-
PDF
- Split View
-
Views
-
Cite
Cite
Martin Steinau, Pamina Gorbach, Beau Gratzer, Jim Braxton, Peter R. Kerndt, Richard A. Crosby, Elizabeth R. Unger, Lauri E. Markowitz, Elissa Meites, Concordance Between Anal and Oral Human Papillomavirus Infections Among Young Men Who have Sex With Men, The Journal of Infectious Diseases, Volume 215, Issue 12, 15 June 2017, Pages 1832–1835, https://doi.org/10.1093/infdis/jix232
Close - Share Icon Share
Abstract
Prevalence of human papillomavirus (HPV) infections was assessed among 1033 young men who have sex with men (MSM) aged 18–26 years. HPV (any type) was detected in 742 (71.8%) anal specimens and 101 (9.8%) oral specimens. Although HPV was detected in specimens from both anatomical sites in 83 (8.0%) participants, type-specific concordance for at least 1 HPV type was found in only 35 (3.4%) participants. HIV and smoking were associated with higher prevalence at both sites and frequency of concordant types. Coinfections of identical HPV types were rare, suggesting independent infection events and/or different modes of clearance.
Human papillomaviruses (HPVs) are ubiquitous in almost all populations worldwide. Although most infections are asymptomatic, a number of genotypes have carcinogenic potential and may cause cervical, anal, and oropharyngeal cancers. Risk of infection increases with sexual behavior (e.g., higher number of lifetime sex partners), but it is often difficult to identify the source of a specific HPV infection. This is often due to the long latency periods between initial infection and development of symptoms, if any. It is not well established whether self-inoculation between anatomic sites might play a role.
Limited data are available about the relationship between HPV infections at different anatomical regions of the same person. Several studies focusing on anal HPV in women with cervical HPV infections showed significant correlations between type-specific detection at both sites, with stronger agreement occurring when cervical intraepithelial neoplasia was present [1–3]. Type-specific comparisons between anogenital and oral samples are challenging and have been scarce due to the low prevalence of oral HPV in the general population and the unavailability of sufficient data. One analysis of cervical and oral HPV prevalence among US women indicated that the infections are generally not independent, but type-specific concordance is low [4]. No nationally representative assessment has been made of the relationship between anal and oral HPV.
Men who have sex with men (MSM) are at high risk for HPV infection and HPV-associated disease. The relative high HPV prevalence in this population provides an opportunity to investigate the correlation of anal and oral HPV types. We aimed to evaluate HPV DNA prevalence in oral rinse and anal swab specimens from young gay, bisexual, and other MSM, and to assess type-specific concordance of HPV infections and factors that are associated with their prevalence.
MATERIALS AND METHODS
Criteria for enrollment of study participants, specimen collection methods, and laboratory assays have been described in detail previously [5]. Briefly, the Young Men’s HPV (YMHPV) study enrolled consenting gay, bisexual, and other MSM who had been assigned male sex at birth and who were within the target age range for HPV vaccination (aged 18–26 years) at health-care centers serving MSM in the United States. Two clinics in Los Angeles, CA and one in Chicago, IL, enrolled participants between July 2012 and July 2014. Participants completed a standardized computer-assisted survey to capture demographics, sexual behavior, HIV status and HPV vaccination. Participants were considered HIV positive if they reported ever having a positive HIV test result; all others were considered “not HIV positive.” Each participant also submitted self-collected oral and anal specimens. Oral rinse specimens were collected by swishing and gargling 10 mL saline for 30 seconds. Anal specimens were collected with a Dacron swab stored in Digene specimen transport media. All specimens were shipped to the HPV laboratory at the Centers for Disease Control and Prevention in Atlanta. HPV detection and typing was achieved with a commercial line blot assay as previously described (5).
Descriptive analysis combined demographic and health data collected via survey with HPV genotyping results from anal and oral specimens. We analyzed the prevalence of any HPV at oral and anal sites, as well as type-specific prevalence and concordance. Relative risks (RRs) were calculated from a 2 × 2 matrix (anal/oral HPV positive/negative) to assess the chance of detecting HPV in 1 site with regard to the HPV status at the other site, as well as 95% confidence intervals (CIs). Independence of categorical variables was tested with χ2 or Fisher exact test, and differences in number of HPV types among groups were assessed with the Mann–Whitney U test. Differences with a P value < .05 were considered significant. All statistical calculations were performed with SPSS v.19 software (IBM, Armonk, NY).
RESULTS
Complete survey responses and adequate HPV typing results from both anal and oral specimen types were received from 1033 participants. Their median age was 23 years (range, 18–26 years). Self-reported HIV prevalence was 10.6%. In total, 111 (10.7%) reported having received at least 1 dose of HPV vaccine by the time of enrollment. Study participants reported being sexually active for an average of 6 years, with a median of 15 (range, 1–500) lifetime sex partners, and 622 of 899 (69.2%) respondents reported multiple sex partners in the previous 3 months.
Any type of HPV DNA was detected in 742 (71.8%) anal samples and 101 (9.8%) oral samples. In 83 (8.0%) participants, HPV was found at both anatomical sites, although types were not necessarily concordant (see below). HPV prevalence was not independent between anal and oral sites. Participants were significantly more likely to have oral HPV if they also had any anal HPV (RR, 1.16; CI, 1.05–1.28); the converse was also true, as participants were significantly more likely to have anal HPV if they also had any oral HPV (RR, 1.81; CI, 1.11–2.96).
The 3 HPV types with the highest prevalence among anal specimens were HPV6 (16.9%), HPV16 (15.1%), and HPV59 (13.4%) (Figure 1). Among oral specimens, the 3 types with the highest prevalence were also HPV16 (1.7%), HPV59 (1.1%) and HPV6 (1.1%). Although multiple HPV types were frequently detected among anal specimens, oral specimens rarely had more than 2 types (Table 1).
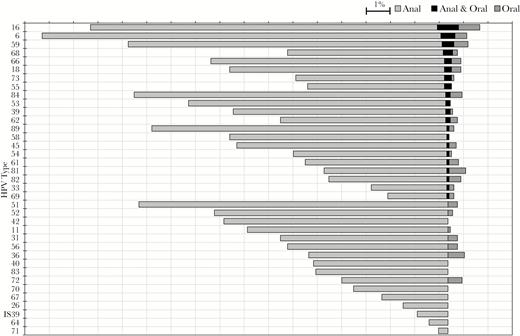
Anal, oral and concordant type-specific HPV prevalence in specimens from 1033 young gay, bisexual, and other MSM participating in the YMHPV study, 2 cities, United States, 2012–2014.
HPV types (left border) are shown in the order of concordant prevalence. The bars are centered on the midpoints of the anal and oral categories.
Abbreviations: HPV, human papillomavirus; MSM, men who have sex with men; YMHPV, Young Men’s HPV study.
HPV Types Detected in Anal and Oral Specimens and Concordance, Overall and by Number of HPV Types Detected, Among 1033 Young Gay, Bisexual, and Other MSM Participating in the YMHPV Study, Two Cities, United States, 2012–2014
| . | Anal Specimens With any HPV Detected . | Oral Specimens With any HPV Detected . | Paired Anal and Oral Specimens With Type-Specific Concordant HPV . | |||
|---|---|---|---|---|---|---|
| n . | % . | n . | % . | n . | % . | |
| Overall | 742 | 71.8 | 101 | 9.8 | 35 | 3.4 |
| Number of HPV types detected | ||||||
| 1 | 181 | 24.4 | 73 | 72.3 | 26 | 74.2 |
| 2 | 156 | 21.0 | 18 | 17.8 | 7 | 20.0 |
| 3 | 120 | 16.2 | 7 | 6.9 | 0 | 0.0 |
| 4 | 68 | 9.2 | 1 | 1.0 | 0 | 0.0 |
| 5 | 61 | 8.2 | 0 | 0.0 | 0 | 0.0 |
| 6 | 42 | 5.7 | 1 | 1.0 | 1 | 2.9 |
| 7 | 25 | 3.4 | 1 | 1.0 | 1 | 2.9 |
| 8 | 25 | 3.4 | 0 | 0.0 | 0 | 0.0 |
| 9 | 15 | 2.0 | 0 | 0.0 | 0 | 0.0 |
| 10 | 19 | 2.6 | 0 | 0.0 | 0 | 0.0 |
| >10 | 30 | 4.0 | 0 | 0.0 | 0 | 0.0 |
| . | Anal Specimens With any HPV Detected . | Oral Specimens With any HPV Detected . | Paired Anal and Oral Specimens With Type-Specific Concordant HPV . | |||
|---|---|---|---|---|---|---|
| n . | % . | n . | % . | n . | % . | |
| Overall | 742 | 71.8 | 101 | 9.8 | 35 | 3.4 |
| Number of HPV types detected | ||||||
| 1 | 181 | 24.4 | 73 | 72.3 | 26 | 74.2 |
| 2 | 156 | 21.0 | 18 | 17.8 | 7 | 20.0 |
| 3 | 120 | 16.2 | 7 | 6.9 | 0 | 0.0 |
| 4 | 68 | 9.2 | 1 | 1.0 | 0 | 0.0 |
| 5 | 61 | 8.2 | 0 | 0.0 | 0 | 0.0 |
| 6 | 42 | 5.7 | 1 | 1.0 | 1 | 2.9 |
| 7 | 25 | 3.4 | 1 | 1.0 | 1 | 2.9 |
| 8 | 25 | 3.4 | 0 | 0.0 | 0 | 0.0 |
| 9 | 15 | 2.0 | 0 | 0.0 | 0 | 0.0 |
| 10 | 19 | 2.6 | 0 | 0.0 | 0 | 0.0 |
| >10 | 30 | 4.0 | 0 | 0.0 | 0 | 0.0 |
Abbreviations: HPV, human papillomavirus; MSM, men who have sex with men; YMHPV, Young Men’s HPV study.
HPV Types Detected in Anal and Oral Specimens and Concordance, Overall and by Number of HPV Types Detected, Among 1033 Young Gay, Bisexual, and Other MSM Participating in the YMHPV Study, Two Cities, United States, 2012–2014
| . | Anal Specimens With any HPV Detected . | Oral Specimens With any HPV Detected . | Paired Anal and Oral Specimens With Type-Specific Concordant HPV . | |||
|---|---|---|---|---|---|---|
| n . | % . | n . | % . | n . | % . | |
| Overall | 742 | 71.8 | 101 | 9.8 | 35 | 3.4 |
| Number of HPV types detected | ||||||
| 1 | 181 | 24.4 | 73 | 72.3 | 26 | 74.2 |
| 2 | 156 | 21.0 | 18 | 17.8 | 7 | 20.0 |
| 3 | 120 | 16.2 | 7 | 6.9 | 0 | 0.0 |
| 4 | 68 | 9.2 | 1 | 1.0 | 0 | 0.0 |
| 5 | 61 | 8.2 | 0 | 0.0 | 0 | 0.0 |
| 6 | 42 | 5.7 | 1 | 1.0 | 1 | 2.9 |
| 7 | 25 | 3.4 | 1 | 1.0 | 1 | 2.9 |
| 8 | 25 | 3.4 | 0 | 0.0 | 0 | 0.0 |
| 9 | 15 | 2.0 | 0 | 0.0 | 0 | 0.0 |
| 10 | 19 | 2.6 | 0 | 0.0 | 0 | 0.0 |
| >10 | 30 | 4.0 | 0 | 0.0 | 0 | 0.0 |
| . | Anal Specimens With any HPV Detected . | Oral Specimens With any HPV Detected . | Paired Anal and Oral Specimens With Type-Specific Concordant HPV . | |||
|---|---|---|---|---|---|---|
| n . | % . | n . | % . | n . | % . | |
| Overall | 742 | 71.8 | 101 | 9.8 | 35 | 3.4 |
| Number of HPV types detected | ||||||
| 1 | 181 | 24.4 | 73 | 72.3 | 26 | 74.2 |
| 2 | 156 | 21.0 | 18 | 17.8 | 7 | 20.0 |
| 3 | 120 | 16.2 | 7 | 6.9 | 0 | 0.0 |
| 4 | 68 | 9.2 | 1 | 1.0 | 0 | 0.0 |
| 5 | 61 | 8.2 | 0 | 0.0 | 0 | 0.0 |
| 6 | 42 | 5.7 | 1 | 1.0 | 1 | 2.9 |
| 7 | 25 | 3.4 | 1 | 1.0 | 1 | 2.9 |
| 8 | 25 | 3.4 | 0 | 0.0 | 0 | 0.0 |
| 9 | 15 | 2.0 | 0 | 0.0 | 0 | 0.0 |
| 10 | 19 | 2.6 | 0 | 0.0 | 0 | 0.0 |
| >10 | 30 | 4.0 | 0 | 0.0 | 0 | 0.0 |
Abbreviations: HPV, human papillomavirus; MSM, men who have sex with men; YMHPV, Young Men’s HPV study.
Type-Specific Concordance
Type-specific HPV concordance was present in specimens from both anal and oral sites in only 35 (3.4%) participants. One HPV type was concordant at both sites in 26 participants, and 2 HPV types were concordant at both sites in 7 participants.
Concordance of more than 2 HPV types at both anatomical sites was rare. Six HPV types were concordant in 1 participant, and 7 HPV types were concordant in 1 participant. Both of these participants reported having had ≥5 sex partners in the past 3 months, and 1 reported being HIV positive, but both were nonsmokers.
Among the 35 participants with any concordance detected at anal and oral sites, complete concordance of all HPV genotypes was observed in 4 (11.4%) participants; the other 31 (88.6%) participants also tested positive for at least 1 additional HPV type at either anatomical site. Participants with any concordance had an average of 5.3 types detected in anal specimens, significantly more than the average of 2.5 HPV types detected in anal specimens from other YMHPV study participants (P < .001). Similarly, participants with any concordance had an average of 1.9 HPV types detected in oral specimens, significantly more than the average of 0.1 HPV types detected in oral specimens from other YMHPV study participants (P < .001).
Type-specific HPV prevalence was similar with or without concordance: concordant types occurred in similar order as their prevalence at each anatomical site, with HPV types 16, 6, and 59 occurring most frequently, although some types (ie, HPV51) with considerable prevalence in either specimen alone, were not found concordantly at both sites of the same person (Figure 1). The 53 concordant types made up 36.1% of all oral types but only 1.9% of all anal types.
Factors Associated With Concordance
HIV and smoking were found to be significantly associated with more concordant anal–oral HPV infections. Among the 110 participants who reported being HIV positive, prevalence compared to other YMHPV study participants was significantly higher for both any anal HPV (92.7% vs 69.3%; P < .001) and any oral HPV (21.8% vs 8.3%; P < .001). Congruently, the frequency of any HPV found at both anatomical sites (20.0% vs 6.6%; P < .001) and type-specific concordance at anal and oral sites (10.0% vs 3.4%; P < .001) were also significantly higher when comparing HIV-positive participants to others. Also, among the 379 participants who were current smokers, the prevalence compared with other YMHPV study participants was significantly higher for any anal HPV (77.6% vs 68.5%; P < .002) and any oral HPV (12.7% vs 8.1%; P < .022), as well as for frequency of any HPV found at both anatomical sites (10.3% vs 6.7%; P < .029) and type-specific concordance (5.0% vs 3.4%; P < .033).
DISCUSSION
Anal HPV prevalence was high among this high-risk population of young, sexually active, gay, bisexual, and other MSM compared with other recent studies of MSM [6, 7]. However, prevalence of oral HPV was less than 10% and only marginally higher than in the general male population [8]. Accordingly, concurrent anal–oral HPV infections were not common overall, but the vast majority of participants with oral HPV were also positive for anal HPV.
The results demonstrated that type-specific anal–oral concordance was higher among participants who reported being HIV positive or current smokers. Both of these risk factors are well known to be associated with high HPV prevalence, and thus the frequency of concordance detected could be largely attributable to the higher HPV prevalence found overall in these groups.
Among the 83 participants with concurrent anal and oral HPV infections, type-specific concordance was present in less than half, and in most cases, additional nonconcurrent types were also detected. The small group with type-specific concordance at both anatomical sites also had higher prevalences of any type of HPV overall, thereby increasing the likelihood of detecting concordance for at least 1 HPV type. Of note, HIV prevalence was also higher among this group than in the other study participants, which may explain the higher site-specific prevalence.
These findings are subject to at least 3 limitations. First, this study population reported high sexual activity overall, as most participants had multiple sex partners in the past 3 months. It is therefore not feasible to assess the correlation between sexual activity and coinfections at the 2 anatomical sites using this dataset. Second, HIV status was self-reported, allowing the possibility of misclassification of some individuals who do not know their HIV status or chose not to disclose it. Third, the overall high prevalence of anal HPV and low prevalence of oral HPV in this study population limited the extent to which additional factors that contributed to coinfections and type-specific concordance could be assessed. Other published studies with smaller sample sizes have found no identical HPV types in anal and oral specimen pairs [9], or did not report performing this analysis presumably due to the lack of noteworthy results [10, 11]. An assessment of concordant types among MSM in Peru reported somewhat higher oral prevalence (30%) and concordant types in 5% [7]. That is the only study to report results comparable to our study, although its sample size of 66 was substantially smaller.
In summary, we found a significant correlation between oral and anal HPV infection status in a population of highly sexually active young gay, bisexual, and other MSM. However, concordance of specific HPV types was rare, similar to the low observed concordance of oral and cervical HPV in studies of females [4]. This suggests either that initial exposure occurred in separate events, and/or that the 2 tissues have different susceptibility to HPV infection. Faster clearance from oral epithelia, or tissue-specific differences to detect HPV DNA in exfoliated cell samples could also explain the general differences in prevalence between the 2 sites. It is also possible that some latent infections stayed undetected due to limitations of exfoliated cell sampling. Nevertheless, the low concordance observed here weakens assumptions that self-inoculation between oral and genital sites play an essential role in incident HPV infections.
Notes
Acknowledgments. We thank the following persons for their contributions to the overall success of the YMHPV study: all study participants; Gitika Panicker, PhD; Adam Parrish, PhD; Tom Collins, BS; Danielle Miller, BS; William Lonergan, MBA; Cody Randel, MMS; Mark McGrath, MPH; Steven Carrasco, MPH; Janell Moore, MPH; and Akbar Zaidi, PhD.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Financial support. This work was supported by the Centers for Disease Control and Prevention (CDC).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
Author notes
Correspondence: E. Meites, MD, MPH, Division of Viral Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, 1600 Clifton Rd, Atlanta, GA 30329 ([email protected]).




