | 320 | 640 | 160 | < | < | < | < | < | < | < | < | < | < | < |
| CHI/83 | 40 | 160 | 80 | < | < | < | < | < | < | < | < | < | < | < |
| TW/86 | < | 10 | 40 | 320 | 320 | 20 | < | < | < | < | < | < | < | < |
| TX/91 | < | < | 10 | 320 | 160 | 40 | 20 | < | < | < | < | < | < | < |
| BAY/95 | < | < | < | 320 | 320 | 40 | 20 | < | < | < | < | < | < | < |
| NC/99 | < | < | < | < | < | 1280 | 80 | 160 | < | < | < | < | < | < |
| SI/06 | < | < | < | < | < | 160 | 640 | 320 | < | < | < | < | < | < |
| BR/07 | < | < | < | < | < | 80 | 320 | 640 | < | < | < | < | < | < |
| X-179A | < | < | < | < | < | < | < | < | 2560 | 2560 | 1280 | 2560 | 2560 | 2560 |
| CA/09 | < | < | < | < | < | < | < | < | 1280 | 1280 | 640 | 1280 | 640 | 640 |
| MI/15e | < | < | < | < | < | < | < | < | 2560 | 2560 | 1280 | 2560 | 2560 | 2560 |
| MI/15c | < | < | < | < | < | < | < | < | 2560 | 2560 | 1280 | 2560 | 2560 | 2560 |
| IW/15e | < | < | < | < | < | < | < | < | 1280 | 1280 | 1280 | 2560 | 1280 | 1280 |
| IW/15c | < | < | < | < | < | < | < | < | 1280 | 1280 | 1280 | 2560 | 1280 | 1280 |
| RG-K163 | < | < | < | < | < | < | < | < | 5120 | 2560 | 2560 | 2560 | 2560 | 2560 |
| RG-K163Q | < | < | < | < | < | < | < | < | 2560 | 2560 | 1280 | 2560 | 2560 | 2560 |
| RG-D127T | < | < | < | < | < | < | < | < | 1280 | 640 | 640 | 1280 | 640 | 640 |
| Virusb . | HI Titer with Reference Ferret Antiseraa . | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| USSR/77 . | ENG/80 . | CHI/83 . | TW/86 . | TX/91 . | NC/99 . | SI/06 . | BR/07 . | X-179A . | CA/09 . | MI/15e . | MI/15c . | IW/15e . | IW/15c . | |
| USSR/77 | 640 | 320 | 160 | < | < | < | < | < | < | < | < | < | < | < |
| ENG/80 | 320 | 640 | 160 | < | < | < | < | < | < | < | < | < | < | < |
| CHI/83 | 40 | 160 | 80 | < | < | < | < | < | < | < | < | < | < | < |
| TW/86 | < | 10 | 40 | 320 | 320 | 20 | < | < | < | < | < | < | < | < |
| TX/91 | < | < | 10 | 320 | 160 | 40 | 20 | < | < | < | < | < | < | < |
| BAY/95 | < | < | < | 320 | 320 | 40 | 20 | < | < | < | < | < | < | < |
| NC/99 | < | < | < | < | < | 1280 | 80 | 160 | < | < | < | < | < | < |
| SI/06 | < | < | < | < | < | 160 | 640 | 320 | < | < | < | < | < | < |
| BR/07 | < | < | < | < | < | 80 | 320 | 640 | < | < | < | < | < | < |
| X-179A | < | < | < | < | < | < | < | < | 2560 | 2560 | 1280 | 2560 | 2560 | 2560 |
| CA/09 | < | < | < | < | < | < | < | < | 1280 | 1280 | 640 | 1280 | 640 | 640 |
| MI/15e | < | < | < | < | < | < | < | < | 2560 | 2560 | 1280 | 2560 | 2560 | 2560 |
| MI/15c | < | < | < | < | < | < | < | < | 2560 | 2560 | 1280 | 2560 | 2560 | 2560 |
| IW/15e | < | < | < | < | < | < | < | < | 1280 | 1280 | 1280 | 2560 | 1280 | 1280 |
| IW/15c | < | < | < | < | < | < | < | < | 1280 | 1280 | 1280 | 2560 | 1280 | 1280 |
| RG-K163 | < | < | < | < | < | < | < | < | 5120 | 2560 | 2560 | 2560 | 2560 | 2560 |
| RG-K163Q | < | < | < | < | < | < | < | < | 2560 | 2560 | 1280 | 2560 | 2560 | 2560 |
| RG-D127T | < | < | < | < | < | < | < | < | 1280 | 640 | 640 | 1280 | 640 | 640 |
Abbreviations: BAY/95, A/Bayern/7/1995; BR/07, A/Brisbane/59/2007; CA/09, A/California/07/2009; CHI/83, A/Chile/1/1983; ENG/80, A/England/333/1980; HI, hemagglutination inhibition; IW/15c and IW/15e, cell- and egg-propagated A/Iowa/53/2015, respectively; MI/15c and MI/15e, cell- and egg-propagated A/Michigan/45/2015, respectively; NC/99, A/New Caledonia/20/1999; RG-D127T, A/California/7/2009-PR8-D127T; RG-K163K, A/California/7/2009-PR8; RG-K163Q, A/California/7/2009-PR8-K163Q; SI/06, A/Solomon Islands/3/2006; TW/86, A/Taiwan/1/1986; TX/91, A/Texas/36/1991; USSR/77, A/USSR/90/1977; X-179A, A/California/07/2009-PR8.
aAntisera were serum samples from ferrets infected with a single dose of A(H1N1) virus. Less-than symbols (<) denote HI titers <10. Titers in bold represent reactions to the homologous virus.
bDashed line separates H1N1pdm09 viruses from prior-2009 sH1N1 viruses. e denotes viruses propagated in eggs; c denotes viruses propagated in cells. Viruses were egg-propagated unless otherwise specified.
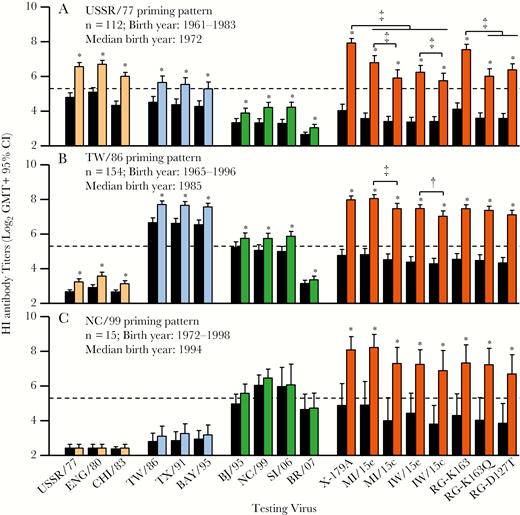
Hemagglutination inhibition (HI) antibody response patterns in prevaccination and postvaccination human serum samples. Pre- and postvaccination paired human serum samples from US adults (n = 281; born in 1961–1998; aged 18–49 years) who received inactivated influenza vaccine (IIV) were collected during 6 influenza seasons after the 2009 A(H1N1) pandemic and tested using HI assays. These individuals show 3 age-related HI antibody patterns, the USSR/77 (A), TW/86 (B), and NC/99 (C) priming patterns. Black bars represent prevaccination geometric mean titers (GMTs); colored bars, postvaccination GMTs for viruses from the USSR/77 group (light orange), the TW/86 group (blue), the group with HA 130-deletion (BJ/95, NC/99, SI/06, BR/07, green), and A(H1N1)pdm09 viruses (orange). Dashed lines denote an HI titer of 40. Pre- and postvaccination HI titers were compared for each of the testing viruses, with asterisks denoting significant increases (P < .01). Postvaccination HI titers were also compared between the A(H1N1)pdm09 viruses (†P < .05; ‡P < .01). CI, confidence interval.
A total of 112 individuals from the oldest cohort (BY, 1961–1983; median BY [MBY], 1972) elicited strong HI antibodies (titer ≥40) to USSR/77, ENG/80, or CHI/83 viruses after CA/09 vaccination. GMTs to these viruses were higher than to 1986–2009 sH1N1 viruses (Figure 1A). There was also a significant rise in GMTs to all the prior-2009 sH1N1 viruses with IIV vaccination, with the higher rate of seroconversion (52 of 112; 46%) being to USSR/77, ENG/80, and CHI/83 viruses; a lower proportion (≤27%) of individuals seroconverted to the 1986–2009 sH1N1 viruses. Collectively, based on these results with human serum samples (Figure 1A), antigenic characteristics from ferret antisera (Table 2) and epidemic periods of these viruses (1977–1986; Supplementary Figure 1), USSR/77, ENG/80, and CHI/83 viruses were designated as the USSR/77-like virus group. These 112 individuals were more than likely first infected with USSR/77-like viruses and thus designated as the USSR/77-primed cohort (Figure 2B).
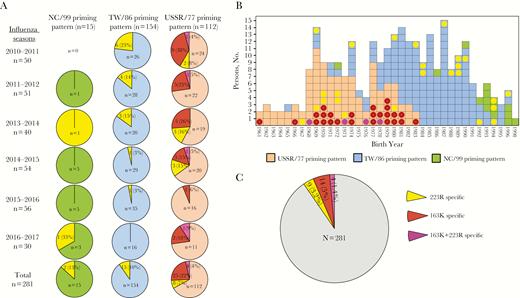
Distribution of individuals with reduced hemagglutination inhibition (HI) antibody response to R223Q or K163Q viruses after inactivated influenza vaccine vaccination. A, Proportions of the individuals carrying 223R-, 163K-, or 163K+223R-specific HI antibody by priming patterns from each of the 6 seasons. Pie graphs are color coded for NC/99 (green), TW/86 (blue), and USSR/77 (light orange) priming patterns. The 223R-specific HI antibodies (yellow) are defined those with as ≥4-fold reduced titers to 6B.1 and 6B.2 cell-grown A(H1N1)pdm09 viruses versus egg-grown pairs, and 163K-specific HI antibodies (dark red) as ≥4-fold reduced titers to RG-K163Q versus RG-163K virus; 163K+223R-specific HI antibodies (pink) contain both of the 2 antibody populations. B, Distribution of the study individuals by birth year. Each square represents an individual with the priming pattern color coded as in A. Colored dots represent individuals showing HI antibody specific to hemagglutinin (HA) 223R, 163K, or 223R+163K epitope (color coding as in A). C, Proportions of those individuals with HI titers <40 to 6B.1 cell-propagated virus by the specificity to HA 223R, 163K, or 223R+163K epitopes.
In contrast, 154 individuals (BY, 1965–1996; MBY, 1985) showed uniformly similar HI titers to TW/86, TX/91, and BAY/95 viruses (GMT, 190–210) after vaccination. Postvaccination titers to these 3 viruses were significantly higher (≥4-fold) than titers to the other 7 sH1N1 viruses (P < .01) (Figure 1B and Supplementary Figure 1). The majority of this cohort showed undetectable HI titers to the 3 USSR/77-like viruses. Based on the responses with human serum samples (Figure 1B), ferret antisera (Table 2), and epidemic periods of these viruses (1986–2001; Supplementary Figure 1), TW/86, TX/91, and BAY/95 viruses were designated as TW/86-like virus group. These 154 individuals were probably first exposed to TW/86-like viruses and thus designated as the TW/86-primed cohort (Figure 2B).
Finally, after IIV vaccination, 15 individuals (BY, 1972–1998; MBY, 1994; 13 were born in 1990–1998) mounted the highest responses (HI titers, ≥40) to NC/99 virus (GMT, 88) or similar titers (≤2 fold differences) to SI/06, BJ/95, and BR/07 viruses which all contain a deletion at position 130 on the HA (Supplemental Figure 1), significantly higher (≥4 fold) than titers to TW/86-group viruses (P < .01). None showed detectable HI titers to USSR/77-like viruses. This cohort was probably first exposed to NC/99-like viruses after birth and thus designated as the NC/99-primed cohort (Figure 2B). Interestingly, significantly reduced GMTs to 6B.1 and 6B.2 viruses compared with those against X-179A, and significantly reduced GMTs to RG-K163Q and RG-D127T viruses compared with those against RG-K163, were noted only among the USSR-primed cohort (Figure 1).
Reduced Vaccine Responses to Circulating H1N1 Viruses Associated With K163Q and Q223R Substitutions in HA as Detected by Human Post Vaccination Sera but not by Ferret Sera
Comparing HI titers to X-179A and titers to egg- and cell-propagated circulating 6B.1 and 6B.2 viruses after vaccination, we identified 3 groups of low responders: (1) individuals who mounted 163K-specific responses (defined as a ≥4-fold reduction in HI titers to RG-K163Q vs RG-163K virus); (2) those who had 223R-specific antibodies targeting the egg-adapted Q223R change (defined as ≥4-fold reduction in titers to cell-propagated 6B.1 and 6B.2 viruses compared with the corresponding egg-propagated viruses); and (3) those with both 163K+223R-specific antibodies (demonstrating both 1 and 2). Proportions of the low responders among the 3 priming cohorts (total N = 281) were analyzed for all 6 seasons (Figure 2A). A total of 54 low responders were identified, of whom 25 showed 223R-specific, 25 showed 163K-specific, and 4 showed 163K+223R-specific HI antibodies. Figure 2B illustrates the age and priming pattern distribution of the low responders among all 281 individuals analyzed. 223R-specific HI antibodies were detected in 7%, 10%, and 13% of the USSR/77-, TW/86- and NC/99-primed cohorts, respectively. In contrast, 163K-specific HI antibodies were detected only in the USSR/77-primed cohort (22% targeting 163K-specific epitope only and 4% recognizing epitopes involving both 163K+223R). Among the 54 low responders, 27 had HI titers to MI/15c virus (representing circulating viruses) below the protective threshold of 40, accounting for approximately 10% of the total cohorts (Figure 2C).
Significantly reduced HI titers to viruses with HA 163Q (n = 29) or 223Q (n = 25) epitopes after vaccination were also confirmed by reactivity of neutralizing antibodies (Figure 3). It is noteworthy that ferret antisera to A(H1N1)pdm09 viruses failed to detect antigenic changes caused by either K163Q or Q223R substitution (Table 2).
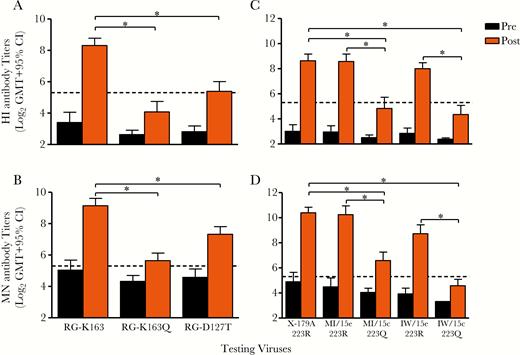
Reduced postvaccination serum antibody response to R223Q or K163Q viruses were confirmed by both hemagglutination inhibition (HI) and microneutralization (MN) assays. A, B, The 29 USSR/77-primed individuals show significantly reduced HI titers to both RG-K163Q and RG-D127T viruses compared with RG-163K virus by HI (A) and MN (B). C, D, The 25 individuals showing significantly reduced HI titers to 223Q viruses (C) were also confirmed by MN assay (D). Black bars represent prevaccination (Pre) geometric mean titers (GMTs); orange bars, postvaccination (Post) GMTs. Dashed lines denotes titers of 40. *P < .05. CI, confidence interval.
We then tested 163K-specific postvaccination human serum pools from 3 seasons with additional A(H1N1)pdm09 viruses circulated since 2009 from genetic groups 5, 6A, 6C, and 7 (163K) and additional 6B.1 and 6B.2 viruses (163Q). Human serum sample pools containing 163K-specific antibodies all had reduced antibody responses to 6B.1 and 6B.2 viruses (≥8 fold reduction compared with responses to egg- and cell-propagated CA/09), but not to the other A(H1N1)pdm09 genetic groups. These antigenic differences were not recognized by ferret antiserum to CA/09 (Table 3).
HI Antibody Reactions to Influenza A(H1N1)pdm Viruses Using Ferret Antiserum and Postvaccination Human Serum Samples
| Strain Designationa . | HA Groupb . | Amino Acid at 163 . | Ferret Antiserum to Egg-Propagated CA/09 . | Postvaccination Human Serum Samplesc . | |||
|---|---|---|---|---|---|---|---|
| Pool 1 (2014–2015) . | Pool 2 (2013–2014) . | Pool 3 (2011–2012) . | Pool 4 (2015–2016) . | ||||
| A/California/7/2009e | H1pdm09 | K | 2560d | 320 | 640 | 640 | 640 |
| A/California/7/2009c | H1pdm09 | K | 2560 | 320 | 640 | 320 | 640 |
| A/England/195/2009c | H1pdm09 | K | 2560 | 320 | 640 | 640 | 640 |
| A/Florida/27/2011e | 5 | K | 2560 | 320 | 640 | 320 | 640 |
| A/Maryland/13/2012c | 6A | K | 5120 | 160 | 640 | 320 | 640 |
| A/Dominican Republic/7293/2013c | 6C | K | 2560 | 320 | 1280 | 320 | 640 |
| A/Washington/24/2012e | 7 | K | 1280 | 160 | 640 | 320 | 640 |
| A/Washington/24/2012c | 7 | K | 2560 | 160 | 640 | 320 | 640 |
| A/Massachusetts/15/2013c | 6B | Q | 1280 | 20 | 20 | 20 | 320 |
| A/Bolivia/559/2013c | 6B | Q | 5120 | 40 | 10 | 10 | 640 |
| A/Florida/63/2014c | 6B | Q | 1280 | 20 | 10 | 10 | 640 |
| A/Michigan/45/2015c | 6B.1 | Q | 2560 | 40 | 10 | 10 | 1280 |
| A/Panama/318595/2016c | 6B.1 | Q | 1280 | 40 | 10 | 10 | 640 |
| A/Iowa/53/2015c | 6B.2 | Q | 1280 | 20 | 10 | 10 | 160 |
| Strain Designationa . | HA Groupb . | Amino Acid at 163 . | Ferret Antiserum to Egg-Propagated CA/09 . | Postvaccination Human Serum Samplesc . | |||
|---|---|---|---|---|---|---|---|
| Pool 1 (2014–2015) . | Pool 2 (2013–2014) . | Pool 3 (2011–2012) . | Pool 4 (2015–2016) . | ||||
| A/California/7/2009e | H1pdm09 | K | 2560d | 320 | 640 | 640 | 640 |
| A/California/7/2009c | H1pdm09 | K | 2560 | 320 | 640 | 320 | 640 |
| A/England/195/2009c | H1pdm09 | K | 2560 | 320 | 640 | 640 | 640 |
| A/Florida/27/2011e | 5 | K | 2560 | 320 | 640 | 320 | 640 |
| A/Maryland/13/2012c | 6A | K | 5120 | 160 | 640 | 320 | 640 |
| A/Dominican Republic/7293/2013c | 6C | K | 2560 | 320 | 1280 | 320 | 640 |
| A/Washington/24/2012e | 7 | K | 1280 | 160 | 640 | 320 | 640 |
| A/Washington/24/2012c | 7 | K | 2560 | 160 | 640 | 320 | 640 |
| A/Massachusetts/15/2013c | 6B | Q | 1280 | 20 | 20 | 20 | 320 |
| A/Bolivia/559/2013c | 6B | Q | 5120 | 40 | 10 | 10 | 640 |
| A/Florida/63/2014c | 6B | Q | 1280 | 20 | 10 | 10 | 640 |
| A/Michigan/45/2015c | 6B.1 | Q | 2560 | 40 | 10 | 10 | 1280 |
| A/Panama/318595/2016c | 6B.1 | Q | 1280 | 40 | 10 | 10 | 640 |
| A/Iowa/53/2015c | 6B.2 | Q | 1280 | 20 | 10 | 10 | 160 |
Abbreviation: HA, hemagglutinin.
bDashed line separates HA groups 6B, 6B.1, and 6B.2 viruses with K163Q change from the other groups. e denotes virus propagated in eggs; c denotes virus propagated in MDCK cells.
cPostvaccination human serum sample pools were prepared using serum samples from middle-aged adults vaccinated in the corresponding seasons, in order to have sufficient volume to test with a boarder set of viruses: pools 1 (n = 2), 2 (n = 2), and 3 (n = 3) were from those with ≥8 fold reduced hemagglutination inhibition (HI) antibody titers to RG-K163Q compared with titers to RG-K163 virus. Pool 4 (n = 2) was from those with similar HI antibody titers to RG-K163Q virus and RG-K163 virus.
dReaction to the homologous virus.
HI Antibody Reactions to Influenza A(H1N1)pdm Viruses Using Ferret Antiserum and Postvaccination Human Serum Samples
| Strain Designationa . | HA Groupb . | Amino Acid at 163 . | Ferret Antiserum to Egg-Propagated CA/09 . | Postvaccination Human Serum Samplesc . | |||
|---|---|---|---|---|---|---|---|
| Pool 1 (2014–2015) . | Pool 2 (2013–2014) . | Pool 3 (2011–2012) . | Pool 4 (2015–2016) . | ||||
| A/California/7/2009e | H1pdm09 | K | 2560d | 320 | 640 | 640 | 640 |
| A/California/7/2009c | H1pdm09 | K | 2560 | 320 | 640 | 320 | 640 |
| A/England/195/2009c | H1pdm09 | K | 2560 | 320 | 640 | 640 | 640 |
| A/Florida/27/2011e | 5 | K | 2560 | 320 | 640 | 320 | 640 |
| A/Maryland/13/2012c | 6A | K | 5120 | 160 | 640 | 320 | 640 |
| A/Dominican Republic/7293/2013c | 6C | K | 2560 | 320 | 1280 | 320 | 640 |
| A/Washington/24/2012e | 7 | K | 1280 | 160 | 640 | 320 | 640 |
| A/Washington/24/2012c | 7 | K | 2560 | 160 | 640 | 320 | 640 |
| A/Massachusetts/15/2013c | 6B | Q | 1280 | 20 | 20 | 20 | 320 |
| A/Bolivia/559/2013c | 6B | Q | 5120 | 40 | 10 | 10 | 640 |
| A/Florida/63/2014c | 6B | Q | 1280 | 20 | 10 | 10 | 640 |
| A/Michigan/45/2015c | 6B.1 | Q | 2560 | 40 | 10 | 10 | 1280 |
| A/Panama/318595/2016c | 6B.1 | Q | 1280 | 40 | 10 | 10 | 640 |
| A/Iowa/53/2015c | 6B.2 | Q | 1280 | 20 | 10 | 10 | 160 |
| Strain Designationa . | HA Groupb . | Amino Acid at 163 . | Ferret Antiserum to Egg-Propagated CA/09 . | Postvaccination Human Serum Samplesc . | |||
|---|---|---|---|---|---|---|---|
| Pool 1 (2014–2015) . | Pool 2 (2013–2014) . | Pool 3 (2011–2012) . | Pool 4 (2015–2016) . | ||||
| A/California/7/2009e | H1pdm09 | K | 2560d | 320 | 640 | 640 | 640 |
| A/California/7/2009c | H1pdm09 | K | 2560 | 320 | 640 | 320 | 640 |
| A/England/195/2009c | H1pdm09 | K | 2560 | 320 | 640 | 640 | 640 |
| A/Florida/27/2011e | 5 | K | 2560 | 320 | 640 | 320 | 640 |
| A/Maryland/13/2012c | 6A | K | 5120 | 160 | 640 | 320 | 640 |
| A/Dominican Republic/7293/2013c | 6C | K | 2560 | 320 | 1280 | 320 | 640 |
| A/Washington/24/2012e | 7 | K | 1280 | 160 | 640 | 320 | 640 |
| A/Washington/24/2012c | 7 | K | 2560 | 160 | 640 | 320 | 640 |
| A/Massachusetts/15/2013c | 6B | Q | 1280 | 20 | 20 | 20 | 320 |
| A/Bolivia/559/2013c | 6B | Q | 5120 | 40 | 10 | 10 | 640 |
| A/Florida/63/2014c | 6B | Q | 1280 | 20 | 10 | 10 | 640 |
| A/Michigan/45/2015c | 6B.1 | Q | 2560 | 40 | 10 | 10 | 1280 |
| A/Panama/318595/2016c | 6B.1 | Q | 1280 | 40 | 10 | 10 | 640 |
| A/Iowa/53/2015c | 6B.2 | Q | 1280 | 20 | 10 | 10 | 160 |
Abbreviation: HA, hemagglutinin.
bDashed line separates HA groups 6B, 6B.1, and 6B.2 viruses with K163Q change from the other groups. e denotes virus propagated in eggs; c denotes virus propagated in MDCK cells.
cPostvaccination human serum sample pools were prepared using serum samples from middle-aged adults vaccinated in the corresponding seasons, in order to have sufficient volume to test with a boarder set of viruses: pools 1 (n = 2), 2 (n = 2), and 3 (n = 3) were from those with ≥8 fold reduced hemagglutination inhibition (HI) antibody titers to RG-K163Q compared with titers to RG-K163 virus. Pool 4 (n = 2) was from those with similar HI antibody titers to RG-K163Q virus and RG-K163 virus.
dReaction to the homologous virus.
Low Responses to K163Q Change: Association With USSR/77 Priming and Evidence of Diverse Serum Antibody Repertoire
All 29 individuals showing 163K specificity were from the USSR/77-primed cohort (Figure 2). Ninety percent (26 of 29) seroconverted to X-179A (163K) and 81% (21/26) seroconverted to the 3 USSR/77-like viruses (163K) (Supplementary Figure 2B), indicating responses to shared dominant epitopes on these viruses. USSR/77-primed cohorts were probably imprinted with 163K-specific epitopes from first infection with a USSR/77-like virus (Supplementary Figure 2). In contrast, reduced titers and lower seroconversions were observed to both 1986–2009 sH1N1 (Supplementary Figure 2B) and RG-D127T viruses (Figure 3A and 3B). This is consistent with a previous study, which proposed that although 163K was also present in 1986–2009 sH1N1 viruses, glycosylation at position 125 could conformationally shield 163 and render 163K epitope less dominant when primed by these viruses [4]. It is noteworthy that the antibody response was very poor (HI titers, ≤20) to MI/15c (163Q) in 62% of USSR/77-primed individuals (18 of 29) (Supplementary Figure 2A), indicating that antibodies targeting 163K dominate in these persons. These data provide further immune basis for the age-specific low VE to A(H1N1) viruses observed in middle-aged adults [7, 8].
Finally, we explored the antibody repertoire in USSR/77-primed low responders using antibody adsorption techniques. Corresponding to the lower HI titers detected against 163Q viruses (Figure 4A), adsorption results for 3 representative USSR/77-primed individuals clearly illustrated that 2-way cross-reactivity was specifically observed between the USSR/77-group viruses (163K) and X-179A/RG-163K virus but not with MI/15e (163Q) and RG-K163Q viruses (Figure 4B), supporting the antigenic basis of shared 163K epitope. In addition, for 2 individuals (Figure 4A; I-2 and I-3) with undetectable or low titers to 223Q virus (MI/15c), serum adsorption with 223Q virus did not significantly reduce titers to X-179A and MI/15e (223R; Figure 4B; I-2 and I-3), confirming 223R specificity. In 1 individual (Figure 4; I-3), antibody to MI/15c was removed by adsorption with multiple viruses, suggesting the presence of cross-reactive antibodies that are not targeting 163 and 223 epitopes.
![Postvaccination serum hemagglutination inhibition (HI) antibody repertoire to A(H1N1)pdm09 viruses demonstrated by serum adsorption assay. A small subset of postvaccination sera of the 29 USSR/77-primed persons showing 163K specificity were adsorbed respectively with purified A(H1N1)pdm09 and pre-2009 sH1N1 viruses. The reduction of preadsorption HI titer to the homologous virus is evaluated in HI by testing the recovered serum after adsorption with heterologous viruses. Cross-reactive antibodies are determined as ≥4-fold reduced titer to the homologous virus after adsorption with heterologous viruses. A, Prevaccination (Pre) and postvaccination (Post) HI titers from 3 representative individuals (I-1 to I-3) to the X-179A, RG-163K, RG-K163Q, 6B.1, and prior-2009 sH1N1 viruses. BY, year of birth. B, HI titers in postadsorption serum samples to the testing virus below each panel of grouped bars for the 3 individuals respectively. Black bars (phosphate-buffered saline [PBS]; mock adsorption) stand for the preadsorption homologous titers to each of the testing viruses. The other color-coded bars represent HI titers to the testing virus after adsorption with different respective heterologous viruses as indicated in the figure. Dashed lines denote the 4-fold reduction in HI titers compared with PBS (mock). C, Proportions of HI antibody populations in postvaccination serum by the specificity to 163K, 223R, or other epitope(s) in the hemagglutinin (HA) of A(H1N1)pdm09 virus. 163K- or 223R-specific antibodies are defined as described in the legend to Figure 2. CR, HI antibodies cross-reactive with X-179A, 6B.1, and some of the prior-2009 sH1N1 viruses.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jid/218/10/10.1093_infdis_jiy376/1/m_jiy37604.jpeg?Expires=1750046106&Signature=0oOWJtQUqZ7ytn~MkW6tRfCQxCzbm6gYIUdZ4SIC2lf9XiulMSSNX0pY0hlgQCot9VN~edy04dSf14Y48dJE36OvlGO1P-Q~oUVa4DKbJkcWUb8UggOfDeDO-5l-vYZG3SMaVcri7jIDy7uqdHT64zpK9gAa7WwaEgT74bgqZo9-rrEPgRcx7r2ovJvIbP13cOWeKJYE5awuglXsE9hRTZa4Rv1jVQjq1cysV4lAqBeZzAf~Xkbq~1hqgXYJsP-LbFvIlt5DdAAs4EZfgb6TmaYqmg0HVgiy9DBlZeKRz0YPlSBEVYAcfMOZMLS-EPBJs9LrTddvslQLjEy-QdPiWw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Postvaccination serum hemagglutination inhibition (HI) antibody repertoire to A(H1N1)pdm09 viruses demonstrated by serum adsorption assay. A small subset of postvaccination sera of the 29 USSR/77-primed persons showing 163K specificity were adsorbed respectively with purified A(H1N1)pdm09 and pre-2009 sH1N1 viruses. The reduction of preadsorption HI titer to the homologous virus is evaluated in HI by testing the recovered serum after adsorption with heterologous viruses. Cross-reactive antibodies are determined as ≥4-fold reduced titer to the homologous virus after adsorption with heterologous viruses. A, Prevaccination (Pre) and postvaccination (Post) HI titers from 3 representative individuals (I-1 to I-3) to the X-179A, RG-163K, RG-K163Q, 6B.1, and prior-2009 sH1N1 viruses. BY, year of birth. B, HI titers in postadsorption serum samples to the testing virus below each panel of grouped bars for the 3 individuals respectively. Black bars (phosphate-buffered saline [PBS]; mock adsorption) stand for the preadsorption homologous titers to each of the testing viruses. The other color-coded bars represent HI titers to the testing virus after adsorption with different respective heterologous viruses as indicated in the figure. Dashed lines denote the 4-fold reduction in HI titers compared with PBS (mock). C, Proportions of HI antibody populations in postvaccination serum by the specificity to 163K, 223R, or other epitope(s) in the hemagglutinin (HA) of A(H1N1)pdm09 virus. 163K- or 223R-specific antibodies are defined as described in the legend to Figure 2. CR, HI antibodies cross-reactive with X-179A, 6B.1, and some of the prior-2009 sH1N1 viruses.
DISCUSSION
Our study aimed to identify the immune basis of the low antibody responses to circulating A(H1N1)pdm09 viruses observed in a proportion of adults after IIV vaccination containing a CA/09-like A(H1N1)pdm09 component, and to further explore how immune priming shapes antibody responses to vaccination. Although IIV induced robust HI antibody responses to the vaccine virus X-179A in our study cohorts, >20% of the individuals had reduced antibodies to cell-propagated 6B.1 and 6B.2 viruses (Table 1). Our study suggests that the low responses to circulating A(H1N1)pdm09 viruses in these adults after vaccination were primarily driven by dominant antibody responses to the 163K-specific epitope and/or to the 223R-specific egg adaptation present in the vaccine virus but absent in the contemporary circulating viruses.
Furthermore, we showed that reduced responses to 163Q on 6B.1, 6B.2 viruses were detected only among older adults who were primed with USSR/77-like viruses. This is consistent with the lower VE observed in similar age cohorts (22%–25%) during the 2015–2016 season when 6B.1 A(H1N1)pdm09 viruses (163Q) predominated [7, 8]. In contrast, ferret antisera used in influenza virological surveillance failed to identify the antigenic difference in these viruses. Indeed, our analyses using human serum samples provided evidence to support the World Health Organization recommendation to update the A(H1N1) vaccine component to a subclade 6B.1 virus (A/Michigan/45/2015-like, 163Q) for the 2017 southern hemisphere [23] and 2017–2018 northern hemisphere seasons [24]. These results underscore the value of human serology in influenza surveillance and the vaccine virus selection process.
With the analysis of antibody profiles to a large panel of A(H1N1) viruses, we were able to construct 3 age-specific priming patterns based on responses to sH1N1 viruses the individual may have been exposed to earlier in life. This is consistent with the concept termed “original antigenic sin” (OAS), which was first described in the 1950s [25–27]. In addition to stimulating antibody responses to the vaccine virus, our results demonstrated that IIV also induced antibodies to historical sH1N1 viruses, with a significant boost to the sH1N1 virus the individual was probably first infected with in childhood (Figure 1). This effect was more pronounced among the 2 older cohorts in our study (TW/86-primed and USSR/77-primed). Similar patterns were also observed with the A(H3N2) antibody landscape study [28]. It is evident from our study that OAS can exist without significantly compromising the antibody response to the contemporary vaccine virus, a point recently readdressed [29]. It is also noteworthy that our study does not include elderly persons who could have been exposed to seasonal H1N1 viruses circulated before 1957 and does not include children; both warrant further investigation.
Linderman et al [4] first described the lower response to K163Q among a small number of adults born between 1965 and 1979. Our study used a systematic analysis to characterize the sH1N1 virus priming history of individuals and further demonstrated that the reduced vaccine antibody responses to viruses bearing 163Q were detected only in a subset of individuals (29 of 112; 22%) that were primed with USSR/77-like viruses. Memory B cells targeting the HA 163K epitope may be first imprinted in these individuals through infection with USSR/77-like viruses, and then preferentially enriched on repeated exposures to viruses also bearing 163K later in life [1]. The vast majority of the individuals showing 163K specificity in our study seroconverted simultaneously to both X-179A and USSR/77-group viruses, suggesting that shared HA epitopes is fundamental for the OAS effect. This is in line with the findings by Li et al [21] that demonstrated the sH1N1 imprinting effect was due to a shared epitope near the RBS.
Nine percent of our study cohorts (25 of 281) mounted antibodies specific to 223R, an egg-adapted mutation located near the RBS of the vaccine virus, indicating that egg-adapted changes in the HA can be a target of human antibody responses to vaccination with egg-based vaccines [11–13]. In a recent study, somatic hypermutation analysis of the 223R-specific antibodies indicated a recalled response, suggesting a history of vaccination with a previous egg-adapted vaccine virus or A(H1N1) virus infection [10]. Our study identified low responders to 223Q A(H1N1)pdm09 viruses among all 3 birth cohorts. It remains unclear whether immune priming also influences responses to 223Q. The observation that the antigenic change caused by Q223R substitution was detected only by human immune serum samples and not by ferret antisera again highlights the need to use human serology to improve antigenic characterization of influenza viruses.
Our study also provided further insight into the diversity of the immunologic responses to vaccination, even in low responders. Using adsorption techniques [18, 19], we identified serum antibody repertoires that included 163K-specific, 223R-specific, and cross-reactive antibodies targeting shared HA epitopes in the USSR/77-primed individuals with low responses to contemporary cell-propagated 6B.1 viruses. Our findings not only confirmed the association of the 163K epitope with USSR/77 priming but also suggested that the influenza vaccination induces polyclonal antibody responses comprising antibodies specific for the respective epitopes. The diverse serum antibody responses detected in this study are consistent with those of other studies conducted at molecular level [1, 30].
Finally, this study provided a plausible immune basis to the birth cohort effects reported from VE observational studies [7, 8], bridging the gap between the birth cohort and immune priming. The majority of our study cohort that was born before the reemergence of sH1N1 in 1977, during the period when only H2N2 (1957–1967) or H3N2 (1968–1976) viruses circulated demonstrated a USSR/77 priming pattern. Furthermore, our detailed analysis of the antibody profiles to historic sH1N1 viruses in 281 individuals revealed that BY alone may not be sufficient to define priming (Figure 2B). Overlapping priming patterns during periods of antigenic transitions were detected, probably due in part to longer intervals between birth and the first A(H1N1) infection in some individuals. In a few instances, the priming virus defined by antibody profiles for a small number of individuals emerged >20 years after their birth (Figure 2B). Both OAS-oriented (163K) responses and egg-adapted epitopes (Q223R) on vaccine virus could contribute to suboptimal VE in a specific population.
An improved VE to A(H1N1)pdm09 virus was observed during the 2017–2018 season (67%) in the United States compared with the 2015–2016 season (47%) after the A(H1N1)pdm09 component in the vaccines was updated to a 6B.1 virus [7, 31]. This study also demonstrated that egg adaptation in A(H1N1)pdm09 vaccine virus can be a target for human antibody responses, which still remains a challenge for influenza vaccines. Continued efforts are needed to improve our understanding of the fine specificity of the immune response to influenza virus infection and vaccination, and ultimately to develop vaccines that can offer broader and longer-lasting immunity.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank the Virology Surveillance and Diagnostics Branch, Influenza Division, CDC, for providing ferret antisera and seeds of influenza viruses. We are also grateful to the Influenza Genomics Team, Influenza Division of CDC, for sequencing assistance.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the CDC.
Financial support. This work was supported by the CDC.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.




