-
PDF
- Split View
-
Views
-
Cite
Cite
Atis Muehlenbachs, Olimpia de la Rosa Vázquez, Daniel G. Bausch, Ilana J. Schafer, Christopher D. Paddock, Jean Paul Nyakio, Papys Lame, Eric Bergeron, Andrea M. McCollum, Cynthia S. Goldsmith, Brigid C. Bollweg, Miriam Alía Prieto, Robert Shongo Lushima, Benoit Kebela Ilunga, Stuart T. Nichol, Wun-Ju Shieh, Ute Ströher, Pierre E. Rollin, Sherif R. Zaki, Ebola Virus Disease in Pregnancy: Clinical, Histopathologic, and Immunohistochemical Findings, The Journal of Infectious Diseases, Volume 215, Issue 1, 1 January 2017, Pages 64–69, https://doi.org/10.1093/infdis/jiw206
Close - Share Icon Share
Here we describe clinicopathologic features of Ebola virus disease in pregnancy. One woman infected with Sudan virus in Gulu, Uganda, in 2000 had a stillbirth and survived, and another woman infected with Bundibugyo virus had a live birth with maternal and infant death in Isiro, the Democratic Republic of the Congo in 2012. Ebolavirus antigen was seen in the syncytiotrophoblast and placental maternal mononuclear cells by immunohistochemical analysis, and no antigen was seen in fetal placental stromal cells or fetal organs. In the Gulu case, ebolavirus antigen localized to malarial parasite pigment–laden macrophages. These data suggest that trophoblast infection may be a mechanism of transplacental ebolavirus transmission.
Ebola virus disease (EVD) and Marburg virus disease are caused by viruses of the Ebolavirus and Marburgvirus genera (family Filoviridae). Here, we collectively refer to Ebola virus (EBOV), Sudan virus (SUDV) and Bundibugyo virus (BDBV) all within the Ebolavirus genus as ebolaviruses. Filovirus infection during pregnancy is associated with maternal hemorrhage, preterm labor, miscarriage, and maternal and neonatal death [1, 2]. Supplementary Table 1 presents a summary of the scientific literature to date; maternal death occurred in 102 of 125 reported cases (82%), and there was uniform loss of offspring, whether by miscarriage, stillbirth, or neonatal death. Of the 18 live births, the longest survival was 19 days [3].
With the exception of liver biopsies performed for patients with Marburg virus disease in Marburg, Germany, in 1967 [4] and a biopsy to evaluate a periorbital mucormycete fungus coinfection in a patient who survived EVD in the Democratic Republic of the Congo (DRC) in 1995 [5], human pathological studies on patients with filovirus infection have been almost entirely limited to postmortem samples at the end stage of disease [6], largely confined to skin punch biopsies and core needle biopsies of the liver and spleen.
Despite the severity of filovirus infection in pregnancy for both mother and child, very little is known regarding pathogenesis. Fetal-placental viral tropism has been hypothesized due to recent observations during the 2013–2016 West Africa EBOV outbreak: pregnant women were noted to survive EVD and clear virus from the blood without fetal loss during acute infection and to deliver stillbirths in the subsequent weeks and months with relatively high EBOV RNA levels in placental and fetal tissue swab specimens [7–10]. We report clinical, histopathologic, and immunohistochemical findings of SUDV and BDBV infections in 2 pregnant women and their offspring that help shed light on the pathogenesis of fetal infection and loss in EVD.
METHODS
Patients
Two pregnant women with EVD were cared for in Ebola treatment centers during ebolavirus outbreaks in Gulu, Uganda, in 2000 [11, 12] and Isiro, DRC, in 2012 [13] (Schafer, unpublished data). Specimens were collected and evaluated during the course of the outbreak responses.
Ebolavirus Diagnostic Testing
SUDV reverse transcription–polymerase chain reaction (RT-PCR) and enzyme-linked immunosorbent assays (ELISAs) in Gulu and BDBV RT-PCR assays in Isiro were performed as previously described [14, 15] in field laboratories run by the Viral Special Pathogens Branch (VSPB), Centers for Disease Control and Prevention (CDC; Atlanta, Georgia). BDBV immunoglobulin M (IgM) and immunoglobulin G (IgG) ELISAs were performed by the VSPB in Atlanta.
Histopathologic Analysis, Immunohistochemical Analysis, and Transmission Electron Microscopy
Placenta (Gulu and Isiro), fetal tissues (Gulu), and a postmortem skin biopsy (Isiro) were collected and placed in 10% neutral buffered formalin and transported to the CDC, where the samples were processed using standard histological methods. The identification and scoring of malarial parasite pigment was performed as previously described [16]. Immunohistochemical analysis for ebolavirus antigens was performed using a polymer-based indirect immunoalkaline phosphatase detection system for colorimetric detection (Biocare Medical, Concord, California). Rabbit polyclonal antisera against EBOV, SUDV, and Reston virus and EBOV hyperimmune mouse ascitic fluid (courtesy of Thomas Ksiazek, VSPB, CDC), previously shown to detect SUDV and BDBV antigens, were each used at a 1:1000 dilution with appropriate positive and negative controls [17]. On-slide embedding and transmission electron microscopy was performed as previously described [18].
RESULTS
Patient 1, Gulu, Uganda
Case Presentation
A 30-year-old housewife in the Gulu District of northern Uganda presented with a 1-day history of asthenia, anorexia, abdominal pain, nausea, vomiting, diarrhea, and dry cough. She reported previous contact with patients who had EVD in her village. Based on information provided during her antenatal clinic visits, she had been pregnant for 28 weeks but reported feeling no fetal movements in the past few days. Vital signs were not taken at admission, owing to minimal staffing, but the next day her axillary temperature was 36.7°C, here heart rage was 120 beats/minute, and her respiratory rate was 24 breaths/minute, with an oxygen saturation level of 92% by pulse oximetry. Physical examination revealed conjunctival injection, diffuse abdominal tenderness, and slight pulmonary rales. The patient was clearly pregnant, but no formal obstetric examination was performed. She was administered intravenous fluids and oral amoxicillin. Her blood tested positive for SUDV by both ELISA antigen assay and nested RT-PCR.
On day four of illness, the patient spontaneously delivered a dead but apparently morphologically normal fetus and placenta. The degree of vaginal bleeding did not seem abnormal for a stillbirth. Oral and written consent was obtained from the patient to have placenta and fetal tissues submitted for pathologic examination. Over the next 3 days, the patient complained of joint pain and swelling, throat and chest pain, persistent dry cough, dyspnea, and, briefly, hiccups. Her wrists and knees were visibly swollen and tender to the touch, and pulmonary rales persisted. She was consistently febrile. Disease severity peaked at day 7 of illness, when vital signs were an axillary temperature of 37.8°C, a heart rate of 128 beats/minute, a respiratory rate of 30 breaths per minute, and an oxygen saturation level of 90%. She gradually improved, and she was discharged on day 13 with normal vital signs and all symptoms resolved.
Pathologic Findings
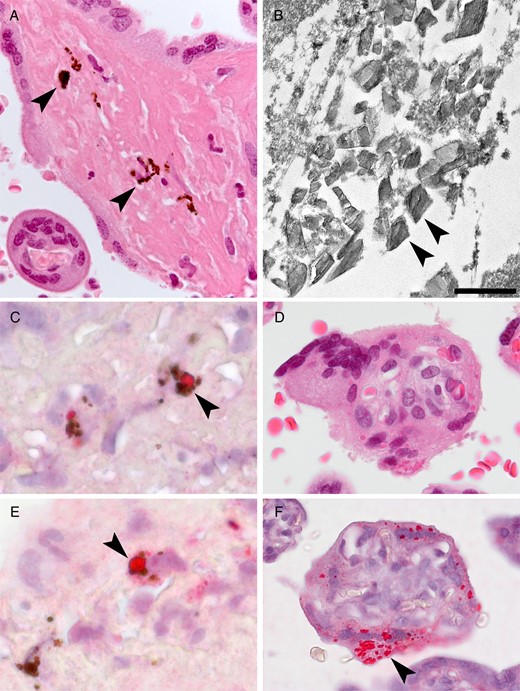
Placental findings from patient 1. A, Hemozoin (malarial parasite pigment) in fibrin (arrowheads). B, Transmission electron microscopy showing malarial parasite hemozoin crystallites (arrowheads); no ebolavirus virions were identified. Scale bar = 500 nm. C, Colocalization of ebolavirus antigen (arrowheads) with malarial parasite pigment. D, Serial sections by hematoxylin-eosin staining (upper) and immunohistochemical staining (lower) showing ebolavirus antigen (arrowhead) localized to the syncytiotrophoblast.
Immunohistochemical analysis revealed ebolavirus antigen in the placenta, primarily within areas of fibrin deposition, localized to embedded maternal mononuclear cells, including malarial parasite pigment–laden macrophages (Figure 1C). Focal immunostaining was seen within the syncytiotrophoblast (Figure 1D). The decidua, fetal placental villous stroma, amnion, and umbilical cord were negative by immunohistochemical analysis, and no tissue necrosis or viral inclusions were noted.
Fetal tissues (lung, heart, liver, spleen, kidney, skin, and bone marrow) were well preserved with minimal autolysis, were normal for gestational age, and had no necrosis or viral inclusions. All fetal tissues were negative by immunohistochemical analysis.
Patient 2, Isiro, DRC
Case Presentation
Several clinical details have been previously published about this patient [13], a 29-year-old housewife, gravida 8 and para 7, who was transferred from a health center because of suspicion of EVD by a local clinician who was aware that her relative died recently. She was admitted to the Ebola treatment center on day 4 of illness with fever, fatigue, headache, abdominal pain (with uterine contractions), anorexia, dysphagia, vomiting, diarrhea, and muscle and joint pain. Her last menstrual period date was unknown, but she was initially estimated to be 7 months pregnant. Conjunctival injection was noted. Her heart rate was 80 beats/minute, and her respiratory rate was 20 breaths/minute. Her cervix was 50% effaced with a 4-cm dilation, and fetal movement was normal.
Before admission, she was treated with oral artemether-lumefantrine (AL), intravenous quinine, ampicillin, diazepam, cimetidine, and scopolamine. At the Ebola treatment center, she was treated with oral rehydration and antibiotics (cefixime, presumably), and AL was continued. On the day of admission, she tested positive for BDBV by RT-PCR and IgM ELISA. On day 5 of illness, her cervix was 100% effaced with an 8-cm dilation, and she was treated with oxytocin. A malaria rapid diagnostic test was positive, and AL was continued. That night (day 6 of illness), spontaneous vaginal delivery of a live-born male infant occurred without assistance. The degree of vaginal bleeding did not seem abnormal for a normal delivery, although she had had an episode of black stool some hours later. She was treated with oxytocin, ergometrine, intravenous fluids, and cefixime, and Plumpy′nut (Nutriset) was provided. On day 7, the mother's condition rapidly deteriorated, with wheezing, drowsiness, weakness, and a temperature of 38.5°C. Antibiotics were switched to ceftriaxone. On day 8, she became comatose and died. A postmortem skin sample was collected from the mother as part of the routine outbreak response protocol [17].
The infant appeared healthy at birth, with Apgar scores of 8/10/10, and was clinically assessed to be at term on the basis of examination of the nails and soles of the feet. Infant formula was provided, although the baby may have briefly breastfed immediately after delivery. A placental sample was collected to evaluate for BDBV. Blood collected at 1 day of age (the second day of life) was positive for BDBV by RT-PCR, with a cycle threshold (Ct) of 29.2. Over the next few days, the baby was noted to be quiet and inactive. He became febrile (temperature, 38.5°C) on day 4 of age, and repeat testing of the blood revealed an RT-PCR Ct of 17.9 with negative IgM and IgG ELISA results. Over the next few days, the baby had hematemesis and bloody stools. He developed respiratory distress and coma and died on the seventh day of age (eighth day of life). No postmortem specimens were collected from the infant.
Pathologic Findings
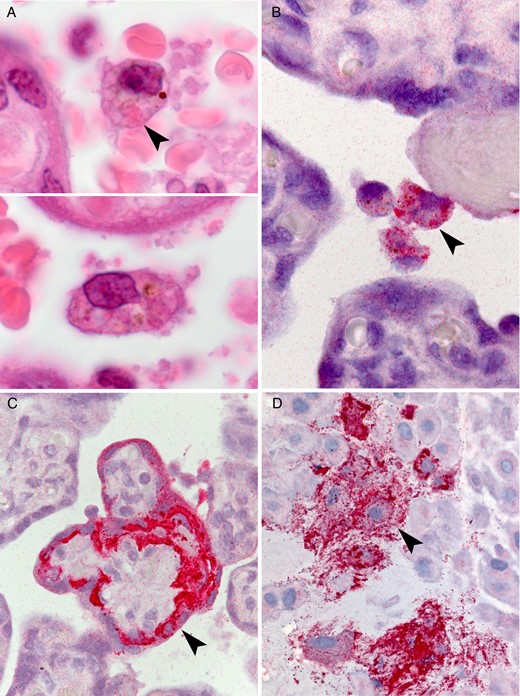
Placental findings from patient 2. A, Circulating atypical maternal macrophages with vacuolated cytoplasm and eosinophilic cytoplasmic granules suggestive of viral inclusions (arrowhead). By immunohistochemical staining, ebolavirus antigen was found to localize to circulating maternal macrophages (B), syncytiotrophoblast (C), and intermediate trophoblast within the basal plate (D; arrowheads).
Ebolavirus antigen was seen by immunohistochemical analysis within the circulating large atypical maternal mononuclear cells (Figure 2B). Antigen was also present in multiple foci within the villous syncytiotrophoblast (Figure 2C), frequently most intense at the basal aspect. Fetal stromal and endothelial cells were negative by immunohistochemical analysis. In the basal plate, immunostaining was prominent within the extravillous trophoblast (Figure 2D) ,with scattered additional cell types likely representing decidual and maternal mononuclear cells. Focally, the lining cells of the maternal vessels of the basal plate (likely endovascular trophoblasts) were positive. Within the placenta, fetal stromal tissue, including villous blood vessels, was negative by immunohistochemical analysis. The postmortem maternal skin specimen was morphologically normal and immunohistochemically negative.
DISCUSSION
Vertical transmission of pathogens can be by transplacental, transvaginal, or by breastfeeding routes. Placenta sampling provides the opportunity to study disease processes in living patients and gain insights regarding the mode and mechanism of vertical transmission. In this study, SUDV or BDBV antigen was noted in fetal trophoblast cells, suggesting that these viruses can infect and potentially cross the placental epithelial barrier, resulting in transplacental infection of the fetus. Transplacental infection of the fetus by EBOV has been previously documented in stillbirths by PCR analysis of amniotic fluid, fetal blood, and fetal swab specimens [7, 8]. The immunoprotective role of the placenta may promote the persistence of virus observed in these cases even after virus has been cleared from maternal blood [8, 9].
Several human pathogens can efficiently penetrate the placental barrier and infect the fetus, including some herpesviruses, human immunodeficiency virus, Zika virus, Treponema, and Toxoplasma. The trophoblast is the major cellular barrier to fetal infection, and it comprises 2 major types: the villous trophoblast, which is directly exposed to maternal blood, and the extravillous trophoblast, which invades the maternal decidua and directly contacts maternal cells, including lymphocytes and decidual stromal cells. In this study, both the syncytiotrophoblast (in both patients) and the extravillous trophoblast (in the patient from Isiro) demonstrated ebolavirus antigen by immunohistochemical analysis.
Findings from the 2, cases reported here together with the recent reports of EBOV RNA RT-PCR–positive stillbirths in women who have recovered from EVD [7, 8], suggest that ebolaviruses have a degree of placental tropism. Ebolavirus entry into cells involves endocytosis and macropinocytosis [19]—both mechanisms are important for the placental acquisition of maternal nutrients for fetal growth [20]. The NPC1 gene, which is required for ebolavirus cellular infection [21], is expressed in the placental syncytiotrophoblast [22].
Unexpectedly, fetal tissue from the Gulu patient showed no features of ebolavirus infection, suggesting that fetal demise was attributable to processes that occurred early in the course of maternal infection (eg, a systemic inflammatory response), which is consistent with the patient's note of the lack of fetal movement in the days prior to presentation and delivery. Postmortem tissue was not available from the baby of the Isiro patient, but he was RT-PCR positive for BDBV by day 1, with a qualitative increase by day 4 of age, suggesting that the infant died of EVD. Although the finding of the immunohistochemically positive trophoblast suggests potential transplacental BDBV transmission in the Isiro case, the baby appeared healthy at birth, and fetal stromal tissue within the placenta was immunohistochemically negative, so transvaginal infection cannot be excluded. Transplacental and transvaginal ebolavirus infection would not be mutually exclusive, such as in vertical transmission of human immunodeficiency virus, in which one third is thought to be intrauterine-transplacental and the remainder transvaginal in the absence of preventive efforts [23].
Identifying infections that occur near or at the time of delivery is particularly important because these may be suitable targets for prevention through procedural or chemotherapeutic interventions. The World Health Organization currently recommends that asymptomatic infants born to mothers with EVD be separated and formula fed. However, if the infant is confirmed or suspected to be infected, the benefits of breastfeeding are thought to outweigh the risks, and breastfeeding is thus recommended if the mother is able [24].
The colocalization of malarial parasite pigment and ebolavirus antigen in the placenta of the Gulu patient is a novel finding and suggests that these 2 pathogens may interact at a cellular level. Filoviruses target monocyte-macrophages, and monocyte-macrophage infiltrates are a hallmark of active placental Plasmodium falciparum infection. Similar infiltrates are seen in other organs (particularly the liver and spleen) in nonpregnant individuals with severe malaria, further raising consideration of the potential for pathogen interaction. Of note, peripheral blood from the Isiro patient had a positive result of a malaria rapid diagnostic test but no evidence of malaria-related placental pathology, perhaps because she received antimalarial treatment with AL; up to one third of documented cases of antenatal malaria do not show evidence of malaria in the placenta [25].
In contrast to the human infections described here, vertical transmission does not appear to occur in Egyptian fruit bats (Rousettus aegyptiacus), which are thought to be the natural reservoir of Marburg virus [26]; placentas of 4 naturally captured Marburg virus RNA–positive Egyptian fruit bats were all PCR negative. This can perhaps be explained by recognition that zoonotic pathogens often have unique maintenance mechanisms in distinct hosts. The cellular structure of placentas is markedly diverse across mammalian species, including whether fetal trophoblasts are directly exposed to maternal blood. Such structural differences may influence the likelihood of pathogen vertical transmission and/or placental tropism in natural versus incidental hosts.
Future sampling of placental tissue is necessary to fully understand the pathogenesis of EVD in pregnant women and their offspring and to ultimately develop ways to prevent or treat infection. In addition, given the very high rates of malaria in many areas where filovirus outbreaks occur and EBOV–malaria parasite coinfections were frequent during the 2013–2016 West African outbreak [27], future investigation of the interaction and clinical outcomes associated with this coinfection should be a priority.
Supplementary Data
Supplementary materials are available at http://jid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We thank Felix Kaducu (Gulu Regional Hospital, Uganda), for his contributions to the case; Jonathan Towner (Centers for Disease Control and Prevention [CDC]), for discussion; and Diane Morof (CDC), for manuscript review.
Disclaimer. The findings and conclusions herein are those of the authors and do not necessarily represent the official position of the CDC.
Financial support. This work was supported by the CDC.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
Author notes
Presented in part: American Society of Tropical Medicine and Hygiene Annual Meeting, Philadelphia, Pennsylvania, November 2015.
Present affiliation: Pandemic and Epidemic Diseases, World Health Organization, Geneva, Switzerland (D. G. B.); and Bacterial Special Pathogens Branch, Division of High-Consequence Pathogens and Pathology, Centers for Disease Control and Prevention, Atlanta, Georgia (I. J. S.).




