-
PDF
- Split View
-
Views
-
Cite
Cite
Kathryn L McDonald, Sarah Garland, Carolee A Carson, Kimberly Gibbens, E Jane Parmley, Rita Finley, Melissa C MacKinnon, Measures used to assess the burden of ESBL-producing Escherichia coli infections in humans: a scoping review, JAC-Antimicrobial Resistance, Volume 3, Issue 1, March 2021, dlaa104, https://doi.org/10.1093/jacamr/dlaa104
Close - Share Icon Share
Abstract
ESBL-producing bacteria pose a serious challenge to both clinical care and public health. There is no standard measure of the burden of illness (BOI) of ESBL-producing Escherichia coli (ESBL-EC) in the published literature, indicating a need to synthesize available BOI data to provide an overall understanding of the impact of ESBL-EC infections on human health.
To summarize the characteristics of BOI reporting in the ESBL-EC literature to (i) describe how BOI associated with antimicrobial resistance (AMR) is measured and reported; (ii) summarize differences in other aspects of reporting between studies; and (iii) highlight the common themes in research objectives and their relation to ESBL-EC BOI.
Two literature searches, run in 2013 and 2018, were conducted to capture published studies evaluating the BOI associated with ESBL-EC infections in humans. These searches identified 1723 potentially relevant titles and abstracts. After relevance screening of titles and abstracts and review of full texts, 27 studies were included for qualitative data synthesis. This review identified variability in the reporting and use of BOI measures, study characteristics, definitions and laboratory methods for identifying ESBL-EC infections.
Decision makers often require BOI data to make science-based decisions for the implementation of surveillance activities or risk reduction policies. Similarly, AMR BOI measures are important components of risk analyses and economic evaluations of AMR. This review highlights many limitations to current ESBL-EC BOI reporting, which, if improved upon, will ensure data accessibility and usefulness for ESBL-EC BOI researchers, decision makers and clinicians.
1. Introduction
ESBL-producing bacteria pose a serious challenge to both clinical care and public health. First described in 1983, the emergence of ESBL Gram-negative Enterobacteriaceae has significantly contributed to the global public health problem of antimicrobial resistance (AMR).1,2 The β-lactamase enzymes produced by ESBL-producing bacteria are classified as Ambler class A and Bush–Jacoby–Medeiros class 2be.3–5 The ESBLs are plasmid-mediated β-lactamases that are capable of hydrolysing β-lactams, including penicillins, cephalosporins and monobactams, and they may be inhibited by β-lactamase inhibitors, including clavulanic acid, vaborbactam and avibactam.6–9 As a consequence of the frequent medical use of β-lactam antimicrobials to treat many human infections, the prevalence of ESBL-producing β-lactam-resistant infections continues to rise.10 ESBL-producing Enterobacteriaceae have likewise been found to possess genes that confer resistance to other classes of antimicrobials, including fluoroquinolones and aminoglycosides, and therefore can be MDR.11,12
Escherichia coli represent a major group of ESBL-producing Gram-negative bacteria, responsible for a range of problematic nosocomial and community-acquired infections in humans, including, but not limited to, urinary tract infections and bloodstream infections.13 Current literature on ESBL-producing E. coli (herein denoted ESBL-EC) inconsistently defines ESBL infections and inconsistently describes how these infections are microbiologically confirmed, contributing to a lack of shared understanding of the frequency and importance of associated illnesses.14
It has been demonstrated that infections caused by ESBL-EC result in excess burdens to patients and to healthcare systems in part because of their impact on antimicrobial treatment efficacy.2,15 A burden of illness (BOI) measure for AMR is an indicator that captures the impact of a resistant bacterial infection on human health and the healthcare system.15 To assess the burden of ESBL-EC infections in humans, studies frequently evaluate differences in morbidity, mortality and healthcare costs associated with resistant versus susceptible E. coli infections.2,15 Variability in methods used to assess the impacts of ESBL-EC infections challenges our ability to understand the BOI attributed to these resistant infections.16,17 The lack of standardization of BOI measures is not unique to ESBL-EC infections and is also seen in the BOI literature for other infectious diseases.
There is a need for a synthesis of the available literature to provide decision makers with better information about ESBL-EC infections and their impact on BOI. The objective of this scoping review is to summarize how ESBL-EC infections are characterized in the literature by (i) describing how BOI associated with AMR is measured and reported; (ii) summarizing differences in other aspects of reporting between studies, including reporting of study characteristics, as well as characteristics of ESBL definitions and confirmation methods; and (iii) highlighting the common themes in research objectives and their relation to ESBL-EC BOI. This scoping review will contribute to Canada’s Integrated Assessment Model for Antimicrobial Resistance (iAM.AMR) Project, which integrates data on the BOI associated with ESBL-EC infections in humans. Quantitative data are not presented in this review; as such, this paper does not report whether or not ESBL-EC infections are associated with a higher BOI than non-ESBL-EC infections. A larger systematic review of the health and health systems burden of AMR E. coli infections is being completed in parallel to this scoping review and it will provide a quantitative synthesis of the BOI measures using a meta-analysis.18
2. Methods
2.1 Literature search
This scoping review was guided by PRISMA-ScR methodology.19,20 A literature search was designed to capture published BOI data on ESBL-EC infections in humans. Six databases were searched: PubMed; Embase in Ovid; Scopus; Global Health in CAB Direct; CAB Abstracts; and Core Collection in Web of Science. Search strings were developed by epidemiologists working with the Canadian Integrated Program for Antimicrobial Resistance Surveillance (CIPARS) (Table S1, available as Supplementary data at JAC-AMR Online) and were validated by ensuring that known relevant articles were captured. An initial literature search was conducted on 31 May 2013, without date restrictions, and was updated on 7 June 2018 to include literature published since the initial 2013 search.
2.2 Inclusion criteria
To be included in this review, articles needed to be published in English and use an analytical observational study design (including but not limited to cross-sectional, cohort, case-control or hybrid), as defined by Dohoo, Martin and Stryhn.21 Included articles had to originate from countries listed as very high development index (HDI) in the United Nations 2018 Human Development Index Report, in order to ensure high relevance to a Canadian context.22 Only studies pertaining to human infections with ESBL-EC (exposed group) and non-ESBL-EC comparator groups (control group) and that analysed at least one BOI measure were considered for this review. Examples of BOI measures include mortality, morbidity, length of hospital stay and healthcare costs23 as well as indicators of patient, payer and provider burdens associated with ESBL-EC and non-ESBL-EC infections.15
2.3 Relevance screening
After removal of duplicates, relevance screening of titles and abstracts was conducted at the time of the literature searches by a single reviewer, screening for language, HDI status, study design, study population and infection type (ESBL-EC or non-ESBL-EC infections) to identify eligible studies for full text review. Articles that failed to meet one or more primary screening inclusion criteria were excluded from this review.
Articles that met title and abstract inclusion criteria proceeded to full-text review, which was conducted independently by two reviewers (K.L.M., M.C.M.), using web-based systematic review software (Distiller SR).24 Full text review used additional criteria for inclusion, including the presence of ESBL-EC infections and non-ESBL-EC comparator groups, and BOI outcomes with extractable E. coli-specific data.
2.4 Qualitative data extraction
Data extraction was performed by two reviewers individually, using a pre-determined custom data extraction form in the web-based software (Distiller SR).24 Qualitative data of interest included study characteristics (country, study period, type of data, study design, site description, study objectives and themes, description of ESBL and ESBL confirmation methods), characteristics of the study participants (description of ESBL and non-ESBL groups, age and gender of participants) and information related to outcomes used to measure the BOI of ESBL-EC.
3. Results
3.1 Characteristics of included studies
This literature search identified 1723 (de-duplicated) potentially relevant articles for screening; 27 references met all inclusion criteria and reported BOI outcome data for ESBL-EC (Figure 1).
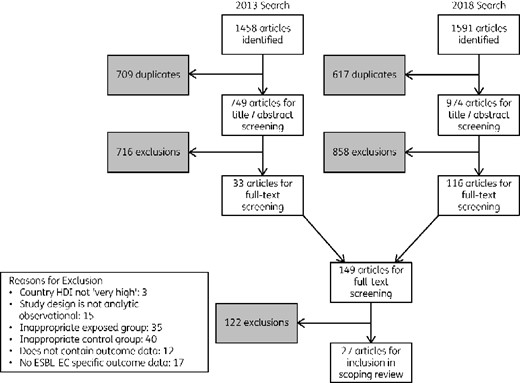
Literature search and screening process, adapted from the Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR)20 for a scoping review of BOI measures related to ESBL-producing E. coli infections in humans.
Study characteristics
All (n = 27) references used hospital-based data in their analyses.25–51 The majority of studies (93%, n = 25) utilized data specifically captured for the purposes of their studies, while two studies utilized data collected for different surveillance purposes.39,40 Data collected for surveillance purposes included single-site surveillance39 and multisite nationwide surveillance.40
The earliest data included in this review were collected in 1991,41 with the latest data extending to the end of 201627,51 (Figure 2). With the exception of one reference by Ho et al.,43 which was published in 2002, all references were published between 2007 and 2018.
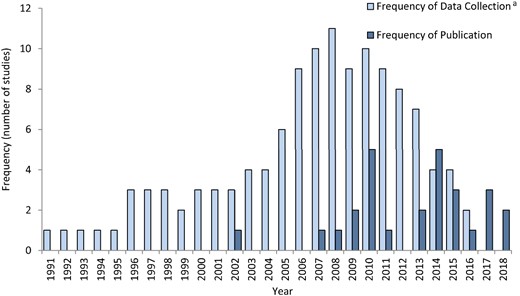
Years of data collection and publication period for data included in the scoping review of BOI measures related to ESBL-producing E. coli infections in humans. aFrequency of Data Collection: each year of data during a multi-year collection period is recorded as one value.
References most commonly reported on the burden of ESBL-EC exclusively, however, several references (n = 4) also reported burden data for other organisms, such as Klebsiella spp. and all Gram-negative bacteria.26,39,47,50 Studies were predominantly conducted in South Korea (n = 7) and Spain (n = 6). Author-reported study designs were predominantly cohort (44%, n = 12) and case-control (26%, n = 7) (Table 1). Multiple studies originated from the same medical facility,29,30,33,35,40,46,47 and also the same cohort.30,47
Characteristics of peer-reviewed references included in scoping review of BOI measures related to ESBL-producing E. coli infections in humans
| Reference . | Author reported study design . | No. of sites . | Study type . | Country of study . | Bacterial species . | Underlying common disease process . | ESBL-EC group . | No. of ESBL-EC participants . | Non-ESBL- EC group . | No. of non- ESBL-EC participants . | Details of ESBL-EC and non-ESBL-EC group selection . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Namikawa H et al. (2017)25 | not reported | single-site | retrospective | Japan | E. coli | N/A | ESBL-EC BSI | 31 | Non-ESBL- EC BSI | 98 | No selection. |
| Maslikowska JA et al. (2016)26 | case-control | single-site | retrospective | Canada | E. coli, Klebsiella spp. | N/A | ESBL-EC infection | 61 | Non-ESBL-EC infection | 50 | Cases and controls matched 1:1. Criteria: sex, age (± 5 years), type of infection, bed allocation. |
| Lee H et al. (2018)27 | case-control | single-site | retrospective | Republic of Korea | E. coli | All from the emergency department | ESBL-EC UTI | 50 | Non-ESBL-EC UTI | 100 | Cases and controls matched 1:2. Criteria: age (± 5 years) and sex. |
| Artero A et al. (2017)28 | cohort | single-site | prospective | Spain | E. coli | All elderly (≥65 years) and had to have community-onset pyelonephritis and/or urosepsis and were in a high rate setting of ESBL-EC | ESBL-EC UTI | 85 | Non-ESBL-EC UTI | 225 | No selection. |
| Leistner R et al. (2014)29 | cohort | single-sitea | retrospective | Germany | E. coli | N/A | ESBL-EC BSId | 115 | Non-ESBL-EC BSI | 983 | No selection. |
| Leistner R et al. (2014)30 | case-control | single-sitea | retrospective | Germany | E. coli | N/A | ESBL-EC BSId | 92 | Non-ESBL-EC BSI | 92 | Cases and controls matched 1:1. Criteria: age (± 5 years), sex, CCI (± 2), discharge year. LOS of the controls before the onset of BSI had to be at least as long as the respective case. |
| Van Aken S et al. (2014)31 | case-control | multisite (≥3) | retrospective | Sweden | E. coli | N/A | ESBL-EC BSI | 70 | Non-ESBL-EC BSI | 140 | Cases and controls matched 1:2. Criteria: time period, bacterial species, the occurrence of bacteraemia and study location. |
| Alotaibi et al. (2017)32 | not reported | single-site | prospective | Saudi Arabia | E. coli | N/A | ESBL-EC BSI | 66 | Non-ESBL-EC BSI | 105 | No selection. |
| Trecarichi EM et al. (2009)33 | cohort | single-siteb | retrospective | Italy | E. coli | All have haematological malignancies | ESBL-EC BSI | 26 | Non-ESBL-EC BSI | 36 | No selection. |
| Rodríguez‐Baño J et al. (2010)34 | case- control- control | multisite (≥3) | retrospective and prospective | Spain | E. coli | All community-onset | ESBL-EC BSI | 95 | Non-ESBL-EC BSI | 188 | Cases and controls matched 1:2. Criteria: hospital and time period. |
| Park SH et al. (2011)35 | cohort | single-sitec | retrospective | South Korea | E. coli | All community-onset | ESBL-EC BSI | 50 | Non-ESBL-EC BSI | 100 | Cases and controls matched 1:2. Criteria: diagnosis time period. |
| Peña C et al. (2008)36 | cohort | single-site | retrospective | Spain | E. coli | All hospitalized patients | ESBL-EC infection | 100 | Non-ESBL-EC infection | 100 | Cases and controls matched 1:1. Criteria: site of infection and closest date of admission to the case. |
| Esteve-Palau E et al. (2015)37 | cohort | single-site | retrospective | Spain | E. coli | All required hospitalization, were community onset (community acquired or healthcare-associated) and had to be symptomatic UTI | ESBL-EC UTI | 60 | Non-ESBL-EC UTI | 60 | Cases and controls matched 1:1. Criteria: age, sex and date of admission. |
| Melzer M, Peterson I. (2007)38 | not reported | single-site | prospective | UK | E. coli | N/A | ESBL-EC BSI | 46 | Non-ESBL-EC BSI | 308 | No selection. |
| Khan FY et al. (2010)39 | observational | single-site | prospective | Qatar | E. coli, Klebsiella spp., Enterobacter cloacae, all bacteraemias included Gram-positive and -negative. | N/A | ESBL-EC BSI | 27 | Non-ESBL-EC BSI | 70 | No selection. |
| Tumbarello M et al. (2010)40 | cohort | single-siteb | retrospective | Italy | E. coli | All were adult inpatients | ESBL-EC BSI | 37 | Non-ESBL-EC BSI | 97 | No selection. |
| Ortega M et al. (2009)41 | other: surveillance | single-site | prospective | Spain | E. coli | N/A | ESBL-EC BSI | 211 | Non-ESBL-EC BSI | 4547 | No selection. |
| Kang CI et al. (2010)42 | cohort | multisite (≥3) | retrospective | South Korea | E. coli | All community-onset | ESBL-EC BSI | 82 | Non-ESBL-EC BSI | 783 | No selection. |
| Ho PL et al. (2002)43 | case-control | single-site | retrospective | Hong Kong | E. coli | N/A | ESBL-EC BSI | 50 | Non-ESBL-EC BSI | 100 | Cases and controls matched 1:2. Criteria: specialty, sex, age (± 10 years) and the closest date to isolation of the corresponding case. |
| Gudiol C et al. (2009)44 | observational | single-site | prospective | Spain | E. coli | All were hospitalized cancer patients or HSCT recipients | ESBL-EC BSI | 17 | Non-ESBL-EC BSI | 118 | No selection. |
| Denis B et al. (2015)45 | case-control | single-site | retrospective | France | E. coli | N/A | ESBL-EC BSI | 41 | Non-ESBL-EC BSI | 41 | Cases and controls matched 1:1. Criteria: date of a case. |
| Park SH et al. (2015)46 | case-control | single-sitec | retrospective | Republic of Korea | E. coli | N/A | Community-associated ESBL-EC APN | 75 | Community-associated non-ESBL-EC APN | 225 | Cases and controls were matched 1:3. Criteria: date of treatment. |
| Leistner R et al. (2014)47 | cohort | single-sitea | retrospective | Germany | E. coli, Klebsiella spp. | N/A | ESBL-EC BSI | 178 | Non-ESBL-EC BSI | 1321 | No selection. |
| Ha YE et al. (2013)48 | cohort | single-site | retrospective | South Korea | E. coli | All patients had cancer. | ESBL-EC BSI | 95 | Non-ESBL-EC BSI | 255 | No selection. |
| Lee S et al. (2014)49 | cohort | single-site | retrospective | South Korea | E. coli | All treated with empirical ceftriaxone | ESBL-EC APN | 26 | Non-ESBL-EC APN | 52 | Cases and Controls matched 1:2. Criteria: bacteraemia, age, sex, CCI, SAPS II and modified APN score. There was a 16-point scoring system for the matching. |
| Kim SH et al. (2013)50 | not reported | single-site | retrospective | South Korea | E. coli, Klebsiella spp. | Neutropenic fever in patients with haematological disease that received chemotherapy or stem cell transplantation | ESBL-EC BSI | 15 | Non-ESBL-EC BSI | 72 | No selection. |
| Haruki Y et al. (2018)51 | cohort | single-site | retrospective | Japan | E. coli | Critically ill patients in the ICU | ESBL-EC BSI | 24 | Non-ESBL-EC BSI | 77 | No selection. |
| Reference . | Author reported study design . | No. of sites . | Study type . | Country of study . | Bacterial species . | Underlying common disease process . | ESBL-EC group . | No. of ESBL-EC participants . | Non-ESBL- EC group . | No. of non- ESBL-EC participants . | Details of ESBL-EC and non-ESBL-EC group selection . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Namikawa H et al. (2017)25 | not reported | single-site | retrospective | Japan | E. coli | N/A | ESBL-EC BSI | 31 | Non-ESBL- EC BSI | 98 | No selection. |
| Maslikowska JA et al. (2016)26 | case-control | single-site | retrospective | Canada | E. coli, Klebsiella spp. | N/A | ESBL-EC infection | 61 | Non-ESBL-EC infection | 50 | Cases and controls matched 1:1. Criteria: sex, age (± 5 years), type of infection, bed allocation. |
| Lee H et al. (2018)27 | case-control | single-site | retrospective | Republic of Korea | E. coli | All from the emergency department | ESBL-EC UTI | 50 | Non-ESBL-EC UTI | 100 | Cases and controls matched 1:2. Criteria: age (± 5 years) and sex. |
| Artero A et al. (2017)28 | cohort | single-site | prospective | Spain | E. coli | All elderly (≥65 years) and had to have community-onset pyelonephritis and/or urosepsis and were in a high rate setting of ESBL-EC | ESBL-EC UTI | 85 | Non-ESBL-EC UTI | 225 | No selection. |
| Leistner R et al. (2014)29 | cohort | single-sitea | retrospective | Germany | E. coli | N/A | ESBL-EC BSId | 115 | Non-ESBL-EC BSI | 983 | No selection. |
| Leistner R et al. (2014)30 | case-control | single-sitea | retrospective | Germany | E. coli | N/A | ESBL-EC BSId | 92 | Non-ESBL-EC BSI | 92 | Cases and controls matched 1:1. Criteria: age (± 5 years), sex, CCI (± 2), discharge year. LOS of the controls before the onset of BSI had to be at least as long as the respective case. |
| Van Aken S et al. (2014)31 | case-control | multisite (≥3) | retrospective | Sweden | E. coli | N/A | ESBL-EC BSI | 70 | Non-ESBL-EC BSI | 140 | Cases and controls matched 1:2. Criteria: time period, bacterial species, the occurrence of bacteraemia and study location. |
| Alotaibi et al. (2017)32 | not reported | single-site | prospective | Saudi Arabia | E. coli | N/A | ESBL-EC BSI | 66 | Non-ESBL-EC BSI | 105 | No selection. |
| Trecarichi EM et al. (2009)33 | cohort | single-siteb | retrospective | Italy | E. coli | All have haematological malignancies | ESBL-EC BSI | 26 | Non-ESBL-EC BSI | 36 | No selection. |
| Rodríguez‐Baño J et al. (2010)34 | case- control- control | multisite (≥3) | retrospective and prospective | Spain | E. coli | All community-onset | ESBL-EC BSI | 95 | Non-ESBL-EC BSI | 188 | Cases and controls matched 1:2. Criteria: hospital and time period. |
| Park SH et al. (2011)35 | cohort | single-sitec | retrospective | South Korea | E. coli | All community-onset | ESBL-EC BSI | 50 | Non-ESBL-EC BSI | 100 | Cases and controls matched 1:2. Criteria: diagnosis time period. |
| Peña C et al. (2008)36 | cohort | single-site | retrospective | Spain | E. coli | All hospitalized patients | ESBL-EC infection | 100 | Non-ESBL-EC infection | 100 | Cases and controls matched 1:1. Criteria: site of infection and closest date of admission to the case. |
| Esteve-Palau E et al. (2015)37 | cohort | single-site | retrospective | Spain | E. coli | All required hospitalization, were community onset (community acquired or healthcare-associated) and had to be symptomatic UTI | ESBL-EC UTI | 60 | Non-ESBL-EC UTI | 60 | Cases and controls matched 1:1. Criteria: age, sex and date of admission. |
| Melzer M, Peterson I. (2007)38 | not reported | single-site | prospective | UK | E. coli | N/A | ESBL-EC BSI | 46 | Non-ESBL-EC BSI | 308 | No selection. |
| Khan FY et al. (2010)39 | observational | single-site | prospective | Qatar | E. coli, Klebsiella spp., Enterobacter cloacae, all bacteraemias included Gram-positive and -negative. | N/A | ESBL-EC BSI | 27 | Non-ESBL-EC BSI | 70 | No selection. |
| Tumbarello M et al. (2010)40 | cohort | single-siteb | retrospective | Italy | E. coli | All were adult inpatients | ESBL-EC BSI | 37 | Non-ESBL-EC BSI | 97 | No selection. |
| Ortega M et al. (2009)41 | other: surveillance | single-site | prospective | Spain | E. coli | N/A | ESBL-EC BSI | 211 | Non-ESBL-EC BSI | 4547 | No selection. |
| Kang CI et al. (2010)42 | cohort | multisite (≥3) | retrospective | South Korea | E. coli | All community-onset | ESBL-EC BSI | 82 | Non-ESBL-EC BSI | 783 | No selection. |
| Ho PL et al. (2002)43 | case-control | single-site | retrospective | Hong Kong | E. coli | N/A | ESBL-EC BSI | 50 | Non-ESBL-EC BSI | 100 | Cases and controls matched 1:2. Criteria: specialty, sex, age (± 10 years) and the closest date to isolation of the corresponding case. |
| Gudiol C et al. (2009)44 | observational | single-site | prospective | Spain | E. coli | All were hospitalized cancer patients or HSCT recipients | ESBL-EC BSI | 17 | Non-ESBL-EC BSI | 118 | No selection. |
| Denis B et al. (2015)45 | case-control | single-site | retrospective | France | E. coli | N/A | ESBL-EC BSI | 41 | Non-ESBL-EC BSI | 41 | Cases and controls matched 1:1. Criteria: date of a case. |
| Park SH et al. (2015)46 | case-control | single-sitec | retrospective | Republic of Korea | E. coli | N/A | Community-associated ESBL-EC APN | 75 | Community-associated non-ESBL-EC APN | 225 | Cases and controls were matched 1:3. Criteria: date of treatment. |
| Leistner R et al. (2014)47 | cohort | single-sitea | retrospective | Germany | E. coli, Klebsiella spp. | N/A | ESBL-EC BSI | 178 | Non-ESBL-EC BSI | 1321 | No selection. |
| Ha YE et al. (2013)48 | cohort | single-site | retrospective | South Korea | E. coli | All patients had cancer. | ESBL-EC BSI | 95 | Non-ESBL-EC BSI | 255 | No selection. |
| Lee S et al. (2014)49 | cohort | single-site | retrospective | South Korea | E. coli | All treated with empirical ceftriaxone | ESBL-EC APN | 26 | Non-ESBL-EC APN | 52 | Cases and Controls matched 1:2. Criteria: bacteraemia, age, sex, CCI, SAPS II and modified APN score. There was a 16-point scoring system for the matching. |
| Kim SH et al. (2013)50 | not reported | single-site | retrospective | South Korea | E. coli, Klebsiella spp. | Neutropenic fever in patients with haematological disease that received chemotherapy or stem cell transplantation | ESBL-EC BSI | 15 | Non-ESBL-EC BSI | 72 | No selection. |
| Haruki Y et al. (2018)51 | cohort | single-site | retrospective | Japan | E. coli | Critically ill patients in the ICU | ESBL-EC BSI | 24 | Non-ESBL-EC BSI | 77 | No selection. |
BSI, bloodstream infection; UTI, urinary tract infection; APN, acute pyelonephritis; CCI, Charlson comorbidity index; LOS, length of stay; N/A, not applicable (indicating that information for this field was not provided in the article).
Originating from Charité University Hospital, Berlin, Germany.
Originating from Catholic University Hospital, Milano, Italy.
Originating from Daejeon St. Mary’s Hospital, Daejeon, South Korea.
Originating from the same cohort of patients.
Characteristics of peer-reviewed references included in scoping review of BOI measures related to ESBL-producing E. coli infections in humans
| Reference . | Author reported study design . | No. of sites . | Study type . | Country of study . | Bacterial species . | Underlying common disease process . | ESBL-EC group . | No. of ESBL-EC participants . | Non-ESBL- EC group . | No. of non- ESBL-EC participants . | Details of ESBL-EC and non-ESBL-EC group selection . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Namikawa H et al. (2017)25 | not reported | single-site | retrospective | Japan | E. coli | N/A | ESBL-EC BSI | 31 | Non-ESBL- EC BSI | 98 | No selection. |
| Maslikowska JA et al. (2016)26 | case-control | single-site | retrospective | Canada | E. coli, Klebsiella spp. | N/A | ESBL-EC infection | 61 | Non-ESBL-EC infection | 50 | Cases and controls matched 1:1. Criteria: sex, age (± 5 years), type of infection, bed allocation. |
| Lee H et al. (2018)27 | case-control | single-site | retrospective | Republic of Korea | E. coli | All from the emergency department | ESBL-EC UTI | 50 | Non-ESBL-EC UTI | 100 | Cases and controls matched 1:2. Criteria: age (± 5 years) and sex. |
| Artero A et al. (2017)28 | cohort | single-site | prospective | Spain | E. coli | All elderly (≥65 years) and had to have community-onset pyelonephritis and/or urosepsis and were in a high rate setting of ESBL-EC | ESBL-EC UTI | 85 | Non-ESBL-EC UTI | 225 | No selection. |
| Leistner R et al. (2014)29 | cohort | single-sitea | retrospective | Germany | E. coli | N/A | ESBL-EC BSId | 115 | Non-ESBL-EC BSI | 983 | No selection. |
| Leistner R et al. (2014)30 | case-control | single-sitea | retrospective | Germany | E. coli | N/A | ESBL-EC BSId | 92 | Non-ESBL-EC BSI | 92 | Cases and controls matched 1:1. Criteria: age (± 5 years), sex, CCI (± 2), discharge year. LOS of the controls before the onset of BSI had to be at least as long as the respective case. |
| Van Aken S et al. (2014)31 | case-control | multisite (≥3) | retrospective | Sweden | E. coli | N/A | ESBL-EC BSI | 70 | Non-ESBL-EC BSI | 140 | Cases and controls matched 1:2. Criteria: time period, bacterial species, the occurrence of bacteraemia and study location. |
| Alotaibi et al. (2017)32 | not reported | single-site | prospective | Saudi Arabia | E. coli | N/A | ESBL-EC BSI | 66 | Non-ESBL-EC BSI | 105 | No selection. |
| Trecarichi EM et al. (2009)33 | cohort | single-siteb | retrospective | Italy | E. coli | All have haematological malignancies | ESBL-EC BSI | 26 | Non-ESBL-EC BSI | 36 | No selection. |
| Rodríguez‐Baño J et al. (2010)34 | case- control- control | multisite (≥3) | retrospective and prospective | Spain | E. coli | All community-onset | ESBL-EC BSI | 95 | Non-ESBL-EC BSI | 188 | Cases and controls matched 1:2. Criteria: hospital and time period. |
| Park SH et al. (2011)35 | cohort | single-sitec | retrospective | South Korea | E. coli | All community-onset | ESBL-EC BSI | 50 | Non-ESBL-EC BSI | 100 | Cases and controls matched 1:2. Criteria: diagnosis time period. |
| Peña C et al. (2008)36 | cohort | single-site | retrospective | Spain | E. coli | All hospitalized patients | ESBL-EC infection | 100 | Non-ESBL-EC infection | 100 | Cases and controls matched 1:1. Criteria: site of infection and closest date of admission to the case. |
| Esteve-Palau E et al. (2015)37 | cohort | single-site | retrospective | Spain | E. coli | All required hospitalization, were community onset (community acquired or healthcare-associated) and had to be symptomatic UTI | ESBL-EC UTI | 60 | Non-ESBL-EC UTI | 60 | Cases and controls matched 1:1. Criteria: age, sex and date of admission. |
| Melzer M, Peterson I. (2007)38 | not reported | single-site | prospective | UK | E. coli | N/A | ESBL-EC BSI | 46 | Non-ESBL-EC BSI | 308 | No selection. |
| Khan FY et al. (2010)39 | observational | single-site | prospective | Qatar | E. coli, Klebsiella spp., Enterobacter cloacae, all bacteraemias included Gram-positive and -negative. | N/A | ESBL-EC BSI | 27 | Non-ESBL-EC BSI | 70 | No selection. |
| Tumbarello M et al. (2010)40 | cohort | single-siteb | retrospective | Italy | E. coli | All were adult inpatients | ESBL-EC BSI | 37 | Non-ESBL-EC BSI | 97 | No selection. |
| Ortega M et al. (2009)41 | other: surveillance | single-site | prospective | Spain | E. coli | N/A | ESBL-EC BSI | 211 | Non-ESBL-EC BSI | 4547 | No selection. |
| Kang CI et al. (2010)42 | cohort | multisite (≥3) | retrospective | South Korea | E. coli | All community-onset | ESBL-EC BSI | 82 | Non-ESBL-EC BSI | 783 | No selection. |
| Ho PL et al. (2002)43 | case-control | single-site | retrospective | Hong Kong | E. coli | N/A | ESBL-EC BSI | 50 | Non-ESBL-EC BSI | 100 | Cases and controls matched 1:2. Criteria: specialty, sex, age (± 10 years) and the closest date to isolation of the corresponding case. |
| Gudiol C et al. (2009)44 | observational | single-site | prospective | Spain | E. coli | All were hospitalized cancer patients or HSCT recipients | ESBL-EC BSI | 17 | Non-ESBL-EC BSI | 118 | No selection. |
| Denis B et al. (2015)45 | case-control | single-site | retrospective | France | E. coli | N/A | ESBL-EC BSI | 41 | Non-ESBL-EC BSI | 41 | Cases and controls matched 1:1. Criteria: date of a case. |
| Park SH et al. (2015)46 | case-control | single-sitec | retrospective | Republic of Korea | E. coli | N/A | Community-associated ESBL-EC APN | 75 | Community-associated non-ESBL-EC APN | 225 | Cases and controls were matched 1:3. Criteria: date of treatment. |
| Leistner R et al. (2014)47 | cohort | single-sitea | retrospective | Germany | E. coli, Klebsiella spp. | N/A | ESBL-EC BSI | 178 | Non-ESBL-EC BSI | 1321 | No selection. |
| Ha YE et al. (2013)48 | cohort | single-site | retrospective | South Korea | E. coli | All patients had cancer. | ESBL-EC BSI | 95 | Non-ESBL-EC BSI | 255 | No selection. |
| Lee S et al. (2014)49 | cohort | single-site | retrospective | South Korea | E. coli | All treated with empirical ceftriaxone | ESBL-EC APN | 26 | Non-ESBL-EC APN | 52 | Cases and Controls matched 1:2. Criteria: bacteraemia, age, sex, CCI, SAPS II and modified APN score. There was a 16-point scoring system for the matching. |
| Kim SH et al. (2013)50 | not reported | single-site | retrospective | South Korea | E. coli, Klebsiella spp. | Neutropenic fever in patients with haematological disease that received chemotherapy or stem cell transplantation | ESBL-EC BSI | 15 | Non-ESBL-EC BSI | 72 | No selection. |
| Haruki Y et al. (2018)51 | cohort | single-site | retrospective | Japan | E. coli | Critically ill patients in the ICU | ESBL-EC BSI | 24 | Non-ESBL-EC BSI | 77 | No selection. |
| Reference . | Author reported study design . | No. of sites . | Study type . | Country of study . | Bacterial species . | Underlying common disease process . | ESBL-EC group . | No. of ESBL-EC participants . | Non-ESBL- EC group . | No. of non- ESBL-EC participants . | Details of ESBL-EC and non-ESBL-EC group selection . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Namikawa H et al. (2017)25 | not reported | single-site | retrospective | Japan | E. coli | N/A | ESBL-EC BSI | 31 | Non-ESBL- EC BSI | 98 | No selection. |
| Maslikowska JA et al. (2016)26 | case-control | single-site | retrospective | Canada | E. coli, Klebsiella spp. | N/A | ESBL-EC infection | 61 | Non-ESBL-EC infection | 50 | Cases and controls matched 1:1. Criteria: sex, age (± 5 years), type of infection, bed allocation. |
| Lee H et al. (2018)27 | case-control | single-site | retrospective | Republic of Korea | E. coli | All from the emergency department | ESBL-EC UTI | 50 | Non-ESBL-EC UTI | 100 | Cases and controls matched 1:2. Criteria: age (± 5 years) and sex. |
| Artero A et al. (2017)28 | cohort | single-site | prospective | Spain | E. coli | All elderly (≥65 years) and had to have community-onset pyelonephritis and/or urosepsis and were in a high rate setting of ESBL-EC | ESBL-EC UTI | 85 | Non-ESBL-EC UTI | 225 | No selection. |
| Leistner R et al. (2014)29 | cohort | single-sitea | retrospective | Germany | E. coli | N/A | ESBL-EC BSId | 115 | Non-ESBL-EC BSI | 983 | No selection. |
| Leistner R et al. (2014)30 | case-control | single-sitea | retrospective | Germany | E. coli | N/A | ESBL-EC BSId | 92 | Non-ESBL-EC BSI | 92 | Cases and controls matched 1:1. Criteria: age (± 5 years), sex, CCI (± 2), discharge year. LOS of the controls before the onset of BSI had to be at least as long as the respective case. |
| Van Aken S et al. (2014)31 | case-control | multisite (≥3) | retrospective | Sweden | E. coli | N/A | ESBL-EC BSI | 70 | Non-ESBL-EC BSI | 140 | Cases and controls matched 1:2. Criteria: time period, bacterial species, the occurrence of bacteraemia and study location. |
| Alotaibi et al. (2017)32 | not reported | single-site | prospective | Saudi Arabia | E. coli | N/A | ESBL-EC BSI | 66 | Non-ESBL-EC BSI | 105 | No selection. |
| Trecarichi EM et al. (2009)33 | cohort | single-siteb | retrospective | Italy | E. coli | All have haematological malignancies | ESBL-EC BSI | 26 | Non-ESBL-EC BSI | 36 | No selection. |
| Rodríguez‐Baño J et al. (2010)34 | case- control- control | multisite (≥3) | retrospective and prospective | Spain | E. coli | All community-onset | ESBL-EC BSI | 95 | Non-ESBL-EC BSI | 188 | Cases and controls matched 1:2. Criteria: hospital and time period. |
| Park SH et al. (2011)35 | cohort | single-sitec | retrospective | South Korea | E. coli | All community-onset | ESBL-EC BSI | 50 | Non-ESBL-EC BSI | 100 | Cases and controls matched 1:2. Criteria: diagnosis time period. |
| Peña C et al. (2008)36 | cohort | single-site | retrospective | Spain | E. coli | All hospitalized patients | ESBL-EC infection | 100 | Non-ESBL-EC infection | 100 | Cases and controls matched 1:1. Criteria: site of infection and closest date of admission to the case. |
| Esteve-Palau E et al. (2015)37 | cohort | single-site | retrospective | Spain | E. coli | All required hospitalization, were community onset (community acquired or healthcare-associated) and had to be symptomatic UTI | ESBL-EC UTI | 60 | Non-ESBL-EC UTI | 60 | Cases and controls matched 1:1. Criteria: age, sex and date of admission. |
| Melzer M, Peterson I. (2007)38 | not reported | single-site | prospective | UK | E. coli | N/A | ESBL-EC BSI | 46 | Non-ESBL-EC BSI | 308 | No selection. |
| Khan FY et al. (2010)39 | observational | single-site | prospective | Qatar | E. coli, Klebsiella spp., Enterobacter cloacae, all bacteraemias included Gram-positive and -negative. | N/A | ESBL-EC BSI | 27 | Non-ESBL-EC BSI | 70 | No selection. |
| Tumbarello M et al. (2010)40 | cohort | single-siteb | retrospective | Italy | E. coli | All were adult inpatients | ESBL-EC BSI | 37 | Non-ESBL-EC BSI | 97 | No selection. |
| Ortega M et al. (2009)41 | other: surveillance | single-site | prospective | Spain | E. coli | N/A | ESBL-EC BSI | 211 | Non-ESBL-EC BSI | 4547 | No selection. |
| Kang CI et al. (2010)42 | cohort | multisite (≥3) | retrospective | South Korea | E. coli | All community-onset | ESBL-EC BSI | 82 | Non-ESBL-EC BSI | 783 | No selection. |
| Ho PL et al. (2002)43 | case-control | single-site | retrospective | Hong Kong | E. coli | N/A | ESBL-EC BSI | 50 | Non-ESBL-EC BSI | 100 | Cases and controls matched 1:2. Criteria: specialty, sex, age (± 10 years) and the closest date to isolation of the corresponding case. |
| Gudiol C et al. (2009)44 | observational | single-site | prospective | Spain | E. coli | All were hospitalized cancer patients or HSCT recipients | ESBL-EC BSI | 17 | Non-ESBL-EC BSI | 118 | No selection. |
| Denis B et al. (2015)45 | case-control | single-site | retrospective | France | E. coli | N/A | ESBL-EC BSI | 41 | Non-ESBL-EC BSI | 41 | Cases and controls matched 1:1. Criteria: date of a case. |
| Park SH et al. (2015)46 | case-control | single-sitec | retrospective | Republic of Korea | E. coli | N/A | Community-associated ESBL-EC APN | 75 | Community-associated non-ESBL-EC APN | 225 | Cases and controls were matched 1:3. Criteria: date of treatment. |
| Leistner R et al. (2014)47 | cohort | single-sitea | retrospective | Germany | E. coli, Klebsiella spp. | N/A | ESBL-EC BSI | 178 | Non-ESBL-EC BSI | 1321 | No selection. |
| Ha YE et al. (2013)48 | cohort | single-site | retrospective | South Korea | E. coli | All patients had cancer. | ESBL-EC BSI | 95 | Non-ESBL-EC BSI | 255 | No selection. |
| Lee S et al. (2014)49 | cohort | single-site | retrospective | South Korea | E. coli | All treated with empirical ceftriaxone | ESBL-EC APN | 26 | Non-ESBL-EC APN | 52 | Cases and Controls matched 1:2. Criteria: bacteraemia, age, sex, CCI, SAPS II and modified APN score. There was a 16-point scoring system for the matching. |
| Kim SH et al. (2013)50 | not reported | single-site | retrospective | South Korea | E. coli, Klebsiella spp. | Neutropenic fever in patients with haematological disease that received chemotherapy or stem cell transplantation | ESBL-EC BSI | 15 | Non-ESBL-EC BSI | 72 | No selection. |
| Haruki Y et al. (2018)51 | cohort | single-site | retrospective | Japan | E. coli | Critically ill patients in the ICU | ESBL-EC BSI | 24 | Non-ESBL-EC BSI | 77 | No selection. |
BSI, bloodstream infection; UTI, urinary tract infection; APN, acute pyelonephritis; CCI, Charlson comorbidity index; LOS, length of stay; N/A, not applicable (indicating that information for this field was not provided in the article).
Originating from Charité University Hospital, Berlin, Germany.
Originating from Catholic University Hospital, Milano, Italy.
Originating from Daejeon St. Mary’s Hospital, Daejeon, South Korea.
Originating from the same cohort of patients.
ESBL-EC and non-ESBL-EC group characteristics
Studies frequently restricted enrolment to specific types of E. coli infection (ESBL and non-ESBL) with the most common infection types being bloodstream infection (n = 20), urinary tract infection (n = 3) and acute pyelonephritis (n = 2) (Table 1). Two studies enrolled E. coli infections in general without restricting to a specific type of E. coli infection (Table 1).
ESBL-EC groups ranged in size from 15–211 participants, with a mean group size of 68 (median 60) participants. By contrast, non-ESBL-EC groups had a much larger range in size (36–4547), with a mean size of 383 (median 100) participants. Matching was used in ESBL and non-ESBL selection for 44% (n = 12) of studies. Characteristics used for matching included age, sex, type of infection, date of admission or discharge, length of stay and disease severity (Table 1). Of studies that reported selection details, a ratio of either 1:1 or 2:1 of non-ESBL-EC to ESBL-EC patients was used during selection, however, no selection details were reported for the remaining references (n = 15).
Reporting of age characteristics varied between references (Table S2). Most (n = 21) reported either group median or mean age, with a measure of variation, however, the lack of consistent reporting made age characteristics challenging to summarize. Six references did not report age details for ESBL-EC or non-ESBL-EC groups. The most commonly used age restrictions were adults and elderly (n = 7), or other (>14–16 years of age) (n = 5), indicating that the majority of studies collected data from adults and/or elderly populations. No age restrictions were reported for 14 references.
Gender was reported for ESBL-EC and non-ESBL-EC groups in most references (81%, n = 22) (Table S3). Participant group gender ratios varied, however, the proportion of females ranged from 34%–88% in case groups and 32%–94% in control groups. No gender demographics were reported in five references.
3.2 ESBL reporting in the literature
Definitions of ESBL
Definitions of ESBLs were infrequently reported among references. The majority of references did not use any form of definition for what constituted an ESBL-producing organism (70%, n = 19). Of the eight references that included some form of definition, five common attributes were identified. Six studies mentioned plasmid-mediated resistance, six specified resistance to specific groups of antimicrobials, four discussed co-resistance, two referred to hydrolysis as a mechanism of resistance, and two referred to resistance genes (Table S4). Four articles cited Paterson and Bonomo8 in their definition of ESBLs: ‘ESBLs are β-lactamases capable of conferring bacterial resistance to the penicillins, first-, second-, and third-generation cephalosporins, and aztreonam (but not the cephamycins or carbapenems) by hydrolysis of these antibiotics, and which are inhibited by β-lactamase inhibitors such as clavulanic acid.’ Additionally, two articles cited a definition used by Pitout and Laupland:13 ‘One group of β lactamases, extended-spectrum β lactamases (ESBLs), have the ability to hydrolyse and cause resistance to various types of the newer β-lactam antibiotics, including the expanded-spectrum (or third-generation) cephalosporins (e.g., cefotaxime, ceftriaxone, ceftazidime) and monobactams (e.g., aztreonam), but not the cephamycins (e.g., cefoxitin and cefotetan) and carbapenems (e.g., imipenem, meropenem, and ertapenem)’.
Methods for ESBL confirmation
Methods for ESBL confirmation varied between studies. The largest percentage of studies (41%, n = 11) used double disc synergy tests as confirmation of ESBL. Other methods included broth microdilution (n = 6) or disc diffusion (n = 1). Four studies reported the use of CLSI guidelines,26,41,42,44 yet only three provided citations for the CLSI informational supplement version that was used to guide ESBL confirmation methodology.26,41,44 Two additional studies reported other confirmation methods: Ambler class C identification set25 and cefotaxime/amoxicillin/clavulanic acid plate.31 Three studies failed to report confirmation methods.37,39,50
3.3 Common themes in ESBL-EC research
Twenty five (93%) studies reported investigating outcomes of ESBL-EC infections as a main theme in study research objectives.25,26,29–51 Examples include assessment of clinical characteristics and clinical outcome,25,26,37,40,48–51 evaluation of impact on treatment outcomes45,46 and estimation of differences in mortality.38,47 The remaining two studies reported outcome data in study results, yet outcomes were not included as a theme in study research objectives.27,28 Most studies also reported on identification of risk factors (n = 15), analysis of prevalence/incidence (n = 4), molecular epidemiology (n = 3), analysis of antimicrobial susceptibility profiles (n = 3), analysis of clinical characteristics (n = 1) and analysis of treatments (n = 1) as main themes present in research objectives.
3.4 Burden of illness
Studies reported data on 12 different outcome measures to analyse the BOI of ESBL-EC infections in humans (Table 2). In total, among the 27 studies, 113 results for BOI measures were reported. Outcome data that were general to all E. coli infections, and not specific for ESBLs, or that reported combined data for Klebsiella pneumoniae and E. coli were excluded (n = 21 BOI data points). The following 92 results are based on BOI measures with outcome data specific to ESBL-EC from the 27 studies included in this scoping review.
| BOI measures category . | No. of peer-reviewed references . | No. of peer-reviewed references with ESBL-EC specific data for BOI measures . |
|---|---|---|
| Mortality | 27 | 27 |
| Inappropriate initial antimicrobial therapy | 21 | 16 |
| Length of stay | 13 | 11 |
| Sepsis or shock | 12 | 7 |
| Treatment failure | 8 | 7 |
| ICU care | 8 | 6 |
| Disease severity | 8 | 6 |
| Cost | 5 | 3 |
| Time to appropriate antimicrobial therapy | 5 | 5 |
| Time to clinical stability | 2 | 1 |
| Relapse | 2 | 2 |
| Duration of IIAT | 2 | 1 |
| Total number of results for BOI measures | 113 | 92 |
| BOI measures category . | No. of peer-reviewed references . | No. of peer-reviewed references with ESBL-EC specific data for BOI measures . |
|---|---|---|
| Mortality | 27 | 27 |
| Inappropriate initial antimicrobial therapy | 21 | 16 |
| Length of stay | 13 | 11 |
| Sepsis or shock | 12 | 7 |
| Treatment failure | 8 | 7 |
| ICU care | 8 | 6 |
| Disease severity | 8 | 6 |
| Cost | 5 | 3 |
| Time to appropriate antimicrobial therapy | 5 | 5 |
| Time to clinical stability | 2 | 1 |
| Relapse | 2 | 2 |
| Duration of IIAT | 2 | 1 |
| Total number of results for BOI measures | 113 | 92 |
| BOI measures category . | No. of peer-reviewed references . | No. of peer-reviewed references with ESBL-EC specific data for BOI measures . |
|---|---|---|
| Mortality | 27 | 27 |
| Inappropriate initial antimicrobial therapy | 21 | 16 |
| Length of stay | 13 | 11 |
| Sepsis or shock | 12 | 7 |
| Treatment failure | 8 | 7 |
| ICU care | 8 | 6 |
| Disease severity | 8 | 6 |
| Cost | 5 | 3 |
| Time to appropriate antimicrobial therapy | 5 | 5 |
| Time to clinical stability | 2 | 1 |
| Relapse | 2 | 2 |
| Duration of IIAT | 2 | 1 |
| Total number of results for BOI measures | 113 | 92 |
| BOI measures category . | No. of peer-reviewed references . | No. of peer-reviewed references with ESBL-EC specific data for BOI measures . |
|---|---|---|
| Mortality | 27 | 27 |
| Inappropriate initial antimicrobial therapy | 21 | 16 |
| Length of stay | 13 | 11 |
| Sepsis or shock | 12 | 7 |
| Treatment failure | 8 | 7 |
| ICU care | 8 | 6 |
| Disease severity | 8 | 6 |
| Cost | 5 | 3 |
| Time to appropriate antimicrobial therapy | 5 | 5 |
| Time to clinical stability | 2 | 1 |
| Relapse | 2 | 2 |
| Duration of IIAT | 2 | 1 |
| Total number of results for BOI measures | 113 | 92 |
Seventeen references reported one to three outcomes, six reported four to six outcomes, and four reported seven to nine outcomes. The three most commonly reported BOI measures were mortality (n = 27), inappropriate initial antimicrobial therapy (IIAT) (n = 16) and length of stay (n = 11) (Table 2).
3.4.1 Mortality
All studies evaluated at least one measure of mortality to assess the BOI in patients with ESBL-EC infections.25–51 The majority (n = 26) of studies provided a description of how mortality was measured,25–31,33–51 while one study did not.32 The most commonly reported measures of mortality include 30 day (n = 13), in-hospital (n = 7), 14 day (n = 4) and 7 day (n = 4) (Table S5). Despite commonalities in timeframes for reporting all-cause and infection-attributable mortalities, the reporting of how mortality was defined within these timeframes varied between studies. Most studies (70%, n = 19) did not define whether the timeframe of reporting was measured from disease onset, discontinuation of antibiotics or discharge. Only eight studies reported when mortality was measured from. Six studies reported mortality following the onset of infection or from the first positive blood culture,31,36,41,43,44 and two studies reported mortality following the discontinuation of antibiotics.26,46
3.4.2 Inappropriate initial antimicrobial therapy
Sixteen studies reported a measure of IIAT or inappropriate empirical antimicrobial therapy.28,31,33–35,37,38,40–45,48,49,51 An example of a general definition of IIAT, as used by Gudiol et al.,44 was: ‘if the treatment regimen did not include at least one antibiotic that is active in vitro against the infecting microorganism’. In 10 studies, no timeframe was associated with an assessment of IIAT.28,31,37,38,40–42,44,49,51 The remaining studies (n = 6) included the timeframes of within 24 h of blood culture (n = 5)34,35,43,45,48 and within 48 h of blood culture (n = 1).33 An example of the former was reported by Denis et al.45 as: ‘active antibiotic not initiated within 24 h after blood sample was drawn’. Additional components of IIAT definitions included dose of antimicrobial, the timing of administration and adherence to antimicrobial recommendations.31,40–42
3.4.3 Length of stay
Eleven studies reported length of stay in the hospital as a measure of BOI.27–31,37,38,40,45,46,49 The majority, 64% (n = 7), reported the total length of stay,27–30,37,46,49 while others reported length of stay post-infection (n = 5).30,31,38,40,45 One study also reported pre-infection length of stay and the length of stay in the ICU and in a normal ward.30 Definitions of post-infection length of stay varied, however, most studies that used this measure (n = 4) analysed the period from first positive blood culture or onset of infection until the patient was discharged, transferred to an alternative facility, or until patient death.31,38,40,45
3.4.4 Sepsis or shock
Seven studies reported severe sepsis, septic shock or shock as BOI measures.31,34,35,40,44,46,48 Septic shock was most commonly reported (n = 6) and was reported in conjunction with severe sepsis in four studies (Table S6).31,34,35,48 Of the four studies which reported both severe sepsis and septic shock, only one reported individual results for each of the two measures.31
3.4.5 Treatment failure
Seven studies reported treatment failure as a measure of BOI.26,37,40,42,43,46,49 Treatment failure was commonly categorized as clinical or microbiological failures (Table S7). Clinical failure was reported in six studies, and was often grouped as early clinical failure (timeframes varied from 72 h to 7 days),26,40,42,43,46 or overall clinical failure (without time constraints) (Table S7).37,46 Definitions for clinical failure varied between studies. Most studies included persisting signs of infection, requiring a change of therapy or cessation of therapy, and death in their definition of clinical failure.26,37,40,42,43,46 An example of a definition of clinical failure, as used by Esteve-Palau et al.,37 was: ‘when the patient showed no improvement or at least one of the initial symptoms worsened, or needed a switch of antimicrobial therapy, or died’.
Three studies reported microbiological failure, with a consensus definition of failure to achieve negative culture, though there was variability in time frames (Table S7).26,46,49 One study reported all three measures of treatment failure.46
3.4.6 Other BOI measures
Additional BOI measures reported in references included ICU stay, disease severity, cost, measures of antimicrobial therapy efficacy and time to stability and relapse. Six studies reported ICU stay as a measure of BOI, however, this measure was not explicitly attributed to infection in any study.27,35,37,38,44,49 Six studies also reported disease severity as a measure of BOI.34,35,40,46,48,51 APACHE II score and Pitt bacteraemia score were the most common measures of disease severity reported (n = 3 and n = 2, respectively). Disease severity was also measured with the SOFA score (n = 1).
Three studies reported cost as a measure of BOI.30,37,40 All three studies reported on elements of total (or overall) costs, which included direct and indirect costs (Table S8). One study also reported on the cost per episode, which included the cost of hospitalization and outpatient antimicrobial therapy.37
BOI measures of antimicrobial efficacy included time to appropriate antimicrobial therapy, duration of IIAT and delay in appropriate antimicrobial therapy. Five studies reported time to appropriate antimicrobial therapy as a BOI measure.37,38,44,46,49 Of these, four reported time to appropriate antimicrobial therapy recorded in days.37,44,46,49 One study by Park et al.46 reported the BOI measure of duration of IIAT within 48 h. Moreover, one study reported the delay in appropriate antimicrobial therapy, dichotomizing as more or less than 24 h.38
Lastly, patient response to antimicrobial therapy was evaluated with the additional BOI measures of time to stability and relapse. One study reported time to clinical stability as a measure of BOI, which was evaluated in reference to the time to fever resolution.49 Two studies reported relapse as a measure of BOI.46,49 A 2015 study from Park et al.46 provided a detailed definition of relapse as: ‘a positive culture of baseline pathogen during the follow-up period after microbiological eradication had been confirmed’. By contrast, Lee et al.49 defined relapse using a 3 month time period, with no further specification.
4. Discussion
ESBL-EC infections place a burden on health systems and the health of patients. Our review has shown inconsistencies in how ESBL-EC research is reported, particularly the inconsistent reporting of ESBL definitions and variability in ESBL confirmation methods reported in the literature. We likewise identified variation in reporting of research objective themes in relation to BOI, and the use of BOI measures. Together, these inconsistencies made it challenging to synthesize the literature and improve our understanding of the BOI attributable to ESBL-EC infections.
A key finding of this review was the identification of several gaps in ESBL-EC reporting. Only 8/27 studies reported a definition of ESBL-EC. The infrequent use of definitions observed in this review could contribute to a lack of shared understanding of ESBL-producing organisms in terms of their mechanisms and spectrum of resistance.8 This may result in notable differences in parameters used to define ESBLs, which could compromise the validity of inter-study comparisons. The absence of standardized confirmation methods may also pose challenges for drawing inter-study conclusions about ESBLs if parameters for screening and confirmation lack standardization.
Variability in the reporting of research themes within study objectives was likewise observed. Most studies (n = 25) reported an analysis of outcomes or prognosis (morbidity and mortality) as a theme in the title and/or objectives. This review was aimed at describing BOI attributable to ESBL-EC infections in humans, and so the reporting of outcomes and prognosis within study objectives was a component of primary literature screening. The two studies that did not report outcomes or prognosis instead reported risk factor analysis as a research theme.27,28 This was an interesting finding that demonstrated the importance of considering the variability in research themes when designing search strategies for future reviews. Our search string had sufficient sensitivity to capture these studies and to identify their relevance to our review, however, more focused search strategies might not have been able to capture studies which reported variable research themes.
In the present study, 12 types of BOI measure were identified in 27 studies reporting 92 results specific to ESBL-EC BOI, highlighting the complexity of ESBL-EC reporting. The most commonly reported BOI measures included mortality, IIAT, length of stay, sepsis, shock and treatment failure. Measures that were less frequently reported included ICU care, disease severity, cost, time to appropriate antimicrobial therapy, time to stability, relapse and duration of IIAT. Differences in the frequency of reporting of the different BOI measures was a key finding of this review and is likely reflective of differences in the availability of retrospectively collected outcome data from hospital-based data sources. Other measures of BOI exist, but were not observed in this review, including disability-adjusted life years (DALYs), quality-adjusted life years, and number of years lost due to disease and lost productivity.52 Recently, Cassini et al. (2019)53 published DALYs for third-generation cephalosporin-resistant E. coli among other antimicrobial-resistant bacterial infections. This publication should stimulate future publication of DALYs for ESBL-EC infections from different populations.
A previous review by Naylor et al. (2018)15 noted differences in the use of specific patient and health system BOI measures to evaluate differences between susceptible and resistant infections. In the current review, all studies reported mortality as a measure of patient burden, however, morbidity measures were less frequently used. Similarly, length of stay in hospital and ICU care were commonly used to summarize the burden on health systems yet measures of cost were infrequently reported in literature. The lack of cost BOI data observed in this review is consistent with previous syntheses of economic burden literature.15,54 Nine studies failed to report outcome data specific to ESBL-EC (data were presented combined with multiple bacterial species) for certain BOI measures, causing 21 BOI measures to be excluded from the synthesis.
Different measures of BOI may be of greater importance for different public health applications. For example, analyses of the economic burden of ESBL-EC, for use in policy development might require more detailed data on length of stay, or treatment and disease-related healthcare costs from ESBL-EC literature. By contrast, analyses of patient burdens from ESBL-EC for use in health research and to inform patient care might require a higher level of detail on treatment failure, relapse, mortality and other measures of morbidity and treatment efficacy from ESBL-EC literature. These differences in applications and data availability drive the variability in the types of BOI measures reported by individual studies and they all have the ability to provide insight into various aspects of the BOI attributable to ESBL-EC.
We also observed that the application of a given BOI measure differed between studies. For example, mortality was defined by six different parameters in the literature, including infection-attributable mortality, in-hospital mortality, and 7, 14, 21 and 30 day mortality. Within these six parameters, measurement of mortality (from disease onset, discharge or discontinuation of antibiotics) was further varied. In one instance, no definition of mortality was provided.32 The distinction between all-cause versus disease-specific mortality has important implications on BOI analyses, as does the parameter of time used in mortality definitions. Therefore, mortality measures should be appropriately defined to minimize risk of misinterpretation.55 This variability was observed in many other BOI measures, including sepsis, shock, treatment failure, and length of stay, which makes data synthesis challenging. The variability or lack of definitions for BOI measures used in the studies highlights an opportunity for improved reporting. This would allow knowledge users to clearly appreciate the differences between studies when they are comparing results and facilitate synthesis of results in meta-analyses.
The majority of ESBL-EC BOI data in this study originated from hospital-based data collection sources. This finding was not surprising but demonstrates that data used in BOI analyses are predominantly based on more severe infections that required hospitalization. Our review is therefore unable to describe ESBL-EC BOI in the community as there was no available research to describe the BOI of less severe ESBL-EC infections that were not treated in hospital. We likewise identified variability in reporting of patient demographics such as the measure of central tendency used to describe age, the use and reporting of age restrictions and the reporting of gender, which challenged the analysis of potential vulnerabilities such as age and gender. This is an important finding, as the identification of at-risk subgroups are a major concern for policy makers, and so the consistent reporting of patient demographics is vital for accurately defining the scope of public health problems and for informing the objectives to be achieved through policy activities.
This review has several limitations, including the small number of references included for synthesis, the lack of recent ESBL-EC BOI data, and the lack of Canadian ESBL-EC BOI literature. This may be due in part to publication delay, and also the exclusion of studies from countries which were not in the very high HDI category of the United Nations 2018 Human Development Index Report.22 This exclusion criterion was applied to generate results with high relevance to the Canadian context of BOI attributable to ESBL-EC, however, it may have impacted the capture of other relevant ESBL-EC BOI references. Similarly, the majority of references captured in this review included data collected in 2013 or earlier, with only one reference reporting on Canadian ESBL-EC BOI data.26 There is need for more Canadian ESBL-EC BOI research and information sharing of Canadian BOI measures captured through existing Canadian surveillance programmes and activities, as well as more recent data to provide a more complete analysis of the Canadian context of ESBL-EC BOI. Another potential limitation of this review was the lack of quality analysis. A quality analysis was intentionally absent from this review, as this will be completed in a parallel systematic review through a risk of bias assessment.18
In conclusion, an integrated understanding of the clinical and public health impacts of ESBL-EC infections is vital for healthcare priority setting and planning. Policy-makers require summarized and clear information regarding the BOI related to ESBL-EC and this necessitates standardized or harmonized ways of reporting such information. This information is also needed for risk analysis, economic analysis, and to inform future surveillance activities. Harmonization of data collection, analysis and reporting of information on the BOI of AMR is needed. This could be achieved through guideline research, drafting or development and sharing by academia, national surveillance organizations, health professional societies or international standard-setting organizations. This review highlighted many limitations of current ESBL-EC reporting, which, if improved upon, will ensure data accessibility and usefulness for ESBL-EC BOI researchers, surveillance epidemiologists, risk assessors and clinicians.
Acknowledgements
The authors thank Dr Richard Reid-Smith (Public Health Agency of Canada) for his supervision and support while conducting this review, and Dr Jan Sargeant (University of Guelph) for web-based software support.
Funding
This work was supported by the Genome Research Development Initiative and also by the Ontario Ministry of Agriculture, Food and Rural Affairs.
Transparency declarations
None to declare.
Supplementary data
Tables S1 to S8 are available as Supplementary data at JAC-AMR Online.
References




Comments