-
PDF
- Split View
-
Views
-
Cite
Cite
Mangala Rao, Sayali Onkar, Kristina K Peachman, Yohann White, Hung V Trinh, Ousman Jobe, Yingjun Zhou, Peter Dawson, Michael A Eller, Gary R Matyas, Carl R Alving, Liposome-Encapsulated Human Immunodeficiency Virus-1 gp120 Induces Potent V1V2-Specific Antibodies in Humans, The Journal of Infectious Diseases, Volume 218, Issue 10, 15 November 2018, Pages 1541–1550, https://doi.org/10.1093/infdis/jiy348
Close - Share Icon Share
Abstract
In the RV144 trial, human immunodeficiency virus (HIV)-1 gp120 V1V2 antibodies correlated inversely with risk of HIV-1 infection; however, the titers waned quickly. We hypothesized that a more potent adjuvant might enhance the magnitude and durability of V1V2 antibodies.
We examined archived sera from a phase I randomized, double-blind placebo-controlled trial, conducted in HIV-1-uninfected individuals, vaccinated with HIV-1SF-2 rgp120 either adsorbed to aluminum hydroxide (aluminum hydroxide arm) or encapsulated in liposomes containing monophosphoryl lipid A (MPL®) and then adsorbed to aluminum hydroxide (liposomal arm).
The median immunoglobulin G antibody titers across weeks 10–112 were higher in the liposomal arm against subtypes B (2- to 16-fold), AE (4- to 8-fold), and C (4- to 16-fold) V1V2 proteins. High titers were maintained even at 10 months after last boost in the liposomal compared with the aluminum hydroxide arm. The antibodies exhibited antibody-dependent cellular cytotoxicity (ADCC) and α4β7-integrin receptor inhibition-binding functions.
Inclusion of 2 adjuvants in the vaccine formulation, aluminum hydroxide, and liposomal MPL®, induced robust, durable, and functional antibodies. Based on the magnitude of antibody responses and the percentage of coiled and β-sheet in the predicted V2/V3-peptide structure, we speculate that liposomal gp120 was presented in a conformation that favored the induction of robust antibody responses.
The RV144 phase III human immunodeficiency virus (HIV)-1 vaccine trial demonstrated modest but statistically significant vaccine efficacy (31.2% after 42 months [1], with 60% efficacy at 12 months post vaccination [2]), suggesting an early but nondurable effect. Immune correlates analysis identified antibodies against the envelope gp120V1V2 region and antibody-dependent cellular cytotoxicity (ADCC)-mediating immunoglobulin (Ig)G with low anti-gp120 IgA antibodies as being associated with lower infection risk [3–7]. Sieve analysis of breakthrough infections identified sites of immune pressure in the V2 region [5]. Potential approaches to improving the magnitude and durability of the V2 antibody response include additional boosts of the vaccine and/or combining the antigen with more potent adjuvants. To examine these approaches, available archived samples from the AVEG015 (ClinicalTrials.gov NCT00001042) phase I trial [8–10] conducted in 1992 in HIV-1-uninfected individuals were assessed.
AVEG015 compared the safety and immunogenicity of HIV-1SF-2 rgp120 combined separately with 7 adjuvants [8–10]. Based on recent advances in the development of liposomal adjuvants for vaccines [11, 12], 2 arms of AVEG015 were of special interest; gp120 adsorbed to aluminum hydroxide ([AH] arm) and AH-adsorbed liposomes containing encapsulated gp120 and monophosphoryl lipid A (L[gp120 + MPL®] + AH) (liposomal arm). Volunteers in the liposomal arm showed much greater HIV-1 neutralizing and lymphoproliferative responses [8–10]. To evaluate the influence of potent adjuvant formulations, archived sera were analyzed for more recently identified correlates of protection [3] including V1V2-specific binding antibody responses, ADCC, and α4β7-integrin receptor binding inhibition.
METHODS
Study Design
AVEG015 was a phase I randomized double-blind, placebo- controlled, clinical trial conducted in 1992 in the United States, sponsored by the National Institute of Allergy and Infectious Diseases, National Institutes of Health ([NIH] Bethesda, MD), to compare the safety and immunogenicity of CHO-expressed HIV-1SF-2 rgp120 (Biocine), individually combined with 7 adjuvants in a total of 112 HIV-1-uninfected low-risk individuals (18–60 years) [8].
Vaccines
Archived sera from the AH arm (HIV-1SF-2 rgp120 adsorbed to Alhydrogel®) and the liposomal arm (L[HIV-1SF-2 rgp120 + MPL®] adsorbed to Alhydrogel®) were used. Volunteers (15 subjects/arm) were vaccinated at weeks 0, 8, and 24 with an optional boost at week 72 with 50 μg of gp120 and 0.5mg of aluminum (AH arm) or 37.5 μg of encapsulated gp120, 2.2 mg of MPL® (RIBI Immunochem Research, Inc.), and 0.5 mg of aluminum (liposomal arm). The multilamellar liposomes consisted of dimyristoyl phosphatidylcholine, dimyristoyl phosphatidylglycerol, cholesterol, and MPL® [12–14]. Cryopreserved archived serum samples were obtained under an HIV Vaccine Trials Network (HVTN) ancillary study as a limited data set with no identifiers. The in vitro assays were carried out under the Institutional Review Board approval (Walter Reed Army Institute of Research [WRAIR] no. 1741/RV313).
Human Immunodeficiency Virus-1 Envelope-Specific Antibodies
Serial 2-fold dilutions of individual serum samples were added in triplicate to wells of Immulon-2 96-well U-bottom polystyrene plates coated with protein/peptide (1 μg/mL, 0.1 mL/well), and antigen-specific binding antibodies were assessed [7]. Horseradish peroxidase (HRP)-linked sheep antihuman IgG (1:1000) or antihuman IgA (1:10000) or HRP-mouse antihuman IgG1 (1:1000) was used as the secondary antibody. The secondary antibodies were specific and did not cross-react with other Ig classes or IgG subclasses. The arithmetic mean absorbance of the 3 replicates for each sample was calculated and used for the determination of endpoint titers, which is defined as the highest reciprocal serum dilution that yielded an absorbance twice the background values (wells without antigen) [15, 16]. The data for all the figures are plotted as median endpoint titers with the interquartile range. Each individual dot in the figure represents the arithmetic mean endpoint titer for each sample. HIVIG/monoclonal antibody CH58 and CH38 were used, respectively, as positive controls for the IgG and IgA assays. Details are in the Supplementary File.
Circular Dichroism
Eight to 10 scans of cV2 or cV3 peptides (0.1mg/mL) in 5 mM phosphate buffer, pH 6.5 at 25°C in a Hellma quartz cell (1-mm path length) were recorded between 190 and 250 nm in 0.5-nm increments on a JASCO-J-815 circular dichroism spectrometer (10 nm/minutes scan speed; 1-nm bandpass), and the arithmetic mean values were calculated and corrected for buffer blank. The percentage of secondary structure (coil, alpha-helix, and β-sheet) was calculated using CDFIT Program [17].
α4β7 Adhesion Assay
The inhibition of binding of V2 peptide to α4β7 receptors on Roswell Park Memorial Institute RPMI8866 cells (Sigma-Aldrich, St. Louis, MO) in the presence or absence of AVEG015 serum was determined as previously described [18]. Streptavidin-biotinylated-cV2 peptide-coated 96-well Immulon 2HB round-bottom plates were washed and blocked, and individual serum samples (1:50; 1:200) were added in triplicate, followed by the addition of cells. Bound cells were quantitated by fluorescence units released by the reduction of AlamarBlue dye (Fisher Scientific, Pittsburgh, PA) and read on a M2 plate reader. Binding inhibition was determined by dividing the fluorescence obtained in the presence of serum by the fluorescence obtained in the absence of serum. ACT-1, CH58, and normal human sera (NHS) served as the positive/negative controls. Mucosal vascular addressin cell adhesion molecule (MAdCAM)-1, the natural ligand of α4β7-integrin receptor, served as a positive assay control. The data expressed as arithmetic mean percentage inhibition is from 1 to 3 independent experiments with an 8% cutoff value based on the arithmetic mean percentage inhibition of preimmunization sera. Responders (percent) whose sera at 2 dilutions showed inhibition were calculated. Details are in the Supplementary File.
Rapid Fluorometric Antibody-Dependent Cellular Cytotoxicity Assay
The ADCC responses were measured using CEM.NKRCCR5+ cells (NIH AIDS Reagent Program, provided by Dr. Alexandra Trkola) pulsed with 30 μg/mL rgp120 SF162 (Immune Technology Corp., NY) and then labeled with PKH26 (Sigma-Aldrich, St. Louis, MO) and carboxyfluorescein diacetate succinimidyl ester ([CFSE] Life Technologies Corp., Carlsbad, CA) [19–22]. Serum from each arm and HIV-negative peripheral blood mononuclear cells were added to the labeled cells at an effector to target ratio of 10:1 for 4 hours. Cells were acquired on a LSRII flow cytometer (Becton Dickenson, San Jose, CA) and analyzed using Flow Jo version 10.0.8 (TreeStar, Inc., Ashland, OR). Percentage of lysis was determined using the following formula: (CFSE−PKH26+/[CFSE−PKH26+ + CFSE+PKH26+]) × 100. The mean of the highest lysis (6.6%; 5.3%) in the 2 arms at week 0 was used to determine the 6% cutoff and is represented by a dotted line. The HIVIG and NHS served as the positive/negative controls.
Statistical Analyses
The enzyme-linked immunosorbent assay endpoint titers within a group at an individual time point are expressed as median with interquartile range and as geometric mean titer (GMT). The 2 arms were compared using Mann-Whitney U test for discrete/continuous variables and Barnard’s unconditional exact tests for binary responses. Spearman’s correlation was used to test the monotonic trend between ADCC and antibody endpoint titers. The half-life of each antibody was calculated for each group assuming an exponential decay [23, 24]. The observed arithmetic mean endpoint titers from each individual were used to fit a generalized linear model, and generalized estimating equations procedure were used to account for repeated measurements of a subject over time. All tests were 2-sided, and P ≤ .05 was considered statistically significant and was performed using Graph Pad Prism software version 6.0a for Mac OS X, JMP version 10.0.0. and SAS version 9.4 (SAS Institute, Inc., Cary, NC).
RESULTS
Study Design
AVEG015 serum samples from AH and liposomal arms were used to evaluate the effect of adjuvants on the magnitude and durability of V2-specific antibody responses. Fifteen volunteers/arm were vaccinated at weeks 0, 8, and 24. Nine subjects in the liposomal and 12 subjects in the AH arm received an additional boost at week 72 (Figure 1A). Two subjects from the liposomal and 1 from the AH arm dropped out at various time points for reasons unrelated to vaccination. Sera for some individuals were either limited or nonexistent. Cryopreserved cells were also nonexistent, and mucosal samples were not collected.
![AVEG015 study design and antibody responses to gp120 proteins. (A) Vaccination schedule (hollow arrows) and bleeds (bold arrows). B–D show the immunoglobulin (Ig)G and IgA endpoint titers against gp120 proteins. Each dot depicts the arithmetic mean endpoint titer for an individual sample assayed in triplicate (red, liposomal; blue, aluminum hydroxide [AH]). Gray bars represent median endpoint titers and error bars represent interquartile range. The numbers on top represent the median titer for each time point. Significance between the 2 arms are noted above the bars (*, P ≤ .05;**, P ≤ .005; ***, P ≤ .0005; 2-tailed Mann-Whitney U test).](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jid/218/10/10.1093_infdis_jiy348/1/m_jiy34801.jpeg?Expires=1749976682&Signature=tKWRN70AxUDCrgTHqdxeh58H9LWx2pNLRSLxgkdj44aNmC7Oa63Co8ZxmZvinhKssc40hkF6m4dgdNhQyh~6dpW8IkwysOtbc9zyxjEPDvYO~x4UnyyhdsfWi-kE9WJ493-o4prsKU0NRjfU5RQGW-yoS6bJKcBK9Fh~FDWYbizCUPWu8Yk6bZLNSe1pqlaatvHYdxmUh33VKDQya-r6oAinTX1aV5U1m6xCKYUKGY0iTVL9Gxoye-B2HTJ3OjqCRRL18dGGRjGDCAnuauqfpUx2tMUSeDHKsKLSGckT~Pv~GnTAcHOcV~e2uglIn5gb6m5ZEZrIDOuHUDaiOCQUwA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
AVEG015 study design and antibody responses to gp120 proteins. (A) Vaccination schedule (hollow arrows) and bleeds (bold arrows). B–D show the immunoglobulin (Ig)G and IgA endpoint titers against gp120 proteins. Each dot depicts the arithmetic mean endpoint titer for an individual sample assayed in triplicate (red, liposomal; blue, aluminum hydroxide [AH]). Gray bars represent median endpoint titers and error bars represent interquartile range. The numbers on top represent the median titer for each time point. Significance between the 2 arms are noted above the bars (*, P ≤ .05;**, P ≤ .005; ***, P ≤ .0005; 2-tailed Mann-Whitney U test).
Antibody Responses and Durability
Antibody responses are shown in Figure 1B and C. Two weeks after the 2nd, 3rd, and 4th immunizations (weeks 10, 26, and 74), and also 10 months after the last boost (week 112), significantly higher (P ≤ .05 at all individual time points) gp120IIIB-specific IgG antibody binding titers were induced in the liposomal compared with the AH arm (Figure 1B). Antibodies were cross-reactive to subtype A/E gp120A244 (Figure 1C) with significantly higher responses in the liposomal arm (P ≤ .05 at all individual time points). The GMTs and the percentage of responders in the 2 arms are shown in Supplementary Table 1. In the liposomal arm, there was a 4-fold drop in GMTs (94810 vs 21945) between weeks 74 and 112 compared with a 6-fold drop for the same time period in the AH arm (24163 vs 4117). However, when the median values were calculated, a 4-fold drop in median gp120IIIB was observed in the liposomal arm compared with an 8-fold drop in median gp120IIIB in the AH arm. The changes in log10-transformed endpoint titer between weeks 74 and 112 were calculated at individual level and compared between the 2 groups using Mann-Whitney U test. The same score values were assigned to the tied observations. The difference seen between the 2 arms was not significant (P = .896), likely due to small sample sizes. Compared with the IgG titers, gp120IIIB-specific IgA responses were lower in both arms with median endpoint titers of 800 for the liposomal and 400 for the AH arms at week 26 (P = .022) (Figure 1D).
Antibody responses to V1V2 proteins, including CaseA2 (subtype B, the same protein used in the RV144 correlate analysis), were determined. The liposomal arm of AVEG015 showed between 2- and 16-fold higher responses to gp70V1V2 proteins (Figures 2A and B and 3, Supplementary Table 1). Two immunizations were sufficient to induce between 7- and 9-fold higher GMTs against the 3 gp70V1V2 proteins in the liposomal arm. Even after 4 immunizations, the GMTs at week 74 was between 3- and 6-fold lower in the AH arm (Supplementary Table 1). The response rate of gp70V1V2 Consensus C was significantly higher in the liposomal than the AH arm (100% vs 57%; P = .017; Supplementary Table 1) at week 10.
![Binding and durability of antibodies to proteins. A and B show the immunoglobulin (Ig)G endpoint titers against gp70V1V2 proteins (subtypes B and A/E). The data are represented as described in Figure 1 legend. Significance between the 2 arms are noted above the bars (*, P ≤ .05; **, P ≤ .005; ***, P ≤ .0005; 2-tailed Mann-Whitney U test). The durability of the antibody responses is plotted as an exponential decay curve for gp70V1V2 CaseA2 (C) and gp70V1V2 A/E (D). The respective antibody half-lives are shown. Each dot (red, liposomal; blue, aluminum hydroxide [AH]) represents the endpoint titer observed for each individual at a specific time point; line and the band show predicted decay curve with 95% confidence interval.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jid/218/10/10.1093_infdis_jiy348/1/m_jiy34802.jpeg?Expires=1749976682&Signature=M4t0pNJHnoWoINkO1DTIvjahZV0yFSF2u9iQeaQlSlRfYT-tQHZbjnnNeeRzd5W-2j5GuTyZS6GibHEYuE1Hj8NKbzjQudubJQy1cUV9DJgg7FPnlxDZdt8ATsmlAThufkLjmRsIco-cK11qYKyW6zzqsiCi6oLZNCcaIOkMLg8XAmffXMnjpBS9ImBo3x7sCGRBXifq4r~O~6r8ZjADPLiZSakmlGKXS1Mo34Ia89XVJgC8bZm6iVG0cyK-IkCQc8K2SvvgMisF6RJxSj9QDRwkqfmMoBZQ~iJBcOG-cuPsZYJuHygKC8vlLxPoz4HbXflml7gdPGy71CHWlf0Juw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Binding and durability of antibodies to proteins. A and B show the immunoglobulin (Ig)G endpoint titers against gp70V1V2 proteins (subtypes B and A/E). The data are represented as described in Figure 1 legend. Significance between the 2 arms are noted above the bars (*, P ≤ .05; **, P ≤ .005; ***, P ≤ .0005; 2-tailed Mann-Whitney U test). The durability of the antibody responses is plotted as an exponential decay curve for gp70V1V2 CaseA2 (C) and gp70V1V2 A/E (D). The respective antibody half-lives are shown. Each dot (red, liposomal; blue, aluminum hydroxide [AH]) represents the endpoint titer observed for each individual at a specific time point; line and the band show predicted decay curve with 95% confidence interval.
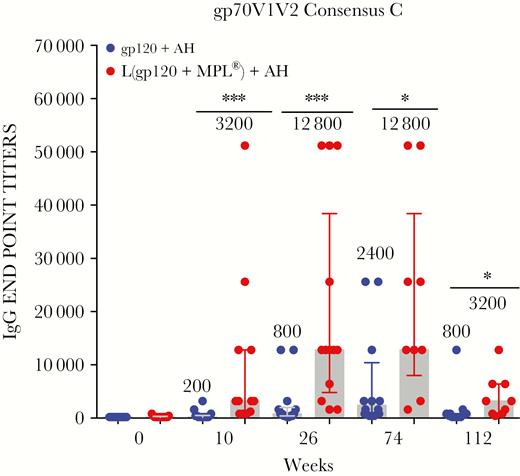
Antibodies to gp70V1V2 Consensus C protein. Each dot depicts the immunoglobulin (Ig)G arithmetic mean endpoint titer against gp70V1V2 Consensus C (subtype C) for an individual sample assayed in triplicate. The data are represented as described in Figure 1 legend (*, P ≤ .05; **, P ≤ .005; ***, P ≤ .0005; 2-tailed Mann-Whitney U test).
Durability of the V1V2 antibody response specific to gp70V1V2 Case A2 (Figure 2C) and gp70V1V2 A244 (Figure 2D) over a period of 40 weeks after last boost were examined. The estimated antibody half-life in the liposomal arm was approximately twice that of the AH arm; 20 vs 11 weeks for gp70V1V2 CaseA2 (P = .129) and 24 vs 10 weeks for gp70V1V2 A/E (P = .009). We also determined gp70V1V2 CaseA2-specific IgG1 responses, because several effector functions are associated with IgG1. The IgG1 titers were significant in the liposomal arm at weeks 10 (P < .0001) and 74 (P = .02) (Figure 4).
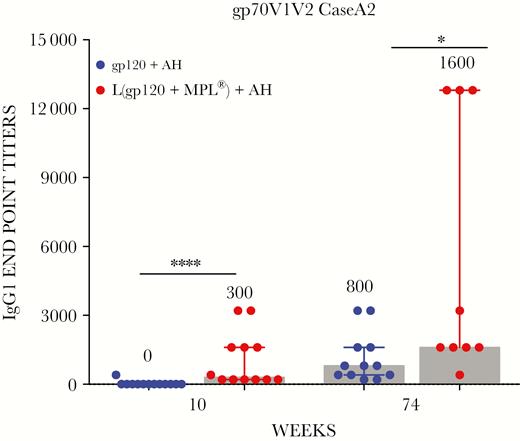
Immunoglobulin (Ig)G1 antibodies specific to gp70V1V2 CASEA2 protein. Individual serum at weeks 10 and 74 in triplicate were analyzed for IgG1 antibodies specific to gp70V1V2 CaseA2. The median endpoint titers are plotted as described above (*, P = .02; ****, P < .0001; 2-tailed Mann-Whitney U test).
Antibodies to cV2 and cV3 Peptides
The liposomal adjuvant also induced significantly higher cV2 and cV3-specific IgG antibodies (P ≤ .05) (Supplementary Figures 1 and 2). Antibodies to acute subtype C were the highest in magnitude and significantly higher at all time points (Supplementary Figure 1). Antibodies to cV2 MN peptide were also 4-fold higher in the liposomal arm (Supplementary Figure 1), but they were weak in the AH arm and could not be boosted (Supplementary Table 1). The magnitude of antibody responses to cV3 MN (median titers 76800 vs 1200; GM titers 45614 vs 1189) and acute cV3 C peptides (median titers 25600 vs 400; GM titers 17540 vs 800) were significantly higher (P ≤ .0005) in the liposomal arm after 2 immunizations (Supplementary Figure 2, and Table 1), whereas antibody responses to cV3 A/E peptide were poor in both arms. The IgG1 antibodies were also induced against cV2 and cV3 peptides. The median IgG1 titers in the liposomal arm were statistically significant (P ≤ .005) compared with the median IgG1 titers in the AH arm at week 10 (Supplementary Figure 3).
Peptides used for analyzing the immune responses and differences in the amino acid sequence compared with SF2 are shown in Supplementary Figure 4. Major differences in amino acids between V2 regions of SF2 and acute C were at positions 169 (site of immune pressure in RV144) (I to R), 177 (R to N), 183 (P to Q), 187 (A to N), and 190 (N to L) in the C strand (Supplementary Figure 4), regions that were probably better exposed or presented in the liposomal formulation.
Circular Dichroism of cV2 and cV3 Peptides
Circular dichroism analyses of cV2 and cV3 peptides were performed to determine whether secondary structural conformations might have influenced antibody responses (Figure 5A and B). Subtype C cV2 peptide, which showed the highest magnitude of antibodies (Supplementary Table 1 and Figure 1), exhibited 67% random coil structure, 32% alpha-helix, and less than 1% β-sheet. In contrast, cV2 MN peptide had the highest proportion of β-sheet (56%) and showed the weakest antibody response (Supplementary Table 1 and Figure 1). Similarly, cV3 (A/E, acute C, and MN) peptides exhibited, respectively, 32%, 23.6%, and 17% β-sheet (Figure 5B), and the antibody titers were lowest against AE, followed by acute C, and highest against MN V3 peptides (Supplementary Table 1 and Figure 2).
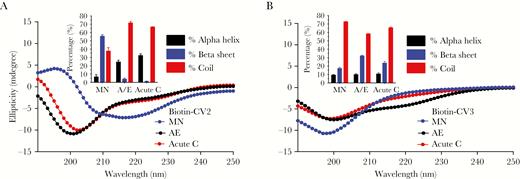
Circular dichrosim. (A and B) depict the arithmetic mean ellipticity of 8–10 scans for each of the cV2 (A) and cV3 (B) peptides tested. The inset in each panel depicts the percentage of secondary structure (coil, alpha-helix, and beta-sheet) that was calculated using the CDFIT Program.
Rapid Fluorometric Antibody-Dependent Cellular Cytotoxicity Activity
We determined whether the antibodies exhibited ADCC functional activity. The gating strategy and assay controls are shown in Supplementary Figure 5. Week 26 sera were analyzed for ADCC activity (Figure 6A). Sera (4/arm) that exhibited ADCC activity above the background were then examined at additional time points including week 26 in the same experiment, to reduce interassay variability (Figure 6B). Varying degrees of ADCC activity were observed in both groups. Peak lysis observed at week 26 declined at week 74 in the liposomal arm, but it remained stable or continued to rise in the AH arm (Figure 6B). The ADCC median response was slightly higher in the AH arm than the liposomal arm, but the difference was not statistically significant (Mann-Whitney U test, P = .42) at week 26, but it was significant at week 74 (P = .03). A positive correlation was observed between gp120-specific antibody binding titers and ADCC activity in both arms (ρ = .712, P = .008; ρ = .640, P = .021) (Figure 6C and D). An increasing monotonic trend between ADCC and IgG endpoint titers was observed in the liposomal arm against gp70V1V2 CaseA2 and gp70V1V2 A/E (Supplementary Figure 6), but not against cV2 peptides (Supplementary Figure 7).
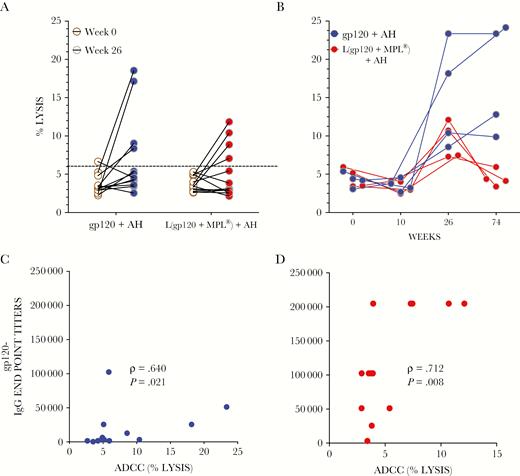
Antibody-dependent cellular cytotoxicity (ADCC) reactivity. Individual serum samples (1:500 dilution) in duplicate from liposomal (red) and aluminum hydroxide (AH) (blue) arms at weeks 0 and 26 (A) were analyzed for ADCC activity using SF162-gp120-pulsed target cells. Four samples per arm were then examined at additional time points (B). Percentage of lysis was determined as described in Methods. Dotted line represents 6% cutoff (see Methods). The differences in the ADCC median response between the 2 arms was not statistically significant (Mann-Whitney U test, P = .42) at week 26, but it was significant at week 74 (P = .03). C and D show the Spearman correlation between gp120IIIB immunoglobulin (Ig)G endpoint titers and ADCC activity at week 26 (red, liposomal: blue, AH). Data are from at least 2 experiments.
α4β7 Adhesion Activity
The gut mucosal homing receptor α4β7 is thought to play a role in HIV-1 infection [25, 26] and in simian immunodeficiency virus protective immune responses [27]. Individual serum samples at weeks 0, 26, and 74 were analyzed for their ability to inhibit the binding of cV2 peptides to α4β7 receptors on RPMI8866 cells using the assay we developed [18]. The magnitude of inhibition (0%–67%) was similar to that observed with purified IgG in the RV144 study (0% to 50%) [18]. Significant inhibition of α4β7 binding to cV2 AE peptide was obtained in the liposomal arm compared with the AH arm at week 26 (Figure 7A, middle panel; P = .019), with a serum dilution of 1:200 but not with 1:50 (Supplementary Figure 8). Positive and negative controls are shown in Figure 7B. Although a higher response rate of α4β7 inhibition was observed in the liposomal arm at weeks 26 and 74, it was not significant (Supplementary Table 2).
![α4β7 inhibition. Individual serum (1:200 dilution; red, liposomal; blue, aluminum hydroxide [AH]) at weeks 0, 26, and 74 (A) were analyzed for inhibition of binding of cV2 peptides to α4β7-integrin receptor as described in Methods. Dotted line shows the cutoff inhibition (8%). Significantly higher inhibition (P = .019; 2-tailed test Mann-Whitney U test) against cV2 AE was seen at week 26 in the liposomal arm. CH58 (10 μg/mL), normal human sera ([NHS] 1:50), and ACT-1 (0.6 μg/well) were used as positive/negative controls (B). In addition, the natural ligand of α4β7-integrin receptor, mucosal vascular addressin cell adhesion molecule (MAdCAM)-1, was also used as a positive assay control. Median with interquartile range is shown from at least 3 experiments with each sample tested in triplicate at each dilution.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jid/218/10/10.1093_infdis_jiy348/1/m_jiy34807.jpeg?Expires=1749976682&Signature=SwEeK6YcGGzMen56YAXMgfMuyrOOtB-JJSjr8qRnwiDX~Td3BRKQQVrmfoEPakt53rN3NvJhGdEVBWEV40P~su7FonyKC8mTUa69nEgL3V~GXnXlBi8IOnQHzyrIUwFSBswziTv3bEgPeezE2HXn-XJ3AlCxCvvQF4KRO6j96U7eMZOFefPL5yLAlqs~I92~Qgd9UcNZ~yMFca1JETfUMaJSqa8eGQv87Om11pDK9iy7m23CJVfspcLgN0Khz2qIFcjZBlJ2E796B9-0Bp30PLh6gXUAGL8C2NSusYcUgvL4Hy~j0zhQzIW~ySgWdEyW96tUB6lfnBfQBxZuwnp7JA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
α4β7 inhibition. Individual serum (1:200 dilution; red, liposomal; blue, aluminum hydroxide [AH]) at weeks 0, 26, and 74 (A) were analyzed for inhibition of binding of cV2 peptides to α4β7-integrin receptor as described in Methods. Dotted line shows the cutoff inhibition (8%). Significantly higher inhibition (P = .019; 2-tailed test Mann-Whitney U test) against cV2 AE was seen at week 26 in the liposomal arm. CH58 (10 μg/mL), normal human sera ([NHS] 1:50), and ACT-1 (0.6 μg/well) were used as positive/negative controls (B). In addition, the natural ligand of α4β7-integrin receptor, mucosal vascular addressin cell adhesion molecule (MAdCAM)-1, was also used as a positive assay control. Median with interquartile range is shown from at least 3 experiments with each sample tested in triplicate at each dilution.
DISCUSSION
The AVEG015 clinical trial compared the safety and immunogenicity of HIV-1SF-2 rgp120 combined with 7 adjuvants, in HIV-1 uninfected low-risk behavior individuals [8, 9]. This trial [9, 10] demonstrated the following: (1) in contrast to the other adjuvants that induced moderate/severe pain and/or tenderness at the site of injection, the liposomal and AH arms exhibited none/or mild local and systemic toxicity; (2) the gp120-specific T-cell proliferation was much greater in the liposomal arm; (3) week 10 sera showed neutralizing antibodies to the homologous virus in 87% of the volunteers (liposomal arm) compared with 21% (AH arm); (4) antibody and cellular immune responses in the liposomal arm were superior; and finally, (5) major histocompatibility complex class I-restricted cytotoxic T lymphocyte responses were inconsistent in both arms. At the time of the AVEG015 trial, the correlates of immune protection had not been identified. Twenty years later, the RV144 trial demonstrated that IgG binding antibodies to the V1V2 region of HIV-1 envelope correlated inversely with risk of infection [3], and post hoc analyses elucidated the importance of V2 and V3 antibodies and other nonneutralizing mechanisms [3–7, 28]. These discoveries prompted us to examine archived AVEG015 sera from the 2 arms, namely, the liposomal and the AH arm for binding antibody responses and their durability.
Both arms induced antigen-specific IgG with low IgA antibodies. Higher gp120IIIB (P ≤ .05) and cross-reactive gp120 A/E-specific IgG antibodies were induced in the liposomal compared with AH arm even at 40 weeks after last boost. These responses were in contrast to the RV144 study [1]. In the follow-on trial RV305, despite additional immunizations administered to some of the RV144 vaccinees 6–8 years later, in an attempt to boost the magnitude and durability of the immune responses, the antibodies waned rapidly after vaccination [7, 29]. Two additional boosts in RV305 generated gp120-specific GMTs of 67986 and 19279, respectively, which were higher than post-RV144 titers but were short-lived [29]. In 2 other HIV-1 vaccine trials, VAX003 [30] and VAX004 [31], where the adjuvant was AH and the immunization regimen consisted only of AIDSVAXB/E protein (same proteins used in RV144) or AIDSVAXB/B, respectively, the antibodies to gp120, gp70V1V2 A/E, and gp70V1V2 CaseA2 proteins were low, short-lived, and declined significantly between vaccinations [32].
In contrast, in AVEG015, gp120 subtype B-specific IgG GMTs (Supplementary Table 1) at weeks 26 (82732) and 74 (94810) were much higher than RV144 (10383) or RV305 peak titers (67986). Compared to 14-fold and 19-fold decrease in the GMTs at 24 weeks after last boost in RV144 and RV305 studies, respectively [29], only a 4-fold drop (94810 to 21945) in the titers was observed at 40 weeks after final boost in the liposomal arm of AVEG015 (Supplementary Table 1). Furthermore, the GMTs for gp70V1V2 A/E and gp70V1V2 CaseA2 proteins declined rapidly over a 6-month period in RV144 (3- and 14-fold decrease) and RV305 (15- and 29-fold decrease) and could not be enhanced or sustained even after an additional immunization even though higher antibody responses were generated in RV305 compared with RV144 [29]. The half-life of gp70V1V2 CaseA2 and gp70V1V2 A/E antibodies in AVEG015 were 20 and 24 weeks, respectively, for the liposomal arm compared with 11 and 10 weeks, respectively, for the AH arm. The decay of antibody was significantly different between the 2 arms for gp70V1V2 A/E. Based on the above data from the various trials, the responses observed in the liposomal arm of the AVEG015 demonstrated that an additional immunization and the adjuvant influenced the magnitude and the durability of the immune response.
In addition to V1V2-specific antibody responses, significantly higher antibodies were also obtained to cyclic peptides belonging to subtypes B and C in the liposomal arm. This is important because in the RV144 vaccinees, V2 and V3-specific antibodies also exerted immune pressure on breakthrough virus [5, 28]. One could speculate that certain portions of the C-strand were exposed in the liposomal formulation resulting in higher magnitude of antibody responses. In a previous study, we showed that palmitoylated-cV2, which displayed a mixture of alpha-helical and random coil structure, when incorporated in liposomes, showed 100% random coil and induced extremely high antibody titers in mice [33]. In the liposomal arm of AVEG015, based on antibodies specific to cyclic peptides, the prediction of the secondary structure from the circular dichroism data, and our previous observations [33], we speculate that the percentage of coil and β-sheet might influence the magnitude of the antibody response with the liposomal formulation favoring a random coil conformation. From crystal structures of CH58 and CH59 bound to V2 peptide, it appears that the antibodies in RV144 were also directed against the helix/coil structures [34].
In AVEG015, the peak ADCC activity and inhibition of α4β7 binding to cV2 AE peptide were both observed at week 26. In view of the RV144 studies, which revealed that the vaccine did not induce effective neutralizing antibodies, but did elicit functional antibodies, which might have played a key role in preventing HIV infection, our results from the AVEG015 study showed that ADCC activity and α4β7 inhibition could be important in guiding vaccine design.
CONCLUSIONS
In summary, AVEG015 highlights the importance of adjuvant combinations in generating robust, durable binding and functional antibodies. Antibodies of this magnitude and durability were not achieved in any of the previous phase III or follow-on HIV-1 vaccine trials [1, 7, 29]. Precedence for adjuvant combinations in vaccine formulations [35–37] is now being increasingly appreciated for durable and functional antibody responses. Based on the results of AVEG015, incorporation of adjuvant combinations, an additional boost, and longer duration between boosts should prove to be promising in the design and development of an effective HIV-1 vaccine.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank the study participants, Christopher Lauver for technical assistance, and HIV Vaccine Trials Network (HVTN) and National Institute of Allergy and Infectious Diseases/Division of AIDS for providing the archived serum.
Author contributions. M. R., C. R. A., and G. R. M. conceived the studies. S. O., H. V. T., K. K. P., O. J., Y. W., and M. A. E. performed the experiments and analyzed the results. Y. Z. and P. D. generated graphs and performed the statistical analysis. M. R. also designed and oversaw all experiments, analyzed and interpreted the results, and wrote the manuscript. All authors edited the manuscript.
Disclaimer. The views expressed are those of the authors and should not be construed to represent the positions of the US Army or the Department of Defense.
Financial support. This work was funded by a cooperative agreement (W81XWH-11-2-0174) between Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc. and US Army Medical Research and Materiel Command.
Potential conflicts of interest. Y. Z. and P. D. are employees of Emmes Corporation and provided statistical support for this manuscript under a contract with the Henry Jackson Foundation and the Military HIV Research Program. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part. Keystone Symposium on HIV Vaccines: Adaptive Immunity and Beyond in Banff, March 9–14, 2014, Alberta, Canada. Abstract 2049 Poster P03.68LB.




