-
PDF
- Split View
-
Views
-
Cite
Cite
Truc T Tran, Nicolo L Cabrera, Anne J Gonzales-Luna, Travis J Carlson, Faris Alnezary, William R Miller, Aki Sakurai, An Q Dinh, Kirsten Rydell, Rafael Rios, Lorena Diaz, Blake M Hanson, Jose M Munita, Claudia Pedroza, Samuel A Shelburne, Samuel L Aitken, Kevin W Garey, Ryan Dillon, Laura Puzniak, Cesar A Arias, Clinical characteristics, microbiology and outcomes of a cohort of patients treated with ceftolozane/tazobactam in acute care inpatient facilities, Houston, Texas, USA, JAC-Antimicrobial Resistance, Volume 5, Issue 1, February 2023, dlac131, https://doi.org/10.1093/jacamr/dlac131
Close - Share Icon Share
Abstract
Ceftolozane/tazobactam is a β-lactam/β-lactamase inhibitor combination with activity against a variety of Gram-negative bacteria, including MDR Pseudomonas aeruginosa. This agent is approved for hospital-acquired and ventilator-associated bacterial pneumonia. However, most real-world outcome data come from small observational cohorts. Thus, we sought to evaluate the utilization of ceftolozane/tazobactam at multiple tertiary hospitals in Houston, TX, USA.
We conducted a multicentre retrospective study of patients receiving at least 48 h of ceftolozane/tazobactam therapy from January 2016 through to September 2019 at two hospital systems in Houston. Demographic, clinical and microbiological data were collected, including the infecting bacterial isolate, when available. The primary outcome was composite clinical success at hospital discharge. Secondary outcomes included in-hospital mortality and clinical disposition at 14 and 30 days post ceftolozane/tazobactam initiation. Multivariable logistic regression analysis was used to identify predictors of the primary outcome and mortality. Recovered isolates were tested for susceptibility to ceftolozane/tazobactam and underwent WGS.
A total of 263 patients were enrolled, and composite clinical success was achieved in 185 patients (70.3%). Severity of illness was the most consistent predictor of clinical success. Combination therapy with ceftolozane/tazobactam and another Gram-negative-active agent was associated with reduced odds of clinical success (OR 0.32, 95% CI 0.16–0.63). Resistance to ceftolozane/tazobactam was noted in 15.4% of isolates available for WGS; mutations in ampC and ftsI were common but did not cluster with a particular ST.
Clinical success rate among this patient cohort treated with ceftolozane/tazobactam was similar compared with previous experiences. Ceftolozane/tazobactam remains an alternative agent for treatment of susceptible isolates of P. aeruginosa.
Introduction
Pseudomonas aeruginosa has long been recognized as a serious therapeutic challenge due to the presence of multiple antimicrobial resistance mechanisms,1 which has led to its classification as a serious threat.2 Inappropriate empirical antimicrobials, delays in initiation of appropriate agents and limited effective treatment options possibly contribute to poor patient outcomes,3 particularly when last-resort antimicrobials such as polymyxins have been used.4–7
Ceftolozane/tazobactam is a combination of a novel cephalosporin and a β-lactamase inhibitor, which can retain activity in the face of multiple pseudomonal resistance mechanisms.8,9 The FDA initially approved ceftolozane/tazobactam for complicated intraabdominal and complicated urinary tract infection at a dose of 1.5 g every 8 h.10 In 2019, ceftolozane/tazobactam was approved for nosocomial pneumonia at a dose of 3 g every 8 h.11,12
However, the ‘real-life’ use of ceftolozane/tazobactam is likely to differ from approved indications. Most outcome data on such use are from small observational studies that need to be corroborated using larger cohorts. We sought to evaluate our collective clinical experiences when using this agent in highly complex hospitalized patients in a multicentre study in Houston, TX, USA. We aimed to describe the clinical outcomes and their predictors in patients treated with ceftolozane/tazobactam as well as genomic determinants of antimicrobial resistance in the P. aeruginosa isolates derived from this cohort.
Methods
Study design and population
The protocol of this study was approved by the local institutional review boards of participating institutions, which waived the requirement for written or verbal consent based on the retrospective nature of the study.
Patients who received inpatient ceftolozane/tazobactam therapy from January 2016 through to September 2019 were identified using pharmacy databases in two healthcare systems (nine academic or community inpatient care centres) in the greater Houston metropolitan area. Patients ≥18 years old who received ceftolozane/tazobactam for ≥48 h during their hospitalization were included. Ten patients may overlap with a previously reported multicentre cohort.13 The electronic medical record (EMR) was retrospectively reviewed to collect demographic, diagnostic, treatment and outcome data. For patients who received multiple courses of ceftolozane/tazobactam, only the first course of ≥48 h of inpatient ceftolozane/tazobactam therapy was included in the analysis as the index episode. A gap of ≥72 h between ceftolozane/tazobactam doses was considered a new course of ceftolozane/tazobactam. Susceptibility testing for ceftolozane/tazobactam was retrieved from clinical notes (prior to May 2017) and laboratory reports in the EMR (after May 2017).
The index event or episode was defined as the infection episode for which ceftolozane/tazobactam was initiated. The index culture was the first culture obtained during the index episode and/or the culture taken from the site of the primary infectious diagnosis (e.g. respiratory tract culture with a pneumonia diagnosis). P. aeruginosa isolates were deemed MDR or XDR as previously described.14,15
Use of ceftolozane/tazobactam was deemed culture-guided or empirical based on microbiological results. The doses considered optimal were 3 g every 8 h for respiratory infections and 1.5 g every 8 h for all other infections, with respective adjustments for renal function as derived from FDA-approved product labelling.16 Suboptimal dosing was based on total ceftolozane/tazobactam dose received within the first 24 h. Detailed description of the clinical data is described in the Supplementary Methods (available as Supplementary data at JAC-AMR Online).
Outcomes
The primary outcome was composite clinical success, defined as fulfilment of all three criteria: (a) recovery from or improvement of acute infection-related signs and symptoms; (b) absence of new signs and symptoms of infection from ceftolozane/tazobactam initiation until discharge; and (c) absence of additional systemic Gram-negative antibacterial therapy >48 h of ceftolozane/tazobactam initiation, not including use of additional agents for de-escalation or after discharge from hospitalization. Antimicrobials were deemed de-escalated if the treating physician documented clinical response to ceftolozane/tazobactam and selected a narrower-spectrum agent for definitive therapy. If the patient did not respond to ceftolozane/tazobactam and required further systemic Gram-negative therapy with another agent or if new infectious signs or symptoms prompted the treating physician to restart a Gram-negative antimicrobial, this was considered additional therapy. Secondary outcomes included in-hospital mortality, infection-related mortality (as documented by treating physician), and clinical disposition at 14 and 30 days after ceftolozane/tazobactam initiation. The categories of clinical disposition are described in the Supplementary Methods.
Microbiological and genomic investigation
Available isolates were tested for ceftolozane/tazobactam susceptibility using gradient diffusion strips (ETEST®, bioMérieux) on Mueller–Hinton agar (Becton Dickinson). Genomic DNA extraction was performed as previously described17 with WGS on a MiSeq (Illumina) using 2 × 300 paired-end reads. Genome assembly, MLST, resistance gene screening, mutational resistance and genome annotation are described in the Supplementary Methods.
Statistical analysis
Characteristics of the cohort were recorded using frequencies and percentages for categorical data and medians and IQRs for continuous data. Chi-squared analysis was used for categorical variables and two-sample t test with equal variance was used for continuous variables, with a significance threshold of P ≤ 0.05. A univariate analysis was performed to identify factors significantly associated with the primary and secondary outcomes from a list of pre-specified variables of interest (the use of empirical systemic antibiotics, time to initiation of ceftolozane/tazobactam, concurrent additional Gram-negative antimicrobial therapy, suboptimal ceftolozane/tazobactam dosing, and infectious diseases consultation for the index event). Multivariable logistic regression models were constructed with Charlson comorbidity index (CCI) score, APACHE II score and immunocompromised state included as independent variables to account for patient baseline risk. For missing values on APACHE II variables, normal or non-deranged values were assumed, contributing zero points to the patient’s APACHE II score. The final models also included covariates with P < 0.2 in the univariate analysis. Model assumptions were evaluated with diagnostic plots (i.e. standardized residuals) and other appropriate measures (e.g. variance inflation factor for multicollinearity). Results are reported as the adjusted OR with 95% CI.
Results
Patient characteristics and clinical microbiological data
A total of 263 patients were included in the study; patient characteristics are given in Table 1. The median age was 61 years (IQR 48–69). Females composed 33.5% of the cohort and 59.3% of patients identified as non-white. Median BMI was 26 kg/m2 (IQR 22–32). The most common comorbidity was diabetes mellitus (41%), followed by congestive heart failure (27%) and cerebrovascular disease (26.2%). Immunocompromised patients made up 14.8% of the patient population, including patients with HIV, patients on ≥15 mg/day prednisone, patients on cancer chemotherapy or with a history of a solid organ transplant. Prior to their hospitalization, 44.5% of patients had been in the community and 32.3% had resided in skilled nursing, long-term acute care or inpatient rehabilitation facilities. The majority of patients (64.6%) had a previous hospitalization within 90 days and 3% had a known ceftolozane/tazobactam exposure prior to the index episode.
| . | Total cohort . | No clinical success . | Clinical success . |
|---|---|---|---|
| Characteristica . | (n = 263) . | (n = 78) . | (n = 185) . |
| Age (years) | 61 (48–69) | 60 (48–67) | 62 (48–71) |
| Female sex | 88 (33.5) | 25 (32.1) | 63 (34.1) |
| Race | |||
| Asian | 5 (1.9) | 2 (2.6) | 3 (1.6) |
| Hispanic/Latin | 9 (3.4) | 5 (6.4) | 4 (2.2) |
| Non-Hispanic black | 61 (23.2) | 22 (28.2) | 39 (21.1) |
| Non-Hispanic white | 107 (40.7) | 22 (28.2) | 85 (45.9) |
| Other/not declared | 81 (30.8) | 27 (34.6) | 54 (29.2) |
| CCI score | 4 (2–6) | 5 (3–6) | 4 (2–6) |
| Comorbidities | |||
| Myocardial infarction | 20 (7.6) | 8 (10.3) | 12 (6.5) |
| Congestive heart failure | 71 (27.0) | 27 (34.6) | 44 (23.7) |
| Cerebrovascular disease | 69 (26.2) | 20 (25.6) | 49 (26.5) |
| Chronic obstructive lung disease | 31 (11.8) | 11 (14.1) | 20 (10.8) |
| Connective tissue disease | 13 (4.9) | 4 (5.1) | 9 (4.9) |
| Hemiplegia/paraplegia | 43 (16.3) | 12 (15.4) | 31 (16.8) |
| Lymphoma | 5 (1.9) | 3 (3.8) | 2 (1.1) |
| Solid cancer | 32 (12.2) | 8 (10.3) | 24 (13.0) |
| Diabetes | 108 (41.1) | 33 (42.3) | 75 (40.5) |
| Chronic liver disease | 16 (6.1) | 5 (6.4) | 11 (5.9) |
| Immunocompromised statusb | 39 (14.8) | 16 (20.5) | 23 (12.4) |
| Neutropenia | 3 (1.1) | 3 (3.8) | 0 (0) |
| HIV | 3 (1.1) | 2 (2.6) | 1 (0.54) |
| Prednisone at ≥15 mg daily or equivalent | 18 (6.8) | 5 (6.4) | 13 (7.0) |
| Cancer chemotherapy within prior 6 months | 3 (1.1) | 2 (2.6) | 1 (0.54) |
| History of solid organ transplant | 23 (8.7) | 9 (11.5) | 14 (7.6) |
| Heart | 11 (4.2) | 6 (7.7) | 5 (2.7) |
| Lung | 6 (2.3) | 1 (1.3) | 5 (2.7) |
| Kidney | 4 (1.5) | 1 (1.3) | 3 (1.6) |
| Liver | 2 (0.76) | 1 (1.3) | 1 (0.54) |
| BMI | 26 (22–32) | 26 (22–30) | 26 (22–32) |
| Prior hospitalization in the prior 90 days | 170 (64.6) | 51 (65.4) | 119 (64.3) |
| Known C/T use within prior 90 days | 8 (3.0) | 4 (5.1) | 4 (2.2) |
| History of a resistant Gram-negative bacterial culture isolate | 159 (60.5) | 40 (51.3) | 119 (64.3) |
| Pre-hospitalization residence | |||
| Community | 117 (44.5) | 24 (30.8) | 93 (50.3) |
| Other acute care hospital | 35 (13.3) | 16 (20.5) | 19 (10.3) |
| SNF/LTAC/inpatient rehabilitation facility | 85 (32.3) | 25 (32.1) | 60 (32.4) |
| Other | 26 (9.9) | 13 (16.7) | 13 (7.0) |
| Source of infection | |||
| Blood/endovascular | 13 (4.9) | 4 (5.1) | 9 (4.9) |
| Bone/joint | 28 (10.6) | 5 (6.4) | 23 (12.4) |
| CNS | 1 (0.38) | 0 (0) | 1 (0.54) |
| Intraabdominal infection | 12 (4.6) | 4 (5.1) | 8 (4.3) |
| Respiratory | 133 (50.6) | 48 (61.5) | 85 (45.9) |
| Skin and soft tissue infection | 6 (2.3) | 0 (0) | 6 (3.2) |
| Urinary tract | 41 (15.6) | 7 (9.0) | 34 (18.4) |
| Other | 11 (4.2) | 3 (3.8) | 8 (4.3) |
| Unknown/not reported | 18 (6.8) | 7 (9.0) | 11 (5.9) |
| Bacteraemia | 22 (8.4) | 10 (12.8) | 12 (6.5) |
| APACHE II score | 17 (11–25) | 22 (15–28) | 15 (9–23) |
| Systemic inflammatory response syndrome | 198 (75.3) | 67 (85.9) | 131 (70.8) |
| ICU admission within 24 h of index episode onset | 121 (46.0) | 50 (64.1) | 71 (38.4) |
| Index event occurred >48 h after admission | 92 (35.0) | 37 (47.4) | 55 (29.7) |
| Mechanical ventilation within 24 h of index episode onset | 101 (38.4) | 44 (56.4) | 57 (30.8) |
| Need for vasopressor support for ≥12 h within 24 h of index episode onset | 58 (22.1) | 23 (29.5) | 35 (18.9) |
| ID inpatient consultation | 257 (97.7) | 74 (94.9) | 183 (98.9) |
| Index culture results | |||
| Positive index culture | 247 (93.9) | 73 (93.5) | 174 (94.1) |
| Negative index culture | 8 (3.0) | 5 (6.4) | 3 (1.6) |
| No culture obtained | 8 (3.0) | 0 (0) | 8 (4.3) |
| Index culture P. aeruginosa growth | 227 (86.3) | 68 (87.2) | 159 (85.9) |
| MDR/XDR | 214 (81.4) | 63 (80.8) | 151 (81.6) |
| Index culture polymicrobial growth | 107 (40.7) | 28 (35.9) | 79 (42.7) |
| Other index culture isolates | |||
| Acinetobacter spp. | 6 (2.3) | 1 (1.3) | 5 (2.7) |
| E. coli | 19 (7.2) | 7 (9.0) | 12 (6.5) |
| Klebsiella spp. | 14 (5.3) | 3 (3.8) | 11 (5.9) |
| Other Enterobacterales | 19 (7.2) | 3 (3.8) | 16 (8.6) |
| Other pathogens | 54 (20.5) | 15 (19.2) | 39 (21.1) |
| Empirical antimicrobial use prior to C/T | 222 (84.4) | 64 (82.1) | 158 (85.4) |
| Duration of active Gram-negative therapyc | 10 (6–15) | 10 (7–16) | 10 (6–14) |
| C/T duration | 7 (4–12) | 7.5 (5–13) | 7.0 (4–12) |
| Reason for C/T therapy | |||
| Culture- and AST-guided | 150 (57.0) | 44 (56.4) | 106 (57.3) |
| Empirical given history of prior resistant Gram-negative isolate or negative cultures | 93 (35.4) | 26 (33.3) | 67 (36.2) |
| Clinical non-response to AST-guided therapy | 13 (4.9) | 7 (9.0) | 6 (3.2) |
| Other | 7 (2.7) | 1 (1.3) | 6 (3.2) |
| Suboptimal C/T dosingd | 89 (33.8) | 31 (39.7) | 58 (31.4) |
| Reason for inpatient C/T discontinuation | |||
| Completion of therapy | 114 (43.3) | 29 (37.2) | 85 (45.9) |
| De-escalation | 36 (13.7) | 10 (12.8) | 26 (14.1) |
| Death | 11 (4.2) | 11 (14.1) | 0 (0) |
| Discharge with plan to continue as outpatient | 79 (30.0) | 12 (15.4) | 67 (36.2) |
| Escalation | 8 (3.0) | 5 (6.4) | 3 (1.6) |
| Adverse event | 3 (1.1) | 0 (0) | 3 (1.6) |
| Transition to comfort care | 12 (4.6) | 11 (14.1) | 1 (0.54) |
| Concurrent systemic Gram-negative-active therapy | 55 (20.9) | 27 (34.6) | 28 (15.1) |
| Aminoglycoside | 21 (8.0) | 8 (10.3) | 13 (7.0) |
| Carbapenem | 5 (1.9) | 1 (1.3) | 4 (2.2) |
| Cephalosporin | 2 (0.76) | 1 (1.3) | 1 (0.54) |
| Polymyxin | 13 (4.9) | 9 (11.5) | 4 (2.2) |
| Tetracycline | 3 (1.1) | 1 (1.3) | 2 (1.1) |
| Trimethoprim/sulfamethoxazole | 1 (0.38) | 1 (1.3) | 0 (0) |
| Fluoroquinolone | 13 (4.9) | 7 (9.0) | 6 (3.2) |
| . | Total cohort . | No clinical success . | Clinical success . |
|---|---|---|---|
| Characteristica . | (n = 263) . | (n = 78) . | (n = 185) . |
| Age (years) | 61 (48–69) | 60 (48–67) | 62 (48–71) |
| Female sex | 88 (33.5) | 25 (32.1) | 63 (34.1) |
| Race | |||
| Asian | 5 (1.9) | 2 (2.6) | 3 (1.6) |
| Hispanic/Latin | 9 (3.4) | 5 (6.4) | 4 (2.2) |
| Non-Hispanic black | 61 (23.2) | 22 (28.2) | 39 (21.1) |
| Non-Hispanic white | 107 (40.7) | 22 (28.2) | 85 (45.9) |
| Other/not declared | 81 (30.8) | 27 (34.6) | 54 (29.2) |
| CCI score | 4 (2–6) | 5 (3–6) | 4 (2–6) |
| Comorbidities | |||
| Myocardial infarction | 20 (7.6) | 8 (10.3) | 12 (6.5) |
| Congestive heart failure | 71 (27.0) | 27 (34.6) | 44 (23.7) |
| Cerebrovascular disease | 69 (26.2) | 20 (25.6) | 49 (26.5) |
| Chronic obstructive lung disease | 31 (11.8) | 11 (14.1) | 20 (10.8) |
| Connective tissue disease | 13 (4.9) | 4 (5.1) | 9 (4.9) |
| Hemiplegia/paraplegia | 43 (16.3) | 12 (15.4) | 31 (16.8) |
| Lymphoma | 5 (1.9) | 3 (3.8) | 2 (1.1) |
| Solid cancer | 32 (12.2) | 8 (10.3) | 24 (13.0) |
| Diabetes | 108 (41.1) | 33 (42.3) | 75 (40.5) |
| Chronic liver disease | 16 (6.1) | 5 (6.4) | 11 (5.9) |
| Immunocompromised statusb | 39 (14.8) | 16 (20.5) | 23 (12.4) |
| Neutropenia | 3 (1.1) | 3 (3.8) | 0 (0) |
| HIV | 3 (1.1) | 2 (2.6) | 1 (0.54) |
| Prednisone at ≥15 mg daily or equivalent | 18 (6.8) | 5 (6.4) | 13 (7.0) |
| Cancer chemotherapy within prior 6 months | 3 (1.1) | 2 (2.6) | 1 (0.54) |
| History of solid organ transplant | 23 (8.7) | 9 (11.5) | 14 (7.6) |
| Heart | 11 (4.2) | 6 (7.7) | 5 (2.7) |
| Lung | 6 (2.3) | 1 (1.3) | 5 (2.7) |
| Kidney | 4 (1.5) | 1 (1.3) | 3 (1.6) |
| Liver | 2 (0.76) | 1 (1.3) | 1 (0.54) |
| BMI | 26 (22–32) | 26 (22–30) | 26 (22–32) |
| Prior hospitalization in the prior 90 days | 170 (64.6) | 51 (65.4) | 119 (64.3) |
| Known C/T use within prior 90 days | 8 (3.0) | 4 (5.1) | 4 (2.2) |
| History of a resistant Gram-negative bacterial culture isolate | 159 (60.5) | 40 (51.3) | 119 (64.3) |
| Pre-hospitalization residence | |||
| Community | 117 (44.5) | 24 (30.8) | 93 (50.3) |
| Other acute care hospital | 35 (13.3) | 16 (20.5) | 19 (10.3) |
| SNF/LTAC/inpatient rehabilitation facility | 85 (32.3) | 25 (32.1) | 60 (32.4) |
| Other | 26 (9.9) | 13 (16.7) | 13 (7.0) |
| Source of infection | |||
| Blood/endovascular | 13 (4.9) | 4 (5.1) | 9 (4.9) |
| Bone/joint | 28 (10.6) | 5 (6.4) | 23 (12.4) |
| CNS | 1 (0.38) | 0 (0) | 1 (0.54) |
| Intraabdominal infection | 12 (4.6) | 4 (5.1) | 8 (4.3) |
| Respiratory | 133 (50.6) | 48 (61.5) | 85 (45.9) |
| Skin and soft tissue infection | 6 (2.3) | 0 (0) | 6 (3.2) |
| Urinary tract | 41 (15.6) | 7 (9.0) | 34 (18.4) |
| Other | 11 (4.2) | 3 (3.8) | 8 (4.3) |
| Unknown/not reported | 18 (6.8) | 7 (9.0) | 11 (5.9) |
| Bacteraemia | 22 (8.4) | 10 (12.8) | 12 (6.5) |
| APACHE II score | 17 (11–25) | 22 (15–28) | 15 (9–23) |
| Systemic inflammatory response syndrome | 198 (75.3) | 67 (85.9) | 131 (70.8) |
| ICU admission within 24 h of index episode onset | 121 (46.0) | 50 (64.1) | 71 (38.4) |
| Index event occurred >48 h after admission | 92 (35.0) | 37 (47.4) | 55 (29.7) |
| Mechanical ventilation within 24 h of index episode onset | 101 (38.4) | 44 (56.4) | 57 (30.8) |
| Need for vasopressor support for ≥12 h within 24 h of index episode onset | 58 (22.1) | 23 (29.5) | 35 (18.9) |
| ID inpatient consultation | 257 (97.7) | 74 (94.9) | 183 (98.9) |
| Index culture results | |||
| Positive index culture | 247 (93.9) | 73 (93.5) | 174 (94.1) |
| Negative index culture | 8 (3.0) | 5 (6.4) | 3 (1.6) |
| No culture obtained | 8 (3.0) | 0 (0) | 8 (4.3) |
| Index culture P. aeruginosa growth | 227 (86.3) | 68 (87.2) | 159 (85.9) |
| MDR/XDR | 214 (81.4) | 63 (80.8) | 151 (81.6) |
| Index culture polymicrobial growth | 107 (40.7) | 28 (35.9) | 79 (42.7) |
| Other index culture isolates | |||
| Acinetobacter spp. | 6 (2.3) | 1 (1.3) | 5 (2.7) |
| E. coli | 19 (7.2) | 7 (9.0) | 12 (6.5) |
| Klebsiella spp. | 14 (5.3) | 3 (3.8) | 11 (5.9) |
| Other Enterobacterales | 19 (7.2) | 3 (3.8) | 16 (8.6) |
| Other pathogens | 54 (20.5) | 15 (19.2) | 39 (21.1) |
| Empirical antimicrobial use prior to C/T | 222 (84.4) | 64 (82.1) | 158 (85.4) |
| Duration of active Gram-negative therapyc | 10 (6–15) | 10 (7–16) | 10 (6–14) |
| C/T duration | 7 (4–12) | 7.5 (5–13) | 7.0 (4–12) |
| Reason for C/T therapy | |||
| Culture- and AST-guided | 150 (57.0) | 44 (56.4) | 106 (57.3) |
| Empirical given history of prior resistant Gram-negative isolate or negative cultures | 93 (35.4) | 26 (33.3) | 67 (36.2) |
| Clinical non-response to AST-guided therapy | 13 (4.9) | 7 (9.0) | 6 (3.2) |
| Other | 7 (2.7) | 1 (1.3) | 6 (3.2) |
| Suboptimal C/T dosingd | 89 (33.8) | 31 (39.7) | 58 (31.4) |
| Reason for inpatient C/T discontinuation | |||
| Completion of therapy | 114 (43.3) | 29 (37.2) | 85 (45.9) |
| De-escalation | 36 (13.7) | 10 (12.8) | 26 (14.1) |
| Death | 11 (4.2) | 11 (14.1) | 0 (0) |
| Discharge with plan to continue as outpatient | 79 (30.0) | 12 (15.4) | 67 (36.2) |
| Escalation | 8 (3.0) | 5 (6.4) | 3 (1.6) |
| Adverse event | 3 (1.1) | 0 (0) | 3 (1.6) |
| Transition to comfort care | 12 (4.6) | 11 (14.1) | 1 (0.54) |
| Concurrent systemic Gram-negative-active therapy | 55 (20.9) | 27 (34.6) | 28 (15.1) |
| Aminoglycoside | 21 (8.0) | 8 (10.3) | 13 (7.0) |
| Carbapenem | 5 (1.9) | 1 (1.3) | 4 (2.2) |
| Cephalosporin | 2 (0.76) | 1 (1.3) | 1 (0.54) |
| Polymyxin | 13 (4.9) | 9 (11.5) | 4 (2.2) |
| Tetracycline | 3 (1.1) | 1 (1.3) | 2 (1.1) |
| Trimethoprim/sulfamethoxazole | 1 (0.38) | 1 (1.3) | 0 (0) |
| Fluoroquinolone | 13 (4.9) | 7 (9.0) | 6 (3.2) |
APACHE II, Acute Physiology and Chronic Health Evaluation II; AST, antimicrobial susceptibility testing; C/T, ceftolozane/tazobactam; LTAC, long-term acute care; SNF, skilled nursing facility.
Values are expressed as median (IQR) for continuous data or frequency (%) for categorical data.
Categories of immune compromise were not mutually exclusive.
Duration includes C/T therapy.
C/T optimal dose: 3 g every 8 h for respiratory infections and 1.5 g every 8 h for all other infections, adjusted for renal function.
| . | Total cohort . | No clinical success . | Clinical success . |
|---|---|---|---|
| Characteristica . | (n = 263) . | (n = 78) . | (n = 185) . |
| Age (years) | 61 (48–69) | 60 (48–67) | 62 (48–71) |
| Female sex | 88 (33.5) | 25 (32.1) | 63 (34.1) |
| Race | |||
| Asian | 5 (1.9) | 2 (2.6) | 3 (1.6) |
| Hispanic/Latin | 9 (3.4) | 5 (6.4) | 4 (2.2) |
| Non-Hispanic black | 61 (23.2) | 22 (28.2) | 39 (21.1) |
| Non-Hispanic white | 107 (40.7) | 22 (28.2) | 85 (45.9) |
| Other/not declared | 81 (30.8) | 27 (34.6) | 54 (29.2) |
| CCI score | 4 (2–6) | 5 (3–6) | 4 (2–6) |
| Comorbidities | |||
| Myocardial infarction | 20 (7.6) | 8 (10.3) | 12 (6.5) |
| Congestive heart failure | 71 (27.0) | 27 (34.6) | 44 (23.7) |
| Cerebrovascular disease | 69 (26.2) | 20 (25.6) | 49 (26.5) |
| Chronic obstructive lung disease | 31 (11.8) | 11 (14.1) | 20 (10.8) |
| Connective tissue disease | 13 (4.9) | 4 (5.1) | 9 (4.9) |
| Hemiplegia/paraplegia | 43 (16.3) | 12 (15.4) | 31 (16.8) |
| Lymphoma | 5 (1.9) | 3 (3.8) | 2 (1.1) |
| Solid cancer | 32 (12.2) | 8 (10.3) | 24 (13.0) |
| Diabetes | 108 (41.1) | 33 (42.3) | 75 (40.5) |
| Chronic liver disease | 16 (6.1) | 5 (6.4) | 11 (5.9) |
| Immunocompromised statusb | 39 (14.8) | 16 (20.5) | 23 (12.4) |
| Neutropenia | 3 (1.1) | 3 (3.8) | 0 (0) |
| HIV | 3 (1.1) | 2 (2.6) | 1 (0.54) |
| Prednisone at ≥15 mg daily or equivalent | 18 (6.8) | 5 (6.4) | 13 (7.0) |
| Cancer chemotherapy within prior 6 months | 3 (1.1) | 2 (2.6) | 1 (0.54) |
| History of solid organ transplant | 23 (8.7) | 9 (11.5) | 14 (7.6) |
| Heart | 11 (4.2) | 6 (7.7) | 5 (2.7) |
| Lung | 6 (2.3) | 1 (1.3) | 5 (2.7) |
| Kidney | 4 (1.5) | 1 (1.3) | 3 (1.6) |
| Liver | 2 (0.76) | 1 (1.3) | 1 (0.54) |
| BMI | 26 (22–32) | 26 (22–30) | 26 (22–32) |
| Prior hospitalization in the prior 90 days | 170 (64.6) | 51 (65.4) | 119 (64.3) |
| Known C/T use within prior 90 days | 8 (3.0) | 4 (5.1) | 4 (2.2) |
| History of a resistant Gram-negative bacterial culture isolate | 159 (60.5) | 40 (51.3) | 119 (64.3) |
| Pre-hospitalization residence | |||
| Community | 117 (44.5) | 24 (30.8) | 93 (50.3) |
| Other acute care hospital | 35 (13.3) | 16 (20.5) | 19 (10.3) |
| SNF/LTAC/inpatient rehabilitation facility | 85 (32.3) | 25 (32.1) | 60 (32.4) |
| Other | 26 (9.9) | 13 (16.7) | 13 (7.0) |
| Source of infection | |||
| Blood/endovascular | 13 (4.9) | 4 (5.1) | 9 (4.9) |
| Bone/joint | 28 (10.6) | 5 (6.4) | 23 (12.4) |
| CNS | 1 (0.38) | 0 (0) | 1 (0.54) |
| Intraabdominal infection | 12 (4.6) | 4 (5.1) | 8 (4.3) |
| Respiratory | 133 (50.6) | 48 (61.5) | 85 (45.9) |
| Skin and soft tissue infection | 6 (2.3) | 0 (0) | 6 (3.2) |
| Urinary tract | 41 (15.6) | 7 (9.0) | 34 (18.4) |
| Other | 11 (4.2) | 3 (3.8) | 8 (4.3) |
| Unknown/not reported | 18 (6.8) | 7 (9.0) | 11 (5.9) |
| Bacteraemia | 22 (8.4) | 10 (12.8) | 12 (6.5) |
| APACHE II score | 17 (11–25) | 22 (15–28) | 15 (9–23) |
| Systemic inflammatory response syndrome | 198 (75.3) | 67 (85.9) | 131 (70.8) |
| ICU admission within 24 h of index episode onset | 121 (46.0) | 50 (64.1) | 71 (38.4) |
| Index event occurred >48 h after admission | 92 (35.0) | 37 (47.4) | 55 (29.7) |
| Mechanical ventilation within 24 h of index episode onset | 101 (38.4) | 44 (56.4) | 57 (30.8) |
| Need for vasopressor support for ≥12 h within 24 h of index episode onset | 58 (22.1) | 23 (29.5) | 35 (18.9) |
| ID inpatient consultation | 257 (97.7) | 74 (94.9) | 183 (98.9) |
| Index culture results | |||
| Positive index culture | 247 (93.9) | 73 (93.5) | 174 (94.1) |
| Negative index culture | 8 (3.0) | 5 (6.4) | 3 (1.6) |
| No culture obtained | 8 (3.0) | 0 (0) | 8 (4.3) |
| Index culture P. aeruginosa growth | 227 (86.3) | 68 (87.2) | 159 (85.9) |
| MDR/XDR | 214 (81.4) | 63 (80.8) | 151 (81.6) |
| Index culture polymicrobial growth | 107 (40.7) | 28 (35.9) | 79 (42.7) |
| Other index culture isolates | |||
| Acinetobacter spp. | 6 (2.3) | 1 (1.3) | 5 (2.7) |
| E. coli | 19 (7.2) | 7 (9.0) | 12 (6.5) |
| Klebsiella spp. | 14 (5.3) | 3 (3.8) | 11 (5.9) |
| Other Enterobacterales | 19 (7.2) | 3 (3.8) | 16 (8.6) |
| Other pathogens | 54 (20.5) | 15 (19.2) | 39 (21.1) |
| Empirical antimicrobial use prior to C/T | 222 (84.4) | 64 (82.1) | 158 (85.4) |
| Duration of active Gram-negative therapyc | 10 (6–15) | 10 (7–16) | 10 (6–14) |
| C/T duration | 7 (4–12) | 7.5 (5–13) | 7.0 (4–12) |
| Reason for C/T therapy | |||
| Culture- and AST-guided | 150 (57.0) | 44 (56.4) | 106 (57.3) |
| Empirical given history of prior resistant Gram-negative isolate or negative cultures | 93 (35.4) | 26 (33.3) | 67 (36.2) |
| Clinical non-response to AST-guided therapy | 13 (4.9) | 7 (9.0) | 6 (3.2) |
| Other | 7 (2.7) | 1 (1.3) | 6 (3.2) |
| Suboptimal C/T dosingd | 89 (33.8) | 31 (39.7) | 58 (31.4) |
| Reason for inpatient C/T discontinuation | |||
| Completion of therapy | 114 (43.3) | 29 (37.2) | 85 (45.9) |
| De-escalation | 36 (13.7) | 10 (12.8) | 26 (14.1) |
| Death | 11 (4.2) | 11 (14.1) | 0 (0) |
| Discharge with plan to continue as outpatient | 79 (30.0) | 12 (15.4) | 67 (36.2) |
| Escalation | 8 (3.0) | 5 (6.4) | 3 (1.6) |
| Adverse event | 3 (1.1) | 0 (0) | 3 (1.6) |
| Transition to comfort care | 12 (4.6) | 11 (14.1) | 1 (0.54) |
| Concurrent systemic Gram-negative-active therapy | 55 (20.9) | 27 (34.6) | 28 (15.1) |
| Aminoglycoside | 21 (8.0) | 8 (10.3) | 13 (7.0) |
| Carbapenem | 5 (1.9) | 1 (1.3) | 4 (2.2) |
| Cephalosporin | 2 (0.76) | 1 (1.3) | 1 (0.54) |
| Polymyxin | 13 (4.9) | 9 (11.5) | 4 (2.2) |
| Tetracycline | 3 (1.1) | 1 (1.3) | 2 (1.1) |
| Trimethoprim/sulfamethoxazole | 1 (0.38) | 1 (1.3) | 0 (0) |
| Fluoroquinolone | 13 (4.9) | 7 (9.0) | 6 (3.2) |
| . | Total cohort . | No clinical success . | Clinical success . |
|---|---|---|---|
| Characteristica . | (n = 263) . | (n = 78) . | (n = 185) . |
| Age (years) | 61 (48–69) | 60 (48–67) | 62 (48–71) |
| Female sex | 88 (33.5) | 25 (32.1) | 63 (34.1) |
| Race | |||
| Asian | 5 (1.9) | 2 (2.6) | 3 (1.6) |
| Hispanic/Latin | 9 (3.4) | 5 (6.4) | 4 (2.2) |
| Non-Hispanic black | 61 (23.2) | 22 (28.2) | 39 (21.1) |
| Non-Hispanic white | 107 (40.7) | 22 (28.2) | 85 (45.9) |
| Other/not declared | 81 (30.8) | 27 (34.6) | 54 (29.2) |
| CCI score | 4 (2–6) | 5 (3–6) | 4 (2–6) |
| Comorbidities | |||
| Myocardial infarction | 20 (7.6) | 8 (10.3) | 12 (6.5) |
| Congestive heart failure | 71 (27.0) | 27 (34.6) | 44 (23.7) |
| Cerebrovascular disease | 69 (26.2) | 20 (25.6) | 49 (26.5) |
| Chronic obstructive lung disease | 31 (11.8) | 11 (14.1) | 20 (10.8) |
| Connective tissue disease | 13 (4.9) | 4 (5.1) | 9 (4.9) |
| Hemiplegia/paraplegia | 43 (16.3) | 12 (15.4) | 31 (16.8) |
| Lymphoma | 5 (1.9) | 3 (3.8) | 2 (1.1) |
| Solid cancer | 32 (12.2) | 8 (10.3) | 24 (13.0) |
| Diabetes | 108 (41.1) | 33 (42.3) | 75 (40.5) |
| Chronic liver disease | 16 (6.1) | 5 (6.4) | 11 (5.9) |
| Immunocompromised statusb | 39 (14.8) | 16 (20.5) | 23 (12.4) |
| Neutropenia | 3 (1.1) | 3 (3.8) | 0 (0) |
| HIV | 3 (1.1) | 2 (2.6) | 1 (0.54) |
| Prednisone at ≥15 mg daily or equivalent | 18 (6.8) | 5 (6.4) | 13 (7.0) |
| Cancer chemotherapy within prior 6 months | 3 (1.1) | 2 (2.6) | 1 (0.54) |
| History of solid organ transplant | 23 (8.7) | 9 (11.5) | 14 (7.6) |
| Heart | 11 (4.2) | 6 (7.7) | 5 (2.7) |
| Lung | 6 (2.3) | 1 (1.3) | 5 (2.7) |
| Kidney | 4 (1.5) | 1 (1.3) | 3 (1.6) |
| Liver | 2 (0.76) | 1 (1.3) | 1 (0.54) |
| BMI | 26 (22–32) | 26 (22–30) | 26 (22–32) |
| Prior hospitalization in the prior 90 days | 170 (64.6) | 51 (65.4) | 119 (64.3) |
| Known C/T use within prior 90 days | 8 (3.0) | 4 (5.1) | 4 (2.2) |
| History of a resistant Gram-negative bacterial culture isolate | 159 (60.5) | 40 (51.3) | 119 (64.3) |
| Pre-hospitalization residence | |||
| Community | 117 (44.5) | 24 (30.8) | 93 (50.3) |
| Other acute care hospital | 35 (13.3) | 16 (20.5) | 19 (10.3) |
| SNF/LTAC/inpatient rehabilitation facility | 85 (32.3) | 25 (32.1) | 60 (32.4) |
| Other | 26 (9.9) | 13 (16.7) | 13 (7.0) |
| Source of infection | |||
| Blood/endovascular | 13 (4.9) | 4 (5.1) | 9 (4.9) |
| Bone/joint | 28 (10.6) | 5 (6.4) | 23 (12.4) |
| CNS | 1 (0.38) | 0 (0) | 1 (0.54) |
| Intraabdominal infection | 12 (4.6) | 4 (5.1) | 8 (4.3) |
| Respiratory | 133 (50.6) | 48 (61.5) | 85 (45.9) |
| Skin and soft tissue infection | 6 (2.3) | 0 (0) | 6 (3.2) |
| Urinary tract | 41 (15.6) | 7 (9.0) | 34 (18.4) |
| Other | 11 (4.2) | 3 (3.8) | 8 (4.3) |
| Unknown/not reported | 18 (6.8) | 7 (9.0) | 11 (5.9) |
| Bacteraemia | 22 (8.4) | 10 (12.8) | 12 (6.5) |
| APACHE II score | 17 (11–25) | 22 (15–28) | 15 (9–23) |
| Systemic inflammatory response syndrome | 198 (75.3) | 67 (85.9) | 131 (70.8) |
| ICU admission within 24 h of index episode onset | 121 (46.0) | 50 (64.1) | 71 (38.4) |
| Index event occurred >48 h after admission | 92 (35.0) | 37 (47.4) | 55 (29.7) |
| Mechanical ventilation within 24 h of index episode onset | 101 (38.4) | 44 (56.4) | 57 (30.8) |
| Need for vasopressor support for ≥12 h within 24 h of index episode onset | 58 (22.1) | 23 (29.5) | 35 (18.9) |
| ID inpatient consultation | 257 (97.7) | 74 (94.9) | 183 (98.9) |
| Index culture results | |||
| Positive index culture | 247 (93.9) | 73 (93.5) | 174 (94.1) |
| Negative index culture | 8 (3.0) | 5 (6.4) | 3 (1.6) |
| No culture obtained | 8 (3.0) | 0 (0) | 8 (4.3) |
| Index culture P. aeruginosa growth | 227 (86.3) | 68 (87.2) | 159 (85.9) |
| MDR/XDR | 214 (81.4) | 63 (80.8) | 151 (81.6) |
| Index culture polymicrobial growth | 107 (40.7) | 28 (35.9) | 79 (42.7) |
| Other index culture isolates | |||
| Acinetobacter spp. | 6 (2.3) | 1 (1.3) | 5 (2.7) |
| E. coli | 19 (7.2) | 7 (9.0) | 12 (6.5) |
| Klebsiella spp. | 14 (5.3) | 3 (3.8) | 11 (5.9) |
| Other Enterobacterales | 19 (7.2) | 3 (3.8) | 16 (8.6) |
| Other pathogens | 54 (20.5) | 15 (19.2) | 39 (21.1) |
| Empirical antimicrobial use prior to C/T | 222 (84.4) | 64 (82.1) | 158 (85.4) |
| Duration of active Gram-negative therapyc | 10 (6–15) | 10 (7–16) | 10 (6–14) |
| C/T duration | 7 (4–12) | 7.5 (5–13) | 7.0 (4–12) |
| Reason for C/T therapy | |||
| Culture- and AST-guided | 150 (57.0) | 44 (56.4) | 106 (57.3) |
| Empirical given history of prior resistant Gram-negative isolate or negative cultures | 93 (35.4) | 26 (33.3) | 67 (36.2) |
| Clinical non-response to AST-guided therapy | 13 (4.9) | 7 (9.0) | 6 (3.2) |
| Other | 7 (2.7) | 1 (1.3) | 6 (3.2) |
| Suboptimal C/T dosingd | 89 (33.8) | 31 (39.7) | 58 (31.4) |
| Reason for inpatient C/T discontinuation | |||
| Completion of therapy | 114 (43.3) | 29 (37.2) | 85 (45.9) |
| De-escalation | 36 (13.7) | 10 (12.8) | 26 (14.1) |
| Death | 11 (4.2) | 11 (14.1) | 0 (0) |
| Discharge with plan to continue as outpatient | 79 (30.0) | 12 (15.4) | 67 (36.2) |
| Escalation | 8 (3.0) | 5 (6.4) | 3 (1.6) |
| Adverse event | 3 (1.1) | 0 (0) | 3 (1.6) |
| Transition to comfort care | 12 (4.6) | 11 (14.1) | 1 (0.54) |
| Concurrent systemic Gram-negative-active therapy | 55 (20.9) | 27 (34.6) | 28 (15.1) |
| Aminoglycoside | 21 (8.0) | 8 (10.3) | 13 (7.0) |
| Carbapenem | 5 (1.9) | 1 (1.3) | 4 (2.2) |
| Cephalosporin | 2 (0.76) | 1 (1.3) | 1 (0.54) |
| Polymyxin | 13 (4.9) | 9 (11.5) | 4 (2.2) |
| Tetracycline | 3 (1.1) | 1 (1.3) | 2 (1.1) |
| Trimethoprim/sulfamethoxazole | 1 (0.38) | 1 (1.3) | 0 (0) |
| Fluoroquinolone | 13 (4.9) | 7 (9.0) | 6 (3.2) |
APACHE II, Acute Physiology and Chronic Health Evaluation II; AST, antimicrobial susceptibility testing; C/T, ceftolozane/tazobactam; LTAC, long-term acute care; SNF, skilled nursing facility.
Values are expressed as median (IQR) for continuous data or frequency (%) for categorical data.
Categories of immune compromise were not mutually exclusive.
Duration includes C/T therapy.
C/T optimal dose: 3 g every 8 h for respiratory infections and 1.5 g every 8 h for all other infections, adjusted for renal function.
The median APACHE II score was 17 (IQR 11–25). A total of 46% of patients were located in the ICU, 38.4% required mechanical ventilation and 22.1% needed ≥12 h of vasopressor support within 24 h of the index episode. The most common clinically diagnosed source of infection was respiratory (50.6%) followed by urinary (15.6%) and bone and joint (10.6%). Twenty-two patients (8.4%) had concomitant bacteraemia.
Index cultures were positive in 93.9% of patients who received ceftolozane/tazobactam therapy, the most frequent organism identified was P. aeruginosa (86.3% of cultures) and polymicrobial infections were identified in 107 patients (40.7%). Escherichia coli, Klebsiella spp. and other Enterobacterales were isolated on index cultures of 19 (7.2%), 14 (5.3%) and 19 patients (7.2%), respectively. Other clinically relevant pathogens isolated during the index episode included Staphylococcus aureus (32 patients, 12.2%), Enterococcus spp. (55, 20.9%), CoNS (5, 1.9%) and Candida spp. (30, 11.4%).
Among the 227 patients with P. aeruginosa isolated in their index culture, 214 of the isolates (81.4%) were MDR or XDR, which were predominantly from respiratory sources. The clinically reported MIC of the index culture isolate to ceftolozane/tazobactam was retrievable from either clinical notes or laboratory reporting in 107 patients for 119 isolates (108 P. aeruginosa, three E. coli, three Klebsiella spp., two Acinetobacter spp., one Achromobacter spp., one Serratia marcescens and one Proteus mirabilis). For the remaining patients with positive cultures, their index episodes predated standardization of laboratory reporting for ceftolozane/tazobactam susceptibility testing and these MICs were not documented in clinical notes. The median ceftolozane/tazobactam MIC was 1 mg/L (IQR 0.75–1.5). Four isolates were considered resistant to ceftolozane/tazobactam [two P. aeruginosa (MIC 16 and ≥ 256 mg/L), one E. cloacae (MIC 8 mg/L) and one Klebsiella spp. (MIC 6 mg/L)]. Two isolates of Acinetobacter spp. (ceftolozane/tazobactam MICs 8 and 16 mg/L) and one Achromobacter spp. (ceftolozane/tazobactam MIC ≥256 mg/L) also exhibited elevated ceftolozane/tazobactam MICs.
Clinical therapy and outcomes
The infectious diseases (ID) inpatient consult service was involved in the care of 257 (97.7%) patients. Empirical systemic Gram-negative-active antimicrobials were given to 84.4% of patients. Use of ceftolozane/tazobactam was culture-guided [i.e. isolate(s) susceptible to ceftolozane/tazobactam] in 57% of patients, utilized empirically (i.e. no information on susceptibility to ceftolozane/tazobactam or negative cultures) in 35.4% episodes and as escalation after clinical non-response to initial culture-guided antimicrobial therapy in 4.9% of cases. Median duration of inpatient ceftolozane/tazobactam treatment was 7 days (IQR 4–12). In the first 24 h of therapy, 33.8% of patients received a suboptimal dose of ceftolozane/tazobactam based on renal function and origin of infection. Median duration of active Gram-negative therapy, including ceftolozane/tazobactam, was 10 days (IQR 6–15). Concurrent use of additional systemic Gram-negative-active antimicrobial therapy for ≥72 h occurred in 55 patients (20.9%). The most frequently used antibiotic classes were aminoglycosides (21 patients), polymyxins (13 patients) and quinolones (13 patients).
For the overall study population, the composite clinical success was achieved in 70.3% of episodes and all-cause in-hospital mortality occurred in 13.7% of patients, with 29 of the 36 deaths classified as infection-related by the treating physician (Figure 1). Of these deaths, 24 had a respiratory source. Survival to discharge occurred in 49.4% of the episodes at 14 days and 57.4% of the episodes at 30 days. A total of 13.7% of patients required readmission within 30 days of the index episode.
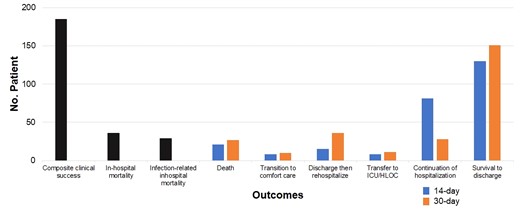
Clinical outcomes and deposition of patients treated with ceftolozane/tazobactam. HLOC, higher level of care.
In univariate analysis, the primary outcome of composite clinical success was significantly associated with lower APACHE II scores (P < 0.001), patients who were not in the ICU within 24 h of the index episode (P < 0.001), those not on mechanical ventilation (P < 0.001), ID consultation (P = 0.045) and patients who did not receive concurrent systemic Gram-negative-active therapy (P < 0.001). Age, CCI score, time between index culture and ceftolozane/tazobactam initiation, immunocompromised status, source of infection, isolation of an MDR organism in the index culture, vasopressor requirement, use of empirical antimicrobials prior to ceftolozane/tazobactam and suboptimal initial ceftolozane/tazobactam dosing were not significantly associated with clinical success (P > 0.05). In the multivariable logistic regression model (Table 2), APACHE II score [adjusted OR (aOR) 0.95; 95% CI 0.92–0.98; P = 0.001] and concurrent Gram-negative active therapy (aOR 0.32; 95% CI 0.16–0.63; P = 0.001) remained significant predictors of lack of clinical success. While ID consultation was significantly associated with clinical success (aOR 7.9; 95% CI 1.20–51.81; P = 0.031), the association of ID consultation with the primary outcome is uncertain due to the very small number of patients for which the ID service was not consulted.
Multivariable logistic regression model of predictors for composite clinical success and in-hospital mortality
| Outcome . | Predictor . | Adjusted OR . | 95% CI . |
|---|---|---|---|
| Composite clinical success | |||
| APACHE II score | 0.95 | 0.92–0.98 | |
| CCI score | 0.94 | 0.85–1.04 | |
| Immunocompromiseda | 0.68 | 0.31–1.46 | |
| Concurrent GN therapy | 0.32 | 0.16–0.63 | |
| Suboptimal C/T doseb | 0.86 | 0.47–1.58 | |
| In-hospital mortality | |||
| APACHE II score | 1.12 | 1.07–1.17 | |
| CCI score | 1.18 | 1.03–1.35 | |
| Immunocompromiseda | 2.98 | 1.12–7.87 | |
| Time to C/T therapy | 1.08 | 1.01–1.17 | |
| Outcome . | Predictor . | Adjusted OR . | 95% CI . |
|---|---|---|---|
| Composite clinical success | |||
| APACHE II score | 0.95 | 0.92–0.98 | |
| CCI score | 0.94 | 0.85–1.04 | |
| Immunocompromiseda | 0.68 | 0.31–1.46 | |
| Concurrent GN therapy | 0.32 | 0.16–0.63 | |
| Suboptimal C/T doseb | 0.86 | 0.47–1.58 | |
| In-hospital mortality | |||
| APACHE II score | 1.12 | 1.07–1.17 | |
| CCI score | 1.18 | 1.03–1.35 | |
| Immunocompromiseda | 2.98 | 1.12–7.87 | |
| Time to C/T therapy | 1.08 | 1.01–1.17 | |
Bolded numbers are significant with P < 0.05.
APACHE II, Acute Physiology and Chronic Health Evaluation II; C/T, ceftolozane/tazobactam; GN, Gram negative.
HIV, prednisone ≥15 mg/day (or equivalent), cancer chemotherapy in preceding 6 months, history of solid organ transplant.
Optimal dose: 3 g every 8 h for respiratory infections and 1.5 g every 8 h for all other infections, adjusted for renal function.
Multivariable logistic regression model of predictors for composite clinical success and in-hospital mortality
| Outcome . | Predictor . | Adjusted OR . | 95% CI . |
|---|---|---|---|
| Composite clinical success | |||
| APACHE II score | 0.95 | 0.92–0.98 | |
| CCI score | 0.94 | 0.85–1.04 | |
| Immunocompromiseda | 0.68 | 0.31–1.46 | |
| Concurrent GN therapy | 0.32 | 0.16–0.63 | |
| Suboptimal C/T doseb | 0.86 | 0.47–1.58 | |
| In-hospital mortality | |||
| APACHE II score | 1.12 | 1.07–1.17 | |
| CCI score | 1.18 | 1.03–1.35 | |
| Immunocompromiseda | 2.98 | 1.12–7.87 | |
| Time to C/T therapy | 1.08 | 1.01–1.17 | |
| Outcome . | Predictor . | Adjusted OR . | 95% CI . |
|---|---|---|---|
| Composite clinical success | |||
| APACHE II score | 0.95 | 0.92–0.98 | |
| CCI score | 0.94 | 0.85–1.04 | |
| Immunocompromiseda | 0.68 | 0.31–1.46 | |
| Concurrent GN therapy | 0.32 | 0.16–0.63 | |
| Suboptimal C/T doseb | 0.86 | 0.47–1.58 | |
| In-hospital mortality | |||
| APACHE II score | 1.12 | 1.07–1.17 | |
| CCI score | 1.18 | 1.03–1.35 | |
| Immunocompromiseda | 2.98 | 1.12–7.87 | |
| Time to C/T therapy | 1.08 | 1.01–1.17 | |
Bolded numbers are significant with P < 0.05.
APACHE II, Acute Physiology and Chronic Health Evaluation II; C/T, ceftolozane/tazobactam; GN, Gram negative.
HIV, prednisone ≥15 mg/day (or equivalent), cancer chemotherapy in preceding 6 months, history of solid organ transplant.
Optimal dose: 3 g every 8 h for respiratory infections and 1.5 g every 8 h for all other infections, adjusted for renal function.
A similar pattern of predictors was seen in secondary outcome analyses. A longer time to initiation of ceftolozane/tazobactam therapy from the index culture was significantly associated with increased all-cause in-hospital mortality. However, timing of therapy did not predict infection-related mortality. Interestingly, suboptimal ceftolozane/tazobactam dosing was not associated with mortality. In the multivariable logistic regression analysis, APACHE II score, CCI score and immunocompromised status were all associated with increased in-hospital mortality (Table 2). Increased time from index culture to initiation of ceftolozane/tazobactam therapy also remained a significant predictor of in-hospital mortality (aOR 1.08; 95% CI 1.01–1.17; P = 0.016).
There were 10 adverse events (3.8%) in 10 unique patients that the treatment team suspected were due to or contributed to by ceftolozane/tazobactam therapy. This included six patients with Clostridioides difficile infection, two patients with peripheral blood eosinophilia, one patient with non-C. difficile diarrhoea and one patient with fever. C. difficile infections were diagnosed by nucleic acid amplification test while patients received ceftolozane/tazobactam or shortly after discontinuation. Ceftolozane/tazobactam was discontinued in three patients (1.1%) due to an adverse event.
Genomic investigation
Nine non-pseudomonal isolates were collected (five Klebsiella spp., two Achromobacter spp., one E. coli and one Acinetobacter spp.). A total of 66 P. aeruginosa isolates from 52 patients were recovered from the clinical laboratory, including isolates recovered after the index hospitalization as part of the surveillance protocol for MDR pathogens. Ten patients had multiple cultures positive for P. aeruginosa. A phylogenetic evaluation of the study strains showed that there was a large degree of heterogeneity observed in the population (Figure 2). No clustering of isolates by healthcare system was noted. ST235 was the most frequent ST, representing approximately 23% of unique patient episodes (i.e. following exclusion of subsequent positive cultures). Sets of isolates from patients who had ≥1 isolate showed minimal differences between the strains. This finding suggests that infections were due to one predominant strain for each patient.
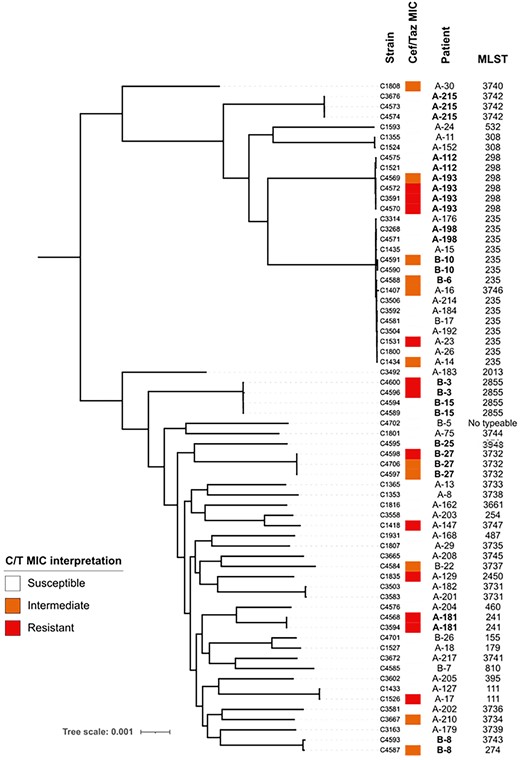
Core-genome phylogenetic tree of recovered P. aeruginosa isolates from patients treated with ceftolozane/tazobactam. Branch length is proportional to the number of differences between strains. MIC interpretive criteria established by CLSI M100, 31st edition. Patients were numbered sequentially at enrolment, A/B denotes the hospital system.
Elevated ceftolozane/tazobactam MICs were not specifically associated with any particular genomic background, suggesting that ceftolozane/tazobactam resistance was not due to clonal expansion of a resistant isolate or high-risk clone. Five patients showed evidence of emergence of resistance (i.e. recovery of ≥1 resistant isolate after ceftolozane/tazobactam exposure while the index isolates were susceptible as reported by the clinical laboratory). The majority of these patients (four of five) received ceftolozane/tazobactam monotherapy and had a respiratory origin of infection. Unfortunately, index isolates for these cases were not available for comparison. In the ceftolozane/tazobactam-resistant isolates, five of eight had mutations in ampC, the gene encoding the chromosomal Pseudomonas-derived cephalosporinases (PDCs), and six of eight had mutations in ftsI, which encodes the binding site for ceftolozane/tazobactam PBP3 (Table S1). Mutations in these genes have been previously associated with ceftolozane/tazobactam resistance and may have contributed to the phenotype observed in these strains.18
Discussion
In this study, we describe a cohort of patients treated with ceftolozane/tazobactam across two large urban hospital systems. This cohort reflects the complexity of patients in which ceftolozane/tazobactam is utilized in clinical practice, namely those with a significant burden of comorbidities and a large proportion of whom presented with severe illness (i.e. 46% in the ICU, 38.4% on mechanical ventilation, 22.1% requiring vasopressor support). The majority of the recipients (86.3%) had a documented P. aeruginosa infection, most of which displayed an MDR phenotype. Notably, a large number of patients (40.7%) had multiple organisms recovered in the index culture, about half of which were other potentially pathogenic Gram-negative bacteria.
The reported composite clinical success rate of 70.3% in our cohort is within the range of cure rates reported in prior series using ceftolozane/tazobactam for P. aeruginosa and nosocomial pneumonia.13,19–25 The ASPECT-NP randomized trial reported a lower overall clinical cure of 63.8% for ceftolozane/tazobactam in the clinically evaluable population, which most closely aligns to the real-world population of the current study, but had a high proportion of patients in the ICU (92%) at the time of study enrolment.12 In a case–control study by Pogue et al.,26 with 100 cases of drug-resistant P. aeruginosa infections, 64% of which were ventilator-associated or hospital-acquired pneumonias, treatment with ceftolozane/tazobactam compared favourably with 100 controls treated with a polymyxin- or aminoglycoside-based regimen. However, the clinical cure rate of 81% among those cases appeared higher than the clinical success rate observed in many of the prior smaller series or in the present cohort. A retrospective cohort published by Jorgensen et al.27 in 2020 included 259 patients treated from 2015 through to 2019 in multiple medical centres in the USA and reported a composite clinical failure rate of 37.6% and in-hospital mortality of 17.3%. These rates are slightly worse than the composite clinical success and 30 day mortality in the present cohort, but included patients with advanced age who had significant comorbidities and severe presentations.
In the univariate and multivariable analyses, high severity of illness was one of the most consistent predictors of composite clinical success and mortality. Lower odds of clinical success was associated with increased APACHE II score while higher odds of in-hospital mortality were seen in patients with elevated APACHE II score, CCI score and immunocompromised status. In contrast to prior studies, the dose of ceftolozane/tazobactam given did not predict subsequent clinical success in this cohort. However, the longer times from initial index culture to ceftolozane/tazobactam therapy initiation increased the odds of mortality. Interestingly, concurrent Gram-negative-active therapy in addition to ceftolozane/tazobactam was associated with lower rates of clinical success. This is likely confounded by the severity of patient illness or the presence of co-pathogens, as clinicians may be more likely to use a combination regimen in a patient who is sicker and critically ill. There was not, however, a statistically significant change in mortality associated with combination therapy. The antimicrobials most likely to be used in combination were polymyxins and aminoglycosides, both of which have significant rates of nephrotoxicity. Thus, it may be prudent to weigh the anticipated benefit against the potential harms when deciding if combination therapy with ceftolozane/tazobactam is warranted.
One interesting finding was the difference in susceptibility as reported by the clinical microbiology laboratory and those isolates that were available for subsequent testing and genomic analysis. While only 4 of the 116 index isolates of Pseudomonas and Enterobacterales for which clinical microbiology laboratory testing was available were reported as resistant (3.4%), we collected 28 of 116 index isolates for sequencing and found full resistance in 3 isolates (10.7%), intermediate susceptibility in 4 isolates (14.3%) and an MIC in the sensitive range for 20 isolates (71.4%). One isolate of Acinetobacter was also tested with no interpretative criteria available. Of note, five of the seven patients infected with ceftolozane/tazobactam-resistant isolates met criteria for clinical success. These patients had polymicrobial infections with another organism that was susceptible to ceftolozane/tazobactam, received inhaled aminoglycosides due to respiratory origin of infection, or had an infection of urinary source.
The rates of ceftolozane/tazobactam activity in this cohort are lower than many large surveys of P. aeruginosa isolates, but in line with studies reporting on MDR and XDR phenotypes.28,29 Twenty-nine of 75 recovered isolates had susceptibility results reported by the clinical microbiology laboratory, which included P. aeruginosa, K. pneumoniae and E. coli. Seven instances of discordant ceftolozane/tazobactam interpretation were found, where the clinical microbiology lab reported a susceptible MIC but the isolate had an intermediate or resistant ceftolozane/tazobactam MIC upon re-testing in the research laboratory. Importantly, resistance was seen across a variety of P. aeruginosa STs, likely as a result of mutational resistance rather than dissemination of a high-risk clone or transmissible resistance determinant. The presence of changes in PDC or PBP3 identified in most resistant isolates supports this hypothesis. Due to differences in the availability of testing supplies and interpretive criteria across the study period, uniform testing and reporting of all isolates in the clinical microbiology laboratory was not performed, and thus it was not possible to determine a change in rates of resistance over time or the impact on patient outcomes.
Limitations of this study are related to its observational and non-comparative design. Though the study was multicentre, the cohort was derived from two large healthcare systems from a single metropolitan area and may not be broadly generalizable. The clinical diagnosis of each case was left to the treating physicians and was not systematically confirmed using standardized diagnostic criteria and may be prone to error. Source control was not ascertained. Optimal dosing of ceftolozane/tazobactam was judged based on exposure in the first 24 h of treatment. Due to complexity, we were unable to capture the overall pharmacokinetic exposure in the absence of tracking of dosing changes or therapeutic drug monitoring throughout the treatment course. Follow-up was not standardized and outcome variables were not systematically elicited at the point of care. Components of clinical success were subjective and fidelity of documentation may vary between clinicians. Collection of data points was affected by variations in documentation over time, especially as it relates to clinical laboratory in vitro susceptibility testing for ceftolozane/tazobactam, and physical availability of the isolates for subsequent testing and sequencing. Treatment factors such as ceftolozane/tazobactam dosing and use of combination regimens were at the discretion of the treating physician, and therefore were subject to confounding factors. Data from the univariate analysis were used to control for potential confounders in the multivariable analysis to reduce potential sources of error.
In summary, ceftolozane/tazobactam demonstrated comparable rates of composite clinical success in a real-world cohort of medically complex patients in which P. aeruginosa was the predominant pathogen, as in previously published cohorts. Shorter time to initiation of ceftolozane/tazobactam therapy was associated with improved outcomes. A genomic analysis of available study isolates suggested that resistance arose sporadically and was likely related to mutational changes in the PDC or PBP3 enzyme, as reported previously. Acquired resistance determinants such as β-lactamases did not appear to drive resistance in this cohort. Ceftolozane/tazobactam remains a potential therapy for hospital-acquired and ventilator-associated pneumonia and complex P. aeruginosa infections.
Funding
This work was supported by funding from Merck Sharp and Dohme, a subsidiary of Merck and Co., Inc. (to C.A.A.). C.A.A. was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (Award Numbers K24AI121296, R01AI134637, R01AI48342 and P01AI152999). W.R.M. was funded by the National Institute of Allergy and Infectious Disease of the National Institutes of Health (Award Number K08AI135093). B.M.H. was partially funded by the National Institute of Allergy and Infectious Disease of the National Institutes of Health (Award Number K01AI148593). C.P. was partially funded by the National Institutes of Health (grant R01AI134637).
Transparency declarations
C.A.A. has received grant support from Merck, MeMed Diagnostics and Entasis Therapeutics. W.R.M. has received grants and research support from Merck and Entasis Therapeutics. K.W.G. has received a research grant from Paratek Pharmaceuticals. Merck and Co., Inc had no role in the design or interpretation of the study. All other authors: none to declare.
Supplementary data
Supplementary Methods and Table S1 are available as Supplementary data at JAC-AMR Online.




Comments