-
PDF
- Split View
-
Views
-
Cite
Cite
Giusy Tiseo, Lorenzo Roberto Suardi, Alessandro Leonildi, Cesira Giordano, Simona Barnini, Marco Falcone, Meropenem/vaborbactam plus aztreonam for the treatment of New Delhi metallo-β-lactamase-producing Klebsiella pneumoniae infections, Journal of Antimicrobial Chemotherapy, Volume 78, Issue 9, September 2023, Pages 2377–2379, https://doi.org/10.1093/jac/dkad206
Close - Share Icon Share
Metallo-β-lactamase (MBL)-producing Klebsiella pneumoniae (Kp) is increasingly reported in Europe and the United States, and limited treatment options are available against this superbug.1–6 MBLs hydrolyse all β-lactams except aztreonam, but this latter cannot be used as monotherapy because of the co-production of expanded-spectrum-β-lactamases (ESBL). Novel β-lactam/β-lactamase inhibitors (BLBLIs), including ceftazidime/avibactam, meropenem/vaborbactam and imipenem/relebactam, are inactive against MBL-producing Enterobacterales. The combination ceftazidime/avibactam plus aztreonam displays in vitro synergy and has been associated with lower mortality rate compared with colistin in patients with bloodstream infections by MBL-producing Enterobacterales.7 Limited data about the combination of aztreonam plus other BLBLIs are available.8–10
Here, we report two cases of infections by NDM-producing Kp treated with meropenem/vaborbactam plus aztreonam (MER/VAB+ATM) and report the synergy assessment through the checkerboard assay.
Clinical case 1
A kidney transplant recipient was admitted to the academic Hospital of Pisa (Italy) with a complicated urinary tract infection. She had diabetes mellitus and chronic renal failure and reported a history of allergy to cephalosporins. She presented with fever and hypotension requiring vasopressors. Serum creatinine was 2 mg/dL (estimated glomerular filtration rate 28 mL/min). The urine culture grew NDM-producing Kp (GeneXpert System, Cepheid), resistant to meropenem (MIC 64 mg/L), amikacin (MIC 16 mg/L), fosfomycin (MIC >128 mg/L) and ceftazidime/avibactam (MIC >8/4 mg/L), and susceptible to colistin (MIC 0.5 mg/L). Considering the allergy to cephalosporins, the combination ceftazidime/avibactam plus aztreonam was discarded. Colistin was avoided due to the risk of nephrotoxicity. The combination MER/VAB+ATM was evaluated as a potential therapeutic option. Synergy between meropenem/vaborbactam and aztreonam was explored using checkerboard analysis in Mueller–Hinton broth and defined as an FIC index (FICI) ≤ 0.5. Checkerboards were set up with 2-fold dilutions of aztreonam (0.03 to 128 mg/L) and meropenem/vaborbactam (2 to 128 mg/L). The combination was fully synergistic (FICI = 0.26; Figure 1a) against the NDM-producing Kp. The patient received meropenem/vaborbactam 1/1 g IV q8h plus aztreonam 1 g IV q8h for 14 days. Her clinical conditions improved, she was discharged and no recurrence occurred after 12 months.
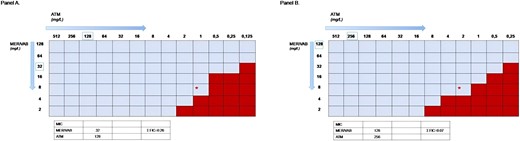
Determination of in vitro synergy by checkerboard broth microdilution against two NDM-producing Klebsiella pneumoniae (Panel A: patient 1. Panel B: patient 2). Optimal inhibition of growth at the susceptibility cut-off MIC value for both antibiotics is marked by a red asterisk: this represents meropenem/vaborbactam (MER/VAB) at a concentration of 8/8 mg/L in the presence of aztreonam (ATM) at 1 mg/L. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Clinical case 2
An 84-year-old woman with multiple comorbidities was admitted to the our hospital because of severe mitral insufficiency. She underwent mitral valve replacement. On Day 50 from cardiac surgery, she developed fever, hypotension and respiratory failure requiring mechanical ventilator support. Laboratory exams showed creatinine 3.5 mg/dL. An NDM-producing Kp was isolated from blood cultures and bronchoalveolar lavage fluid. The Kp isolate was resistant to meropenem (MIC 32 mg/L), ceftazidime/avibactam (MIC >64 mg/L) and colistin (MIC >4 mg/L). Cefiderocol 1 g IV q8h plus fosfomycin 2 g q8h were started, but she developed a severe anaphylactic reaction with acute tongue oedema, and cefiderocol was stopped. The synergy between meropenem/vaborbactam and aztreonam through the checkerboard showed full synergy (FICI = 0.07, Figure 1b). The patient started MER/VAB+ATM, but she had a worsening of inflammatory markers (increase in procalcitonin values) and clinical conditions, and she died 3 days after the start of treatment due to sepsis.
Discussion
Our clinical experience highlights the in vitro synergy between meropenem/vaborbactam plus aztreonam against NDM-Kp. However, we achieved clinical success in only one of the two treated patients, findings that suggest caution in using this combination against NDM-Kp.
Our data are in line with and strengthen the available literature. A microbiological study showed synergy between meropenem/vaborbactam and aztreonam against eight clinical Enterobacterales that co-produced NDM and serine β-lactamases using time–kill experiments.8 In this study, avibactam restored aztreonam more consistently than vaborbactam based on MIC test results, but time–kill experiments did not highlight different bacterial killing of avibactam- or vaborbactam-based combinations.8 Maraki and collaborators9 evaluated the in vitro efficacy of aztreonam in combination with different BLBLI against 40 MBL-producing Kp using the Etest method. Synergy rates were 97.5% for CAZ/AVI/ATM and imipenem/relebactam/aztreoam, and 72.5% for MER/VAB/ATM, suggesting a potential lower efficacy of this latter combination.
The clinical experience of using meropenem/vaborbactam plus aztreonam in infections by MBL-producing Enterobacterales is anecdotal. A retrospective case series including two patients with NDM-producing Kp who received MER/VAB+ATM reported a clinical success in one of the two cases.10 Our findings confirmed the in vitro synergy between meropenem/vaborbactam and aztreonam by checkerboard assay, adding important information about the potential efficacy of this combination against MBL-producing Kp. However, we did not observe clinical success in one of the 2 treated cases, who was a severely ill patient with multiple comorbidities. It is not possible to exclude that the impairment of heart function may be finally responsible for the poor outcome but at the same time we observed a laboratory response consistent with progression of sepsis. Future research is need to assess the clinical efficacy of this antibiotic combination in the real-life clinical practice.
In conclusion, MER/VAB+ATM may be considered to treat seriously ill patients with MBL-producing Kp when no other treatments are suitable. Future studies are needed to compare the MER/VAB+ATM combination with CAZ/AVI+ATM in MBL-producing Kp infections.
Acknowledgements
These data have been presented as Poster P2357 at the 32nd ECCMID Congress (Lisbon, Portugal, 23–26 April 2022).
Funding
This study was supported by internal funding.
Transparency declarations
M.F. received unconditional grants from MSD and grants or speaker honoraria from Angelini, Shionogi, Pfizer, Menarini, MSD, Gilead and Nordic Pharma. G.T. received honoraria for educational meetings from Shionogi. Declared conflicts of interest are outside the submitted work. All other authors: none to declare.



