-
PDF
- Split View
-
Views
-
Cite
Cite
Etienne Simon-Loriere, Ren-Jye Lin, Sita Mint Kalayanarooj, Ampaiwan Chuansumrit, Isabelle Casademont, Shyr-Yi Lin, Han-Pang Yu, Worachart Lert-itthiporn, Wathanee Chaiyaratana, Nattaya Tangthawornchaikul, Kanchana Tangnararatchakit, Sirijitt Vasanawathana, Bi-Lan Chang, Prapat Suriyaphol, Sutee Yoksan, Prida Malasit, Philipe Despres, Richard Paul, Yi-Ling Lin, Anavaj Sakuntabhai, High Anti–Dengue Virus Activity of the OAS Gene Family Is Associated With Increased Severity of Dengue, The Journal of Infectious Diseases, Volume 212, Issue 12, 15 December 2015, Pages 2011–2020, https://doi.org/10.1093/infdis/jiv321
Close - Share Icon Share
Abstract
Dengue is a mosquito-borne viral disease that afflicts millions of individuals worldwide every year. Infection by any of the 4 dengue virus (DENV) serotypes can result in a spectrum of disease severity. We investigated the impact of variants of interferon-regulated innate immunity genes with a potent antiviral effect on the outcome of DENV infection. We compared the effect of OAS gene family variants on 2 DENV serotypes in cell culture. While both OAS1-p42 and p46 showed antiviral activity against DENV-2, only OAS1-p42 presented anti–DENV-1 activity. Conversely, whereas both OAS3_S381 and R381 variants were able to block DENV-1 infection, the anti–DENV-2 activity observed for OAS3_S381 was largely lost for the R381 variant. By means of an allelic association study of a cohort of 740 patients with dengue, we found a protective effect of OAS3_R381 against shock (odds ratio [OR], 0.37; P < .001). This effect was due to DENV-2 infections (OR, 0.13; P = .007) but was absent for DENV-1, in accordance with the serotype-dependent OAS3 activity found in the functional study. Severe dengue has long been associated with a cytokine storm of unclear origin. This work identifies an early innate immunity process that could lead to the immune overreaction observed in severe dengue and could be triggered by a specific host genotype-pathogen genotype interaction.
Dengue is a mosquito-borne viral disease that afflicts millions of individuals yearly. Infection outcome ranges from asymptomatic or mild fever to life-threatening hemorrhagic fever and shock. Human genetics [1, 2], secondary infection, and virus genotype have been shown to be associated with disease severity [3]. Several epidemiological studies have shown that infection outcome can be influenced by human ethnicity [1, 2]. Genetic variation has been linked to disease severity, and genotypic variation among ethnic groups could explain these observations. Alleles of susceptibility or resistance to severe dengue have notably been identified in genes encoding HLA class I and II, tumor necrosis factor α (TNF-α), Fcγ receptor IIa, and CD209, among others [4–9]. More recently, a genome-wide association study of Vietnamese children with severe dengue and cord blood samples identified MICB and PCLE1 as playing an important role in the development of severe disease [10].
There is mounting evidence for a major role of innate immunity in the earliest stages of arbovirus infections. However, little is known about the early innate immune response and the mechanisms that are responsible for virus-induced immune protection. Interferon α/β (IFN-α/β) constitute the very first lines of antiviral innate immune response against arboviral infections. IFN-α/β trigger the activation of IFN-stimulated genes, such as members of the 2′,5′-oligoadenylate synthetase (OAS) family, that play a critical role in the establishment of an antiviral state against different RNA and DNA viruses [11–13].
OAS plays important roles in the antiviral effect of IFN through the activation of a latent endoribonuclease, RNase L [14]. Oas1b, one of the 8 OAS1 genes in the mouse genome, was first identified as a flavivirus resistance gene [15]. In humans, OAS is a family of 10 different isoforms encoded by 3 functional genes (OAS1, OAS2, and OAS3) and an OASL gene. Our group reported that OAS1 p42, OAS1 p46, and OAS3 p100, but not the other gene family members, exhibited anti-DENV activity and that these antiviral effects were largely lost in cells deprived of RNase L expression [16]. Furthermore, RNase L activity measured by ribosomal RNA (rRNA) integrity, indicated that the human OAS1 p42, OAS1 p46, and OAS3 p100 triggered activation of RNase L during DENV replication. Thus, OAS1 p42/p46 and OAS3 p100 are likely to contribute to host defense against DENV infection and to play a role in determining the outcome of DENV infection severity.
The human OAS family was demonstrated to contribute to the susceptibility to West Nile virus (WNV) [17, 18]. Clinical evidence from WNV-seropositive patients showed that the A allele at single-nucleotide polymorphism (SNP) rs10774671 of OAS1 (OAS1 splicing variant), which generates OAS1 p48 and p52 but not p46, is a risk factor for initial infection by WNV [17]. This polymorphism has also been shown to be associated with other infections, such as those due to measles virus [19] or hepatitis C virus [20]. Several studies have shown that cells expressing human OASs could mediate antiviral effects [21–24]. A recent study with a limited sample size could not find association between polymorphism of OAS1, OAS2, and OAS3 and mild dengue or dengue with plasma leakage, but the study suggested that specific haplotypes could be differentially associated with symptomatic dengue [25].
To understand how OAS antiviral activity could lead to different disease outcome when exposed to different DENV serotype, we investigated the effect of OAS variants on viral replication and cytokine production ex vivo. We then performed a genetic association study on a cohort of patients with different degrees of dengue severity, to understand the role of OAS variants on clinical outcome. In addition to the OAS1 splicing variant, we evaluated the role and function of 2 common nonsynonymous polymorphisms of OAS3 found in the world's populations, OAS3_K18R (rs1859330) and OAS3_S381R (rs2285933). We report for the first time 2 serotype-specific associations between OAS variants and dengue severity in Thailand. An OAS1 splicing variant was associated with increased susceptibility to plasma leakage and shock, while an OAS3 missense variant was strongly associated with protection against shock. It is noteworthy that these effects only occurred in DENV-2 infections and not DENV-1 infections. In contrast to other viral infections [17, 20], the risk of severe dengue disease was correlated with the strength of OAS activity.
MATERIALS AND METHODS
Cohort
We enrolled 740 patients (age range, 1–31 years; male to female ratio, 0.989) with symptomatic DENV infection during 2000–2003 from 2 hospitals each in Bangkok, Ramathibodi, and Siriraj, Thailand, and 1 hospital in Khonkaen, Thailand (Supplementary Data). Patients in whom dengue was suspected on the basis of clinical features were admitted to the hospital. The diagnosis of DENV infection was later confirmed by either detection of viral genome or a comparable immunoglobulin G (IgG) and immunoglobulin M (IgM) titers, measured by an enzyme-linked immunosorbent assay, in late acute and/or convalescent sera.
We defined dengue severity according to 1997 and 2009 World Health Organization (WHO) criteria [26, 27]. Severe dengue was defined by the presence of severe plasma leakage and/or bleeding, leading to shock or profound shock (ie, dengue shock syndrome, as defined by 2009 WHO criteria [26]), which is equivalent to grade 3 and 4 dengue hemorrhagic fever, according to 1997 WHO criteria [27]. Cases of nonsevere dengue were defined according to 1997 WHO criteria, based on evidence of plasma leakage (an increase in hematocrit of ≥20%) or pleural effusion. Secondary infection was defined as a DENV-specific IgM to IgG ratio of <1.8.
Ethics Statement
The project protocol and objectives were carefully explained to the patients and their parents or relatives. Written informed consent was individually obtained from all subjects or, for minors, from their parent or tutor. The protocol was approved by the ethics committees from the Faculty of Medicine, Ramathibodi Hospital, Mahidol University; the Faculty of Medicine, Siriraj Hospital, Mahidol University; the Khon Kaen Hospital; and the Thailand Ministry of Public Health.
Viral Serotyping and Genotyping
Viral RNA was extracted from plasma samples stored in ethylenediaminetetraacetic acid, using the QIAamp Viral RNA Mini Kit, and reverse transcribed into complementary DNA, using universal primers. We performed nested PCR and distinguished the virus serotype by using serotype-specific primers (Supplementary Data). Genotyping of OAS1 splice variant and OAS3_S381R polymorphisms was performed using TaqMan probes (identification numbers, C_2567433_10 and C_2567406_20, respectively; Applied Biosystems) according to the manufacturer's recommendations. Genotyping of OAS3-K18R was performed by PCR and restriction fragment length polymorphism analysis, using ACGAAACCAGAAATCCGAAG as a forward primer and CGCCTTCTCTACGAACTCCTT as a reverse primer. The PCR fragment was cut by the restriction enzyme AluI, with products of 152 bp or 120 bp and 32 bp, respectively.
Functional Genetic Study
DENV-1 Hawaii and DENV-2 PL046 strains [28] were propagated in the mosquito C6/36 cell line grown in Roswell Park Memorial Institute 1640 medium containing 5% fetal bovine serum (FBS). The antiviral potential of OAS variants was tested by using lentiviruses expressing OAS1 variants as described previously [16]. Briefly, A549 cells transduced with lentivirus for 3 days were infected with DENV-1 for 24 hours and were analyzed by an immunofluorescence assay. The HEK293 cell line with tetracycline-regulated expression (T-REx 293; Invitrogen) was cultured in Dulbecco's modified Eagle's medium containing 10% FBS and 5 µg/mL blasticidin (InvivoGen). For viral infection, cells were adsorbed with virus at the indicated multiplicity of infection (MOI) for 2 hours at 37°C. Unbound virus was removed by gentle washing with Hanks’ balanced salt solution (Hyclone), and cells were then cultured at 37°C. Culture supernatants were collected at different times after infection and diluted for plaque-forming assays on BHK-21 cells as previously described [16]. Stable T-REx 293 cell lines for production of doxycycline (Dox)–inducible expression of hemagglutinin (HA)–tagged OAS3 variants, were established after transfection with pcDNA5/TO encoding the variant and selection with hygromycin (250 µg/mL; InvivoGen) and blasticidin (5 µg/mL) for 8 days.
Viral protein expression was analyzed by immunoblotting as previously described [14]. The RNase L activity was determined by detecting the 28S and 18S rRNA integrity, using RNA chips as described previously [16]. Briefly, total cellular RNA was extracted by using an RNeasy total RNA kit (Qiagen), separated with an RNA 6000 Nano Chip, and analyzed with a 2100 Bioanalyzer (Agilent Technologies).
The level of gene expression was measured by reverse transcription–quantitative PCR (RT-qPCR). Briefly, total cellular RNA was isolated and assayed using TaqMan primer/probe sets for IL-1β (Hs01555410_m1), IL-6 (Hs00985639_m1), TNF-α (Hs01113624_g1), MCP-1 (Hs00234140_m1), and IFN-β (Hs01077958_s1), using GAPDH (Hs02758991_g1) as an internal control (Applied Biosystems). RT-qPCR amplification and data analysis were performed on an ABI Prism 7500 system (Applied Biosystems).
Statistical Analyses
Allelic association was analyzed by the Pearson χ2 test. Statistically significant results were confirmed by a permutation test. The impact of environmental and genotype variables on the outcome of infection (presence or absence of plasma leakage and presence or absence of shock) was analyzed by logistic regression. Within-factor levels were compared by the t test. The impact of the genetic variants of OAS3 codons 18 and 381 on the in vitro growth of DENV-1 and DENV-2 was analyzed using generalized linear regression, in which the DENV OAS3 haplotype combination was fitted as an explanatory variable. All viral titers of the controls (Dox negative) were normalized to compare DENV-1 and DENV-2 infections. Viral titers (Dox positive) were normalized with respect to the control titer (Dox negative) for each experiment. Comparison of differential effects on viral titer among haplotypes was performed by the t test. Statistical analyses were performed using GenStat, version 14.1.
Population Genetic Analyses
Data were downloaded from the HapMap and 1000 genomes projects. Haplotypes were constructed using the ELB algorithm in ArleQuin, version 3.1 [29]. The population genetic structures were calculated by analysis of molecular variance, classifying each ethnic population as a group and our Thai mild dengue versus dengue with plasma leakage disease categories (for OAS1) or nonshock dengue and Thai severe dengue disease categories (for OAS3) as 2 subpopulations; population comparisons were then performed. Paired population comparisons of Fst values were calculated by the distance method for single-locus comparisons. The level of significance was calculated by a permutation test.
RESULTS
Anti-DENV Activity of Human OAS1 Isoforms
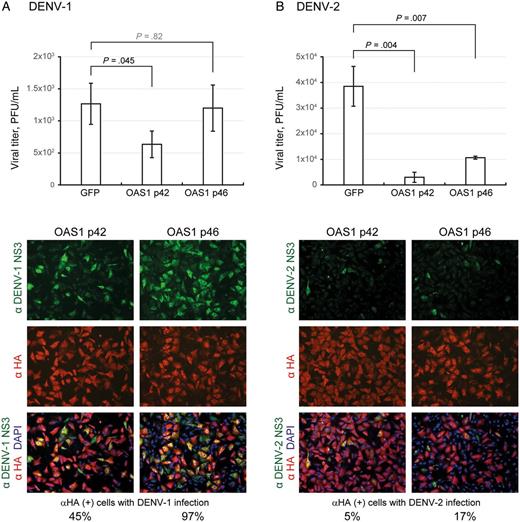
Antiviral activity of human OAS1 p42 and p46 isoforms against dengue virus serotype 1 (DENV-1) and DENV-2 infection. A549 cells were transduced with lentiviral vectors expressing the hemagglutinin (HA)–tagged OAS1 isoforms (p42 or p46). Seventy-two hours after transduction, cells were infected with DENV-1 (A) or DENV-2 (B; multiplicity of infection, 5) for 24 hours. Culture supernatants were collected for viral titration by plaque-forming assays. Viral titers are means ± SD of 3 independent experiments. In parallel, cells were fixed and permeabilized for an immunofluorescence assay. Cells stained with HA-tagged OAS (red), DENV protein NS3 (green), and DAPI (blue) were photographed using a fluorescent microscope. Abbreviations: GFP, green fluorescent protein; PFU, plaque-forming units.
Anti-DENV Activity of Human OAS3 Variants

Anti–dengue virus (DENV) activity of human OAS3 variants with variations at residues 18 and 381. A, Human T-REx 293 cells stably transfected with pcDNA5/TO vector, or pcDNA5/TO with 4 variants of OAS3 depending on amino acid at position 18 and 381, were cultured in the absence (−) or presence (+) of doxycycline (Dox; 1 µg/mL) for 16 hours. The cells were then infected with DENV-1 or DENV-2 (multiplicity of infection, 5) for 24 hours. Culture supernatants were collected for viral titration by plaque-forming assays. Viral titer of the controls (Dox negative), represented as a dashed line, were normalized to compare DENV-1 and DENV-2 infections. Viral titers (Dox positive) were normalized with respect to the control titer (Dox negative) for each experiment and then natural logarithm transformed. Viral titers are means ± SD of 3 independent experiments that have been natural logarithm transformed. Statistically significant differences are indicated. B and C, The cell lysates were harvested for Western blotting with antibodies against DENV NS3, hemagglutinin tag for OAS3 expression, and actin. Band density was quantified with ImageJ, and relative ratios of NS3 to actin are shown. Abbreviation: PFU, plaque-forming units.
Induction of Cytokine Gene Expression
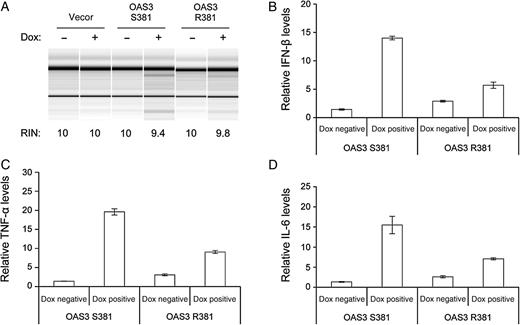
Cytokine induction in cells with human OAS3 S381 versus R381. Human T-REx 293 cells with OAS3 S381 or R381 (on a background of K18)–inducible expression for 24 hours were infected with dengue virus serotype 2 (multiplicity of infection, 5) in medium without (−) or with (+) doxycycline (Dox; 1 µg/mL) for 24 hours. Total cellular RNA was extracted and analyzed for ribosomal RNA integrity by RNA chip (A) and for gene expression levels by quantitative real-time reverse transcription–polymerase chain reaction (B and D). The RNA integrity number (RIN) was determined by Agilent 2100 Expert software. Relative messenger RNA (mRNA) levels of interferon β (IFN-β; B), tumor necrosis factor α (TNF-α; C), and interleukin 6 (IL-6; D) were normalized to GAPDH mRNA levels.
Epidemiological Genetics Study
To investigate genetic mechanism and environmental interaction for the effect of OAS genes on dengue severity, we performed analyses for the 2 infection outcomes: plasma leakage and shock. Hospital had no impact on the occurrence of shock but did influence the occurrence of plasma leakage (P < .001), with increased mild dengue in hospital 1 (Siriraj). Secondary infection increased the risk of plasma leakage 4-fold (P < .001) and shock 3-fold (P = .008). When analyzing all serotypes together, serotype had no impact on either plasma leakage (P = .22) or shock (P = .09). However, when comparing DENV-2 with DENV-1, DENV-2 was associated with an increased risk of shock (P = .039; OR, 1.82; 95% CI, 1.03–3.23). Assumption of a dominant effect of the minor allele (G, resulting in p46 and not p48 and p52) of the OAS1 splicing variant yielded an increased risk of the minor allele genotypes for shock (OR, 1.55; 95% CI, 1.07–2.23; P = .017); this was confirmed by permutation (P = .020). There was no effect on plasma leakage (P = .271; Table 1). While adopting a dominant effect for the minor allele (AGG, resulting in amino acid R) of OAS3_K18R did not yield any significant effect (plasma leakage, P = .61; shock, P = .056), assuming a dominant model for the minor allele (AGG, resulting in amino acid R) of OAS3_S381R revealed a significant negative association with shock (OR, 0.37; 95% CI, .22–.60; P < .001); there was no effect on the risk of plasma leakage (P = .067).
Association of OAS1 and OAS3 Variants With the Risk of Plasma Leakage or the Risk of Shock
| . | . | . | . | . | . | . | . | . | Risk of Plasma Leakage . | Risk of Shock . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variant . | Genotype . | Dominant Effect . | Allele . | Univariate . | Multivariate . | Univariate . | Multivariate . | |||||||||
| OR (95% CI) . | P Value . | OR (95% CI) . | P Value . | OR (95% CI) . | P Value . | OR (95% CI) . | P Value . | |||||||||
| OAS1 splicing | AA | AG | GG | AA | AG+GG | A | G | MAF | ||||||||
| Mild | 132 | 87 | 15 | 132 | 102 | 351 | 117 | 0.25 | … | … | … | … | … | … | … | … |
| Plasma leakage | 196 | 142 | 23 | 196 | 165 | 534 | 188 | 0.26 | 1.22 (.86–1.73) | .27 | 1.70 (1.01–2.86) | .045 | 1.55 (1.07–2.23) | .017 | 1.41 (.97–2.06) | .072 |
| Shock | 58 | 61 | 13 | 58 | 74 | 177 | 87 | 0.33 | … | … | … | … | … | … | … | … |
| OAS3-18 A(A/G)G:K/Ra | AA | AG | GG | AA | AG+GG | A | G | MAF | ||||||||
| Mild | 120 | 91 | 24 | 120 | 115 | 331 | 139 | 0.30 | … | … | … | … | … | … | … | … |
| Plasma leakage | 182 | 144 | 29 | 182 | 173 | 508 | 202 | 0.28 | 1.10 (.77–1.55) | .61 | 0.70 (.32–1.52) | .36 | 1.44 (.99–2.08) | .056 | 0.83 (.35–1.96) | .67 |
| Shock | 57 | 64 | 14 | 57 | 78 | 178 | 92 | 0.34 | … | … | … | … | … | … | … | … |
| OAS3-381 AG(C/G):S/Ra | CC | CG | GG | CC | CG+GG | C | G | MAF | ||||||||
| Mild | 160 | 71 | 7 | 160 | 78 | 391 | 85 | 0.18 | … | … | … | … | … | … | … | … |
| Plasma leakage | 256 | 101 | 7 | 256 | 108 | 613 | 115 | 0.16 | 1.43 (.98–2.08) | .067 | 0.72 (.49–1.07) | .11 | 0.37 (.22–.60) | <.001 | 0.37 (.22–.61) | <.001 |
| Shock | 116 | 17 | 2 | 116 | 19 | 249 | 21 | 0.08 | … | … | … | … | … | … | … | … |
| . | . | . | . | . | . | . | . | . | Risk of Plasma Leakage . | Risk of Shock . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variant . | Genotype . | Dominant Effect . | Allele . | Univariate . | Multivariate . | Univariate . | Multivariate . | |||||||||
| OR (95% CI) . | P Value . | OR (95% CI) . | P Value . | OR (95% CI) . | P Value . | OR (95% CI) . | P Value . | |||||||||
| OAS1 splicing | AA | AG | GG | AA | AG+GG | A | G | MAF | ||||||||
| Mild | 132 | 87 | 15 | 132 | 102 | 351 | 117 | 0.25 | … | … | … | … | … | … | … | … |
| Plasma leakage | 196 | 142 | 23 | 196 | 165 | 534 | 188 | 0.26 | 1.22 (.86–1.73) | .27 | 1.70 (1.01–2.86) | .045 | 1.55 (1.07–2.23) | .017 | 1.41 (.97–2.06) | .072 |
| Shock | 58 | 61 | 13 | 58 | 74 | 177 | 87 | 0.33 | … | … | … | … | … | … | … | … |
| OAS3-18 A(A/G)G:K/Ra | AA | AG | GG | AA | AG+GG | A | G | MAF | ||||||||
| Mild | 120 | 91 | 24 | 120 | 115 | 331 | 139 | 0.30 | … | … | … | … | … | … | … | … |
| Plasma leakage | 182 | 144 | 29 | 182 | 173 | 508 | 202 | 0.28 | 1.10 (.77–1.55) | .61 | 0.70 (.32–1.52) | .36 | 1.44 (.99–2.08) | .056 | 0.83 (.35–1.96) | .67 |
| Shock | 57 | 64 | 14 | 57 | 78 | 178 | 92 | 0.34 | … | … | … | … | … | … | … | … |
| OAS3-381 AG(C/G):S/Ra | CC | CG | GG | CC | CG+GG | C | G | MAF | ||||||||
| Mild | 160 | 71 | 7 | 160 | 78 | 391 | 85 | 0.18 | … | … | … | … | … | … | … | … |
| Plasma leakage | 256 | 101 | 7 | 256 | 108 | 613 | 115 | 0.16 | 1.43 (.98–2.08) | .067 | 0.72 (.49–1.07) | .11 | 0.37 (.22–.60) | <.001 | 0.37 (.22–.61) | <.001 |
| Shock | 116 | 17 | 2 | 116 | 19 | 249 | 21 | 0.08 | … | … | … | … | … | … | … | … |
In each analysis, by logistic regression, a dominant model for the minor allele is used. Multivariate analyses include all environmental and genetic variables, with stepwise backward elimination of least significant variables.
Abbreviations: CI, confidence interval; MAF, minor allele frequency; OR, odds ratio.
a The nucleotides shown correspond to the messenger RNA.
Association of OAS1 and OAS3 Variants With the Risk of Plasma Leakage or the Risk of Shock
| . | . | . | . | . | . | . | . | . | Risk of Plasma Leakage . | Risk of Shock . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variant . | Genotype . | Dominant Effect . | Allele . | Univariate . | Multivariate . | Univariate . | Multivariate . | |||||||||
| OR (95% CI) . | P Value . | OR (95% CI) . | P Value . | OR (95% CI) . | P Value . | OR (95% CI) . | P Value . | |||||||||
| OAS1 splicing | AA | AG | GG | AA | AG+GG | A | G | MAF | ||||||||
| Mild | 132 | 87 | 15 | 132 | 102 | 351 | 117 | 0.25 | … | … | … | … | … | … | … | … |
| Plasma leakage | 196 | 142 | 23 | 196 | 165 | 534 | 188 | 0.26 | 1.22 (.86–1.73) | .27 | 1.70 (1.01–2.86) | .045 | 1.55 (1.07–2.23) | .017 | 1.41 (.97–2.06) | .072 |
| Shock | 58 | 61 | 13 | 58 | 74 | 177 | 87 | 0.33 | … | … | … | … | … | … | … | … |
| OAS3-18 A(A/G)G:K/Ra | AA | AG | GG | AA | AG+GG | A | G | MAF | ||||||||
| Mild | 120 | 91 | 24 | 120 | 115 | 331 | 139 | 0.30 | … | … | … | … | … | … | … | … |
| Plasma leakage | 182 | 144 | 29 | 182 | 173 | 508 | 202 | 0.28 | 1.10 (.77–1.55) | .61 | 0.70 (.32–1.52) | .36 | 1.44 (.99–2.08) | .056 | 0.83 (.35–1.96) | .67 |
| Shock | 57 | 64 | 14 | 57 | 78 | 178 | 92 | 0.34 | … | … | … | … | … | … | … | … |
| OAS3-381 AG(C/G):S/Ra | CC | CG | GG | CC | CG+GG | C | G | MAF | ||||||||
| Mild | 160 | 71 | 7 | 160 | 78 | 391 | 85 | 0.18 | … | … | … | … | … | … | … | … |
| Plasma leakage | 256 | 101 | 7 | 256 | 108 | 613 | 115 | 0.16 | 1.43 (.98–2.08) | .067 | 0.72 (.49–1.07) | .11 | 0.37 (.22–.60) | <.001 | 0.37 (.22–.61) | <.001 |
| Shock | 116 | 17 | 2 | 116 | 19 | 249 | 21 | 0.08 | … | … | … | … | … | … | … | … |
| . | . | . | . | . | . | . | . | . | Risk of Plasma Leakage . | Risk of Shock . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variant . | Genotype . | Dominant Effect . | Allele . | Univariate . | Multivariate . | Univariate . | Multivariate . | |||||||||
| OR (95% CI) . | P Value . | OR (95% CI) . | P Value . | OR (95% CI) . | P Value . | OR (95% CI) . | P Value . | |||||||||
| OAS1 splicing | AA | AG | GG | AA | AG+GG | A | G | MAF | ||||||||
| Mild | 132 | 87 | 15 | 132 | 102 | 351 | 117 | 0.25 | … | … | … | … | … | … | … | … |
| Plasma leakage | 196 | 142 | 23 | 196 | 165 | 534 | 188 | 0.26 | 1.22 (.86–1.73) | .27 | 1.70 (1.01–2.86) | .045 | 1.55 (1.07–2.23) | .017 | 1.41 (.97–2.06) | .072 |
| Shock | 58 | 61 | 13 | 58 | 74 | 177 | 87 | 0.33 | … | … | … | … | … | … | … | … |
| OAS3-18 A(A/G)G:K/Ra | AA | AG | GG | AA | AG+GG | A | G | MAF | ||||||||
| Mild | 120 | 91 | 24 | 120 | 115 | 331 | 139 | 0.30 | … | … | … | … | … | … | … | … |
| Plasma leakage | 182 | 144 | 29 | 182 | 173 | 508 | 202 | 0.28 | 1.10 (.77–1.55) | .61 | 0.70 (.32–1.52) | .36 | 1.44 (.99–2.08) | .056 | 0.83 (.35–1.96) | .67 |
| Shock | 57 | 64 | 14 | 57 | 78 | 178 | 92 | 0.34 | … | … | … | … | … | … | … | … |
| OAS3-381 AG(C/G):S/Ra | CC | CG | GG | CC | CG+GG | C | G | MAF | ||||||||
| Mild | 160 | 71 | 7 | 160 | 78 | 391 | 85 | 0.18 | … | … | … | … | … | … | … | … |
| Plasma leakage | 256 | 101 | 7 | 256 | 108 | 613 | 115 | 0.16 | 1.43 (.98–2.08) | .067 | 0.72 (.49–1.07) | .11 | 0.37 (.22–.60) | <.001 | 0.37 (.22–.61) | <.001 |
| Shock | 116 | 17 | 2 | 116 | 19 | 249 | 21 | 0.08 | … | … | … | … | … | … | … | … |
In each analysis, by logistic regression, a dominant model for the minor allele is used. Multivariate analyses include all environmental and genetic variables, with stepwise backward elimination of least significant variables.
Abbreviations: CI, confidence interval; MAF, minor allele frequency; OR, odds ratio.
a The nucleotides shown correspond to the messenger RNA.
Host Genetics and Viral Serotype Interaction
Because both secondary infection and DENV-2 were associated with risk of plasma leakage and shock, we investigated the role of these variables on the genetic effect observed for OAS1 splicing variants and OAS3_S381R variants. Because there were no shock cases with DENV-3 and DENV-4 for a minor allele, we restricted our analyses to DENV-1 and DENV-2 and again assumed a dominant effect of a minor allele for each locus (Table 2). There was a significant interaction between OAS3_S381R and serotype (P = .023); the minor allele (AGG, resulting in amino acid R) had a protective effect against shock for DENV-2 (OR, 0.13; 95% CI, .03–.56) but not for DENV-1 (OR, 0.83; 95% CI, .33–2.06). To verify that there was no confounding effect of more secondary infections being associated with DENV-2 infections, we analyzed the DENV serotype-specific effect, using only secondary infections. Again, there was no protective effect of the OAS3_S381R dominant minor allele for DENV-1 (P = .19), but the effect was maintained for DENV-2 infections (P = .001).
Serotype-Specific Association of OAS1 and OAS3 Variants With the Risk of Plasma Leakage or the Risk of Shock
| Variant, DENV Serotype . | Nucleotide(s), Outcome . | Univariate . | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) . | P Value . | |||||
| OAS1 splicing | AG+GG | AA | ||||
| Mild Dengue | Plasma Leakage | Mild Dengue | Plasma Leakage | |||
| 1 | 26 | 43 | 26 | 47 | 0.92 (.46–1.81) | .799 |
| 2 | 7 | 49 | 22 | 49 | 3.14 (1.23–8.03) | .017 |
| OAS3_381 | CG+GG | CC | ||||
| Nonshock | Shock | Nonshock | Shock | |||
| 1 | 42 | 6 | 93 | 16 | 0.83 (.30–2.27) | .715 |
| 2 | 37 | 2 | 74 | 31 | 0.13 (.03–.57) | .007 |
| Variant, DENV Serotype . | Nucleotide(s), Outcome . | Univariate . | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) . | P Value . | |||||
| OAS1 splicing | AG+GG | AA | ||||
| Mild Dengue | Plasma Leakage | Mild Dengue | Plasma Leakage | |||
| 1 | 26 | 43 | 26 | 47 | 0.92 (.46–1.81) | .799 |
| 2 | 7 | 49 | 22 | 49 | 3.14 (1.23–8.03) | .017 |
| OAS3_381 | CG+GG | CC | ||||
| Nonshock | Shock | Nonshock | Shock | |||
| 1 | 42 | 6 | 93 | 16 | 0.83 (.30–2.27) | .715 |
| 2 | 37 | 2 | 74 | 31 | 0.13 (.03–.57) | .007 |
In each analysis, by logistic regression, a dominant model for the minor allele is used. Multivariate analyses include all environmental and genetic variables, with stepwise backward elimination of least significant variables.
Abbreviations: CI, confidence interval; DENV, dengue virus; OR, odds ratio.
Serotype-Specific Association of OAS1 and OAS3 Variants With the Risk of Plasma Leakage or the Risk of Shock
| Variant, DENV Serotype . | Nucleotide(s), Outcome . | Univariate . | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) . | P Value . | |||||
| OAS1 splicing | AG+GG | AA | ||||
| Mild Dengue | Plasma Leakage | Mild Dengue | Plasma Leakage | |||
| 1 | 26 | 43 | 26 | 47 | 0.92 (.46–1.81) | .799 |
| 2 | 7 | 49 | 22 | 49 | 3.14 (1.23–8.03) | .017 |
| OAS3_381 | CG+GG | CC | ||||
| Nonshock | Shock | Nonshock | Shock | |||
| 1 | 42 | 6 | 93 | 16 | 0.83 (.30–2.27) | .715 |
| 2 | 37 | 2 | 74 | 31 | 0.13 (.03–.57) | .007 |
| Variant, DENV Serotype . | Nucleotide(s), Outcome . | Univariate . | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) . | P Value . | |||||
| OAS1 splicing | AG+GG | AA | ||||
| Mild Dengue | Plasma Leakage | Mild Dengue | Plasma Leakage | |||
| 1 | 26 | 43 | 26 | 47 | 0.92 (.46–1.81) | .799 |
| 2 | 7 | 49 | 22 | 49 | 3.14 (1.23–8.03) | .017 |
| OAS3_381 | CG+GG | CC | ||||
| Nonshock | Shock | Nonshock | Shock | |||
| 1 | 42 | 6 | 93 | 16 | 0.83 (.30–2.27) | .715 |
| 2 | 37 | 2 | 74 | 31 | 0.13 (.03–.57) | .007 |
In each analysis, by logistic regression, a dominant model for the minor allele is used. Multivariate analyses include all environmental and genetic variables, with stepwise backward elimination of least significant variables.
Abbreviations: CI, confidence interval; DENV, dengue virus; OR, odds ratio.
In a multivariate analysis taking into account variation due to hospital and secondary infections, there was a serotype-specific effect of the OAS1 splicing variant minor dominant allele (G, resulting in p46 and not p48 and 52) on the risk of plasma leakage (P = .019); this minor dominant allele was associated with an increased risk of plasma leakage in DENV-2 infections (OR, 3.17; 95% CI, 1.31–7.68) but not in DENV-1 infections (OR, 0.81; 95% CI, .37–1.75). When we analyzed the serotype-specific effect on plasma leakage by using only secondary infections, the increased risk of the OAS1 splicing variant dominant minor allele was again not significant for DENV-1 (P = .44), and the effect was maintained for DENV-2 infections (P = .003).
Population Genetics Difference
Differences in susceptibility to plasma leakage and severe dengue have been observed, with Europeans appearing more susceptible than Africans in Cuba [30, 31] and Chinese appearing more susceptible than Malays in Malaysia [32, 33]. We investigated the frequency of the OAS polymorphisms relevant to DENV infection outcome in different populations. We obtained genotype data of the 3 SNPs from the HapMap project phase 2 and 3 release 2 (available at: http://www.hapmap.org) and the 1000 genomes project (available at: http://www.1000genomes.org; Supplementary Data). Pairwise comparison of population differentiation for the OAS1 splicing variant revealed little differentiation among European, Chinese, and Thai populations, whether contrasting populations defined by shock versus nonshock or dengue fever versus dengue with plasma leakage. By contrast, Yoruba were strongly differentiated from Japanese (Fst = 0.499; P < .0001) and Thai populations (Fst = 0.34 and 0.30 for mild and severe dengue, respectively; P < .0001; Table 3). Pairwise comparison of OAS3_K18R revealed small but significant differentiation in all populations (mean Fst = 0.066) except among Chinese and Thai populations.
| Population . | Fst Value, by Population . | |||||
|---|---|---|---|---|---|---|
| CEU . | CHB . | JPT . | YRI . | Thai, Mild Dengue . | Thai, Severe Dengue . | |
| CEU | … | 0.022a | 0.010b | 0.121a | 0.010b | 0.097a |
| CHB | … | … | −0.001 | 0.223a | 0.0001 | 0.036c |
| JPT | … | … | … | 0.190a | −0.003 | 0.053a |
| YRI | … | … | … | … | 0.199a | 0.306a |
| Thai, mild dengue | … | … | … | … | … | 0.052a |
| Thai, severe dengue | … | … | … | … | … | … |
| Population . | Fst Value, by Population . | |||||
|---|---|---|---|---|---|---|
| CEU . | CHB . | JPT . | YRI . | Thai, Mild Dengue . | Thai, Severe Dengue . | |
| CEU | … | 0.022a | 0.010b | 0.121a | 0.010b | 0.097a |
| CHB | … | … | −0.001 | 0.223a | 0.0001 | 0.036c |
| JPT | … | … | … | 0.190a | −0.003 | 0.053a |
| YRI | … | … | … | … | 0.199a | 0.306a |
| Thai, mild dengue | … | … | … | … | … | 0.052a |
| Thai, severe dengue | … | … | … | … | … | … |
Abbreviations: CEU, Utah residents with northern and western European ancestry; CHB, Han Chinese residents in Beijing, China; JPT, Japanese residents in Tokyo, Japan; YRI, Yoruban residents in Ibadan, Nigeria.
aP < .000, by the distance method.
bP < .05, by the distance method.
cP = .054, by the distance method.
| Population . | Fst Value, by Population . | |||||
|---|---|---|---|---|---|---|
| CEU . | CHB . | JPT . | YRI . | Thai, Mild Dengue . | Thai, Severe Dengue . | |
| CEU | … | 0.022a | 0.010b | 0.121a | 0.010b | 0.097a |
| CHB | … | … | −0.001 | 0.223a | 0.0001 | 0.036c |
| JPT | … | … | … | 0.190a | −0.003 | 0.053a |
| YRI | … | … | … | … | 0.199a | 0.306a |
| Thai, mild dengue | … | … | … | … | … | 0.052a |
| Thai, severe dengue | … | … | … | … | … | … |
| Population . | Fst Value, by Population . | |||||
|---|---|---|---|---|---|---|
| CEU . | CHB . | JPT . | YRI . | Thai, Mild Dengue . | Thai, Severe Dengue . | |
| CEU | … | 0.022a | 0.010b | 0.121a | 0.010b | 0.097a |
| CHB | … | … | −0.001 | 0.223a | 0.0001 | 0.036c |
| JPT | … | … | … | 0.190a | −0.003 | 0.053a |
| YRI | … | … | … | … | 0.199a | 0.306a |
| Thai, mild dengue | … | … | … | … | … | 0.052a |
| Thai, severe dengue | … | … | … | … | … | … |
Abbreviations: CEU, Utah residents with northern and western European ancestry; CHB, Han Chinese residents in Beijing, China; JPT, Japanese residents in Tokyo, Japan; YRI, Yoruban residents in Ibadan, Nigeria.
aP < .000, by the distance method.
bP < .05, by the distance method.
cP = .054, by the distance method.
Population comparisons using OAS3_S381R revealed population differentiation even between the 2 Thai dengue disease categories (Fst = 0.05; P < .0001), consistent with our genetic statistical analysis revealing an association of this locus with shock. Yoruba again showed the highest population differentiation (Fst = 0.31; P < .0001, compared with Thai severe dengue); the level of differentiation with the other populations, including Thai nonshock dengue, was considerably lower than this (Fst range, 0.12–0.22; Table 3).
DISCUSSION
The study of the antiviral activity of OAS genes variants on DENV infection revealed strong and serotype-specific disparities. We show that, whereas both OAS1-p42 and p46 had antiviral activity against DENV-2 (Figure 1), p46 did not affect DENV-1 replication. All other OAS1 isoforms (p44, p48, and p52) did not affect DENV replication (Supplementary Data and [16]). This suggests that specific properties of DENV-1 allow it to escape or prevent the triggering of OAS1-p46 antiviral activity. Most importantly, the G allele of the OAS1 splicing variant, generating p46, has been associated with higher enzyme activity [34]. Conversely, the OAS3_R381 variant presents reduced antiviral activity against DENV-2, compared with either the OAS3_S381 variant or to the effect of all OAS3 genotypes tested on DENV-1 (Figure 2A).
Despite the relaxed overall sequence specificity of double-stranded RNA (dsRNA) recognition by OAS [35, 36], it is possible that differences in primary sequence or RNA secondary structure between DENV-1 and DENV-2 lead to weaker sensing by OAS1-p46 or OAS3 R381. Indeed, the precise in vivo ligands for OAS are still unknown [14], and conformational differences could result in differential detection efficiency. It is also possible that differences in replication dynamics between genotypes or serotypes could lead to weaker sensing of specific strains. The position of amino acid 381 inside the linker between domains 1 and 2 of OAS3 could result in other aspects of OAS3 activation being specifically affected when interacting with DENV-2 dsRNA.
Most surprisingly, findings of the genetic association study of these OAS gene polymorphisms correlate with an increased risk of severe disease with variants presenting strong antiviral activity in cell culture. The AGG variant (resulting in amino acid R) of OAS3_S381R was associated with dominant protection against shock. Importantly, this protection was only associated with patients infected by DENV-2. This protection was not found for DENV-1, indicating serotype-specific host-pathogen interactions. The protective effect afforded by the AGG variant of OAS3_S381R against shock in DENV-2 infections was strong, 3 times that of the increased susceptibility to shock occurring in secondary infections. Moreover, an OAS1 splicing variant (generating p46 and not p48 and p52) was associated with increased susceptibility to plasma leakage and shock in individuals infected with DENV-2.
The high activity of OAS1-p46 triggered by DENV-2 infection could contribute to the risk of disease, just as there is a protective effect associated with the lower antiviral action of OAS3_R381 on DENV-2. This observation is in contrast with the effect of the OAS1 splicing variant against other viral infections, such as those due to WNV [17] and hepatitis C virus [20], as well as the measles vaccine response [19], in which higher OAS enzymatic activity has been associated with increased protection.
These results suggest that the antiviral actions of OAS (1 and 3) might be a double-edged sword that could contribute to pathogenesis in the process of blocking viral infection. Indeed, small RNAs generated from RNase L cleavage of cellular and viral mRNAs can induce IFN-β expression through RIG-I and/or MDA5 [37, 38]. This process might also lead to the induction of cytokines [39], which have been implicated in the development of plasma leakage and shock in dengue [40]. The cascade of cytokine production proposed to increase vascular permeability resulting from excessive immune activation has been termed a cytokine storm [41]. To test this notion, we measured transcript levels of cytokines in T-REx 293 cells with OAS3_S381 or R381 upon DENV-2 infection. The induction levels of IFN-β and IL-6 were all higher in DENV-2–infected cells with OAS3_S381, compared with that of R381 (Figure 2). Activation of OAS may contribute to the immunopathogenesis involved in severe dengue shock syndrome, because it is an early step in the cascade of cytokine production.
Importantly, these observations also suggest that both human and viral genetics need to be taken into account in virulence assessment, as such genotype-serotype specific interactions may generate important bias in any animal or cellular model used. Furthermore, the functional assay suggests that the protective variant does not strongly reduce the viral titer for DENV-2. Thus, this serotype-specific protective effect may act without influencing the viral titer, which could partially explain the discordant correlations previously observed between clinical severity and viral titer [42–45].
Finally, at the population level, these results could constitute a prime example of the molecular basis of the disparities in the risk of disease severity observed in different genetic backgrounds [1, 46]. There is significant population-based differentiation at the OAS3_S381R locus when comparing African to non-African populations. The higher frequency of the protective allele of OAS3_S381R in African populations might indeed partially explain why, in Cuba, severe dengue occurred less frequently in patients of African ancestry than in patients of European ancestry [30, 31] and why, in Africa, reports of severe dengue are scarce or absent [47].
Our findings have important consequences for the prediction of disease severity and for the development of OAS-based prophylaxis and therapy against infections by this virus and other positive-sense single-stranded RNA viruses of medical importance. Furthermore, they underline the importance of pathogen genetics with regard to genetic studies of susceptibility to infectious diseases.
Notes
Financialsupport. This work was supported by the European Commission Seventh Framework Programme [FP7/2007-2013] for the DENFREE project under Grant Agreement n°282 378; the Agence Nationale de la Recherche (ANR grant 2010-INTB-1601-01); the National Science Council (100-2923-B-001-002-MY3); Academia Sinica; the Office of the Higher Education Commission and Mahidol University, under the National Research Universities Initiative); the Medical Scholars Program, Mahidol University (scholarships to S. M. K. and W. L.); and National Research University (grant through Mahidol University to P. S.).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
Author notes
E. S.-L., R.-J. L., and S. M. K. contributed equally to this work.




