-
PDF
- Split View
-
Views
-
Cite
Cite
Lisa M Dunkle, Ruvim Izikson, Peter A Patriarca, Karen L Goldenthal, Derek Muse, Manon M J Cox, Randomized Comparison of Immunogenicity and Safety of Quadrivalent Recombinant Versus Inactivated Influenza Vaccine in Healthy Adults 18–49 Years of Age, The Journal of Infectious Diseases, Volume 216, Issue 10, 15 November 2017, Pages 1219–1226, https://doi.org/10.1093/infdis/jix478
Close - Share Icon Share
Abstract
Seasonal influenza vaccines are transitioning to quadrivalent formulations including the hemagglutinins of influenza A subtypes H1N1 and H3N2 and B lineages Yamagata and Victoria.
A new quadrivalent recombinant influenza vaccine (RIV4) was compared directly with a standard-dose, egg-grown, quadrivalent-inactivated influenza vaccine (IIV4) for immunogenicity and safety in adults 18–49 years of age. The coprimary endpoints for noninferiority were hemagglutination inhibition seroconversion rates and postvaccination geometric mean titer ratios for each antigen using US regulatory criteria. Reactogenicity solicited for 7 days, other safety events collected for 28 days, and serious or medically attended adverse events collected for 6 months after vaccination comprised the safety evaluation.
The immunogenicity of RIV4 was comparable to that of IIV4; the coprimary noninferiority criteria were met for 3 antigens, and the antibody responses to the fourth antigen, influenza B/Brisbane/60/2008, were low in each group, making comparisons uninterpretable. Systemic and injection site reactions were mild, transient, and similar in each group, whereas none of the spontaneously reported adverse events, serious or nonserious, were considered related to study vaccine.
This first head-to-head comparison of recombinant versus inactivated quadrivalent influenza vaccines in 18–49 year old adults showed comparable immunogenicity, safety, and tolerability for both vaccines.
The commercialization of the trivalent recombinant hemagglutinin (HA) vaccine (RIV3; Flublok, Protein Sciences, Meriden, CT) introduced a novel influenza vaccine produced by modern recombinant technology that yields a highly purified protein vaccine and more importantly assures that the antigenic components of the vaccine are exact genetic matches to the HAs of the wild-type strains selected each year for the seasonal vaccine [1]. By avoiding adaptation of infectious virus to growth in eggs, the recombinant technology assures no mutations in the HA gene that can change the antigenic properties of the HA and reduce the protective efficacy of the vaccine [2, 3, 4, 5].
Clinical efficacy of RIV3 was demonstrated in a placebo-controlled trial in adults 18–49 years of age [6, 7], whereas immunogenicity of RIV3 was shown to be comparable to licensed inactivated vaccines (IIV3; Fluzone, Sanofi Pasteur, Swiftwater, PA) in older adults [8, 9]. This study supported licensure of RIV4 (Flublok Quadrivalent) for adults 18–49 years of age, whereas a concurrent study in adults 50 years of age and older confirmed the efficacy of RIV3 and supported licensure of RIV4 in the older age group [10]. In the latter study, the relative vaccine efficacy (rVE) of RIV4 versus inactivated quadrivalent influenza vaccine (IIV4) against polymerase chain reaction-confirmed influenza-like illness (all strains) was 30% (95% confidence interval [CI], 10–47; P = .006), which satisfied prespecified criteria for both noninferiority and superiority of RIV4 versus IIV4 (Fluarix Quadrivalent, GlaxoSmithKline, Research Triangle Park, NC). In addition, rVE was 43% (95% CI, 21–59) for RIV4 over IIV4 against culture-confirmed, influenza-like illness.
Hemagglutinin antigens produced in insect cells by recombinant baculoviruses have been evaluated and shown to be safe and well tolerated in adults 18 years of age and older [7–9, 11, 12]. Furthermore, recombinant HA (rHA) has been administered in clinical trials using up to 405 µg of protein per dose, assuring that the addition of a fourth antigen in a vaccine that would total 180 µg per dose would be well tolerated [13]. The RIV4 formulation contains the 4 antigens selected annually, including influenza A/H1 and A/H3 subtypes and both type B lineages, Victoria and Yamagata.
Noninferior HAI immunogenicity of the relevant antigens in quadrivalent versus trivalent formulations (one IIV3 with B/Victoria lineage and another with B/Yamagata lineage) has previously supported regulatory approval of new quadrivalent formulations [14], extrapolating protective efficacy from the trivalent products [14–17]. Our study, conducted during the 2014–2015 season, was the first designed to compare 2 quadrivalent vaccines directly. The primary objective was to demonstrate noninferior immunogenicity of all 4 vaccine antigens of RIV4 versus a licensed, IIV4 in subjects 18 to 49 years of age, the age group in which clinical efficacy of RIV3 had already been demonstrated.
METHODS
Vaccines
The RIV4 comprises 4 HA proteins produced by the Baculovirus Expression System Technology. The genes for the HA surface protein of the 4 influenza strains represented in the vaccine were cloned independently into the plasmid baculovirus expression vector pPSC12. The PSC12 plasmid contained the Autographica californica Nuclear Polyhedrosis Virus (AcNPV) baculovirus polyhedrin promoter, the baculovirus 61K signal peptide, and flanking baculovirus deoxyribonucleic acid (DNA) derived from the EcoR1-I fragment of AcNPV. After confirmation of the correct sequence, the DNA sequences were inserted into AcNPV by homologous recombination. Recombinant viruses containing the respective genes for HA were then used to express the HAs in the high-yield insect cell line expresSF+ under serum-free conditions [18].
The rHA proteins produced under these conditions are not cleaved into the HA1 and HA2 domains and are referred to as rHA0; they are competent to form multimeric rosette-like structures visible by electron microscopy. The proteins are purified by a combination of ion-exchange and hydrophobic interaction chromatography and filtration technology to ≥90% purity as assessed by protein gel electrophoresis and scanning densitometry [18]. The final concentration of each rHA was determined by the single radial immunodiffusion assay [19].
The composition of the 2 study vaccines was based on strains recommended for the North American 2014–2015 season [20]. The RIV4 (Flublok Quadrivalent, Batch no. QFCA1401; Protein Sciences Corporation, Meriden, CT) contained 180 µg of rHA (4 × 45 µg) derived from H1N1: A/California/07/2009, H3N2: A/Texas/50/2012, B/Massachusetts/2/2012 (B/Yamagata-lineage) and B/Brisbane/60/2008 (B/Victoria-lineage); commercial IIV4 (Fluarix Quadrivalent, Lot GA22N; GlaxoSmithKline, Research Triangle Park, NC) contained 60 µg of HA (4 × 15 µg), derived from H1N1: A/Christchurch/16/2010 (an A/California/07/2009-like virus), H3N2: A/Texas/50/2012, B/Massachusetts/02/2012 and B/Brisbane/60/2008.
Study Design
The study was an observer-blind, randomized, active-controlled, Phase 3 multicenter clinical trial conducted at 10 clinical sites in the United States in ambulatory, medically stable adults aged 18–49 years with no contraindications to either study vaccine (based on US Food and Drug Admistration [FDA]-approved package inserts). All subjects provided written informed consent to participation before any study procedures; the study was approved and monitored by a single central Institutional Review Board (Quorum IRB, Seattle, WA) and was conducted in accordance with international standards [21]. Subjects and study staff (except designated staff who administered the vaccine) were blinded to vaccine product (ClinicalTrials.gov no. NCT02290509).
A total of 1350 subjects were randomized 3:1 on day 0 to receive a single dose of RIV4 or IIV4 using a block randomization scheme assigned via an interactive web-based response system, and they were observed for approximately 6 months after vaccination. Subjects recorded specifically solicited reactogenicity events on a memory aid, ie, diary card, during the 7 days after vaccination. All other adverse events (AEs) were captured on a separate card from day 0 to day 28. Serious AEs (SAE) and medically attended AEs (MAEs) were collected during the 6 months after vaccination. Medically attended AEs were defined as AEs leading to a visit to or from medical personnel for any reason. A phone call with a healthcare professional was not considered medically attended. If an MAE led to hospitalization (or met other SAE criteria), it was also reported as an SAE.
Serology
Serum samples for hemagglutination inhibition (HAI) serology were collected on day 0 before vaccination and on day 28, maintained at ≤−20°C until transfer to the central laboratory where all samples were tested (Focus Diagnostics, Q2 Solutions, Valencia, CA). The HAI assays were performed using a validated protocol using egg-derived antigens for each strain (National Institute for Biological Standards and Control, Hertfordshire, UK) represented in the vaccines.
Statistical Analysis
The coprimary immunogenicity endpoints were the HAI geometric meant antibody titer (GMT) ratios and HAI seroconversion rates (SCRs). The Immunogenicity Population was comprised of all randomized subjects who received study vaccine, provided pre- and postvaccination serology for HAI titers on days 0 and 28, and had no protocol deviations that might adversely impact their immune response. The Safety Population included all randomized subjects who received a dose of study vaccine and for whom some safety data were available after vaccination. Subjects were analyzed according to the vaccine received regardless of the vaccine to which they were randomized.
Noninferiority of immunogenicity was determined using the differences in HAI SCRs and ratios of HAI GMTs as described in Center for Biologics Evaluation and Research’s (CBER) “Guidance for Industry: Clinical Data Needed to Support the Licensure of Seasonal Inactivated Influenza Vaccines” [22]. Seroconversion rate was defined as the proportion of subjects with either a prevaccination HAI titer <10 and postvaccination titer ≥40 or a prevaccination titer ≥10 and a minimum 4-fold rise in postvaccination titer. The 95% CIs for SCR were calculated using the Clopper-Pearson exact method [23]. The 95% CI for difference in SCR used the Farrington-Manning score method [24]. The 95% CI for GMTs were calculated as the antilog of the 95% CI of the log-transformed titers. The 95% CI for the GMT ratios were calculated as the antilog of the 95% CI of the mean difference between log-transformed titers.
Reactogenicity was determined by tabulating the frequency and severity of subject-reported solicited events characteristic of injection site or systemic reactions to vaccination, and safety was measured by the incidence and severity of unsolicited AEs, all of which were coded using MedDRA, version 17.0, and tabulated by Preferred Term and System Organ Class.
RESULTS
Study Population
The 1350 study participants who were enrolled were randomized and received their dose of study vaccine between October 22 and November 24, 2014. Three individuals (1 assigned to RIV4 and 2 assigned to IIV4) received the wrong study vaccine in error and were not included in further analyses. A total of 1285 participants (>95%) completed the study, and the entire flow of study subjects, with analysis populations, is described in Figure 1. The demographics reflected a population of healthy young adults that were well balanced between the 2 vaccine groups (Table 1).
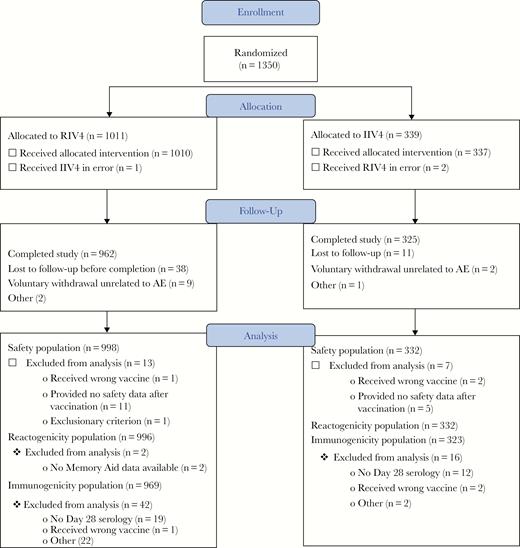
Flow of study participants. AE, adverse events; IIV4, quadrivalent-inactivated influenza vaccine; RIV4, quadrivalent recombinant influenza vaccine.
| Characteristic . | Flublok Quadrivalent N = 998 . | IIV4 N = 332 . |
|---|---|---|
| Age (years) | ||
| Mean | 33.3 | 34.0 |
| Range | 18–50 | 18–49 |
| Sex, n (%) | ||
| Male | 359 (36) | 110 (33) |
| Female | 639 (64) | 222 (67) |
| Race, n (%) | ||
| American Indian or Alaska Native | 7 (1) | 3 (1) |
| Asian | (<1) | 4 (1) |
| Black or African American | 376 (38) | 114 (34) |
| Native Hawaiian/Pacific Islander | 11 (1) | 2 (1) |
| White or Caucasian | 589 (59) | 202 (61) |
| Other | 12 (1) | 7 (2) |
| Ethnicity, n (%) | ||
| Hispanic | 162 (16) | 57 (17) |
| Non-Hispanic | 836 (84) | 275 (83) |
| Characteristic . | Flublok Quadrivalent N = 998 . | IIV4 N = 332 . |
|---|---|---|
| Age (years) | ||
| Mean | 33.3 | 34.0 |
| Range | 18–50 | 18–49 |
| Sex, n (%) | ||
| Male | 359 (36) | 110 (33) |
| Female | 639 (64) | 222 (67) |
| Race, n (%) | ||
| American Indian or Alaska Native | 7 (1) | 3 (1) |
| Asian | (<1) | 4 (1) |
| Black or African American | 376 (38) | 114 (34) |
| Native Hawaiian/Pacific Islander | 11 (1) | 2 (1) |
| White or Caucasian | 589 (59) | 202 (61) |
| Other | 12 (1) | 7 (2) |
| Ethnicity, n (%) | ||
| Hispanic | 162 (16) | 57 (17) |
| Non-Hispanic | 836 (84) | 275 (83) |
Abbreviations: IIV4, quadrivalent-inactivated influenza vaccine.
| Characteristic . | Flublok Quadrivalent N = 998 . | IIV4 N = 332 . |
|---|---|---|
| Age (years) | ||
| Mean | 33.3 | 34.0 |
| Range | 18–50 | 18–49 |
| Sex, n (%) | ||
| Male | 359 (36) | 110 (33) |
| Female | 639 (64) | 222 (67) |
| Race, n (%) | ||
| American Indian or Alaska Native | 7 (1) | 3 (1) |
| Asian | (<1) | 4 (1) |
| Black or African American | 376 (38) | 114 (34) |
| Native Hawaiian/Pacific Islander | 11 (1) | 2 (1) |
| White or Caucasian | 589 (59) | 202 (61) |
| Other | 12 (1) | 7 (2) |
| Ethnicity, n (%) | ||
| Hispanic | 162 (16) | 57 (17) |
| Non-Hispanic | 836 (84) | 275 (83) |
| Characteristic . | Flublok Quadrivalent N = 998 . | IIV4 N = 332 . |
|---|---|---|
| Age (years) | ||
| Mean | 33.3 | 34.0 |
| Range | 18–50 | 18–49 |
| Sex, n (%) | ||
| Male | 359 (36) | 110 (33) |
| Female | 639 (64) | 222 (67) |
| Race, n (%) | ||
| American Indian or Alaska Native | 7 (1) | 3 (1) |
| Asian | (<1) | 4 (1) |
| Black or African American | 376 (38) | 114 (34) |
| Native Hawaiian/Pacific Islander | 11 (1) | 2 (1) |
| White or Caucasian | 589 (59) | 202 (61) |
| Other | 12 (1) | 7 (2) |
| Ethnicity, n (%) | ||
| Hispanic | 162 (16) | 57 (17) |
| Non-Hispanic | 836 (84) | 275 (83) |
Abbreviations: IIV4, quadrivalent-inactivated influenza vaccine.
Immunogenicity
The immunogenicity of the 4 antigens in RIV4 measured by HAI antibody titers, compared with that of the IIV4, proved to be comparable for all antigens (Figures 2 and 3). The differences in SCRs between groups and the ratios of HAI GMT, evaluated using the criteria established by the FDA [22], showed that the RIV4 responses to influenza A/H1, A/H3, and B/Massachusetts all met both noninferiority criteria. Furthermore, HAI antibody responses among RIV4 recipients were significantly higher to influenza A/Texas/50/2012 compared with IIV4 recipients. Although neither criterion for noninferiority was met by RIV4 for B/Brisbane, the responses and GMTs were very low in both vaccine groups, making the comparison difficult to interpret.
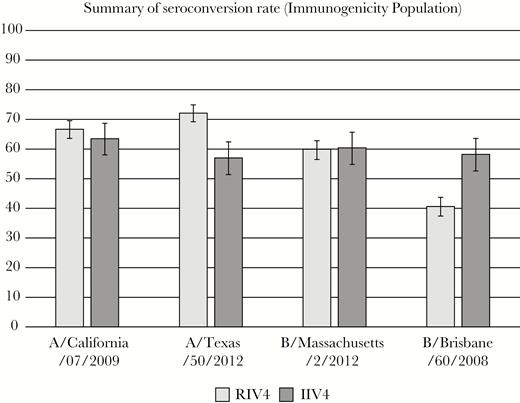
Hemagglutinin inhibition (HAI) seroconversion rates at day 28—immunogenicity population. Seroconversion rate (SCR) defined as percentage of subjects with either a prevaccination HAI titer <10 and day 28 HAI titer ≥40 or a prevaccination HAI titer ≥10 and a day 28 ≥4-fold rise over baseline detectable titer. Noninferiority was defined as the upper bound of the 2-sided 95% confidence interval around the SCR difference (SCRIIV4–SCRRIV4) should not exceed 10 percent. IIV4, quadrivalent-inactivated influenza vaccine; RIV4, quadrivalent recombinant influenza vaccine.
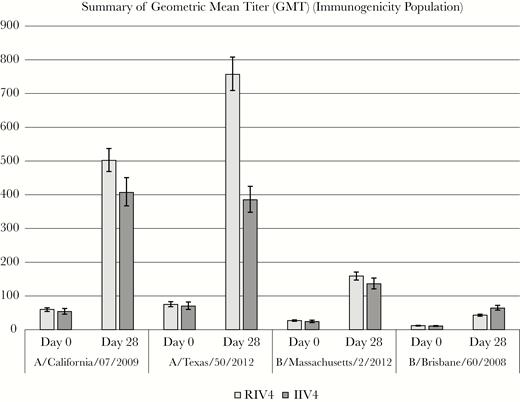
Pre- and postvaccination hemagglutinin inhibition geometric meant antibody titers (GMTs)—immunogenicity population. Noninferiority was defined as the upper bound of 2-sided 95% confidence interval around ratio of GMTIIV4/GMTRIV4 must be <1.5. IIV4, quadrivalent-inactivated influenza vaccine; RIV4, quadrivalent recombinant influenza vaccine.
Reactogenicity
Subject-recorded reports of reactions to study vaccine in the 7 days after vaccination were, in general, of similar frequency and severity in each vaccine group (Table 3). Injection site pain and tenderness, usually of mild severity, were reported in approximately half of the subjects in each vaccine group. Injection site erythema (mostly mild in both groups) was more frequent after RIV4 than IIV4 (P = .002), but it occurred in fewer than 5% of RIV4 recipients. As has been reported with other vaccines [25], women reported local reactions to vaccine injections significantly more frequently than men, but with no difference between the vaccine groups. Likewise, whites and non-Hispanics in this study reported injection site reactions more frequently than non-whites and Hispanics.
Systemic reactions were reported much less frequently than injection site reactions; most were mild-to-moderate in severity, and none were statistically significantly different between treatment groups (Table 2). Gender differences similar to those for injections-site reactions were observed. Fever was rare and in no case >40°C (>104°F).
| Reactogenicity Event . | RIV4 N = 996 n (%) . | IIV4 N = 332 n (%) . | ||||
|---|---|---|---|---|---|---|
| Any Severity . | Grade 3 . | Grade 4 . | Any Severity . | Grade 3 . | Grade 4 . | |
| Any injection site reactiona,b | 51.2 | 1.1 | <1 | 51.8 | 1.5 | 0 |
| Local pain | 367 (36.8) | 9 (0.9) | 0 | 121 (36.4) | 3 (0.9) | 0 |
| Local tenderness | 478 (48.0) | 9 (0.9) | 0 | 155 (46.7) | 4 (1.2) | 0 |
| Redness | 42 (4.2) | 0 | 0 | 3 (0.9) | 0 | 0 |
| Firmness/swelling | 49 (4.9) | 0 | 0 | 10 (3.0) | 0 | 0 |
| Any systemic reactionb | 339 (34.1) | 23 (2.3) | 0 | 119 (35.8) | 9 (2.7) | 1 (0.3) |
| Fatigue | 164 (16.5) | 5 (0.5) | 0 | 55 (16.6) | 4 (1.2) | 0 |
| Shivering/chills | 69 (6.9) | 5 (0.5) | 0 | 20 (6.0) | 4 (1.2) | 0 |
| Joint pain | 94 (9.5) | 9 (0.9) | 0 | 34 (10.2) | 2 (0.6) | 0 |
| Muscle pain | 127 (12.8) | 9 (0.9) | 0 | 39 (11.7) | 3 (0.9) | 0 |
| Headache | 202 (20.3) | 1 (1.3) | 0 | 70 (21.1) | 6 (1.8) | 1 (0.3) |
| Nausea | 89 (9.0) | 6 (0.6) | 1 (0.1) | 31 (9.3) | 4 (1.2) | 0 |
| Feverc | 15 (1.5) | 4 (0.4) | 0 | 2 (0.6) | 1 (0.3) | 0 |
| Reactogenicity Event . | RIV4 N = 996 n (%) . | IIV4 N = 332 n (%) . | ||||
|---|---|---|---|---|---|---|
| Any Severity . | Grade 3 . | Grade 4 . | Any Severity . | Grade 3 . | Grade 4 . | |
| Any injection site reactiona,b | 51.2 | 1.1 | <1 | 51.8 | 1.5 | 0 |
| Local pain | 367 (36.8) | 9 (0.9) | 0 | 121 (36.4) | 3 (0.9) | 0 |
| Local tenderness | 478 (48.0) | 9 (0.9) | 0 | 155 (46.7) | 4 (1.2) | 0 |
| Redness | 42 (4.2) | 0 | 0 | 3 (0.9) | 0 | 0 |
| Firmness/swelling | 49 (4.9) | 0 | 0 | 10 (3.0) | 0 | 0 |
| Any systemic reactionb | 339 (34.1) | 23 (2.3) | 0 | 119 (35.8) | 9 (2.7) | 1 (0.3) |
| Fatigue | 164 (16.5) | 5 (0.5) | 0 | 55 (16.6) | 4 (1.2) | 0 |
| Shivering/chills | 69 (6.9) | 5 (0.5) | 0 | 20 (6.0) | 4 (1.2) | 0 |
| Joint pain | 94 (9.5) | 9 (0.9) | 0 | 34 (10.2) | 2 (0.6) | 0 |
| Muscle pain | 127 (12.8) | 9 (0.9) | 0 | 39 (11.7) | 3 (0.9) | 0 |
| Headache | 202 (20.3) | 1 (1.3) | 0 | 70 (21.1) | 6 (1.8) | 1 (0.3) |
| Nausea | 89 (9.0) | 6 (0.6) | 1 (0.1) | 31 (9.3) | 4 (1.2) | 0 |
| Feverc | 15 (1.5) | 4 (0.4) | 0 | 2 (0.6) | 1 (0.3) | 0 |
Abbreviations: IIV4, quadrivalent-inactivated influenza vaccine; RIV4, quadrivalent recombinant influenza vaccine.
aFor injection-site reactions, the denominator for RIV4 group was 996, and for the IIV4 group it was 332. For systemic reactions, the denominators were 994 and 332 for the RIV4 and IIV4 groups, respectively; for fever, these were 990 and 327 for the RIV4 and IIV4 groups, respectively.
bThe grading system for injection site and system reactogenicity was as follows: Grade 1, mild; Grade 2, moderate; Grade 3:
cThe grading system for fever was as follows: Grade 1, 38–38.4°C (100.4–101.1°F); Grade 2, >38.4–38.9°C (101.2–102.0°F); Grade 3, >38.9–40°C (102.1–104°F); Grade 4, >40°C (>104°F).
| Reactogenicity Event . | RIV4 N = 996 n (%) . | IIV4 N = 332 n (%) . | ||||
|---|---|---|---|---|---|---|
| Any Severity . | Grade 3 . | Grade 4 . | Any Severity . | Grade 3 . | Grade 4 . | |
| Any injection site reactiona,b | 51.2 | 1.1 | <1 | 51.8 | 1.5 | 0 |
| Local pain | 367 (36.8) | 9 (0.9) | 0 | 121 (36.4) | 3 (0.9) | 0 |
| Local tenderness | 478 (48.0) | 9 (0.9) | 0 | 155 (46.7) | 4 (1.2) | 0 |
| Redness | 42 (4.2) | 0 | 0 | 3 (0.9) | 0 | 0 |
| Firmness/swelling | 49 (4.9) | 0 | 0 | 10 (3.0) | 0 | 0 |
| Any systemic reactionb | 339 (34.1) | 23 (2.3) | 0 | 119 (35.8) | 9 (2.7) | 1 (0.3) |
| Fatigue | 164 (16.5) | 5 (0.5) | 0 | 55 (16.6) | 4 (1.2) | 0 |
| Shivering/chills | 69 (6.9) | 5 (0.5) | 0 | 20 (6.0) | 4 (1.2) | 0 |
| Joint pain | 94 (9.5) | 9 (0.9) | 0 | 34 (10.2) | 2 (0.6) | 0 |
| Muscle pain | 127 (12.8) | 9 (0.9) | 0 | 39 (11.7) | 3 (0.9) | 0 |
| Headache | 202 (20.3) | 1 (1.3) | 0 | 70 (21.1) | 6 (1.8) | 1 (0.3) |
| Nausea | 89 (9.0) | 6 (0.6) | 1 (0.1) | 31 (9.3) | 4 (1.2) | 0 |
| Feverc | 15 (1.5) | 4 (0.4) | 0 | 2 (0.6) | 1 (0.3) | 0 |
| Reactogenicity Event . | RIV4 N = 996 n (%) . | IIV4 N = 332 n (%) . | ||||
|---|---|---|---|---|---|---|
| Any Severity . | Grade 3 . | Grade 4 . | Any Severity . | Grade 3 . | Grade 4 . | |
| Any injection site reactiona,b | 51.2 | 1.1 | <1 | 51.8 | 1.5 | 0 |
| Local pain | 367 (36.8) | 9 (0.9) | 0 | 121 (36.4) | 3 (0.9) | 0 |
| Local tenderness | 478 (48.0) | 9 (0.9) | 0 | 155 (46.7) | 4 (1.2) | 0 |
| Redness | 42 (4.2) | 0 | 0 | 3 (0.9) | 0 | 0 |
| Firmness/swelling | 49 (4.9) | 0 | 0 | 10 (3.0) | 0 | 0 |
| Any systemic reactionb | 339 (34.1) | 23 (2.3) | 0 | 119 (35.8) | 9 (2.7) | 1 (0.3) |
| Fatigue | 164 (16.5) | 5 (0.5) | 0 | 55 (16.6) | 4 (1.2) | 0 |
| Shivering/chills | 69 (6.9) | 5 (0.5) | 0 | 20 (6.0) | 4 (1.2) | 0 |
| Joint pain | 94 (9.5) | 9 (0.9) | 0 | 34 (10.2) | 2 (0.6) | 0 |
| Muscle pain | 127 (12.8) | 9 (0.9) | 0 | 39 (11.7) | 3 (0.9) | 0 |
| Headache | 202 (20.3) | 1 (1.3) | 0 | 70 (21.1) | 6 (1.8) | 1 (0.3) |
| Nausea | 89 (9.0) | 6 (0.6) | 1 (0.1) | 31 (9.3) | 4 (1.2) | 0 |
| Feverc | 15 (1.5) | 4 (0.4) | 0 | 2 (0.6) | 1 (0.3) | 0 |
Abbreviations: IIV4, quadrivalent-inactivated influenza vaccine; RIV4, quadrivalent recombinant influenza vaccine.
aFor injection-site reactions, the denominator for RIV4 group was 996, and for the IIV4 group it was 332. For systemic reactions, the denominators were 994 and 332 for the RIV4 and IIV4 groups, respectively; for fever, these were 990 and 327 for the RIV4 and IIV4 groups, respectively.
bThe grading system for injection site and system reactogenicity was as follows: Grade 1, mild; Grade 2, moderate; Grade 3:
cThe grading system for fever was as follows: Grade 1, 38–38.4°C (100.4–101.1°F); Grade 2, >38.4–38.9°C (101.2–102.0°F); Grade 3, >38.9–40°C (102.1–104°F); Grade 4, >40°C (>104°F).
Other Adverse Events
Rates of unsolicited, spontaneously reported AEs during the 28 days after vaccination were similar in both treatment groups (10.3% of RIV4 and 10.5% of IIV4 recipients), with no clinically concerning patterns or unexpected events. The most common unsolicited events (based on preferred terms), regardless of relatedness to study vaccine, reflect the common complaints commonly observed during the fall and winter season (Table 3). In this population of young adults, there were no deaths and no SAEs related to study vaccine or concerning patterns of other clinically significant AEs. Serious AEs were reported by 12 subjects, 10 (1%) RIV4 recipients and 2 (0.6%) IIV4 recipients, and no subject withdrew from study due to an AE. These were not unexpected observations in this overall relatively healthy participating population.
Most Common Unsolicited Adverse Events That Occurred in ≥1% of Subjects in Either Treatment Group: Days 0–28
| Adverse Event . | RIV4 N = 998 N (%) . | IIV4 N = 332 N (%) . |
|---|---|---|
| Headache | 20 (2.0) | 5 (1.5) |
| Cough | 14 (1.4) | 4 (1.2) |
| Nasopharyngitis | 13 (1.3) | 5 (1.5) |
| Upper respiratory tract infection | 10 (1.0) | 5 (1.5) |
| Sinusitis | 6 (0.6) | 5 (1.5) |
| Adverse Event . | RIV4 N = 998 N (%) . | IIV4 N = 332 N (%) . |
|---|---|---|
| Headache | 20 (2.0) | 5 (1.5) |
| Cough | 14 (1.4) | 4 (1.2) |
| Nasopharyngitis | 13 (1.3) | 5 (1.5) |
| Upper respiratory tract infection | 10 (1.0) | 5 (1.5) |
| Sinusitis | 6 (0.6) | 5 (1.5) |
Abbreviations: IIV4, quadrivalent-inactivated influenza vaccine; RIV4, quadrivalent recombinant influenza vaccine.
Most Common Unsolicited Adverse Events That Occurred in ≥1% of Subjects in Either Treatment Group: Days 0–28
| Adverse Event . | RIV4 N = 998 N (%) . | IIV4 N = 332 N (%) . |
|---|---|---|
| Headache | 20 (2.0) | 5 (1.5) |
| Cough | 14 (1.4) | 4 (1.2) |
| Nasopharyngitis | 13 (1.3) | 5 (1.5) |
| Upper respiratory tract infection | 10 (1.0) | 5 (1.5) |
| Sinusitis | 6 (0.6) | 5 (1.5) |
| Adverse Event . | RIV4 N = 998 N (%) . | IIV4 N = 332 N (%) . |
|---|---|---|
| Headache | 20 (2.0) | 5 (1.5) |
| Cough | 14 (1.4) | 4 (1.2) |
| Nasopharyngitis | 13 (1.3) | 5 (1.5) |
| Upper respiratory tract infection | 10 (1.0) | 5 (1.5) |
| Sinusitis | 6 (0.6) | 5 (1.5) |
Abbreviations: IIV4, quadrivalent-inactivated influenza vaccine; RIV4, quadrivalent recombinant influenza vaccine.
DISCUSSION
The transition to quadrivalent seasonal influenza vaccines was motivated by the frequent mismatches of the B lineages in trivalent vaccines versus the predominant circulating wild-type B viruses [26] that could best be avoided by including both B lineages in the seasonal vaccine. Regulatory approval of these new products has been based on immunogenicity and safety compared with the relevant antigens in trivalent formulations, with clinical efficacy extrapolated from studies with earlier trivalent formulations [14, 17]. In the case of RIV3, absolute vaccine efficacy had been demonstrated in a placebo-controlled trial in adults 18–49 years of age [7], so this was applicable only to RIV4 in younger adults. A trial in adults 50 years of age and older was conducted concurrently with this study to confirm the efficacy of RIV4 relative to a comparator IIV4 in older adults [10].
This trial was the first head-to-head comparison of 2 quadrivalent seasonal influenza vaccines, which also compared RIV4 manufactured by modern recombinant technology versus IIV4 manufactured by the decades-old process of inactivating egg-grown influenza virus. The trial was designed to demonstrate noninferior immunogenicity and safety of RIV4 versus IIV4 in adults 18–49 years of age. Noninferior immunogenicity was tested using CBER criteria for licensure of seasonal influenza vaccines [22], and safety in the 6 months after vaccination was assessed by well established procedures [7–9]. The trial enrolled 1350 subjects, randomized 3:1 to RIV4 or IIV4 to assure an adequate assessment of the safety of the new product.
Overall, the immunogenicity of RIV4 was comparable to that of IIV4. The RIV4 met both coprimary endpoints (SCRs and postvaccination HAI GMT ratios) for A/California/07/2009, A/Texas/50/2012, and B/Massachusetts/2/2012. Of interest in light of the clinical efficacy demonstrated in adults ≥50 years of age by RIV4 during the same A/H3-predominant flu season [10], the antibody responses to influenza A/Texas/50/2012 in this study were significantly higher among RIV4 versus IIV4 recipients. Higher immune responses to H3N2 in RIV3 or RIV4 recipients (versus IIV3 and IIV4 recipients, respectively) have consistently been observed in active-controlled trials [8–10].
However, antibody responses to influenza B/Brisbane were very low in recipients of both vaccines, and RIV4 recipients did not meet the criteria for noninferiority to IIV4. Results of HAI assays are more variable when titers are low, and the GMTs of the 2 vaccine groups (43 and 64, respectively) were within the range of sensitivity for the assay [27, 28]. Titers this low may not allow clinically meaningful conclusions to be drawn.
The HAI assay used in this study used antigens derived from egg-grown viruses, raising the question of comparability of the HAI titers for the 2 vaccine groups, because the antigens might interact differently with antibodies induced by recombinant protein vaccine versus the egg-grown vaccine. A small pilot study of postvaccination antisera from RIV4 or IIV4 recipients tested in the HAI assay using egg-grown and mammalian cell-grown HA antigens reported that HAI antibody titers reported from the cell-grown antigen assay were considerably higher than those reported from the egg-grown antigen assay, regardless of the vaccine administered [29]. Nevertheless, the antibodies induced by the 2 different vaccines appeared not to have notably discrepant affinity for the antigens of the 2 different sources.
Safety profiles, including local and systemic reactogenicity and spontaneously reported AEs, were similar between RIV4 and IIV4. Most reactions were mild and transient in nature. Overall, the reactogenicity and safety profile during the 6 months after vaccination was satisfactory for both vaccine groups.
CONCLUSIONS
In summary, in adults 18–49 years of age, safety and immunogenicity of RIV4 were similar to IIV4. The immunogenicity of RIV4 met the prespecified noninferiority criteria for HAI antibody responses for 3 of 4 flu strains represented in the vaccines. Limitations of this study include the unexpectedly low antibody responses to influenza B/Brisbane. Given the greater variability of HAI assays at the lower range of titers, the titers were insufficient to draw clinically meaningful conclusions [28]. However, in the concurrent clinical trial in adults ≥50 years of age, similar protection by RIV4 and IIV4 against the circulating influenza B strains was observed, suggesting adequate immunogenicity, despite the titers, as measured [10]. Both postvaccination reactogenicity and longer-term safety follow-up showed that RIV4 was safe and well tolerated. Overall, this trial demonstrated an acceptable degree of immunogenicity and a satisfactory safety profile of RIV4 in adults 18–49 years of age.
Acknowledgments
Financial support. This work was funded by Protein Sciences Corporation under a contract from the US Health and Human Services Biomedical Advanced Research and Development Authority (Contract no. HSSO 100200900106C).
Potential conflicts of interest. L.M.D., R. I., and M. M. J. C. are employees and shareholders of Protein Sciences Corporation, the manufacturer of Flublok and Flublok Quadrivalent. P. A. P. and K. L. G. are independent consultants who have received consulting fees from Protein Sciences. P. A. P. has also received consulting fees from Altimmune, FluGen, Georgia Institute of Technology, Medicago, Vaxinnate, Vaxart, Biosciences, Moderna Therapeutics, Novavax, Sequirus, and Vistera. K. L. G. has received consulting fees from Pfizer, Johnson and Johnson, Novartis, and the Bill and Melinda Gates Foundation. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References




