-
PDF
- Split View
-
Views
-
Cite
Cite
Sabine Bou-Antoun, Rebecca C Oettle, Alistair Leanord, Ronald Andrew Seaton, Ben S Cooper, Berit Muller-Pebody, Geraldine Conlon-Bingham, Frances Kerr, Kieran S Hand, Jonathan A T Sandoe, Martin J Llewelyn, Naomi Fleming, Nicholas M Brown, Nicholas Reid, Philip Howard, Sarah-Jayne Mckinstry, William Malcolm, Alicia Demirjian, Diane Ashiru-Oredope, Adaptation of the WHO AWaRe (Access, Watch, Reserve) antibiotic classification to support national antimicrobial stewardship priorities in the UK: findings from a modified Delphi approach to achieve expert consensus, JAC-Antimicrobial Resistance, Volume 7, Issue 1, February 2025, dlae218, https://doi.org/10.1093/jacamr/dlae218
Close - Share Icon Share
Abstract
In 2017, the WHO introduced the AWaRe (Access, Watch and Reserve) classification of antibiotics to support antibiotic stewardship (AMS) at local, national and global levels. We assessed the categorization of each of the antibiotics for systemic use for antimicrobial stewardship and quality improvement practice across primary and secondary care in the UK, proposing a nationally adapted UK-AWaRe classification.
A four-stage modified Delphi survey was conducted to review the AWaRe classifications in light of antibiotic resistance profiles, antibiotic use and stewardship practice in the UK. Recommendations were iteratively discussed and consensus reached on how to adapt the WHO AWaRe list. Implications of the proposed new categorization for possible antibiotic usage targets were assessed using England national antibiotic consumption data as a case study.
Sixty-one experts across the four UK nations participated in the modified Delphi process. Consensus was most easily reached, with least between-expert variation, for Access antibiotics. Seventeen antibiotics differed in categorization when comparing proposed adapted UK-AWaRe classification and the 2023 WHO AWaRe classification. Through the focus group discussions, the importance of clear messaging was highlighted for the proposed move of first-generation cephalosporins into the Access category, a step-change from the 2019 England AWaRe classification, where all cephalosporins were in the Watch category. From the case study analysis of national data in England, Access antibiotics accounted for >60% of human antibiotic use between 2018 and 2022, 69.7% when using the WHO 2023 classification and 63.7% with the proposed UK-adapted 2024 classification.
An adapted UK-AWaRe list has been suggested through a consensus-reaching process. This will support national AMS and antibiotic usage targets of the UK antimicrobial resistance 2024–29 national action plan.
Introduction
Antimicrobial resistance (AMR) poses a global threat to human health, with related increasing patient morbidity and mortality, and healthcare costs.1,2 The diminishing effectiveness of current antibiotics is compounded by a limited pipeline and limited investment in the development of new antibiotics.
Tackling AMR, including optimizing the appropriate use of antibiotics, has had global attention and is a key objective of the WHO’s global action plan on AMR, which was first adopted at the 68th World Health Assembly in 2015.1,3–5 In 2015, WHO launched a Global Antimicrobial Resistance and Use Surveillance System (GLASS) reporting annual country-level consumption data.6 In 2017, WHO created a new classification system for indexing of antibiotics, assigning most antibiotics in the Essential Medicines List (EML) to one of three categories known as AWaRe (Figure 1), expressing the importance of global access (Access) to necessary antibiotics (at appropriate indication, dose, duration, quality and price), whilst needing to monitor (Watch) and preserve (Reserve) the effectiveness of antibiotics and reducing the potential development of AMR. The WHO AWaRe classification was revised in 2019, 2021 and again in 2023 and continued to focus on optimizing quantity and quality of antibiotic prescribing, with an emphasis on reducing inappropriate use of Watch and Reserve antibiotics.7,10,11
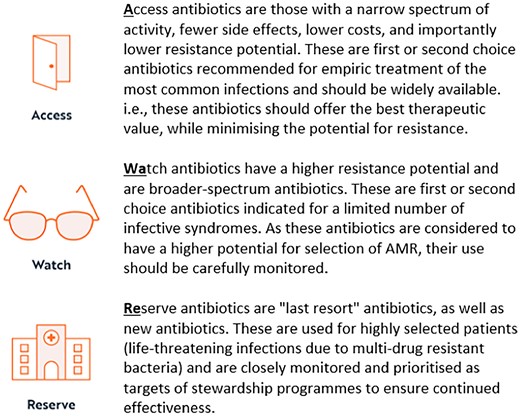
The AWaRe classification has since been used to aid development of quality improvement measures and facilitate AMR stewardship activities, and to evaluate progress towards improvement goals nationally, as well as comparatively analysing global antibiotic consumption trends of the WHO member states, via the GLASS antimicrobial consumption reports.6 The WHO also used this metric to set international measures to contain rising resistance, proposing in its 13th General Programme of Work 2019–23 that antibiotics in the Access group should account for at least 60% of country-level total consumption by 2023.12,13
The WHO AWaRe classification was developed with the intention of providing a basis for supporting countries, regions and districts to develop their own quality indicators and targets for safely reducing inappropriate antibiotic prescribing.8,11 In 2019, antimicrobial stewardship (AMS) experts in England produced a national adaptation of the WHO AWaRe classification to be more reflective of AMS practice, considering levels and trends of antibiotic resistance in the UK, as well as the epidemiology and risk of Clostridioides difficile infection (CDI).11 This 2019 England-AWaRe classification was subsequently integrated into national surveillance of antimicrobial consumption trends, and incorporated into national stewardship strategy, including annual targets set for healthcare provider organizations and the 2019–24 UK AMR National Action Plan (NAP) (which included decreasing Watch and Reserve use in hospitals).14–17
Following the recent amendments to the WHO classification and in preparation for the next 5 year UK AMR NAP (2024–29), the UK Department of Health and Social Care’s Advisory Committee on Antimicrobial Prescribing, Resistance and Healthcare Associated Infections (APRHAI) recommended a review of the AWaRe categories based on the UK’s stewardship approaches.
The aim of this study was to assess the 2023 WHO AWaRe classification for use in the current UK context and collate multidisciplinary expert opinion, representative of the four UK nations, to reach consensus on a recontextualized, UK-wide adapted AWaRe classification (UK-AWaRe). Furthermore, this study aimed to assess the implications that the proposed UK-AWaRe categories could have on setting improvement goals for antimicrobial use for the UK’s AMR NAP (2024–29) compared with international targets based on the WHO AWaRe classification.
Methods
Modified Delphi and nominal group methods
A collaborative multidisciplinary UK-wide (England, Scotland, Northern Ireland and Wales) consensus-reaching approach was utilized, involving iterative rounds of communications via a national survey and two workshops, as well as iterative rounds of communication with APRHAI seeking further endorsement (Figure 2). Further details on the methodology and the ACcurate COnsensus Reporting Document (ACCORD) checklist are provided in the Supplementary data (available at JAC-AMR Online).
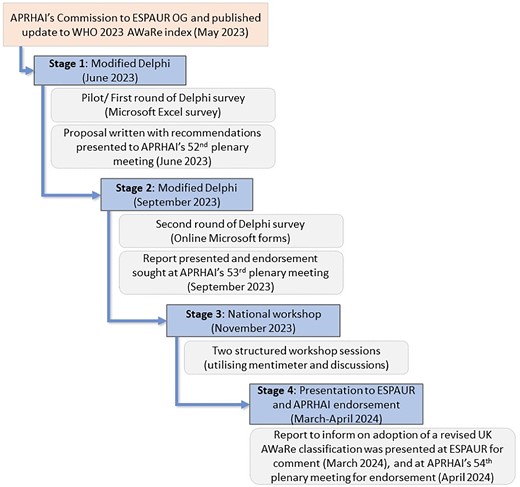
Overview of the UK-AWaRe readaptation process and the modified Delphi method used.
The modified Delphi method combined four stages, which are detailed in Figure 2 and Supplementary information 1. The first stage, which included a pilot survey and virtual meeting, comprised a working group of selected experts and all co-authors (except S.B. and R.O.), identified and invited through the national AMR policy leads in the four UK nations, English Surveillance Programme for Antimicrobial Utilisation and Resistance (ESPAUR) oversight group and APRHAI membership. They have expertise in clinical practice and/or experience in AMS, surveillance, policy, public health practice, pharmacy and microbiology. Following this initial stage, updates were made to the survey questions and format with a subsequent wider-reaching stage asking for individual participant feedback via e-mail, thus reducing the risk of ‘group thinking’, with a link to a developed Microsoft Forms online accessible survey. The survey was cascaded widely across the four UK nations, with further reach achieved by cascading not only to the UK-AWaRe working group but to the ESPAUR oversight group, and further through snowballing via these expert contacts to microbiology and AMS professional networks. Survey responses were extracted to Microsoft Excel to calculate the level of consensus for each antibiotic and to calculate median scores of consensuses by AWaRe category. Survey responses were also analysed to identify if there was requirement for potential alternative categorization from the WHO AWaRe classification, as well as to thematically understand any provided reasoning that accompanied the suggested categorization of antibiotics. Participant-identifiable information was collected on a voluntary basis, with consent, to facilitate ongoing communication and involvement. The APRHAI and ESPAUR oversight national groups oversaw and approved the project methods. The study was not prospectively registered. No honoraria were provided for participating in this initiative.
The third stage involved two workshops held via Microsoft Teams video call to facilitate detailed structured discussion of antibiotics where survey response classifications were inconclusive, with invitations limited to the UK-AWaRe core working group with representation from the four UK nations. Discussions at this stage were informed by previous-stage findings, as well as WHO AWaRe classifications.
Consensus was reached when 70% or more of participants were in agreement. Where the review of antibiotics and their respective AWaRe categories, in the context of the UK, had not led to consensus at the survey stage (i.e. did not reach the agreement threshold of 70% or higher), these antibiotics were specifically re-reviewed in the subsequent stages. The workshop took place over two sessions to provide enough time for thorough structured discussions; initial anonymous answers via private electronic submission of opinions during online video meetings using Mentimeter were followed by open discussions seeking balanced participation from group members, and iterative private reclassifications to converge agreement and consensus. The experts were informed of the agreed conclusion of the participant feedback on the UK-AWaRe classification, at which point no further amendments or differing opinions were made.
The final fourth stage was the presentation of the modified Delphi scientific process and evidence produced, reflecting the recommendations for the readaptation of a UK-wide AWaRe classification to ESPAUR Oversight Group for comment, and then to APRHAI to inform recommendations to government and inform an agreed path forward with the UK-AWaRe classifications of systemic antibiotics (Figure 2).
Case study: assessment of antibiotic prescribing in England using WHO and proposed 2024 UK-AWaRe classification systems
The proposed 2024 AWaRe categories for the UK were used as an illustration to model how this might impact the trend in antibiotic consumption seen in England, between 2018 and 2023 (for national AMS policy interventions) and how different this would be when using the WHO 2023 AWaRe classification for the purposes of international surveillance requirements.18
Antimicrobial dispensing data for England were obtained from the NHS Business Services Authority (via ePACT2) and Rx-info (Define) for primary (including NHS dental prescriptions) and secondary care, respectively, and converted to WHO DDDs (ATC/DDD Index, 2023) for aggregation by UK-AWaRe classification. England mid-year populations (inhabitants) estimates from the Office for National Statistics (ONS) were used for rate calculations of DDDs per 1000 population.
Ethics
Ethical approval was not required for this study according to the NHS Health Research Authority tool. Consent was obtained from all contributors for the collection of their responses, with the aims and objectives of the data collection clearly stated prior to commencing the surveys and workshops. No individuals are identifiable in this manuscript. No patient-level data were used, or relevant.
Results
Consensus approach stage 1 and 2: Delphi survey
Participant characteristics
Sixty-one experts from across the four UK nations completed the national survey (72% of responses were from England, 10% Scotland, 8% Wales and 10% Northern Ireland; the much larger response from England is proportionate in the context of the four UK nations’ population).
The organization types with which participants were associated included teaching hospitals (30%), district general hospitals (20%) and trusts with multiple types of hospitals (18%). Full details are available on the responses of all questions related to participants within Table S1.
Most respondents stated they had expertise in AMS (49%), microbiology (31%) or infectious diseases (8.2%). The primary professions of participants were pharmacists (48%), microbiologists (27%) or hospital doctors (14%). Respondents’ years of experience in specialty covered a wide range, with the greatest proportion (41%) having 10–20 years of experience within specialty.
Consensus at stage 1 and 2
Accumulated views and opinions via the Delphi survey (completed September 2023), seeking recommendations for UK-AWaRe classifications based on experience and expertise, resulted in a consensus for most antibiotics (Table S2).
Consensus was reached at this stage for classification of amoxicillin/clavulanic acid within Watch rather than Access (the WHO 2023 AWaRe classification for this antibiotic).
The box-and-whisker plot (Figure 3) indicates the distribution of percentage agreement amongst the consensus responses at this Delphi survey stage, with medians demonstrating the median percentage agreement within the categorizations amongst experts and the interquartile range (IQR) demonstrating the 50% spread. The median and IQRs were less variable, with the most symmetrical distribution of answers, for antibiotics classified as Access compared with Watch and Reserve antibiotics, i.e. there was higher percentage agreement for antibiotics that reached consensus amongst the Access category, indicated by the higher median value and IQRs for Access antibiotics (median consensus response for antibiotics: 93%; IQR: 89%–98%) and the smaller span of the IQR indicating less between-antibiotic variability in percentage agreement for categorization. This is compared with Watch (median: 82%; IQR: 77%–90%) and Reserve (median: 85%; IQR: 80%–94%) categories. The Access outlier was oral fosfomycin. Further details of the consensus percentages by antibiotic are available in Table S2.
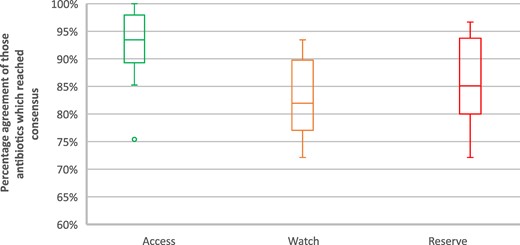
Box-and-whisker plot of all antibiotics that reached consensus at stage 1.
Consensus approach stage 3: workshops
Seventeen experts, with representation from across the four UK nations [8 England (47%), 4 Scotland (24%), 4 Northern Ireland (24%), 1 Wales (6%)] contributed to the workshop split across two sessions. Tables S3 and S4 present the antibiotics for which the 70% threshold for consensus was not reached and where further discussions were held via these two workshops. Twenty-eight antibiotics required further discussion, of which 6 were later classified as Access antibiotics, 10 as Watch, 2 as Reserve and 10 as Other/uncategorized. Table S4 provides details of the proposed UK-AWaRe classification resulting from the stage 3 workshops, and the respective 2023 WHO AWaRe categories.
Final consensus on proposed UK categories for endorsement (Stage 4)
The full list of antibiotics reviewed throughout the process and the final consensus on the proposed 2024 UK-AWaRe classification and corresponding 2023 WHO AWaRe classification, which was presented to ESPAUR and ARPHAI at stage 4, are provided in Table S5. Seventeen antibiotics differed in categorization when comparing the 2023 WHO AWaRe classification and the proposed adapted UK-AWaRe classification. Nine antibiotics differed in categorization from the previous 2019 adapted England-AWaRe classification (Table S4).
Themes from feedback during workshops
Themes that were derived from the modified Delphi process, workshops and stage 4 as rationale for categorizations were often related to: licence status and use/availability of the antibiotic in the UK; agreement that all new antibiotics should be placed in the Reserve category at launch; consideration of the need to reserve use of the antibiotic, or if required as first-line treatment for MDR infections; and associations with CDI. There were also discussions about placement of different generations of cephalosporins in differing categories and the importance of clear messaging to clinicians, especially as all cephalosporins were previously placed in the Watch category. Further insight on the thematic reasoning can be found in Supplementary Information 3.
Case study: assessment of antibiotic prescribing in England based on WHO and proposed 2024 UK-AWaRe classification systems
Assessment applying different AWaRe categories to national antibiotic use data in one of the four UK nations (England) and their impact on trends and national measures are presented in Figure 4 as a case study illustration. Access antibiotics as a proportion of total antibiotic use in England was higher when using the WHO 2023 classification, compared with the proposed UK-adapted 2024 classification; in 2022, 69.66% of total antibiotic use was Access antibiotics where Access antibiotics were defined using the WHO 2023 classification, compared with 63.67% using the nationally adapted 2024 UK-AWaRe classification.

Proportion of total antibiotic use in England by AWaRe classification: (a) 2023 WHO AWaRe classification, and (b) proposed 2024 UK-AWaRe classification.
Assessment of the proposed 2024 UK-AWaRe classification by antibiotic consumption, measured as DDDs per 1000 population per day, is shown in Figure S1. The figure highlights an increase in Access category use as a proportion of total use following the decrease noted during 2020, related to COVID-19.
Discussion
This study reached national consensus on the placement of antibiotics used in the UK among the AWaRe classification categories and represents a feasible method of multidisciplinary involvement and iterative consensus-reaching techniques when considering an update or adaptation of the AWaRe classification. Alongside the strength of the iterative process in eliciting expert knowledge, the recommendations and findings were discussed and endorsed by APRHAI, an advisory committee to the UK Government on AMR and healthcare-associated infections. The largest revision seen to the antibiotics section of the WHO EML was in 2017, when the AWaRe classification was first introduced.19,20 Since the inception of the classification and the subsequent publication of the WHO AWaRe book,8 countries have been encouraged to adapt the AWaRe categorizations to their own AMR and antimicrobial usage patterns, and to develop their own quality targets to safely and appropriately reduce unnecessary antibiotic prescribing. As such, the WHO AWaRe classification can be used as a quality monitoring tool and has been used as such internationally and in the UK. However, whilst recognized by WHO as a potential requirement, defining and adapting the classification for context-specific use and use over time requires complex assessment and decision support with limitations that will remain, particularly around the evidence base for antibiotics placed within different categories.21
We have provided details of a modified approach to reaching consensus that was used to inform a readaptation of a UK-wide AWaRe antibiotic classification. Where there is either an excess or an insufficient amount of information, consensus modified Delphi techniques (often via surveys/questionnaires, and incorporating nominal group method within this process via discussions) permit accumulation of expert opinion and knowledge, and facilitate unbiased summation and agreement.22,23 The multistage approach, across interdisciplinary experts and across the four UK nations, provided a robust and transparent method to re-evaluate the categories for the UK-AWaRe classification. Systematic methods that can be used to review over time are beneficial, with this being the second attempt of adaptation of the WHO AWaRe classification in the UK.11 The proposed adapted classification provides evidence to inform a country-specific (UK-wide) tool for stewardship and quality improvement measures, whilst acknowledging the variation between the four UK nations in AMR profiles, antibiotic consumption, supply chain, CDI risk and national guidance. The anonymized initial stages of the process, and the snowballing technique used, meant unbiased responses and wider reach were achieved. The nominal group design of the latter workshop stage included selected specialists to discuss and represent views of the four UK nations. The discussions were informed by the previous Delphi stages with the findings of them presented to participants; however, rounds of anonymous voting were used alongside discussions to reach consensus for those antibiotics that had proved unclear in categorization, thus attempting to attenuate for potential bias and ‘group thinking’.
Seventy-three antibiotics (81%) within the proposed 2024 UK-AWaRe classification aligned with the 2023 WHO AWaRe classification [17 antibiotics differed (19%)]. Amoxicillin/clavulanic acid, placed as Watch within the 2024 UK-AWaRe classification and Access for the 2023 WHO AWaRe classification, is the most significant of those with differing categories, due to the relatively high rate of consumption in England, particularly in secondary care. The consensus process highlighted the importance of distinguishing between the AWaRe categories assigned to different cephalosporin generations and placed the first-generation cephalosporins into Access, a different AWaRe category from the other generations, based on propensity for resistance, antibiotic use (whether first-line treatment, or importance of this oral option for treatment of community urinary tract infections), CDI risk, or whether used as a valuable alternative for patients with non-severe/unverified penicillin allergy. Unlike the 2019 England-adapted AWaRe classification (which placed all generations of cephalosporins in Watch), this process has resulted in the 2024 UK-AWaRe classification aligning with the 2023 WHO AWaRe classification (which also places first-generation cephalosporins in Access, and second-, third- and fourth-generation cephalosporins in Watch). How this differing cephalosporin categorization may impact messaging and the need to ensure transparency and early communication was discussed at length, as were concerns of the move of first-generation cephalosporins from Watch to Access and how this may be misconstrued as ‘promoting’ the use of cephalosporins, and in so doing opposing the national targets to reduce cephalosporin prescribing. Hence, the importance of clear communication for this cephalosporin classification change and the need for stewardship and support for appropriate antibiotic prescribing messaging (as has been successfully managed for the aminoglycosides gentamicin and amikacin, which have been classified into differing categories of Access and Watch, respectively). While first-generation cephalosporins have been placed within the Access category, this does not translate to mandating this change in guidelines and, as mentioned for the other generations, where there is a CDI risk it would be reasonable to advise avoidance of use of these antibiotics.
Other outcomes included clarity around new antibiotics being automatically placed within the Reserve category and any therapies not licensed in the UK to be placed within the Other/uncategorized category, although there were deliberations on how to engage in supporting licensing of these agents whilst placed within the Other category.
Throughout the Delphi process, the importance of using the WHO AWaRe classification for continuing international evaluations was emphasized, i.e. stewardship at a national level would be better informed with a tailored classification that is context specific; however, for international benchmarking and comparisons, the use of the WHO classification would still be required. Surveillance data of total antibiotic consumption in humans according to the WHO AWaRe classification in 2020 demonstrated similar Access prescribing proportions in England (66.91%) compared with Belgium (67%) and Lithuania (67%) from the European Economic Area (EU/EEA countries) countries,12 and along with 17 of the 26 countries contributing data to the WHO GLASS Antimicrobial Consumption (AMC) surveillance system, England demonstrated Access antibiotic consumption that accounted for greater than 60% of total antibiotic consumption,12 exceeding the international target. It is worth noting that subsequently at the UN General Assembly, High-Level Meeting on Antimicrobial Resistance (AMR) in September 2024, world leaders committed to a more ambitious target, that by 2030, at least 70% of antibiotics used for human health globally should belong to the WHO Access group antibiotics.24
The proportions of total antibiotic prescribing accounted for by the Access, Watch and Reserve categories of the current 2019 England-adapted AWaRe classification have been published as indicators within the UKHSA England data on a public-facing open-access platform, Fingertips (https://fingertips.phe.org.uk/profile/amr-local-indicators). Indicators reporting antibiotic prescribing by DDDs per 1000 admissions for these categories have also been published on this platform. NHS hospitals are therefore able to track progress towards national quality measures and compare amongst peer organizations. Updates to the UK-AWaRe classification will impact currently available monitoring data. When the revised 2024 UK-AWaRe classification becomes ratified and subsequently adopted based on the evidence obtained here, a challenge is presented in that the historical monitoring data will need to be updated to support the reclassifications and ongoing monitoring, with future iterations of the UK-AWaRe adaptations requiring amendments in monitoring data to support national stewardship activities. Furthermore, there may be further policy considerations with adoption of the proposed 2024 UK-AWaRe classification across the UK four nations, with differences in data availability/surveillance, particularly with concerns over the changes to cephalosporin classification from a communications perspective and potential impact on CDI. Although all four UK nations are committed to the targets in the 2024–29 NAP (namely, Target 4b: by 2029 an aim to achieve 70% of total antibiotic use from the Access category across the human healthcare system),25 adoption of the readapted UK-AWaRe classification, where ratified, could be staged or lagged by UK country, regionally and locally, and can be implemented differently based on the most relevant surveillance ‘information for action’ data. This would need to be done in the context of the single target in the 2024–29 NAP, which all four nations committed to [‘Target 4b: by 2029, we aim to achieve 70% of total use of antibiotics from the Access category (new UK category) across the human healthcare system’].25
A limitation of current surveillance and monitoring is the lack of adjustments for age, sex, socioeconomic factors, deprivation and ethnicity. Case-mix adjustments and assessment by population groups to identify disparities and directed stewardship are required for targeted improvements, with the potential that the AWaRe categories could be adapted more specifically by care setting at the least.
Conclusions
The WHO AWaRe classification has been an important tool for AMS across the UK, with publication of adapted classifications used nationally in the UK since 2019. Whilst attempts were made to keep the readapted UK-AWaRe classification aligned with the recently updated WHO 2023 AWaRe classification (including cephalosporin classifications), it was necessary to make modifications based on the UK context (with a key difference being in classification of amoxicillin/clavulanic acid within the Watch rather than the Access category). The data presented highlight the implications the reclassifications could have in England, as a case study, and can guide current antibiotic consumption measures, implementation of current and future NAPs aimed at optimizing and monitoring use of Watch and Reserve antibiotics.
Acknowledgements
With thanks to the wider members of the English Surveillance Programme for Antimicrobial Utilisation and Resistance (ESPAUR) oversight group, Department of Health Advisory Committee on Antimicrobial Prescribing, Resistance and Healthcare Associated Infection (APRHAI) and all external collaborators across the NHS and research communities who contributed to the adapted Delphi method.
We would like to thank Angela Falola, UKHSA, for her assistance with provision of primary care prescribing data, Jordan Charlesworth and Faatimah S. Shaikh for operational support, Emma Budd for insight into previous England adaptation approach, Professor Michael Sharland for presenting at the workshop and sharing insights into the WHO AWaRe classification and EML, and to Connie Longmate, Department of Health and Social Care, and Russell Hope, UKHSA for commenting on the article prior to submission.
Funding
The project did not receive additional funding and was conducted as part of UKHSA’s HCAI and AMR activities. D.A.-O., A.D. and B.M.-P. are supported by the National Institute for Health Research (NIHR) [Health Protection Research Unit (HPRU) in Healthcare Associated Infections and Antimicrobial Resistance, a partnership between UKHSA and (i) Imperial College London (NIHR200876) and (ii) the University of Oxford] (NIHR200915). D.A.-O. is also supported by National Institute for Health and Care Research, Senior Clinical and Practitioner Research Award (NIHR304553).
Transparency declarations
S.B.-A., R.C.O. have no declarations of interest. All other authors were involved in the development of the UK National Action Plan 2019–24. No artificial intelligence tools were used. The views expressed are those of the author(s) and not necessarily those of their employers, NIHR or the Department of Health and Social Care.
Author contributions
D.A.-O. led the study conception and design, with contributions from A.D., B.M.-P. and S.B.-A. D.A.-O. oversaw the project, identified and invited individuals with relevant expertise to form the core author group, and was responsible for the study methods and oversight of project management. S.B.-A. was responsible for the day-to-day project management and project implementation including, data collection, and analysis, and manuscript authorship. R.C.O. supported project implementation and analysis. A.L., R.A.S., B.S.C., B.M.-P., G.C.-B., F.K., K.S.H., J.A.T.S., M.J.L., N.F., N.M.B., N.R., P.H., S.-J.M., W.M., A.D., D.A.-O., who have expertise in clinical practice and/or experience in AMS, surveillance, policy, public health practice, pharmacy and/or microbiology formed the core consensus working group. All authors contributed to drafting subsequent versions of the manuscript and approved the final manuscript.
Supplementary data
Figure S1, Tables S1–S5 and Supplementary information 1–3 are available as Supplementary data at JAC-AMR Online.




Comments