-
PDF
- Split View
-
Views
-
Cite
Cite
Marc Wirden, Cecile Pouderoux, Gilles Peytavin, Basma Abdi, Antoine Fayçal, Romain Palich, Marc Antoine Valantin, Sophie Seang, Christine Katlama, Vincent Calvez, Valerie Pourcher, Anne-Geneviève Marcelin, Ultra-rapid selection of the N74D capsid inhibitor resistance mutation after 3 weeks on lenacapavir, Journal of Antimicrobial Chemotherapy, Volume 79, Issue 7, July 2024, Pages 1706–1707, https://doi.org/10.1093/jac/dkae115
Close - Share Icon Share
Lenacapavir is a very recent first-in-class capsid inhibitor approved for HIV-1 treatment.1 It is a long-acting antiretroviral intended for dosing twice a year by subcutaneous injection. In combination with other antiretrovirals, lenacapavir is indicated in heavily treatment-experienced adults with multidrug resistant HIV-1 infection failing their current antiretroviral regimen due to resistance, intolerance or safety considerations. Because lenacapavir is the first and only compound of its class, this drug is expected to be fully active regardless of previous treatment history. To date, several resistance mutations (RM) have been identified in the capsid protein (L56I, M66I, Q67H, K70N, N74D/S and T107N).2 They have been selected in vitro or in vivo during virological failures in the CAPELLA or CALIBRATE studies.3,4 None of these mutations was found to be polymorphic and therefore likely to be present before lenacapavir intake.5 However, the selection of these mutations is sufficiently frequent in the context of lenacapavir treatment failure to estimate that this drug presents a low genetic barrier to resistance. Therefore, given the risk of developing resistance in situations of functional monotherapy, careful consideration should be given to having good adherence and active drugs in addition to lenacapavir in the treatment regimen. The present clinical case reports the very rapid selection of resistance after only 3 weeks on lenacapavir.
The individual is a man, diagnosed with HIV-1 subtype D in 2015 at the age of 25 with tuberculosis coinfection. The HIV-1 plasma viral load (pVL) was of 4.66 log10 copies/mL and CD4 cells count was 348 cells/mm3. From 2015 to 2023, the patient was regularly experiencing relapses of his tuberculosis and virological failures due to poor adherence and multiple treatment interruptions. During this period, the nadir of CD4 was 14 cells/mm3 and zenith of pVL >7.00 log10 copies/mL. He received six different antiretroviral combinations including NRTI, PI and integrase strand transfer inhibitor. The patient was hospitalized on 21 December 2023 for deterioration in general condition with disseminated tuberculosis. At this time, he had stopped all his treatments, and pVL was 6.54 log10 copies/mL with 18 CD4/mm3. HIV-1 resistance genotyping showed a strain susceptible to all antiretrovirals similar to previous available data. On 3 January 2024, an oral once-daily fixed drug combination bictegravir/emtricitabine/tenofovir alafenamide (BIC/FTC/TAF) was prescribed. Five days later (day 0), due to the patient’s worrying clinical condition and the high risk of new interruption of treatment, lenacapavir was added by subcutaneous injection as the simplified regimen with a 2-day oral loading dose (600 mg on day 0 and on day 1), and 300 mg on day 8. At week 5, oral BIC/FTC/TAF was stopped and a long acting treatment with monthly intramuscular injections of cabotegravir (600 mg) and rilpivirine (900 mg) was started. This strategy was validated at a multidisciplinary council meeting. Faced with the stagnation of pVL between weeks 1 and 6 (−0.33 log10 copies/mL), we asked for determination of drug concentrations and new resistance genotypes. The results, pVL evolution and the therapeutic sequence are illustrated in Figure 1. The lenacapavir resistance mutation N74D on the capsid-gene and the M184V conferring resistance to emtricitabine were detected at week 3 and confirmed at week 6. Among the three samples available between weeks 1 and 4, minimal plasma concentrations of bictegravir, emtricitabine and tenofovir (determined 24 hours after the last drug intake) were detected only once, due to poor adherence. At the same time, lenacapavir plasma concentrations were effective (147 and 90 ng/mL at weeks 1 and 4, respectively), well above the mean trough concentration of 15.5 ng/mL (≥4-fold higher than the in vitro protein-adjusted 95% effective concentration in MT-4 cells).1
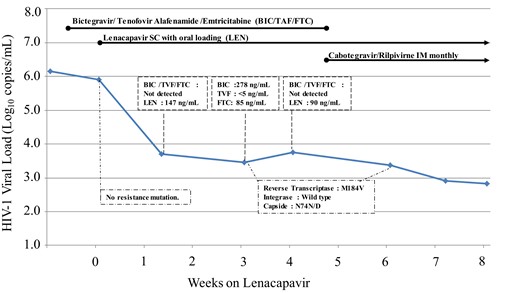
Evolution of HIV-1 viral load, drug concentrations and resistance, during Lenacapavir treatment in association with other antiretroviral compounds. TVF, tenofovir. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
The N74D mutation results in the loss of a direct hydrogen bond and electrostatic repulsions between the capsid and the lenacapavir molecule. Consequently, lenacapavir decreases its binding affinity to capsid by 20-fold and virus susceptibility by 10-fold.2 The ultra-rapid selection of the N74D mutation, in the present case, can be explained by the very poor adherence with oral treatment leading to lenacapavir monotherapy. It is the first time, to the best our knowledge, that ultra-rapid selection of an isolated N74D has been reported. After 26 weeks on lenacapavir in the CAPELLA study, five of the eight patients in virological failure harboured virus with lenacapavir RM at week 4 in a setting of functional lenacapavir monotherapy.6 These RMs were mostly in the M66 position without N74D, which was later found at week 10 in a single patient in association with M66I. This ‘real life’ case confirms the very low genetic barrier of lenacapavir and underlines the need to ensure the effectiveness and perfect adherence to oral associated antiretroviral drugs.
Funding
This work was supported by the Agence Nationale de la Recherche sur le SIDA et les Maladies Infectieuses Emergentes (ANRS MIE).
Transparency declarations
Gilles Peytavin, Basma Abdi, Romain Palich, Sophie Seang, Christine Katlama, Vincent Calvez, Valerie Pourcher and Anne-Geneviève Marcelin have received grants or honoraria from Gilead Sciences, ViiV Healthcare and Merck. All other authors: none to declare.



