-
PDF
- Split View
-
Views
-
Cite
Cite
The 2021 American Burn Association State and Future of Burn Science Working Group , Proceedings of the 2021 American Burn Association State and Future of Burn Science Meeting, Journal of Burn Care & Research, Volume 43, Issue 6, November/December 2022, Pages 1241–1259, https://doi.org/10.1093/jbcr/irac092
Close - Share Icon Share
Abstract
Periodically, the American Burn Association (ABA) has convened a State of the Science meeting on various topics representing multiple disciplines within burn care and research. In 2021 at the request of the ABA President, meeting development was guided by the ABA’s Burn Science Advisory Panel (BSAP) and a subgroup of meeting chairs. The goal of the meeting was to produce both an evaluation of the current literature and ongoing studies, and to produce a research agenda and/or define subject matter-relevant next steps to advance the field(s). Members of the BSAP defined the topics to be addressed and subsequently solicited for nominations of expert speakers and topic leaders from the ABA’s Research Committee. Current background literature for each topic was compiled by the meeting chairs and the library then enhanced by the invited topic and breakout discussion leaders. The meeting was held in New Orleans, LA on November 2nd and 3rd and was formatted to allow for 12 different topics, each with two subtopics, to be addressed. Topic leaders provided a brief overview of each topic to approximately 100 attendees, followed by expert-lead breakout sessions for each topic that allowed for focused discussion among subject matter experts and interested participants. The breakout and topic group leaders worked with the participants to determine research needs and associated next steps including white papers, reviews and in some cases collaborative grant proposals. Here, summaries from each topic area will be presented to highlight the main foci of discussion and associated conclusions.
At the request of American Burn Association (ABA) President Dr. Lucy Wibbenmeyer, the organization’s Burn Science Advisory Panel (BSAP) designed a program for the 2021 State and Future of Burn Science Meeting. While the ABA has historically held a periodic State of the Science meeting, convening experts from various disciplines across the spectrum of burn care and research, the goals of the 2021 meeting were somewhat distinct. Rather than a historical review of the evolution of the subject matter since inception that brings us to present, and an associated update via summary manuscript(s), the request was to focus on current (approximately within the past 5 years only) advances, therapeutics, approaches and research and project forward, producing an agenda and well-defined next steps. The next steps and proposed research needs should be expected to address gaps in knowledge, unmet therapeutic needs and align with the overall mission of the ABA, improving lives of everyone affected by burn injury.
The BSAP membership proposed and iteratively refined the topics to be featured during the meeting. Twelve topics were identified as priorities for inclusion in the meeting, and for each topic, two more focused subtopics (Table 1). A smaller group of BSAP members was identified as meeting chairs/organizers (Jeffrey Shupp, MD FACS; James Holmes, MD FACS; Lauren Moffatt, PhD). This group then proceeded to compile topic-relevant current literature meant to be used as background reading for group leaders and participants. The list of topics and compiled literature was then circulated back to the BSAP and also to the ABA’s Research Committee members for feedback and with two asks: to suggest topic leaders that are subject matter experts and currently productive within the field, and to suggest additional literature references that may be critical to a current understanding of the discipline or topic. From the responses, the meeting chairs worked with the ABA Central Office and sent invitations to the proposed topic leaders. The format of the meeting was developed to allow for all attendees to participate in a topic overview, provided as a presentation from the topic leader and then attend up to four different breakout sessions, where the subtopics were addressed in more detail.
| 2021 ABA State and Future of Burn Science Topics . |
|---|
| 1. Burn injury in the extremes of age |
| 1a. Metabolic considerations |
| 1b. Frailty |
| 2. Aftercare |
| 2a. Return to Work/School |
| 2b. Social and Community Reintegration |
| 3. Burn Shock |
| 3a. Pathophysiology |
| 3b. Treatment |
| 4. Nutrition & Metabolism |
| 4a. Micronutrients and Trace Elements |
| 4b. Meeting Caloric Needs |
| 5. Neuropsychological |
| 5a. Neuropathic Pain and Pruritis |
| 5b. Burn-related ASD and Progression to PTSD |
| 6. Scar Management |
| 6a. Therapeutic Approaches |
| 6b. Dyschromia |
| 7. Multiorgan System Support |
| 7a. Inhalation Injury |
| 7b. Multisystem Support |
| 8. Wound Depth Assessment |
| 8a. Pathophysiology |
| 8b. Diagnostics |
| 9. Advance in Wound Coverage |
| 9a. Autologous |
| 9b. Allogenic and Biosimilar Constructs |
| 10. Incorporating Exercise into the Rehabilitation of the Burn Patient |
| 10a. Rehab in the ICU |
| 10b. Cardiovascular/Strength Training After Burns |
| 11. Infection |
| 11a. Advances in Diagnostics, Defining Sepsis |
| 11b. Microbiome |
| 12. Frostbite |
| 12a. Pathophysiology |
| 12b. Diagnostics and Therapeutics |
| 2021 ABA State and Future of Burn Science Topics . |
|---|
| 1. Burn injury in the extremes of age |
| 1a. Metabolic considerations |
| 1b. Frailty |
| 2. Aftercare |
| 2a. Return to Work/School |
| 2b. Social and Community Reintegration |
| 3. Burn Shock |
| 3a. Pathophysiology |
| 3b. Treatment |
| 4. Nutrition & Metabolism |
| 4a. Micronutrients and Trace Elements |
| 4b. Meeting Caloric Needs |
| 5. Neuropsychological |
| 5a. Neuropathic Pain and Pruritis |
| 5b. Burn-related ASD and Progression to PTSD |
| 6. Scar Management |
| 6a. Therapeutic Approaches |
| 6b. Dyschromia |
| 7. Multiorgan System Support |
| 7a. Inhalation Injury |
| 7b. Multisystem Support |
| 8. Wound Depth Assessment |
| 8a. Pathophysiology |
| 8b. Diagnostics |
| 9. Advance in Wound Coverage |
| 9a. Autologous |
| 9b. Allogenic and Biosimilar Constructs |
| 10. Incorporating Exercise into the Rehabilitation of the Burn Patient |
| 10a. Rehab in the ICU |
| 10b. Cardiovascular/Strength Training After Burns |
| 11. Infection |
| 11a. Advances in Diagnostics, Defining Sepsis |
| 11b. Microbiome |
| 12. Frostbite |
| 12a. Pathophysiology |
| 12b. Diagnostics and Therapeutics |
| 2021 ABA State and Future of Burn Science Topics . |
|---|
| 1. Burn injury in the extremes of age |
| 1a. Metabolic considerations |
| 1b. Frailty |
| 2. Aftercare |
| 2a. Return to Work/School |
| 2b. Social and Community Reintegration |
| 3. Burn Shock |
| 3a. Pathophysiology |
| 3b. Treatment |
| 4. Nutrition & Metabolism |
| 4a. Micronutrients and Trace Elements |
| 4b. Meeting Caloric Needs |
| 5. Neuropsychological |
| 5a. Neuropathic Pain and Pruritis |
| 5b. Burn-related ASD and Progression to PTSD |
| 6. Scar Management |
| 6a. Therapeutic Approaches |
| 6b. Dyschromia |
| 7. Multiorgan System Support |
| 7a. Inhalation Injury |
| 7b. Multisystem Support |
| 8. Wound Depth Assessment |
| 8a. Pathophysiology |
| 8b. Diagnostics |
| 9. Advance in Wound Coverage |
| 9a. Autologous |
| 9b. Allogenic and Biosimilar Constructs |
| 10. Incorporating Exercise into the Rehabilitation of the Burn Patient |
| 10a. Rehab in the ICU |
| 10b. Cardiovascular/Strength Training After Burns |
| 11. Infection |
| 11a. Advances in Diagnostics, Defining Sepsis |
| 11b. Microbiome |
| 12. Frostbite |
| 12a. Pathophysiology |
| 12b. Diagnostics and Therapeutics |
| 2021 ABA State and Future of Burn Science Topics . |
|---|
| 1. Burn injury in the extremes of age |
| 1a. Metabolic considerations |
| 1b. Frailty |
| 2. Aftercare |
| 2a. Return to Work/School |
| 2b. Social and Community Reintegration |
| 3. Burn Shock |
| 3a. Pathophysiology |
| 3b. Treatment |
| 4. Nutrition & Metabolism |
| 4a. Micronutrients and Trace Elements |
| 4b. Meeting Caloric Needs |
| 5. Neuropsychological |
| 5a. Neuropathic Pain and Pruritis |
| 5b. Burn-related ASD and Progression to PTSD |
| 6. Scar Management |
| 6a. Therapeutic Approaches |
| 6b. Dyschromia |
| 7. Multiorgan System Support |
| 7a. Inhalation Injury |
| 7b. Multisystem Support |
| 8. Wound Depth Assessment |
| 8a. Pathophysiology |
| 8b. Diagnostics |
| 9. Advance in Wound Coverage |
| 9a. Autologous |
| 9b. Allogenic and Biosimilar Constructs |
| 10. Incorporating Exercise into the Rehabilitation of the Burn Patient |
| 10a. Rehab in the ICU |
| 10b. Cardiovascular/Strength Training After Burns |
| 11. Infection |
| 11a. Advances in Diagnostics, Defining Sepsis |
| 11b. Microbiome |
| 12. Frostbite |
| 12a. Pathophysiology |
| 12b. Diagnostics and Therapeutics |
The meeting was convened at the University Medical Center in New Orleans on November 2nd and 3rd, 2021 and hosted by their Burn Team and Burn Center Director, Dr. Jeffrey Carter. Experts in each of the 12 topics as well as experts for each of the subtopics/breakout sessions attended. Along with these 40 individuals, over 55 more members of the burn community, and other subject matter experts attended as participants representing surgeons, nurses, rehabilitation specialists, pharmacists, psychologists, nutrition experts, scientists and burn survivors among others. The agenda was structured with topics 1–6 addressed on day 1 of the meeting, and 6–12 covered on day 2. On day 1, each leader of topics 1–6 gave a 15-minute overview presentation of the current state of the topic and potential knowledge or research gaps. This was followed by 1.5 h breakout sessions for each topic, lead by the breakout leaders in collaboration with the overall topic lead. The same breakouts were then repeated, allowing attendees to participate in discussions on 2 different topics. This format was repeated on day 2 for topics 6–12, and the meeting culminated in presentations from each topic/breakout leadership team, reporting the main points from the discussions and proposed next steps.
After action support and resources were offered by the meeting chairs and the ABA central office, including online virtual meeting and file share access, in order to continue sharing literature and working collaboratively on documents and other projects that may have been identified by the participants as next steps. Each topic group has also provided summaries, which will be presented here to capture the meeting proceedings.
TOPIC BRIEFINGS
Topic 1: Burn Injury in the Extremes of Age
Led by Herbert A. Phelan MD FACS, Linda Sousse PhD MBA and Kathleen S. Romanowski MD FACS; Summary composed by Dr. Romanowski and Marc Jeschke MD PhD FACS FCAHS FCCM FRCS(C)
Metabolic Considerations.
When considering the metabolic alteration in extreme of ages, one needs to differentiate between pediatric burn patients and older adult burn patients.1,2 Pediatric burn patients undergo a prototypical ebb and flow-phase with profound hypermetabolism and substantial catabolism lasting years after the initial insult. These findings were confirmed in numerous studies leading to current treatment suggestions, such as adequate nutrition, early excision and grafting, specialized pediatric critical care, early mobilization, anabolic agent administration, eg, oxandrolone, as well as anticatabolic agent administration such as propranolol.3
While the metabolic alterations are very well defined in pediatric burn patients, they are less known in older adult burn patients. Moreover, it appears that current treatment recommendations established for pediatric and adult burn patients are not applicable for older adult burn patients. Aging is usually associated with inflammageing, a condition characterized by elevated levels of inflammatory and stress markers that carry high susceptibility for chronic morbidity, frailty and premature death.4 Inducers and enhancers of inflammageing include glucose and lipid metabolism, cellular senescence, oxidative stress caused by dysfunctional mitochondria, formation of reactive oxygen species, immune cell dysregulation and chronic infections.5 Aging in general leads to increased insulin resistance, lipolysis via infiltration of inflammatory and immune cells. It seems intuitive that the aging induced hyperinflammatory and hypermetabolic responses would be further augmented in older adult burn patients, and these profoundly increased inflammatory and metabolic changes would be the cause of poor outcomes of these patients. Surprisingly, and contrary to this hypothesis, studies showed that older adult burn patients have a markedly reduced inflammatory and attenuated hypermetabolic response when compared to adult burn patients during acute hospitalization. These unexpected findings were confirmed in two recent studies. In one, Rehou et al. looked at the acute phase response in older adult burn patients and found that older adults cannot initiate an appropriate inflammatory and stress response within the first 96 h after burn.6 These hypo-inflammatory and hypo-stress responses were associated with increased organ dysfunction and greater mortality. In the second, Dreckmann et al. conducted a genome-wide study in older adult and adult burn patients.7 Pathway analysis and heat map generation suggest that older adult patients express a distinct hypo-inflammatory response with downregulation of several immune-related pathways, ubiquitination, and proteasome degradation. Cell signaling pathways, such as NF-κB, C-type lectin receptor and T cell receptor signaling, were also significantly down-regulated in older adult burn patients.
These findings strongly suggest that older adult burn patients express blunted inflammatory and hypermetabolic responses associated with substantially increased adverse outcomes. Based on these findings it seems like no surprise that current treatment recommendations for adult and pediatric patients cannot be simply translated to older adults. It is currently not known whether older adults benefit from Parkland resuscitation, early excision, nutritional support, anti-infective strategies, leading to the demand that older adult specific treatment recommendations have to be characterized, studied and implemented.3
Frailty.
The older adult population is growing. As of 2019, 54.1 million Americans are more than 65-years-old and by 2060 this number will grow to almost 95 million people.8 With this population growth there will also be a corresponding increase in the number older burn injured patients. The traditional model for predicting mortality in burn patients is the Baux score which is based on patient age and burn size (%TBSA). Unfortunately, these models fail to fully account for the individual difference between patients. Individuals with the same chronological age can have widely different states of health and functional status making age alone a poor predictor of outcome.9 The idea that age alone is not a good predictor of outcomes has been tested in an older adult burn patients by Alpert et al.10 They found that octogenarians have similar injuries with respect to TBSA compared to the younger patients and that older age was not associated with increased complications or mortality. Frailty has been suggested as an alternative to age alone for predicting outcomes in older adults with burn injury.
More than 70 tools exist for measuring frailty, and there is not a scale that is considered a consensus standard.11 Due to its ease of use and ability to be used in retrospective studies, the most commonly used frailty score in a burn population has been the Canadian Study on Health and Aging Clinical Frailty Score (CSHA CFS) (Figure 1). The CHSA CFS is a seven-point clinical opinion scale.12 Masud et al. examined patients who were more than the age of 65-years-old and had sustained at least 10% TBSA burns.13 In this retrospective study population, patients with better pre-injury functional status (lower frailty scores) were more likely to survive their burn injuries. Romanowski et al. repeated their study but included all patients irrespective of burn size.14 This study also found that patients who died had significantly higher CHSA CFS compared to survivors and that CSHA CFS was higher in patients discharged to a skilled nursing facility (SNF). Similar results were found when younger patients (age 50–64) were studied.15 Again, higher CSHA CFS was found to be associated with increased risk of death and of discharge to a SNF, even more so in patients aged 50–64-years-old (odds ratio for death = 2.5) than those 65 and older (odds ratio for death = 1.63).
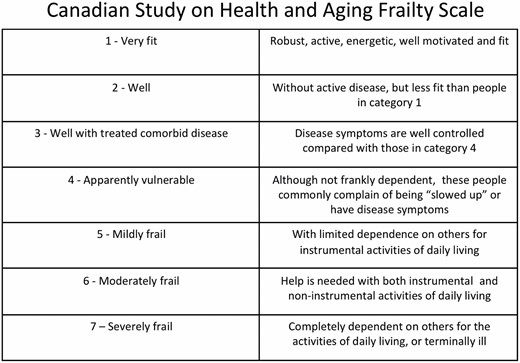
Canadian Study on Health and Aging Clinical Frailty Score (CSHA CFS). CSHA CFS is a seven-point clinical opinion scale which has been used to establish the correlation between frailty and outcomes such as mortality in burn patient populations.
Despite the success of CSHA CFS in predicting outcomes in the burn population, some researchers have started to examine the utility of moving from general frailty scores (such as CSHA CFS) to specialty specific scales designed to be used in a specific patient population. The Burn Frailty Index (BFI) was modeled after the Emergency General Surgery Frailty Index16,17 (Figure 2). Using the BFI, Maxwell et al. found that frail patients had more complications, more discharges to nonhome locations, longer hospital stays and higher mortality at both one and three years (P < .001).
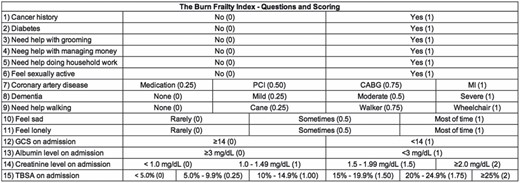
The Burn Frailty Index (BFI). The BFI was modeled after the Emergency General Surgery Frailty Index in an effort to move from general frailty scores (such as CSHA CFS) to specialty specific scales designed to be used in a specific patient population.
In the older adult burn population, there has not been a study that compares the BFI and the CSHA CFS. The BFI includes injury related factors such as TBSA and requires patient interaction to complete. The CSHA CFS is a generic scale that can be used even with minimal patient participation. Depending on the goals associated with the use of the frailty score either BFI or CSHA CFS might be reasonable choices in an older burn patient population.
Given its ability to be used retrospectively, and its ease of use, the CSHA CFS has largely been the frailty score of choice in the burn population and used to establish the correlation between frailty and outcomes such as mortality, and discharge to a SNF. Research now needs to move beyond the correlation of frailty with outcomes to using frailty to plan care and make decisions for patients. Consideration should be given to including both CSHA CFS and the BFI in the Burn Care Quality Program (BCQP) so that evaluation of outcomes and practices can be done at the national level.
Beyond frailty, more work needs to be done to examine the current practices being used to treat older adult burn patients with respect to surgery, therapy and nutrition. Once we understand the current practices being used to treat older patients, new protocols can be developed and tested to improve the outcomes of older adult burn patients.
Widely accepted standards of care for burn injury must be better characterized and subsequently adapted in older adult burn patients and cannot just be translated from pediatric and adult patients.
Age alone, as considered in the Baux score, is not adequate for predicting outcomes in burn patients due to individual variability in terms of health status and function.
Frailty scoring is nonstandard and the applications and goals of using frailty scores to predict outcomes should be considered when selecting a scoring system.
Topic 2: Aftercare
Led by Karen J. Kowalske MD and Karen Badger PhD MSW; Summary composed by Drs. Kowalske and Badger, and Rebekah Allely OTR/L
Return to Work/School.
A review of literature from 1984 to present day offers multiple factors that impede return to work (RTW) for burn survivors. Some of these factors such as TBSA and specific occupations have been consistent over the years. Others appear to have become more common, such as pain, mental health challenges and length of stay (LOS) in the hospital. Other factors found to affect RTW are the patient’s employment status pre-injury, severity, and etiology of burn and predictors of pre-injury work satisfaction and a good relationship with the employer.
Future research with focus on evaluating current interventions to assist with successful RTW as well as developing additional support tools is necessary. Areas to assess include availability of vocational counseling, the effectiveness of work hardening programs, communication opportunities between burn team and employers, and the use of job coaches.
Evaluation of return to school shows that a formal school re-entry program can facilitate this transition. Implementing access to this program is recommended as a priority for all burn centers. Future research is needed to evaluate the role of social skills training for children.
Social and Community Reintegration.
Literature reviews indicate common factors that burn survivors encounter that present challenges for successful social and community reintegration. Poor body image, lack of peer support, pre-existing mental health conditions, social skill challenges, and limited resources are known difficulties. During our SOS discussion group, burn survivors reported that “aftercare” must begin soon after injury, while initially healing physical wounds. Survivors shared that their emotional healing was much more difficult and had greater impact than their physical healing and described aftercare as “quality of life”. The challenge this presents for the burn team is recognizing that each patient is a unique individual with their own experiences and perspective that need to be understood and accounted for in aftercare. Surveys and interviews may assist us in identifying general tendencies for emotional healing, however individual needs must also be assessed. Additionally, the importance of team-based care, team/unit culture, self (team)reflection and patient/family centric practice that includes identifying and mitigating unconscious bias was highlighted to create an inclusive environment supportive of patient/family engagement throughout aftercare.
Interventions to be further evaluated include 1) the “A through F” ICU Bundle (in the acute care setting) which identifies daily patient/family and burn team goals, action plan and desired outcomes and 2) interdisciplinary burn team Social Skills Training (SST) to increase awareness of the emotional challenges patients encounter and how to prepare them for social reintegration that includes an assessment of the burn team’s understanding of social skills tools, comfort level in teaching these tools and perspective of their effectiveness.
It is well documented that peer support has a positive impact in recovery. The challenge to burn units is to provide peer support in a variety of avenues to meet the needs of a diverse patient population. Peer support can be offered across the continuum of care and is available in a variety of ways such as, in person, virtual, one-on-one, in group settings, hobby focused and outdoor recreation activity. Understanding the psychosocial responses/healing components for both patients and family and determining best practices for aftercare programming can help address these needs effectively and result in greater continuity of services across the burn community.
Methods for supporting and identifying individualized aftercare needs of patients need to be developed and integrated into standards of care.
Assessments of current tools and resources available to support return to work and social reintegration should be made routinely and iteratively to determine their effectiveness and to further refine and enhance them.
Involvement of the multidisciplinary burn team as well as the patient family and support structure is critical.
Topic 3: Burn Shock
Led by Robert Cartotto MD FRCS(C), David M. Burmeister PhD and John C. Kubasiak MD
Part 1: Highlights of the Current State of the Science.
Consensus on the optimal fluid regimen for burn shock resuscitation has not been achieved and it is now well established that excessive fluid resuscitation exacerbates edema formation and contributes to increased morbidity and mortality.18,19 A local and systemic endotheliopathy involving a pervasive injury to the microvascular endothelium’s glycocalyx layer (EGL) develops following major burns.18,20,21
The problem of fluid creep continues to stimulate burn shock resuscitation research. Resuscitation based on 2 cc/kg/%TBSA burn/24 h instead of 4 cc/kg/% burn/24 h,22 applying the rule of 10’s,23 employment of customized resuscitation plans, and use of computerized decision support (CDSS)24 for fluid titration have been evaluated. Promising preliminary results from a large multicenter prospective observational study of CDSS have been reported.25
Alternative Resuscitation Endpoints have been considered as a strategy to limit fluid creep. The serum lactate and arterial base deficit are probably good markers of outcome26,27 but have been insufficiently studied in isolation as titratable endpoints. Resuscitation endpoints based on oxygen consumption and oxygen delivery have been associated with delivery of excessive amounts of fluid.28 Studies examining the use of hemodynamic endpoints (eg, using transpulmonary thermal or indicator dilution, and arterial waveform contour analysis) are small and heterogeneous, and generally have found that compared to using urine output alone led to more fluid administration, no improvement in lactate levels and no difference in clinical outcomes.29
Albumin is now frequently used to limit fluid creep. Dated and highly heterogeneous randomized studies comparing albumin to crystalloids have generally found that introduction of albumin resulted in a significant reduction of resuscitation fluid volumes.30–33 The ABRUPT study found that albumin rapidly lowered the in-to-out ratio, indicating albumin’s potential as a volume-sparing agent.34 Currently, the prospective randomized ABRUPT-2 study (Clin Trials.gov NCT0435659) is comparing crystalloid to crystalloid + 5% albumin started at 8 to 12 hours.
Resuscitation using plasma is not a new concept and clinical evidence of FFP’s fluid sparing effect35 and the recent experimental observation that FFP helped reverse endotheliopathy,21 has stimulated new interest in the use of this colloid during acute burn resuscitation. The PROpOLIs study (ClinTrials.gov NCT 04681638) will compare pathogen-reduced plasma to crystalloids for examining fluid resuscitation volumes as well as the incidence of Transfusion-Related Acute Lung Injury (TRALI).
Finally, resuscitation Using High-Dose Vitamin C (HDVC) is an alternative strategy to limit fluid creep. Preclinical evidence identified that high-dose vitamin C (HDVC) greatly reduced resuscitation volume requirements and edema formation.36,37 Human studies have reported mixed results on HDVC’s efficacy in lowering volume requirements,38 clinical outcomes and risks such as acute kidney injury from oxalate.38,39.
Part 2: Key Discussions from the SOS Conference
. A questionnaire was disseminated to guide the discussion (Figure 3). The timing of colloids and role of FFP were the highest ranked research priorities. Nearly all respondents use colloids (mostly albumin), but there was lack of agreement in timing of colloid initiation, thus highlighting the importance of the ABRUPT trial, and the ongoing ABRUPT-2 and PROpOLIs studies. The next most prioritized topics included alternative endpoints to resuscitation, with stroke volume variation and global end diastolic volume index being most employed. Other potentially valuable markers of burn shock quantification included lactate and plasma SDC-1. Extracorporeal life support was also mentioned as a topic of interest. There was little enthusiasm for examining coagulopathy and viscoelastic assays during resuscitation. There was a low priority score given to researching oral resuscitation, despite that fact that 71% of participants indicated a role for oral resuscitation. Lastly, low priority was given to HDVC research and three-quarters of respondents saw no place for Vitamin C in resuscitation.
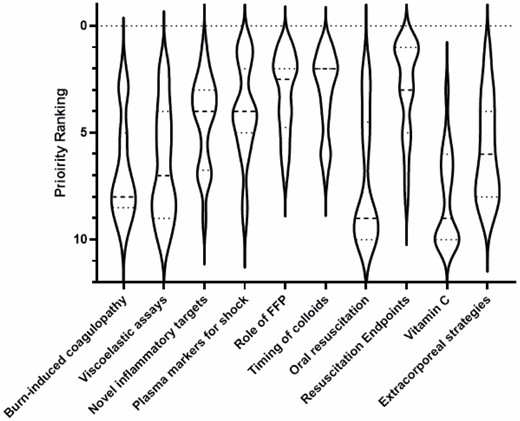
Violin plot showing the priority rank for the 10 topics indicated on the questionnaire. The plot shows distribution of interest from a consistent high ranking given to the use of colloids (FFP), to highly variable enthusiasm for the use of extracorporeal strategies (wide violin plot throughout), to a low priority given to, for example, oral resuscitation with select few individuals ranking this highly (long upper tail).
Part 3: Research Priorities and Future Directions.
Based on the conference discussions, we recommend greater clarity and precision in colloid-directed research, specifically with respect to type of colloid (eg, albumin, fresh frozen plasma, pathogen-reduced plasma, lyophilized plasma), dose, timing, and proposed indication (eg, routine vs rescue). Along this line, future research should continue with multicenter studies to directly compare of albumin and plasma-based regimens in burn shock. Studies evaluating timing and dose of colloids should be prioritized.
Conference participants described varying trigger points for the escalation of therapy and adjunctive approaches to treat worsening burn shock and failing resuscitation. These adjuncts include albumin, plasma, vasopressors, continuous renal replacement therapy (CRRT), plasma exchange and extracorporeal membrane oxygenation (ECMO). Furthermore, it was agreed that a clinical definition of refractory burn shock has not yet been identified. Based on this conference discussion, a preliminary definition for refractory burn shock was proposed as follows: After 4 h of resuscitation the current total fluid infusion rate is >1 L/h OR, 2× the baseline fluid rate at admission, and over 2 consecutive hours UOP is <15 ml/h. Future research is needed to both define and rapidly recognize burn shock and should be directed at the generation of biochemical, plasma-based, phenotypes of burn shock and refractory burn shock, to aid in clinical identification and treatment algorithms.
Hourly urine output remains the gold standard and often the only measure used for fluid titration. Alternate approaches were discussed, but these depend on available resources at each center. Disagreement exists as to alternate markers (eg, blood malperfusion markers, oxygen delivery variables or hemodynamic endpoints) for end organ perfusion in the setting of burn shock. The panel recommends small multicenter studies to directly compare these alternate methods to monitor ongoing resuscitation as well as continued assessment of urinary output using CDSS.
Difficulty with recruitment of patients for multicenter trials was noted by multiple participants. Reduced mortality following major burns over the preceding decades makes mortality a crude and inadequate outcome measure. The panel recommends the convening of an expert consensus panel to define composite organ system-based endpoints for resuscitation in the treatment of burn shock.
Finally, the need to identify a clinical phenotype for burn shock and refractory burn shock emerged in the panel discussions. We recommend studies to characterize burn shock, refractory burn shock and burn-induced coagulopathy, as well as the mechanisms by which albumin and plasma may mitigate these clinical states.
Despite years of research, fluid creep remains a concern in burn shock resuscitation.
A more complete understanding of refractory burn shock, burn-induced coagulopathy and endothelial dysfunction and the role and timing of colloids (specifically plasma) for mitigating these, is needed.
Mortality has become an inadequate measure of outcome, so work is needed to define better endpoints for burn shock resuscitation.
Topic 4: Nutrition and Metabolism
Led by Steven E. Wolf MD FACS, Katherine F. Wallace MS RD LD CNSC and Justin Gillenwater MD MS FACS
All burn providers agree that delivery of nutrition is a key metric in quality of burn care, but few advances in our understanding of the parameters and outcomes surrounding its delivery have occurred in the past decade. Variations in practice are common among institutions and we still cannot answer basic questions surrounding nutritional optimization in burned patients. High-quality, prospective data are lacking on how to best estimate the quantity, quality and mechanism of nutritional delivery to prevent nutritional complications and achieve best outcomes after burn injury.
At the 2021 State of The Science meeting, the existing literature was reviewed and a schema for making progress in this key area of burn care developed. The participants of this meeting included physicians, nurses, therapists and exercise specialists and nutritional researchers and clinical dietitians. A variety of topics were discussed and summarized below.
Initial Nutritional Needs.
While indirect calorimetry (IC) is a standard for estimating energy utilization in the critically ill, some centers indicated difficulty in routinely or consistently obtaining IC. Therefore, predictive equations are commonly used to predict nutritional needs for patients with an acute burn injury. This is also an area in which much variability exists in which formulas are used.
Nutritional Delivery.
Among both groups, the consensus was that enteral is the preferred route for nutritional delivery. This includes the placement of a feeding tube and initiation of tube feeds to achieve prescribed calories and protein if needed. Additional discussion regarding tube positioning (gastric vs postpyloric) and method of feeding (bolus vs continuous) were determined to require further evidence before firm recommendations are proffered. Macronutrient breakdown of nutrition provided was also briefly addressed.
Nutritional Adequacy.
Given burn patients are a unique population, should endpoints differ for these patients to determine nutritional adequacy? Some suggestions in our groups included weight measurements (noting the fluctuations that occur with fluid resuscitation), other anthropometric measurements (mid-arm muscle circumference, skin fold thickness) and frailty scores. Since burned patients often remain in the inpatient setting for some time, how frequently nutritional needs are recalculated and adjusted is another topic of interest. Our groups discussed perhaps to recommend set intervals (ie, weekly) or maybe after a change in clinical condition (including as wounds are healing).
Micronutrients.
Within our group participants, the most commonly monitored and/or supplemented vitamins and micronutrients were copper, selenium, Vitamin C, Vitamin D and zinc. The best way of measuring various vitamins and minerals was also discussed as many are intracellular and the accuracy of some measurements could be questionable. It was also suggested that perhaps routine supplementation without first checking levels may be acceptable. Recommendations for micronutrient supplementation for burn patients do exist in the European Society for Parenteral and Enteral Nutrition (ESPEN) guidelines40 and it was mentioned that perhaps those should be adopted by the ABA as well.
Additional Topics.
Other topics discussed were the opportunities for further research to develop best practices in burned patient care for the following topics: constipation (including prevention and treatment), feeding intolerance (how is it defined and how do we deal with it), enteral feeding while on vasoconstrictors (are there set parameters to be followed) and perioperative feeding practices.
We were unable to reach consensus on these various points of discussion, but all agreed that better quality data are needed to better guide nutritional optimization of our patients. We suggest starting with assessment of current practices in ABA verified burn centers. We will develop and submit two practice-based surveys at the ABA meeting in 2022: one for burn center directors and another for burn center dietitians. Based on the responses to these surveys, our working group will develop recommended data points and outcome measures to be added to the BQIP registry. Some initial suggestions include weight, height, other anthropometric measurements (arm or leg circumference), calories prescribed and delivered, frailty scores, malnutrition diagnosis and measurements of strength or exercise tolerance.
Key Points
To summarize, we formulated the following gaps in scientific knowledge in burned patients regarding nutritional expectations and management.
Clinically useful measurements of nutritional adequacy including optimal frequency of assessment
Ideal substrate for energy utilization in the severely burned, including delineation of best method and timing of delivery
The role of micronutrients in recovery from burn injury
Assessment of nutritional issues at the margin of care, such as enteral feeding intolerance, multiple organ failure, etc.
Topic 5: Neuropsychological
Led by Daniel M. Schneider PsyD, C. Scott Hultman MD MBA FACS and Shelley A. Wiechman PhD ABPP
Neuropathic Pain & Pruritis.
Neuropathic pain and pruritus are common and serious complications of burn injury and persist during the rehabilitation and wound healing process.41,42 The two postburn injury complications severely disrupt the quality of life for patients. Current treatments can be categorized as topical, systematic, extracorporeal shock wave therapy (ESWT), cognitive, mechanical and surgical.41 Understanding the pathophysiology of neuropathic pain & pruritis requires ongoing investigation to best quantify and assess. Further investigation can assess the impact, clinical features, differential diagnoses, mechanisms and attend to ways of measuring neuropathic pain & pruritis. Burn-related neuroanatomic pathways involve differential roles of prelimbic and anterior cingulate cortical region in the modulation of histaminergic and nonhistaminergic itch.42 Spinal endomorphins attenuate burn-injury pain in male mice by inhibiting p38 mitogen-activated protein kinase (MAPK) signaling pathway through the mu-opioid receptor.43
Burn-related neuropathic pain and pruritis predictors and risk factors.
The Burns Registry of Australia and New Zealand (BRANZ) Long-Term Outcomes Project investigated Predictors and risk factors associated with itch and pain in the 12 months following burn injury. The review identified less than 15% of patients that reported moderate or severe pain at 12 months, while approximately one-quarter of the patients reported itch at the same period. Further research is required to better assess and identify individuals reporting high levels of pain and itch to implement appropriate interventions.44
Epidemiology and Mechanisms.
A review of epidemiology and mechanisms highlights decreased peri-traumatic plasma omega-3 fatty acid levels to be associated with increased chronic neuropathic pain.45 Elevated peri-traumatic 17beta-estradiol protective for developing chronic posttraumatic pain in women.46
Patient Supported Outcomes.
Consideration of Burn patients’ pain experiences and perceptions is an additional pathway toward better understanding mechanisms involved in the cognitive and emotional predictors associated with pain. In a review, burn patients reported variable pain experiences and a strong desire to receive additional pain education.47 The applicability of education takes into consideration Burn patients’ perspectives to develop new strategies and content creation for pain-related education materials.
In sum, a framework for rank ordering approaches for pain management in conjunction with precision medicine is needed. Further investigation of precision medicine with genetic testing may generate alternative assessments and treatments to improve quality of life. The use of biomarkers and neuroscience may assist with better identifying individuals’ high risk for pain & pruritis during the rehabilitative phase of burn recovery.
Acute Stress Disorder (ASD) and Posttraumatic Stress Disorder (PTSD).
These conditions are defined by the DSM-V.48 as the development of nine or more symptoms within any of the following categories: Negative mood or alterations of cognition (Dissociation), Avoidance, Intrusion, Arousal. ASD is diagnosed if symptoms occur within 3 days to 1 month after a traumatic event while PTSD is diagnosed if symptoms occur after 1 month. The criteria for a diagnosis of ASD or PTSD are fairly robust and the number of patients who experience symptoms of ASD and PTSD is even higher than those that meet full criteria for a diagnosis. Experiencing some symptoms of ASD in the aftermath of a trauma is typical and we do not want to interfere with a patient’s necessary processing of the trauma. This highlights the importance of screening for PTSD risk factors, as well as symptoms.48
Gaps in the Literature.
In our literature review, we found significant differences in reported prevalence of PTSD, likely due to small sample sizes, differences in timepoints and lack of consistency in reporting symptoms vs diagnosis.49–57 Therefore, determining the course and predictors of ASD/PTSD is needed. To accomplish this, we need evidence-based guidelines for the type of screening tools to use and time of administration, for both adults and children. These guidelines should include reporting standards for identifying and defining symptoms vs an actual DSM-V diagnosis. Gaps also exist in early determination of risk factors for the development of PTSD so that we can intervene with those patients earlier.
Regarding treatment, there are not enough studies in the burn population for a systematic review or meta-analysis, to determine the efficacy of treatment. However, a large body of literature exists that has established several evidence-based treatments for PTSD from all types of traumas.58 There is a need to explore how the unique issues that burn survivors face impact PTSD treatment. All the treatments for PTSD list ongoing pain and continued threat as a contraindication for starting treatment, a gap exists in determining what type of ASD/PTSD treatment is indicated if someone is still experiencing pain. Further, changes in body image may be unique to burn survivors and there is some evidence that body image dissatisfaction mediates the relationship between burn severity and PTSD.59 Future research should examine how body image dissatisfaction intersects with rates of PTSD. Finally, there is a need to examine the intersectionality of posttraumatic growth and resilience on PTSD. The literature is inconclusive on this relationship and is critically important to the quality of life of burn survivors.
Large, multicentered studies are needed to answer these meaningful research questions and gaps in the literature. The field needs to avoid small, single site clinical trials unless it is to establish feasibility or collect pilot data for larger clinical trials.
To refine and develop treatments for neuropathic pain and pruritis, a better understanding of the pathophysiology and associated clinical phenotypes and diagnostic tools is needed.
A role exists for precision medicine in early identification of individuals predisposed to pain or itch to provide new and early opportunities for intervention.
More robust, multicenter research is needed to develop standards for ASD and PTSD risk factors, screening and intervention in terms of timelines and appropriate therapies.
Topic 6: Burn Scar Management and Dyschromia
Led by J. Kevin Bailey MD FACS, Heather M. Powell PhD, Taryn E. Travis MD FACS, Dorothy M. Supp PhD and Bonnie C. Carney PhD
Scar-related disfigurement and loss of function carry significant emotional and financial impacts for patients. Current management of burn scars is multimodal, with a variety of therapies used individually or in combination. Each treatment modality has the potential to contribute to improvement for the patient, but none is a stand-alone solution for all patients. Among the various therapies discussed at the meeting were some that are relatively low cost and low risk, but with little high-quality data supporting efficacy. Examples include silicone gel sheets and massage therapy.60–62
Compression therapy is one of the oldest scar management strategies, with varying reports of efficacy. Most studies report improvements in scar thickness and erythema following compression therapy, although a dearth of well-designed clinical trials, complicated by high levels of patient and scar diversity and lack of quantitative outcome measures, has confounded definitive demonstration of clinical efficacy.63 Recent preclinical studies suggested that compression therapy alters mechano-signaling responses in scars, with significant improvements measured when consistent levels of pressure are delivered for 16–24 h per day, and when therapy is initiated relatively early after injury and continued throughout scar maturation.64–68 Translation of the full benefit of compression therapy is hindered by challenges in patient adherence and low durability of the compression garments.69,70 Burn survivors in attendance at the meeting discussed the discomfort of garments as a deterrent to continuous wear. Survivors and clinicians agreed that improved patient education regarding the potential benefits of compression therapy could improve compliance, as could the use of more comfortable fabrics and garment designs.
Intralesional injections of corticosteroids, such as triamcinolone, remains a relatively common therapy for hypertrophic scars, with improvements in scar thickness and elasticity documented in a recent randomized controlled trial.71 Although intralesional injections are painful, most patients in a recent study still reported satisfaction with the treatment.71 Other injectable drugs used for treatment of scars include 5-fluorouracil, botulinum toxin A, bleomycin and verapamil, which have been used alone or in combinations with each other with variable efficacy.72 Most of these drugs work by reducing inflammation and/or decreasing fibroblast proliferation.72 In addition to pain at the injection site, these therapies are typically limited to relatively small scars, suggesting a need for improved approaches for the delivery of such drugs to larger scar areas.
Among the more invasive therapies currently available, lasers have undergone a fairly rapid deployment for treatment of burn scars. Laser therapies are generally associated with higher costs and greater risks for adverse reactions compared to more conservative scar therapies, but also hold promise for potentially greater benefits. Laser technologies target various features of scars, such as erythema, height, pigmentation and pliability.73 A difficulty in assessing the results of laser therapy is a lack of a clear consensus for the optimal treatment assessment parameters for a given scar type, although efforts towards standardization of treatment paradigms have been undertaken.74 Conference attendees noted that a general acceptance of incremental improvements could lead to a discussion of the cost-benefit and risk-benefit ratios of laser treatment for burn scars. Analysis of risks vs benefits is particularly important for pediatric patients due to the potential risk associated with anesthesia required for multiple laser procedures.75 As with other scar therapies, there is a need for more rigorous studies that include control areas of scars, objective, quantitative measures and larger study populations.
Scar dyschromia continues to be a problem for burn survivors, particularly in patients with skin of color. Although some of the therapies described above can improve pigmentation, variable efficacies have been reported and benefits are often only temporary.76 Surgical treatments, such as dermabrasion/scar area preparation, followed by thin autograft and/or sprayed cell suspension application have been investigated for treatment of hypopigmented scars, although these therapies are generally limited to relatively small areas.77,78 Recent data suggests that melanocytes are present in patient hypopigmented scars and are capable of responding to stimulation with alpha melanocyte stimulating hormone, suggesting potential future approaches for treatment of scar hypopigmentation.79 Development of therapies for scar hyperpigmentation lag behind that for hypopigmentation.
The discussion identified a need for rapid, simple, cost-effective methods for measuring scars. Currently, qualitative and subjective (for patients and clinicians) scar scales are employed due to their ease of use. These scores lack reproducibility, are not burn scar-specific and are highly subjective. Multiple instruments that quantify the physical characteristics of cutaneous scars, such as thickness, pigmentation, erythema, stiffness and elasticity are available, and studies have demonstrated their reliability and reproducibility.80–83 Unfortunately, high instrument costs and the time and expertise required for usage were cited as major impediments to integration of quantitative measurements into clinical practice. However, these drawbacks are outweighed by the need for high-quality studies with quantitative data to demonstrate the efficacy of scar therapies.
Challenges in study design, such as use of appropriate controls, appropriate stratification of patients, ability to collect biopsies and patient adherence to therapy, also hinder development of more effective therapies. The group agreed that scar studies are difficult to carry out in burn patients, emphasizing the need for further preclinical research in this arena. Rigorous study designs that address these challenges in future work will be required to demonstrate efficacy of current treatments and develop novel, targeted, scar-specific therapies.84
The lack of objective and quantitative assessment tools, as well as variability in the implementation of various treatments, have limited the ability to fully characterize the impacts of interventions on scar quality.
Research should include larger populations, appropriate control areas of scar and objective measures of both initial scar characteristics and outcomes.
A role for preclinical studies exists due to the wide ranging characteristics of scars in individual patients, and other issues that make scar therapeutics difficult to study in humans.
Topic 7: Multiorgan System Support
Led by Laura S. Johnson MD FACS FCCP, Kevin K. Chung MD FCCM FACP and Steven A. Kahn MD FACS
Current Knowledge.
Patient responses to critical illness after severe burns proceed through a classically described set of pathophysiologic host responses that are the hallmark of both the acute resuscitative phase and the extended acute hospital stay. Failure to manage inflammation and endotheliopathy results in a pathologic feedback loop of increasing inflammation, immune system hyperreactivity (and sometimes eventual suppression) and hypermetabolism. If these effects cannot be addressed in a timely fashion, patients can quickly develop multisystem organ failure (MSOF).
Despite extensive efforts by the critical care community to study these phenomena, understanding of this problem is still in its infancy; knowledge of pathophysiology, diagnostic modalities, classification systems and treatment strategies continues to evolve.
For patients with severe burns, inhalation and other issues managed on Burn services, the challenge of understanding MSOF is compounded by the challenge of accounting for the impact of injury to the largest organ system in the body (the skin), an organ that is often overlooked in larger critical care studies evaluating MSOF. The general lack of inclusion of burn patients in larger critical care studies can be seen in multiple areas. For example, the more ubiquitous scoring systems to measure organ failure after sepsis are the SOFA and DENVER2 scores, with NEWS and qSOFA showing early promise. However, all of these systems were developed and validated in heterogenous ICU populations and have inherent limitations with a paucity of data in burn patients.
Areas for Improvement and Opportunities for Research.
Several organs of failure have been extensively studied in burn populations, most notably the respiratory and renal systems. Significant work has been done demonstrating the challenges of conventional ventilation management in critically ill burn patients, identifying limits related to the unique physiology of a hypermetabolic patient.85 Additional work is ongoing on characterizing the nature and impact of inhalation injury on both the clinical presentation and inflammatory profiles of patients who present to burn centers.86 Investigation of the utilization of kidney replacement therapy in critically ill burn patients has invited a renewed interest in blood purification as a method to address the inflammatory detritus of thermal injury.87,88
However, many gaps still exist in both the general critical care literature and the burn literature. With a population of patients that routinely lives for an extended period of time in a state of critical illness and survives it, the burn community has an opportunity to add significantly to the understanding of many of the long-term outcomes that are increasingly important to the entire critical care world.89 Neurocognitive changes such as delirium, critical illness weakness (myopathy and polyneuropathy) and the constellation of other outcomes associated with postintensive care syndrome are ripe for investigation given the already standard long-term relationships between burn centers and their patients.90 New understanding of cardiac dysfunction in critical illness offers potential answers to unanswered questions about the ebb and flow of myocardial dysfunction after thermal injury, and novel techniques for infection screening and identification need to be trialed in the thermally injured population to optimize antimicrobial stewardship.91,92 Finally, the burn community has a unique opportunity to contribute to a burgeoning interest in the role of the gastrointestinal tract in critical illness.93
Coupled with the scientific investigations noted above comes an increasingly urgent need for standardization of the language used to discuss patient manifestations of disease both between burn centers and when in conversation with the larger critical care community. Improvements in burn critical care outcomes necessitates a data dictionary just like those in the larger Burn and Trauma Quality Improvement Projects (BQIP/TQIP) in order to pool data across centers that even at their busiest do not see enough large burns for independently run studies. Additionally, while those within the burn community have experiential knowledge from which to share thoughts across centers, translation of that experience to the larger critical care community is fraught with misalignment of priorities and the potential for suboptimal care.
MSOF is poorly understood in burn patient populations, which have been excluded from many studies that developed the most widely used organ failure scoring systems.
Burn patients should be seen as a key population in studying MSOF and critical illness as they represent a group that routinely survives extended critical illness, organ failure, neurocognitive concerns and maintain a long-term relationship with the care providers.
Knowledge capture and translation across burn centers, and from burn centers to the critical care community, is important to gain meaningful data on larger numbers of patients.
Topic 8: Wound Depth Assessment
Led by Angela L.F. Gibson MD PhD FACS, Robert J. Christy PhD and Jeffrey E. Carter MD FACS
Multiple human and animal studies have attempted to improve current standard of care, however the prevailing method to determine whether or not surgery is necessary for wound healing is visual evaluation by a trained burn surgeon.94 It is well acknowledged that surgical decision-making is imprecise and thus patients may undergo unnecessary interventions, delays in surgery and associated risks.
As a first step, our working groups clarified that wound depth assessment is one component of determining healing potential. A discussion ensued regarding the nuances of healing that are not as objectively measured as the histologic depth of injury, such as the impact of pre-existing comorbidities and social determinants of health. Even with the objectiveness provided by histologic depth measurement, the minimal dermal elements (cells and extracellular matrix) necessary for normal wound healing to occur have yet to be well-defined. Furthermore, the determination of wound healing potential requires that we consider technologies that augment or replace traditional autograft techniques. These may offer donor-sparing options and lower the threshold of dermal elements necessary to heal the wound.
A review of the literature on wound depth assessment focused primarily on pathophysiology95–101 and diagnostics.102–109 The majority of studies on depth assessment have been performed in animal models of burn injury, most commonly using rodents or pigs. Through a lively discussion, a number of questions were raised and we collectively put forth potential solutions including where future research is needed as detailed below.
What defines a healed wound in clinical studies?
Recommendation: For clinical studies regarding wound depth, complete wound closure should be reframed in agreement with the FDA statement, “Complete wound closure is defined as skin re-epithelialization without drainage or dressing requirements confirmed at two consecutive study visits 2 weeks apart”.110 Future research should develop methods to measure and define additional characteristics of wound healing.
Are rodents an acceptable model for studying burn depth injury?
Recommendation: Researchers should caution against burn depth studies in rodent models. The group determined that while studies in rodents are necessary first steps, the field should move away from using rodent models exclusively due to the vast differences in the thickness, hair follicle density and absence of eccrine glands in rodent skin. Future research should span multiple models including pig models95 and human subject research. Multicenter clinical trials should be used to enhance generalizability and increase recruitment. Retrospective reviews and prospective observational trials, eg, the ABRUPT study34 can guide protocol development for prospective randomized controlled trials.
What defines the histologic diagnosis of nonhealing burn injury?
Recommendation: A multidisciplinary expert consensus panel should define histological parameters for a nonhealing burn injury for research purposes. Additional research is necessary to further develop and refine histologic parameters. Investigators should consider a multicenter study of patients refusing surgery with nonhealing burn injuries through a retrospective study, which could inform a subsequent prospective study.
What is the unmet need to enhance healing?
Recommendation: A multidisciplinary consensus panel including engineers and patients should define acceptable outcomes for wound healing including both functional and cosmetic outcomes. A working group to develop a list of clinically useful attributes for the development of next generation wound care products would further guide research.
Does burn conversion occur in humans?
Recommendation: A roadmap for initial pilot studies should be designed to study burn conversion in humans. Outcome measures for interventions aimed at treating burn conversion should include analysis of the reduction in both surface area and depth of the wound, as well as improved rate of healing.
What do users need for burn assessment?
Recommendation: An available, adaptable and affordable burn injury assessment tool could improve triage, transfer and treatment algorithms for burn and emergency care providers. Due to resource constraints and dynamic demands in healthcare, principles of implementation science should be considered for new technologies in this field.
Addressing these questions will enable providers to administer appropriate and effective interventions for the early treatment of burn patients. The information gained through appropriate clinical trials outlined above will optimize wound assessment, minimize wound complications and improve the healing potential of wounds from severe burn injury. Due to the limited funding available for clinical trials related to burn wounds, other issues including ease-of-use, cost and the product’s commercial viability and reimbursement status will all need to be addressed during the proposed clinical trials.
While attempts at creating an objective tool for assessing burn depth have been made and published, surgeon visual assessment remains the primary determinant of need for surgery.
Other determinants including comorbidities impact the ability of a burn wound to heal aside from depth.
Most studies on burn depth assessment as well as burn wound progression/conversion have been done in controlled animal models.
Topic 9: Advances in Wound Coverage
Led by Joshua S. Carson MD FACS, Tina L. Palmieri MD FACS MCCM, Nicole M. Kopari MD FACS, Sigrid A. Blome-Eberwein MD FACS and William L. Hickerson MD FACS
Wound coverage, including products available to facilitate wound closure, stimulated discussion. Participants unanimously agreed that the most common burn wound closure technique is autologous split thickness skin grafting. During the discussions, the need for clarity and precision in the definition of skin substitutes became apparent.
Surgical Procedure Definitions.
While burn surgeons tailor wound coverage approach based on the wound bed and patient needs, the language describing those approaches is limited. For coding purposes, operations are deemed either debridement or excision and preparation of the wound bed. Wound bed preparation is completed using various techniques based on wound status: hydro-surgical excision, dermabrasion, a manual or powered dermatome or a chemical agent via either tangential or a fascial excision. The group consensus was that these terms should be standardized and documented.
Wound Coverage.
Wound coverage was the largest identified gap between practice and taxonomy. Surgeons described that their coverage approach and/or product use was based on many factors but was primarily determined by the specific goals for that operation. Although wound coverage products can be employed to accomplish any of a number of objectives, there is no clearly delineated tracking process to assess efficacy or outcomes. This lack of effective language, use-cases and functions of skin substitutes is also a major barrier for industry, as it limits their ability to effectively develop and market their products. Products are approved by the FDA for a narrow set of indications that fail to capture factors and objectives driving product use in clinical practice. Furthermore, the lack of accurate product description limits the clinician’s ability to justify a new product to hospital systems. Particularly challenging is the “wound coverage” indication, which covers almost all substitutes despite differences in characteristics and practical indications.
We offer the following possible solutions: 1) focus on precisely defining the product used, 2) develop a common language for wound coverage operations that captures both the function and goal of the operation. For example, defining surgical objectives in the operative note, which could include: permanent (autologous) wound coverage, preparation of underlying wound bed for future grafting, creation of a neo-dermis or supportive coverage to facilitate re-epithelialization of a partial thickness injury. A predefined practice of identifying objectives prospectively would facilitate effective tracking of outcomes and assessing performance.
Product Categories.
We discussed the dizzyingly broad and unwieldy field of products offered for wound closure. A better way of characterizing skin substitutes that captures their unique properties, although difficult due to evolving technologies, may help. Focusing on functional objectives may be more fruitful than product properties.
Outcome Definitions Limitations in the precision of our current outcome definitions were identified.
While the standard definition of closure as percentage of wound re-epithelialized seems simple, it is deceptively difficult to measure. This seemingly objective measure is actually based on an individual’s subjective estimate from visualizing a wound. These assessments are generally unblinded (made by operating surgeon) with no standardized documentation. Currently, there is no readily available technology that can effectively measure closure independent of human assessment. Possible solutions raised included: 1) Photo documentation. In an age of universal personalized camera ownership, photo documentation of wound closure should be feasible, facilitating on-demand auditing of reporting. 2) Computerized area measurements. Computer programs that segment photos (manually or via artificial intelligence) and then measure the various segments (ie, healed vs nonhealed) could be employed. This approach would combine software solutions with physical time spent executing measurements. 3) A process to control for interobserver variation, enabling assessment of wounds by two judges via a defined protocol.
Outcome Tracking
Outcome tracking for advanced wound closure operations is distinctly limited by the lack of quality, precision and scope.
Regulatory Driven Outcomes.
Outcomes required by regulatory agencies for product approval are often onerous and out of sync with clinical reality. One example is the “100% re-epithelialization” outcome. This end point implies no open areas or dressing need, which is impractical. This single measure is quantifiable, but by no means relevant in capturing clinically effective closure.
Long-Term Outcomes.
A major concern for all in discussions is the limited definitions for long-term outcomes. In most cases the primary goal in the wound coverage process is optimizing function, symptomology and scar appearance. Unfortunately, we have little data on these outcomes. Barriers included—frequent loss to follow up, protracted scar maturation, lack of resources/incentives to facilitate patient compliance. Industry partners might be able to help fund an independent consortium to facilitate this process nationwide by financing and creating an environment where patients meet survivors, linked to participation in follow up studies at the event.
Histology.
Advancing outcome data to include histological assessment of wounds and subsequent scars may be the key to improving care. Although multiple biopsies may pose challenges, burn survivors noted they would be willing to participate and they felt many other survivors would as well. While burn patients in this discussion were eager to participate in research, they may not necessarily represent the total population. Discussion centered around the possibility of industry financially supporting an independent entity to oversee a burn tissue bank of this kind.
Existing Products.
An Excel spreadsheet of the most common products used in the United States today was developed and made available to meeting participants.
There is a lack of clarity in defining the applications, indications, composition and functions of various skin substitute products.
Defining wound closure and a standard means for assessing it would support an improved ability to determine outcomes for various wound coverage approaches.
Long-term outcomes including histologic assessments would aid in our understanding of the various materials and products.
Topic 10: Incorporating Exercise into the Rehabilitation of the Burn Patient
Led by Ingrid Parry MS PT BT-C, Jill M. Cancio OTD OTR/L CHT and Oscar Suman PhD MS
Part 1: Review of the current literature on Exercise in Burn Rehabilitation
Introduction.
Severely burned patients experience a hypermetabolic response that results in catabolic erosion of skeletal muscle and wasting of lean body mass that can persist long after injury.111,112 Patients may also experience periods of joint or whole body immobility secondary to medical instability, mechanical ventilation, postoperative restrictions after multiple skin graft surgeries, sedation, delirium or reduced willingness to move due to fear, anxiety or pain. The combined effect of the body’s stress response to injury and prolonged bed rest has been shown to result in reduced lean body mass,113,114 decreased bone density,115 reduced muscle strength and cardiopulmonary endurance,116–119 increased burn scar contracture120 and diminished functional outcome and health-related quality of life (HRQOL).121 Exercise, in the early and later stages of recovery, is thought to play an important role in mitigating these burn sequelae and improving patient outcomes.
Definition of Terms.
When reviewing the evidence, clarification of three basic terms was necessary to provide a more comprehensive understanding of the state of the science: “exercise,” “rehabilitation” and “outcome.” Although 93% of clinicians worldwide offer exercise as part of burn rehabilitation, the definition of exercise in burn rehabilitation is varied.122,123 An understanding of the types, intensity, and duration of exercise are needed when evaluating exercise efficacy since the physiological benefit of various exercise interventions are different depending on the body systems involved, the manner and intensity of application and level of patient participation.
“Rehabilitation,” including exercise is conducted at all stages of burn recovery from critical care to reintegration to wellness.124 Differentiating the stage of recovery when exercise is implemented allows for a more comprehensive understanding of the applicability, safety and benefit of providing exercise as part of all phases of burn rehabilitation and serves as a foundation for future research.
Lastly, a variety of outcomes are currently represented in the literature to measure the effects of exercise. Outcomes should be chosen with direct relevance to the exercise provided. In addition, selecting consistent outcomes between studies is needed in order to compare studies and build a body of evidence. Currently, there is no consensus on exercise outcome measurements singularly to represent a specific response, or collectively to represent overall wellness.
Exercise and Mobilization during Rehabilitation in ICU/Acute Care Hospital Stay.
Currently, no standard of care regarding the frequency, duration, intensity, progression and types of exercise exists for severely burned patients in the ICU.118 Early mobilization of the critically ill population is generally considered safe125 and has been shown to result in improved physical function,126,127 reduced need for mechanical ventilation,128 and improved health-related quality of life (HRQOL).127 A systematic review of physical rehabilitation in the ICU demonstrated improved physical function and reduced LOS.129 The same review concluded that functional interventions reduced mechanical ventilation and that there may be a dose–response relationship to the amount of physical rehabilitation provided to ICU patients. For the nonburn critically ill population, clinical practice guidelines recommending early mobilization of ICU patients have been published.130–132 It is unclear if these guidelines could be specifically applied to the burn population with their unique medical, surgical and rehabilitative needs.
Exercise during Long-term Follow Up Post Hospital Discharge.
Exercise after discharge from the hospital has been shown to be effective in improving lean body mass, aerobic power, muscle strength, bone mass and domains of mental health.133,134 Defining exercise is key, as there are other activities of physical movement or physical activity that fall under the definition of exercise. Nontraditional exercise studies (ie, yoga, Tai Chi) though uncommon, warrant further study given they are part of the concept of wellness.135 For scientific purposes, it is vital that exercise be described in quantitative terms, while its effect can be both quantitative and qualitative.
Safety considerations of exercise for the burn survivor are similar as those applied to the general population and include relative and absolute contraindications to exercise, or special considerations when exercising. These most likely are conditions where medical consultation is recommended. Persons with conditions such as hypertension, diabetes, kidney disease, osteoporosis, pulmonary diseases to name a few, would benefit from obtaining medical advice before embarking on an exercise program or routine. Attention to heat-intolerance and dangers of hyperthermia should also be considered.
Interplay of Other Factors with Exercise during Burn Recovery.
Exercise applied after a burn involves the interplay of many factors that affect recovery and exercise outcome. Nutrition is an example of a factor that must be considered when implementing and evaluating exercise outcome.136 Further research is warranted to identify barriers in achieving nutrition and physical activity goals in the immediate postburn phase and how to maximize their interplay for optimal outcome. Another acute care consideration for some burn patients is early mobility and exercise and the relationship with coordinated reduction of ventilator support.137 This relationship requires further research.
Expected outcome of exercise and other therapeutic interventions for individuals with burns not only includes optimal physical function in society but also psychological wellness. Burn survivors experience a succession of traumatic assaults to their mind and body which present challenges to psychological resilience. Studies on this topic should include a component of measuring the benefits beyond physical outcome in order to provide a comprehensive picture of recovery and direct patient-focused research. Fear of returning to preburn activities was a message conveyed by burn survivors who attended SOS. A mixed methods study examined the lived experience of burn survivors with respect to fear-avoidance and identified five vital themes: perceived vulnerability to re-injury, others as fear influencers, difficulties and hardships during recovery, engagement in activity and active thoughts.138 It is recommended that this concept be further explored.
Teamwork.
Implementing exercise in the early stages of recovery or the later stages, requires coordination and collaboration of many healthcare team members. For early mobilization to be successful in the ICU, the importance of a structured and collaborative interprofessional approach to building a culture of early mobilization has been described.139 The Society for Critical Care Medicine’s (CSSM) liberation A-F bundle is often used to implement an interdisciplinary team-based approach to early mobilization.140,141
Part 2: Key Discussion Points Regarding Incorporating Exercise into Burn Rehabilitation
. Following the SOS presentations, participants gathered to discuss considerations regarding exercise as part of burn rehabilitation. The group was comprised of various burn team members including at least one burn survivor. The following are some of the key discussion points generated: 1) Promote mobility culture and team coordination to achieve early exercise and mobility; 2) Apply the CCSM liberation A-F bundle to promote early mobilization in the ICU; 3) Consideration of patient delirium, sedation and depression on ability to participate in physical rehabilitation; 4) Determine the safety and feasibility of exercise at different stages of recovery; 5) Consider trauma response and fear-avoidance when resuming activities; 6) Measure the fiscal affect of exercise programs—short and long-term; 7) A wellness profile after burn injury should include home/community, psychological, social, physical, mental/emotional, spiritual and lifestyle factors; 8) Coordinate with community services and organizations to promote long-term activity and wellness; 9) Examine the effect of premorbid lifestyle, motivation and resilience on exercise adherence; 10) Work toward individualized patient-centered approaches to exercise; 11) Use consistent, relevant and validated outcome measures (self-reported and physical performance measures) to determine the efficacy of exercise across all burn centers.
Part 3: Research Priorities and Future Direction.
Future research priorities included the following: 1) Research on exercise with burn survivors should explicitly define the type, intensity and duration of the exercise and the stage of recovery when the exercise is being implemented; 2) Establish relevant exercise outcome measures that are specific to the target benefits of those interventions; 3) Studies on exercise should be interdisciplinary to achieve a holistic understanding of exercise within the framework of burn recovery. For example, role of nutrition in promoting optimal exercise performance and outcome; 4) Define a wellness profile of the burn patient with consideration of psychological, social, physical, mental/emotional, spiritual and lifestyle factors relevant to the individual. For example – a 60-year-old burn survivor who enjoys listening to music and walking can be “trained” with activities to promote walking to more lively music while still being able to sing or converse; 5) Determine optimal team-based care coordination in the inpatient and outpatient settings to maximize the efficiency and effectiveness of exercise programs and patient outcomes; 6) Build on published guidelines for early mobilization and rehabilitation, exercise training and fitness and strengthening exercises when designing and implementing future studies; 7) Evaluate the feasibility and safety of early exercise and mobility with burn patients throughout the stages of burn recovery.
In studying the role for exercise in burn care and rehabilitation, it is important to first define what is being referred to as “exercise”, “rehabilitation” and “outcome”.
While early mobilization of burn ICU patients is generally accepted and has been shown to confer benefits, there is no standard of care for exercise parameters in the ICU.
Research on the interplay of nutritional status/optimization, psychological factors including fear avoidance and appropriate exercise regimen should be conducted. Individualized approaches are needed to account for these and other factors.
Topic 11: Infection
Led by Carl I. Schulman MD MSPH PhD, Regina Lamendella PhD and David M. Hill PharmD BCPS BCCCP FCCM
The infection session of the State and Future Science Meeting was tasked with determining the current state and future research direction for defining and diagnosing sepsis and determining interest and utility in leveraging the microbiome as a prognostic and predictive tool in patients with burn injuries.
Defining and Diagnosing Sepsis.
For sepsis, 5 questions were devised in preparation for the meeting to guide discussion:
What component(s) of the 2007 ABA consensus criteria has the best/worst predictive value?
In your practice, what criteria to you use to trigger further sepsis workup?
What role do biomarkers have in diagnosing sepsis?
Which biomarkers need to be vetted more for potential to aid diagnosis of sepsis?
What role do EHR alerts and sepsis bundles have in patients with severe burn injuries?
There was continued unanimity regarding the disuse of white blood cells (WBC) counts to diagnose sepsis. The collaborative felt strongly temperature, feeding intolerance and acute changes in glycemic variability (esp. patients without a history of diabetes) were all useful variables. However, future studies should focus on adjusting current systems and adding emphasis to others. The new Sepsis-3 guidance should be adjusted to patients with burn injuries, including developing a Burn-specific Sequential Organ Failure Assessment (SOFA) score. The greatest portion of the discussion surrounded what each provider used in his/her own practice to trigger further sepsis workup (eg, tachycardia, hyperglycemia, hyper/ hypothermia, changes in renal function, feeding intolerance, changes in wound appearance, shifts in WBC types, worsening hypotension or pressor use, mental status changes or any unexplained new findings.)
Most notably, there was a strong desire to move away from thresholds. Often overlooked in the ABA 2007 guidance is the key use of the word “progressive.” Arbitrary thresholds are suited for retrospective analysis to delineate sepsis where documentation is lacking, but add little to the day-to-day clinical decisions made at bedside. As such, thresholds should not be used to guide clinical practice.
Biomarkers.
There was agreement in the potential for biomarkers to serve as a diagnostic aid with more focus required, specific to burn patients. However, currently available biomarkers lack sensitivity or specificity or have intrinsically delayed onsets. While timing currently inhibits PCT as an aid to deploy important early interventions for an acute septic episode, there was discussion on the utility of trending daily PCT values to help discern uncontrolled, underlying infectious sources and prevent progression to sepsis. It was felt that lactate is not a good marker for diagnosing sepsis, but it can be used to monitor the progression/resolution of sepsis. Additionally, for both procalcitonin and lactate, it was felt following the trends after postburn day 3 would be most useful. In addition to immediate onset and excellent positive predictive value, the ideal biomarker(s) should be identifiable immediately, even as a point of care test.
EHR alerts. As discussed, SIRS alerts are a disutility in the burn phenotype, leading to overprescribing or worse, alarm fatigue and subsequent neglected cases. Artificial intelligence (AI) is emerging in other aspects of the continuum of care and may have a future with aiding sepsis diagnosis in patients with burn injuries.
Leveraging the Human Microbiome. Several clinical microbiome studies are warranted following questions posed during the ABA State and Future Science Meeting:
What taxonomic microbiome biomarkers in blood, oral, skin, gut and lung are signatures that correlate with patient outcomes?; 2. What techniques for studying microbes should be leveraged for diagnostic and therapeutic purposes?; 3. How can we study and reduce incidence of infections caused by Multi-Drug Resistant bacteria in burn centers?; 4. What microbial taxonomic and functional (ie, antimicrobial resistance genes, biofilm formation genes and other virulence genes) biomarkers will shed light on progression and survival of sepsis?; 5. What microbial genes are expressed response to treatment? And progression of sepsis?; 6. Is antibiotic treatment effective for long-term outlook?; 7. What is the role of supportive care (eg, enteral nutrition, probiotics, fluid resuscitation, antimicrobial therapies) and their link with dynamic microbiota changes to enable us to discover microbial biodiversity that may be beneficial for gut homeostasis in hospitalized patients?
Areas of Opportunity and Future Perspectives. During the session there was robust discussion and enthusiasm regarding the incredible promise studying the microbiome holds for both prognostic and diagnostic potential in the treatment of burn patients.
Studies should aim to collect longitudinal patient samples over the course of burn injury and recovery across multiple locations to include the wound bed (and adjacent uninjured tissue), lung, serum, buccal and stool samples to cover temporal and spatial diversity.
In addition to generating a comprehensive microbial community profile (eg, 16S rRNA gene), studies should provide comprehensive functional microbial and host gene expression profiles (eg, MT) that measure active antibiotic resistance genes, and should investigate the utility of the perioperative use of antibiotics and better inform the antimicrobial stewardship program in burn units.
Apply machine-learning and other AI tools to associate microbiome signatures with clinical data, including patient outcomes. Real-time measurement of microbiome data should leverage both community structure (amplicon sequencing) and shotgun (MG and/or MT) technologies to generate microbial taxa abundances and functional genes (including virulence genes, antimicrobial resistance genes, biofilm genes, etc.) These microbiome data can then be subject to deep learning approaches to identify microbial features that predict and inform patient outcomes and treatment to maximize survival and recovery.
Leverage microbiome technologies to investigate skin microbiota structure and function to generate a “predictive index” that stratifies burn patients on admission according to their resident microbiome and correlation to risk of pneumonia, wound infection, delayed wound healing or multidrug resistance.
Investigate the correlation between microbiota changes and probiotics supplementation, fluid resuscitation and enteral nutrition to identify biomarkers that improve patient prognosis.
Action Items for Sepsis and the Microbiome.
It was unanimous future specific planning meetings should occur to design and determine the resources needed to successfully apply for and fund the research studies required to answer the questions raised by this consensus conference.
Current systems for sepsis guidance should be re-addressed and potentially adjusted to accommodate burn patients, moving away from thresholds and considering a burn-specific SOFA score.
Point of care biomarkers would be a significant benefit to identifying and/or predicting sepsis, however currently available labs and signatures are inadequate in terms of consistency, timeline and specificity.
Microbiome dynamics and signatures are ripe for further study and integration into biomarker development for a variety of aspects of burn pathophysiology and treatment.
Topic 12: Frostbite
Led by Lucy A. Wibbenmeyer MD FACS, Rachel M. Nygaard PhD, Anne Lambert Wagner MD FACS and Damien W. Carter MD FACS
Severe frostbite can result in amputations and chronic pain causing long-term disability ultimately limiting quality of life. The goal of treatment for frostbite injury is to preserve functional limb length. Little progress has been made in advancing treatment modalities following frostbite injury due to the relatively low volume of frostbite injury at each treating center and the seasonality of injury.
Early medical advances in frostbite treatment included rapid rewarming, oral ibuprofen, topical aloe vera and wound care. Rapid rewarming involves placing the injured limb(s) in 40–44℃ water baths until thawing occurs.142–145 In addition to rapid rewarming, oral ibuprofen has been used to disrupt prostaglandin and thromboxane formation following frostbite injury.146–150 A major advancement in the science of frostbite treatment occurred with the use of thrombolytics which halved the amputation rate.151–157 Combining both IV and IA routes of administration, digit salvage rates are reported in excess of 70% (78.7% weighted average and 73.6% average).158,159 While not recommended for monotherapy, many centers use varying combinations of heparin, enoxaparin injections and aspirin for continued anticoagulation needs following frostbite injury.151–153,160 Finally, wound care employing elevation, daily hydrotherapy and bulky dressings is widely practiced.
Preclinical studies of frostbite injury are limited and dated. These models included the use of mice, rats, rabbits and pigs.148,161–176 The methods to establish frostbite injury in these models include: contact, rapid freezing in cold liquid, slow freezing in cold liquid and atmospheric cooling. Various treatment modalities to either prevent or reverse the damage caused by freezing or the reperfusion injury came to mixed results in these models. The most effective preclinical practice to improve frostbite outcomes was rapid rewarming in a 40–44 Celsius water bath.162–164,168,176 Because of inadequate models and recent advances in clinical management, there remains a critical need to establish a reliable preclinical model to assess current and future treatment modalities for use in humans.
Preclinical Research Needs:
Assess current treatment modalities such as thrombolytics and adjuvants
Assess modalities measuring frostbite injury (angiography, Tc99 bone scan, etc.)
Clinical practice guidelines for the treatment and management frostbite injury are limited and rely on experience from high volume centers. However, many frostbite injured patients present to smaller or more rural emergency departments. Time is tissue in frostbite injury making the rapid identification, initiation of therapy and potential transfer to a higher level of care essential to reduce significant morbidity.157,177 To address this critical deficiency, education and outreach efforts should be targeted to the public, first responders and emergency room physicians on best practices in identifying and treating frostbite injury.
Education Needs:
Develop frostbite section for American Burn Association and Advanced Burn Life Support (ABLS) and ATLS
Develop public safety announcements stressing the need to avoid freeze-thaw cycles, rapid rewarming and urgency of transfer to a burn center
While few centers treat a large proportion of severe frostbite injury in the United States, there remain scant studies with high methodological scores to assess current treatment practices. Because of these shortcomings, the scientific support of these promising adjuvant and thrombolytic therapies for the management of frostbite remains incomplete, inconsistent and unclear.
Clinical Management Needs:
Centers of excellence (COE) should be established
Rapid rewarming should be completed and patients sent to COE within 2 h
Initiate multicenter trial to assess outcomes associated with current clinical management of frostbite injury
Determine how to quantify frostbite injury and which tool to use to score it
Determine which method of thrombolytic delivery results in the best outcomes
Determine which adjuvant therapies improve outcomes
Define factors to influence planning and timing of definitive operations
Assess long-term outcomes: pain, mobility, back to work/activities/school
Based on our review of the literature and discussion with experts in the field, we propose the below action items to promote education surrounding the management of severe frostbite injury and to ultimately decrease long lasting disability resulting from severe frostbite.
Opportunities
Partner with COT to help with education; update ATLS; update ABLS Frostbite chapter
Partner with Emergency Medicine Physicians for education of best practices
Engage a basic science researcher to establish a frostbite injury model and assess current practices
Share treatment protocols and clinical education on ABA website
Add variables to BCQP to collect real-time data of frostbite injury
Apply for DOD grants to study frostbite injury
SUMMARY
Mirroring the care of burn patients, the meeting topics and approaches taken to address them were multidisciplinary. Thorough discussions occurred in the breakout sessions and the cross-pollination of various ideas and projects was supported through interactions among diverse experts during and between the meeting agenda items. This has enabled unique approaches and perspectives on next steps. With several groups pursuing projects after the meeting that include grant proposals and manuscripts, the goal of moving the needle on research and diminishing knowledge gaps in these areas is likely to be obtained.
ACKNOWLEDGMENTS
We thank University Medical Center New Orleans and their Burn Center team, as well as all meeting participants and attendees, especially the Phoenix Society representatives and survivors. The authors thank the ABA, in particular Kimberly Hoarle, MBA, CAE (Executive Director) and Maureen Kiley, BBA for their attendance and support of this project. The authors must specifically acknowledge Lori Palfalvi, CRA (Senior Manager Research Administration) from the ABA for her extensive coordination and diligent work in bringing this meeting to fruition, including the ongoing support of the action items and projects described in the proceedings.
The 2021 American Burn Association State and Future of Burn Science Working Group Authorship: Jeffrey W. Shupp, MD FACS, James H. Holmes, MD FACS, Lauren T. Moffatt, PhD, Herbert A. Phelan MD FACS, Linda Sousse PhD MBA, Kathleen S. Romanowski MD FACS, Marc Jeschke MD PhD FACS FCAHS FCCM FRCS(C), Karen J. Kowalske MD, Karen Badger PhD MSW, Rebekah Allely OTR/L, Robert Cartotto MD FRCS(C), David M. Burmeister PhD, John C. Kubasiak MD, Steven E. Wolf MD FACS, Katherine F. Wallace MS RD LD CNSC, Justin Gillenwater MD MS FACS, Daniel M. Schneider PsyD, C. Scott Hultman MD MBA FACS, Shelley A. Wiechman PhD ABPP, J. Kevin Bailey MD FACS, Heather M. Powell PhD, Taryn E. Travis MD FACS, Dorothy M. Supp PhD, Bonnie C. Carney PhD, Laura S. Johnson MD FACS FCCP, Kevin K. Chung MD FCCM FACP, Steven A. Kahn MD FACS, Angela L.F. Gibson MD PhD FACS, Robert J. Christy PhD, Jeffrey E. Carter MD FACS, Joshua S. Carson MD FACS, Tina L. Palmieri MD FACS MCCM, Nicole M. Kopari MD FACS, Sigrid A. Blome-Eberwein MD FACS, William L. Hickerson MD FACS, Ingrid Parry MS, PT, BT-C, Jill M. Cancio, OTD, OTR/L, CHT, Oscar Suman, PhD, MS, Carl I. Schulman MD MSPH PhD, Regina Lamendella PhD, David M. Hill PharmD BCPS BCCCP FCCM, Lucy A. Wibbenmeyer MD FACS, Rachel M. Nygaard PhD, Anne Lambert Wagner MD FACS, and Damien W. Carter MD FACS, David G. Greenhalgh MD FACS, Mary Beth Lawless RN MSN, Deborah L. Carlson PhD, David T. Harrington MD FACS
REFERENCES
DISCLAIMER
The opinions or assertions contained herein are the private views of the authors, and are not to be construed as official or as reflecting the views of the Uniformed Services University, Department of the Army, or the Department of Defense.
There are no disclosures relevant to this manuscript to report.
The meeting serving as the basis for this manuscript was supported by the American Burn Association.



