-
PDF
- Split View
-
Views
-
Cite
Cite
Angel Salgado-Barreira, Jose Seijas-Amigo, Moises Rodriguez-Mañero, María Piñeiro-Lamas, Sonia Eiras, Alberto Cordero, Jose Ramon Gonzalez-Juanatey, Adolfo Figueiras, Effect of dapagliflozin on COVID-19 infection and risk of hospitalization, Journal of Antimicrobial Chemotherapy, Volume 78, Issue 9, September 2023, Pages 2335–2342, https://doi.org/10.1093/jac/dkad241
Close - Share Icon Share
Abstract
Dapagliflozin has been proposed as a potential treatment for coronavirus disease 2019 (COVID-19) by reducing cytokine production and inflammation. However, there are limited data on its effectiveness. We aimed to evaluate the impact of dapagliflozin on COVID-19 severity (including hospitalization risk, ICU admission, in-hospital death and progression to severe COVID-19) and its potential on susceptibility to COVID-19 infection.
We conducted a population-based case-control study. For aim 1, we assessed COVID-19 severity in cases (positive PCR patients requiring hospitalization) and matched controls (negative PCR patients or positive PCR patients not requiring hospitalization). For aim 2, we compared positive PCR cases (hospitalized and non-hospitalized) with controls. Adjusted odds ratios (aORs) were calculated using a generalized linear mixed model.
We analysed 86 602 subjects: 3060 were hospitalized cases, 26 757 were non-hospitalized cases and 56 785 were controls. Among the hospitalized COVID-19 patients, 228 were admitted to the ICU and 413 died. Dapagliflozin had no effect on the risk of hospitalization (aOR 0.98; 95% CI 0.65–1.48; P = 0.915), ICU admissions (aOR 1.21; 95% CI 0.34–4.25; P = 0.767) or in-hospital death (aOR 1.33; 95% CI 0.53–3.30; P = 0.543). Dapagliflozin reduced the risk of progression to severe COVID-19 by 35%, but this was not statistically significant (aOR 0.65; 95% CI 0.40–1.06; P = 0.086). Dapagliflozin was associated with a 30% increased risk of susceptibility to COVID-19 infection (aOR 1.31; 95% CI 1.05–1.62; P = 0.015).
Use of dapagliflozin prior to SARS-CoV-2 infection was not associated with an increased risk of hospitalization, ICU admission, mortality or progression to severe COVID-19. However, it was associated with an increased risk of susceptibility to COVID-19 infection.
Introduction
Coronavirus disease 2019 broke out at the beginning of 2020, and by the end of November 2021 more than 257 million people had been infected with COVID-19 globally, approximately 5.1 million of whom died.1 Worse outcomes from COVID-19 have been associated with increased age, socioeconomic deprivation, male sex and chronic diseases including diabetes.2 The risk of hospital-related COVID-19 mortality is increased 3-fold with type 1 diabetes and 2-fold with type 2 diabetes (T2D).3
Dapagliflozin, a drug that belongs to the sodium-glucose co-transporter-2 inhibitor (SGLT2i) family, is currently widely used in patients with T2D due to its positive effect on cardiovascular and renal function.4–10 Furthermore, a certation degree of anti-inflammatory effect has been shown recently.11 This could explain a beneficial effect in serious infection such as COVID-19 by mitigating cytokine production and inflammatory responses. SGLT2i drugs have also been proved to reduce inflammatory indicators such as C-reactive protein, ferritin and IL-6. Lastly, SGLT2i agents improve the vascular endothelium function. Due to these effects, their use as prophylactic treatment in thrombotic SARS-CoV-2 disease has been suggested.11 To date, observational studies have reported that the use of SGLT2i therapies is not associated with worse outcomes in patients with COVID-19.12,13 In addition, recent results from the Dapagliflozin in Respiratory Failure in Patients With COVID-19 (DARE-19) trial, performed in patients with at least one risk factor, did not result in a significant risk reduction in organ dysfunction or death, or improvement in clinical recovery.14 However, there is a paucity of information on how pre-existing long-term treatment with SGLT2i might affect the severity of COVID-19 disease and the susceptibility to infection.15
For this reason, the objectives of our study were to assess the effect of dapagliflozin on: (i) COVID19 severity, as determined by risk of hospitalization, risk of progression to severe COVID-19, risk of ICU admission and mortality; and (ii) its influence on susceptibility to COVID-19 infection in patients with previous long-term SGLT2i treatment.
Methods
Background context
This population study was conducted in Galicia, a region located in the northwest of Spain, having a population of 2.7 million. In Spain, 98% of citizens are covered by the National Health Service, which is largely funded by taxation. The Galician Health Service (Servicio Gallego de Salud/Servizo Galego de Saúde/SERGAS) comes under Spain’s National Health Service and keeps electronic medical records (EMRs) on all patients. These EMRs register all information on clinical care provided at a primary and hospital level, and show data relating to income, medical visits, emergency visits, diagnostic tests, drug prescriptions, the Minimum Basic Data Set on hospital discharges, and the International Classification of Primary Care Codes.16
Population and study design
We carried out a multiple case-population study, whereby we used all cases (in our instance, exhaustive sampling) in a precisely defined and identified population (in our instance, the population attended by the Public Health System in Galicia), and compared these with data on persons (controls) randomly extracted from the same population as the cases (population-based case controls), something that could be assumed to give a valid estimate of the prevalence of exposure and covariates in the population of origin.17
The selection criteria for cases and controls for each of the outcomes assessed were:
1) COVID19 severity
1.1) Risk of hospitalization: We defined cases as all patients admitted due to COVID-19, with PCR confirmation, to a public hospital in Galicia since the onset of the pandemic, whose clinical course ended before 1 January 2021. As controls, we selected a random sample of subjects who had no positive PCR (not having done any PCR or being PCR negative) during the same period. With the aim of enhancing the efficiency of our analysis of the risk of hospitalization, controls were randomly selected and matched with cases by age, sex and primary healthcare centre. We selected up to 20 controls for each case.
1.2) Risk of ICU admission for COVID-19: Cases were defined as all hospitalized individuals who were diagnosed with COVID-19 and required ICU admission. Controls were selected from the same matched group as those used to determine the risk of hospitalization.
1.3) In-hospital death due to COVID-19: Cases were defined as all hospitalized individuals who were diagnosed with COVID-19 and died from this disease. Controls were selected from the same matched group as those used to determine the risk of hospitalization.
1.4) Risk of progression to severe COVID-19: We defined the group of cases as all cases diagnosed with COVID-19 (the diagnosis had to be confirmed by PCR) who were hospitalized, and the control group as all patients diagnosed with COVID-19 who did not require hospitalization. In both groups, the diagnosis had to be confirmed by PCR in 2020.
2) Susceptibility to COVID-19 infection, defined as the risk of having a positive PCR test. Cases were defined as all persons diagnosed with COVID-19 confirmed by a positive PCR (hospitalized and not hospitalized) across the study period in Galicia. As controls, we used the same persons as those employed to assess the risk of hospitalization (subjects who had no positive PCR). As in the case of progression to severe COVID-19 (see 1.4 above), the cases were unmatched with controls, which does not produce any type of bias, only a decrease in efficiency.
Ethical aspects
The study was conducted in accordance with the Helsinki Declaration principles and current biomedical research legislation, approved by the Galician Clinical Research Ethics Committee (Comité de Ética de Investigación de Galicia), reference 2020/349, and certified by the Spanish Agency of Medicines and Medical Devices (Agencia Española del Medicamento y Productos Sanitarios/AEMPS). The study protocol is registered in the EU electronic Register of Post-Authorization Studies (EUPAS44587) and is available online at https://www.encepp.eu/encepp/viewResource.htm?id=44588.
Data extraction, variables and outcomes
All data were extracted semi-automatically by an independent information technology services company from the Complex Data-Analysis Systems (Sistemas de Información y Análisis Complejos/SIAC) used for SERGAS, which serve as a data warehouse whose designated purpose is to store and organize data by content (dispensing of medications, diagnoses, hospitalizations, among others).16
The exposure variable was the use of dapagliflozin, prescribed and dispensed, in all the study groups over 6 months preceding the index date (with this being defined as the 10 days prior to diagnosis of the disease). For controls it was the same day as the index day of the matched case of the hospitalization model. The indication for dapagliflozin in Spain during 2020 was for adults and children from 10 years of age whose condition is not controlled well enough. It is used with appropriate diet and exercise in patients who cannot take metformin (another diabetes medicine). It can also be used as ‘add-on’ treatment to other diabetes medicines.18
As our study covariates, we recorded demographic variables, anthropometric variables, comorbidities (hypertension, diabetes, chronic obstructive pulmonary disease, obesity, ischaemic heart disease, cerebrovascular accident, heart failure, atrial fibrillation, chronic renal failure, cancer, asthma), and exposure to all other medications prescribed and dispensed to each of the subjects in the 6 months prior to the index date. We used as a proxy for the degree of chronicity of the patients the number of different medications prescribed and dispensed for chronic diseases in the last 6 months before the index day.19
The outcome variables assessed were: (i) COVID19 severity: (a) hospitalization—defined as risk of hospitalization due to COVID-19 versus healthy controls; (b) risk of ICU admission—i.e. risk of ICU admission due to COVID-19 versus healthy controls; (c) mortality—risk of mortality due to COVID-19 versus healthy controls; (d) progression—risk of hospitalization among subjects with PCR+; and (ii) Susceptibility: risk of PCR+ (hospitalized and non-hospitalized) versus not having COVID-19.
Statistical analysis
Qualitative variables were expressed as frequencies and percentages, and quantitative variables as mean and SD or median and IQR. Adjusted odds ratios (aORs) of hospitalization, ICU admission, mortality, progression and susceptibility, and their 95% CIs were calculated using generalized linear mixed models for binomial dependent variables (case versus control).
The independent variable used to construct the models was dispensed versus absence of dapagliflozin treatment. We assumed that the effect of exposure on the probability of being a case (adjusted for covariates) could have been different between the different waves of contagion. For this reason, we included in the model a random effect (unstructured) indexed in each of the waves. Results were expressed in ORs with their 95% CIs, and adjustment was made for the above covariates.
Statistical significance was set at 0.05, and all statistical analyses were performed using the free R Statistical Software environment (version 4.1.0).16
Results
Study participants
In this case-population study, for the two aims, we analysed a total of 86 602 subjects, of whom 3060 were cases (subjects with a positive PCR who required hospitalization); 26 757 were non-hospitalized cases (subjects with a positive PCR who did not require hospitalization) and 56 785 were controls (subjects without a positive PCR). Among the hospitalized COVID-19 patients, 228 were admitted to ICU and 413 died (see Figure 1).

Flowchart. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Demographic and baseline characteristics
Demographic and baseline characteristics from the cohort group are shown in Tables 1 and 2. Mean age was 73 (IQR 59–84) years, 50% of the sample were females and 19% of the patients had T2D, 49% had hypertension, 19% had obesity, 13% had cancer, 10% had atrial fibrillation and 15% were smokers. Regarding SGLT2i drugs, 510 subjects were prescribed dapagliflozin and they represented around 1% of the different groups of this case-population study (see Tables 1 and 2).
Sociodemographic and clinical characteristics of the study population for the outcomes: hospitalization, ICU admission and in-hospital death for COVID-19
| Characteristic . | Hospitalization . | ICU admission . | In-hospital death . | |||
|---|---|---|---|---|---|---|
| Cases (n = 3060) . | Controls (n = 56 785) . | Cases (n = 228) . | Controls (n = 4398) . | Cases (n = 413) . | Controls (n = 7408) . | |
| Sex, n (%) | ||||||
| Male | 1552 (50.7) | 28 729 (50.6) | 160 (70.2) | 3078 (70.0) | 242 (58.6) | 4394 (59.3) |
| Female | 1508 (49.3) | 28 056 (49.4) | 68 (29.8) | 1320 (30.0) | 171 (41.4) | 3014 (40.7) |
| Age, median (IQR) | 74 (59–84) | 73 (59–84) | 69 (60–76) | 69 (60–76) | 85 (77–89) | 84 (75–88) |
| Health-related profession, n (%) | 81 (2.6) | 1260 (2.2) | 8 (3.5) | 84 (1.9) | 0 (0) | 0 (0) |
| Comorbidities, n (%) | ||||||
| Hypertension | 1754 (57.4) | 28 020 (49.3) | 126 (55.8) | 2060 (46.8) | 304 (73.6) | 4870 (65.7) |
| Diabetes | 841 (27.5) | 10 920 (19.2) | 75 (33.2) | 900 (20.5) | 160 (38.7) | 1826 (24.6) |
| COPD | 398 (13.0) | 4569 (8.0) | 34 (15.0) | 392 (8.9) | 89 (21.5) | 904 (12.2) |
| Obesity | 889 (29.1) | 10 817 (19.0) | 82 (36.3) | 809 (18.4) | 115 (27.8) | 1592 (21.5) |
| Ischaemic heart disease | 359 (11.7) | 4768 (8.4) | 32 (14.2) | 408 (9.3) | 91 (22.0) | 942 (12.7) |
| Cerebrovascular accident | 306 (10.0) | 3874 (6.8) | 17 (7.5) | 243 (5.5) | 75 (18.2) | 763 (10.3) |
| Heart failure | 469 (15.3) | 4030 (7.1) | 24 (10.6) | 205 (4.7) | 110 (26.6) | 831 (11.2) |
| Atrial fibrillation | 466 (15.2) | 5769 (10.2) | 33 (14.6) | 329 (7.5) | 90 (21.8) | 1186 (16.0) |
| Chronic renal failure | 437 (14.3) | 4316 (7.6) | 31 (13.7) | 251 (5.7) | 102 (24.7) | 912 (12.3) |
| Cancer | 529 (17.3) | 7770 (13.7) | 34 (15.0) | 620 (14.1) | 104 (25.2) | 1378 (18.6) |
| Asthma | 285 (9.3) | 3388 (6.0) | 16 (7.0) | 227 (5.2) | 26 (6.3) | 387 (5.2) |
| Current smoking | 809 (26.4) | 8532 (15.0) | 82 (36.0) | 875 (19.9) | 88 (21.3) | 890 (12.0) |
| Current medication, n (%) | ||||||
| Antihypertensive | 1590 (52) | 27 068 (47.7) | 129 (56.6) | 2041 (46.4) | 283 (68.5) | 4626 (62.4) |
| NSAIDs | 232 (7.6) | 3662 (6.4) | 19 (8.3) | 301 (6.8) | 19 (4.6) | 389 (5.3) |
| Paracetamol | 522 (17.1) | 7995 (14.1) | 36 (15.8) | 482 (11.0) | 88 (21.3) | 1324 (17.9) |
| Lipid-modifying agents | 1132 (37.0) | 20 288 (35.7) | 103 (45.2) | 1692 (38.5) | 176 (42.6) | 3143 (42.4) |
| Anticoagulants | 497 (16.2) | 6643 (11.7) | 40 (17.5) | 420 (9.5) | 97 (23.5) | 1298 (17.5) |
| Antiplatelet agents | 608 (19.9) | 9582 (16.9) | 58 (25.4) | 772 (17.6) | 139 (33.7) | 1753 (23.7) |
| Glucocorticoids | 426 (13.9) | 3757 (6.6) | 28 (12.3) | 287 (6.5) | 81 (19.6) | 591 (8.0) |
| Characteristic . | Hospitalization . | ICU admission . | In-hospital death . | |||
|---|---|---|---|---|---|---|
| Cases (n = 3060) . | Controls (n = 56 785) . | Cases (n = 228) . | Controls (n = 4398) . | Cases (n = 413) . | Controls (n = 7408) . | |
| Sex, n (%) | ||||||
| Male | 1552 (50.7) | 28 729 (50.6) | 160 (70.2) | 3078 (70.0) | 242 (58.6) | 4394 (59.3) |
| Female | 1508 (49.3) | 28 056 (49.4) | 68 (29.8) | 1320 (30.0) | 171 (41.4) | 3014 (40.7) |
| Age, median (IQR) | 74 (59–84) | 73 (59–84) | 69 (60–76) | 69 (60–76) | 85 (77–89) | 84 (75–88) |
| Health-related profession, n (%) | 81 (2.6) | 1260 (2.2) | 8 (3.5) | 84 (1.9) | 0 (0) | 0 (0) |
| Comorbidities, n (%) | ||||||
| Hypertension | 1754 (57.4) | 28 020 (49.3) | 126 (55.8) | 2060 (46.8) | 304 (73.6) | 4870 (65.7) |
| Diabetes | 841 (27.5) | 10 920 (19.2) | 75 (33.2) | 900 (20.5) | 160 (38.7) | 1826 (24.6) |
| COPD | 398 (13.0) | 4569 (8.0) | 34 (15.0) | 392 (8.9) | 89 (21.5) | 904 (12.2) |
| Obesity | 889 (29.1) | 10 817 (19.0) | 82 (36.3) | 809 (18.4) | 115 (27.8) | 1592 (21.5) |
| Ischaemic heart disease | 359 (11.7) | 4768 (8.4) | 32 (14.2) | 408 (9.3) | 91 (22.0) | 942 (12.7) |
| Cerebrovascular accident | 306 (10.0) | 3874 (6.8) | 17 (7.5) | 243 (5.5) | 75 (18.2) | 763 (10.3) |
| Heart failure | 469 (15.3) | 4030 (7.1) | 24 (10.6) | 205 (4.7) | 110 (26.6) | 831 (11.2) |
| Atrial fibrillation | 466 (15.2) | 5769 (10.2) | 33 (14.6) | 329 (7.5) | 90 (21.8) | 1186 (16.0) |
| Chronic renal failure | 437 (14.3) | 4316 (7.6) | 31 (13.7) | 251 (5.7) | 102 (24.7) | 912 (12.3) |
| Cancer | 529 (17.3) | 7770 (13.7) | 34 (15.0) | 620 (14.1) | 104 (25.2) | 1378 (18.6) |
| Asthma | 285 (9.3) | 3388 (6.0) | 16 (7.0) | 227 (5.2) | 26 (6.3) | 387 (5.2) |
| Current smoking | 809 (26.4) | 8532 (15.0) | 82 (36.0) | 875 (19.9) | 88 (21.3) | 890 (12.0) |
| Current medication, n (%) | ||||||
| Antihypertensive | 1590 (52) | 27 068 (47.7) | 129 (56.6) | 2041 (46.4) | 283 (68.5) | 4626 (62.4) |
| NSAIDs | 232 (7.6) | 3662 (6.4) | 19 (8.3) | 301 (6.8) | 19 (4.6) | 389 (5.3) |
| Paracetamol | 522 (17.1) | 7995 (14.1) | 36 (15.8) | 482 (11.0) | 88 (21.3) | 1324 (17.9) |
| Lipid-modifying agents | 1132 (37.0) | 20 288 (35.7) | 103 (45.2) | 1692 (38.5) | 176 (42.6) | 3143 (42.4) |
| Anticoagulants | 497 (16.2) | 6643 (11.7) | 40 (17.5) | 420 (9.5) | 97 (23.5) | 1298 (17.5) |
| Antiplatelet agents | 608 (19.9) | 9582 (16.9) | 58 (25.4) | 772 (17.6) | 139 (33.7) | 1753 (23.7) |
| Glucocorticoids | 426 (13.9) | 3757 (6.6) | 28 (12.3) | 287 (6.5) | 81 (19.6) | 591 (8.0) |
NSAIDs, non-steroidal anti-inflammatory drugs.
Sociodemographic and clinical characteristics of the study population for the outcomes: hospitalization, ICU admission and in-hospital death for COVID-19
| Characteristic . | Hospitalization . | ICU admission . | In-hospital death . | |||
|---|---|---|---|---|---|---|
| Cases (n = 3060) . | Controls (n = 56 785) . | Cases (n = 228) . | Controls (n = 4398) . | Cases (n = 413) . | Controls (n = 7408) . | |
| Sex, n (%) | ||||||
| Male | 1552 (50.7) | 28 729 (50.6) | 160 (70.2) | 3078 (70.0) | 242 (58.6) | 4394 (59.3) |
| Female | 1508 (49.3) | 28 056 (49.4) | 68 (29.8) | 1320 (30.0) | 171 (41.4) | 3014 (40.7) |
| Age, median (IQR) | 74 (59–84) | 73 (59–84) | 69 (60–76) | 69 (60–76) | 85 (77–89) | 84 (75–88) |
| Health-related profession, n (%) | 81 (2.6) | 1260 (2.2) | 8 (3.5) | 84 (1.9) | 0 (0) | 0 (0) |
| Comorbidities, n (%) | ||||||
| Hypertension | 1754 (57.4) | 28 020 (49.3) | 126 (55.8) | 2060 (46.8) | 304 (73.6) | 4870 (65.7) |
| Diabetes | 841 (27.5) | 10 920 (19.2) | 75 (33.2) | 900 (20.5) | 160 (38.7) | 1826 (24.6) |
| COPD | 398 (13.0) | 4569 (8.0) | 34 (15.0) | 392 (8.9) | 89 (21.5) | 904 (12.2) |
| Obesity | 889 (29.1) | 10 817 (19.0) | 82 (36.3) | 809 (18.4) | 115 (27.8) | 1592 (21.5) |
| Ischaemic heart disease | 359 (11.7) | 4768 (8.4) | 32 (14.2) | 408 (9.3) | 91 (22.0) | 942 (12.7) |
| Cerebrovascular accident | 306 (10.0) | 3874 (6.8) | 17 (7.5) | 243 (5.5) | 75 (18.2) | 763 (10.3) |
| Heart failure | 469 (15.3) | 4030 (7.1) | 24 (10.6) | 205 (4.7) | 110 (26.6) | 831 (11.2) |
| Atrial fibrillation | 466 (15.2) | 5769 (10.2) | 33 (14.6) | 329 (7.5) | 90 (21.8) | 1186 (16.0) |
| Chronic renal failure | 437 (14.3) | 4316 (7.6) | 31 (13.7) | 251 (5.7) | 102 (24.7) | 912 (12.3) |
| Cancer | 529 (17.3) | 7770 (13.7) | 34 (15.0) | 620 (14.1) | 104 (25.2) | 1378 (18.6) |
| Asthma | 285 (9.3) | 3388 (6.0) | 16 (7.0) | 227 (5.2) | 26 (6.3) | 387 (5.2) |
| Current smoking | 809 (26.4) | 8532 (15.0) | 82 (36.0) | 875 (19.9) | 88 (21.3) | 890 (12.0) |
| Current medication, n (%) | ||||||
| Antihypertensive | 1590 (52) | 27 068 (47.7) | 129 (56.6) | 2041 (46.4) | 283 (68.5) | 4626 (62.4) |
| NSAIDs | 232 (7.6) | 3662 (6.4) | 19 (8.3) | 301 (6.8) | 19 (4.6) | 389 (5.3) |
| Paracetamol | 522 (17.1) | 7995 (14.1) | 36 (15.8) | 482 (11.0) | 88 (21.3) | 1324 (17.9) |
| Lipid-modifying agents | 1132 (37.0) | 20 288 (35.7) | 103 (45.2) | 1692 (38.5) | 176 (42.6) | 3143 (42.4) |
| Anticoagulants | 497 (16.2) | 6643 (11.7) | 40 (17.5) | 420 (9.5) | 97 (23.5) | 1298 (17.5) |
| Antiplatelet agents | 608 (19.9) | 9582 (16.9) | 58 (25.4) | 772 (17.6) | 139 (33.7) | 1753 (23.7) |
| Glucocorticoids | 426 (13.9) | 3757 (6.6) | 28 (12.3) | 287 (6.5) | 81 (19.6) | 591 (8.0) |
| Characteristic . | Hospitalization . | ICU admission . | In-hospital death . | |||
|---|---|---|---|---|---|---|
| Cases (n = 3060) . | Controls (n = 56 785) . | Cases (n = 228) . | Controls (n = 4398) . | Cases (n = 413) . | Controls (n = 7408) . | |
| Sex, n (%) | ||||||
| Male | 1552 (50.7) | 28 729 (50.6) | 160 (70.2) | 3078 (70.0) | 242 (58.6) | 4394 (59.3) |
| Female | 1508 (49.3) | 28 056 (49.4) | 68 (29.8) | 1320 (30.0) | 171 (41.4) | 3014 (40.7) |
| Age, median (IQR) | 74 (59–84) | 73 (59–84) | 69 (60–76) | 69 (60–76) | 85 (77–89) | 84 (75–88) |
| Health-related profession, n (%) | 81 (2.6) | 1260 (2.2) | 8 (3.5) | 84 (1.9) | 0 (0) | 0 (0) |
| Comorbidities, n (%) | ||||||
| Hypertension | 1754 (57.4) | 28 020 (49.3) | 126 (55.8) | 2060 (46.8) | 304 (73.6) | 4870 (65.7) |
| Diabetes | 841 (27.5) | 10 920 (19.2) | 75 (33.2) | 900 (20.5) | 160 (38.7) | 1826 (24.6) |
| COPD | 398 (13.0) | 4569 (8.0) | 34 (15.0) | 392 (8.9) | 89 (21.5) | 904 (12.2) |
| Obesity | 889 (29.1) | 10 817 (19.0) | 82 (36.3) | 809 (18.4) | 115 (27.8) | 1592 (21.5) |
| Ischaemic heart disease | 359 (11.7) | 4768 (8.4) | 32 (14.2) | 408 (9.3) | 91 (22.0) | 942 (12.7) |
| Cerebrovascular accident | 306 (10.0) | 3874 (6.8) | 17 (7.5) | 243 (5.5) | 75 (18.2) | 763 (10.3) |
| Heart failure | 469 (15.3) | 4030 (7.1) | 24 (10.6) | 205 (4.7) | 110 (26.6) | 831 (11.2) |
| Atrial fibrillation | 466 (15.2) | 5769 (10.2) | 33 (14.6) | 329 (7.5) | 90 (21.8) | 1186 (16.0) |
| Chronic renal failure | 437 (14.3) | 4316 (7.6) | 31 (13.7) | 251 (5.7) | 102 (24.7) | 912 (12.3) |
| Cancer | 529 (17.3) | 7770 (13.7) | 34 (15.0) | 620 (14.1) | 104 (25.2) | 1378 (18.6) |
| Asthma | 285 (9.3) | 3388 (6.0) | 16 (7.0) | 227 (5.2) | 26 (6.3) | 387 (5.2) |
| Current smoking | 809 (26.4) | 8532 (15.0) | 82 (36.0) | 875 (19.9) | 88 (21.3) | 890 (12.0) |
| Current medication, n (%) | ||||||
| Antihypertensive | 1590 (52) | 27 068 (47.7) | 129 (56.6) | 2041 (46.4) | 283 (68.5) | 4626 (62.4) |
| NSAIDs | 232 (7.6) | 3662 (6.4) | 19 (8.3) | 301 (6.8) | 19 (4.6) | 389 (5.3) |
| Paracetamol | 522 (17.1) | 7995 (14.1) | 36 (15.8) | 482 (11.0) | 88 (21.3) | 1324 (17.9) |
| Lipid-modifying agents | 1132 (37.0) | 20 288 (35.7) | 103 (45.2) | 1692 (38.5) | 176 (42.6) | 3143 (42.4) |
| Anticoagulants | 497 (16.2) | 6643 (11.7) | 40 (17.5) | 420 (9.5) | 97 (23.5) | 1298 (17.5) |
| Antiplatelet agents | 608 (19.9) | 9582 (16.9) | 58 (25.4) | 772 (17.6) | 139 (33.7) | 1753 (23.7) |
| Glucocorticoids | 426 (13.9) | 3757 (6.6) | 28 (12.3) | 287 (6.5) | 81 (19.6) | 591 (8.0) |
NSAIDs, non-steroidal anti-inflammatory drugs.
Sociodemographic and clinical characteristics of the study population for the outcomes: susceptibility to SARS-CoV-2 and COVID-19 progression
| Characteristic . | Susceptibility to SARS-CoV-2 . | COVID-19 progression . | ||
|---|---|---|---|---|
| Cases (n = 29 817) . | Controls (n = 56 785) . | Cases (n = 3060) . | Controls (n = 26 757) . | |
| Sex, n (%) | ||||
| Male | 12 674 (42.5) | 28 729 (50.6) | 1552 (50.7) | 11 122 (41.6) |
| Female | 17 143 (57.5) | 28 056 (49.4) | 1508 (49.3) | 15 635 (58.4) |
| Age, median (IQR) | 49 (34–67) | 73 (59–84) | 74 (59–84) | 47 (33–63) |
| Health-related occupation, n (%) | 1316 (4.4) | 1260 (2.2) | 81 (2.6) | 1235 (4.6) |
| Comorbidities, n (%) | ||||
| Hypertension | 7847 (26.3) | 28 020 (49.3) | 1754 (57.3) | 6093 (22.8) |
| Diabetes | 3301 (11.1) | 10 920 (19.2) | 841 (27.5) | 2460 (9.2) |
| COPD | 1128 (3.8) | 4569 (8.0) | 398 (13.0) | 730 (2.7) |
| Obesity | 4790 (16.1) | 10 817 (19.0) | 889 (29.1) | 3901 (14.6) |
| Ischaemic heart disease | 1191 (4.0) | 4768 (8.4) | 359 (11.7) | 832 (3.1) |
| Cerebrovascular accident | 1144 (3.8) | 3874 (6.8) | 306 (10.0) | 838 (3.1) |
| Heart failure | 1108 (3.7) | 4030 (7.1) | 469 (15.3) | 639 (2.4) |
| Atrial fibrillation | 1501 (5.0) | 5769 (10.2) | 466 (15.2) | 1035 (3.9) |
| Chronic renal failure | 1115 (3.7) | 4316 (7.6) | 437 (14.3) | 678 (2.5) |
| Cancer | 2230 (7.5) | 7770 (13.7) | 529 (17.3) | 1701 (6.4) |
| Asthma | 2437 (8.2) | 3388 (6.0) | 285 (9.3) | 2152 (8.0) |
| Current smoking, n (%) | 4845 (16.2) | 8532 (15.0) | 809 (26.4) | 4036 (15.1) |
| Current medication, n (%) | ||||
| Antihypertensive | 7139 (23.9) | 27 068 (47.7) | 1590 (52.0) | 5549 (20.7) |
| NSAIDs | 2061 (6.9) | 3662 (6.4) | 232 (7.6) | 1829 (6.8) |
| Paracetamol | 2551 (8.6) | 7995 (14.1) | 522 (17.1) | 2029 (7.6) |
| Lipid-modifying agents | 5507 (18.5) | 20 288 (35.7) | 1132 (37.0) | 4375 (16.4) |
| Anticoagulants | 1671 (5.6) | 6643 (11.7) | 497 (16.2) | 1174 (4.4) |
| Antiplatelet agents | 2206 (7.4) | 9582 (16.9) | 608 (19.9) | 1598 (6.0) |
| Glucocorticoids | 1639 (5.5) | 3757 (6.6) | 426 (13.9) | 1213 (4.5) |
| Characteristic . | Susceptibility to SARS-CoV-2 . | COVID-19 progression . | ||
|---|---|---|---|---|
| Cases (n = 29 817) . | Controls (n = 56 785) . | Cases (n = 3060) . | Controls (n = 26 757) . | |
| Sex, n (%) | ||||
| Male | 12 674 (42.5) | 28 729 (50.6) | 1552 (50.7) | 11 122 (41.6) |
| Female | 17 143 (57.5) | 28 056 (49.4) | 1508 (49.3) | 15 635 (58.4) |
| Age, median (IQR) | 49 (34–67) | 73 (59–84) | 74 (59–84) | 47 (33–63) |
| Health-related occupation, n (%) | 1316 (4.4) | 1260 (2.2) | 81 (2.6) | 1235 (4.6) |
| Comorbidities, n (%) | ||||
| Hypertension | 7847 (26.3) | 28 020 (49.3) | 1754 (57.3) | 6093 (22.8) |
| Diabetes | 3301 (11.1) | 10 920 (19.2) | 841 (27.5) | 2460 (9.2) |
| COPD | 1128 (3.8) | 4569 (8.0) | 398 (13.0) | 730 (2.7) |
| Obesity | 4790 (16.1) | 10 817 (19.0) | 889 (29.1) | 3901 (14.6) |
| Ischaemic heart disease | 1191 (4.0) | 4768 (8.4) | 359 (11.7) | 832 (3.1) |
| Cerebrovascular accident | 1144 (3.8) | 3874 (6.8) | 306 (10.0) | 838 (3.1) |
| Heart failure | 1108 (3.7) | 4030 (7.1) | 469 (15.3) | 639 (2.4) |
| Atrial fibrillation | 1501 (5.0) | 5769 (10.2) | 466 (15.2) | 1035 (3.9) |
| Chronic renal failure | 1115 (3.7) | 4316 (7.6) | 437 (14.3) | 678 (2.5) |
| Cancer | 2230 (7.5) | 7770 (13.7) | 529 (17.3) | 1701 (6.4) |
| Asthma | 2437 (8.2) | 3388 (6.0) | 285 (9.3) | 2152 (8.0) |
| Current smoking, n (%) | 4845 (16.2) | 8532 (15.0) | 809 (26.4) | 4036 (15.1) |
| Current medication, n (%) | ||||
| Antihypertensive | 7139 (23.9) | 27 068 (47.7) | 1590 (52.0) | 5549 (20.7) |
| NSAIDs | 2061 (6.9) | 3662 (6.4) | 232 (7.6) | 1829 (6.8) |
| Paracetamol | 2551 (8.6) | 7995 (14.1) | 522 (17.1) | 2029 (7.6) |
| Lipid-modifying agents | 5507 (18.5) | 20 288 (35.7) | 1132 (37.0) | 4375 (16.4) |
| Anticoagulants | 1671 (5.6) | 6643 (11.7) | 497 (16.2) | 1174 (4.4) |
| Antiplatelet agents | 2206 (7.4) | 9582 (16.9) | 608 (19.9) | 1598 (6.0) |
| Glucocorticoids | 1639 (5.5) | 3757 (6.6) | 426 (13.9) | 1213 (4.5) |
NSAIDs, non-steroidal anti-inflammatory drugs.
Sociodemographic and clinical characteristics of the study population for the outcomes: susceptibility to SARS-CoV-2 and COVID-19 progression
| Characteristic . | Susceptibility to SARS-CoV-2 . | COVID-19 progression . | ||
|---|---|---|---|---|
| Cases (n = 29 817) . | Controls (n = 56 785) . | Cases (n = 3060) . | Controls (n = 26 757) . | |
| Sex, n (%) | ||||
| Male | 12 674 (42.5) | 28 729 (50.6) | 1552 (50.7) | 11 122 (41.6) |
| Female | 17 143 (57.5) | 28 056 (49.4) | 1508 (49.3) | 15 635 (58.4) |
| Age, median (IQR) | 49 (34–67) | 73 (59–84) | 74 (59–84) | 47 (33–63) |
| Health-related occupation, n (%) | 1316 (4.4) | 1260 (2.2) | 81 (2.6) | 1235 (4.6) |
| Comorbidities, n (%) | ||||
| Hypertension | 7847 (26.3) | 28 020 (49.3) | 1754 (57.3) | 6093 (22.8) |
| Diabetes | 3301 (11.1) | 10 920 (19.2) | 841 (27.5) | 2460 (9.2) |
| COPD | 1128 (3.8) | 4569 (8.0) | 398 (13.0) | 730 (2.7) |
| Obesity | 4790 (16.1) | 10 817 (19.0) | 889 (29.1) | 3901 (14.6) |
| Ischaemic heart disease | 1191 (4.0) | 4768 (8.4) | 359 (11.7) | 832 (3.1) |
| Cerebrovascular accident | 1144 (3.8) | 3874 (6.8) | 306 (10.0) | 838 (3.1) |
| Heart failure | 1108 (3.7) | 4030 (7.1) | 469 (15.3) | 639 (2.4) |
| Atrial fibrillation | 1501 (5.0) | 5769 (10.2) | 466 (15.2) | 1035 (3.9) |
| Chronic renal failure | 1115 (3.7) | 4316 (7.6) | 437 (14.3) | 678 (2.5) |
| Cancer | 2230 (7.5) | 7770 (13.7) | 529 (17.3) | 1701 (6.4) |
| Asthma | 2437 (8.2) | 3388 (6.0) | 285 (9.3) | 2152 (8.0) |
| Current smoking, n (%) | 4845 (16.2) | 8532 (15.0) | 809 (26.4) | 4036 (15.1) |
| Current medication, n (%) | ||||
| Antihypertensive | 7139 (23.9) | 27 068 (47.7) | 1590 (52.0) | 5549 (20.7) |
| NSAIDs | 2061 (6.9) | 3662 (6.4) | 232 (7.6) | 1829 (6.8) |
| Paracetamol | 2551 (8.6) | 7995 (14.1) | 522 (17.1) | 2029 (7.6) |
| Lipid-modifying agents | 5507 (18.5) | 20 288 (35.7) | 1132 (37.0) | 4375 (16.4) |
| Anticoagulants | 1671 (5.6) | 6643 (11.7) | 497 (16.2) | 1174 (4.4) |
| Antiplatelet agents | 2206 (7.4) | 9582 (16.9) | 608 (19.9) | 1598 (6.0) |
| Glucocorticoids | 1639 (5.5) | 3757 (6.6) | 426 (13.9) | 1213 (4.5) |
| Characteristic . | Susceptibility to SARS-CoV-2 . | COVID-19 progression . | ||
|---|---|---|---|---|
| Cases (n = 29 817) . | Controls (n = 56 785) . | Cases (n = 3060) . | Controls (n = 26 757) . | |
| Sex, n (%) | ||||
| Male | 12 674 (42.5) | 28 729 (50.6) | 1552 (50.7) | 11 122 (41.6) |
| Female | 17 143 (57.5) | 28 056 (49.4) | 1508 (49.3) | 15 635 (58.4) |
| Age, median (IQR) | 49 (34–67) | 73 (59–84) | 74 (59–84) | 47 (33–63) |
| Health-related occupation, n (%) | 1316 (4.4) | 1260 (2.2) | 81 (2.6) | 1235 (4.6) |
| Comorbidities, n (%) | ||||
| Hypertension | 7847 (26.3) | 28 020 (49.3) | 1754 (57.3) | 6093 (22.8) |
| Diabetes | 3301 (11.1) | 10 920 (19.2) | 841 (27.5) | 2460 (9.2) |
| COPD | 1128 (3.8) | 4569 (8.0) | 398 (13.0) | 730 (2.7) |
| Obesity | 4790 (16.1) | 10 817 (19.0) | 889 (29.1) | 3901 (14.6) |
| Ischaemic heart disease | 1191 (4.0) | 4768 (8.4) | 359 (11.7) | 832 (3.1) |
| Cerebrovascular accident | 1144 (3.8) | 3874 (6.8) | 306 (10.0) | 838 (3.1) |
| Heart failure | 1108 (3.7) | 4030 (7.1) | 469 (15.3) | 639 (2.4) |
| Atrial fibrillation | 1501 (5.0) | 5769 (10.2) | 466 (15.2) | 1035 (3.9) |
| Chronic renal failure | 1115 (3.7) | 4316 (7.6) | 437 (14.3) | 678 (2.5) |
| Cancer | 2230 (7.5) | 7770 (13.7) | 529 (17.3) | 1701 (6.4) |
| Asthma | 2437 (8.2) | 3388 (6.0) | 285 (9.3) | 2152 (8.0) |
| Current smoking, n (%) | 4845 (16.2) | 8532 (15.0) | 809 (26.4) | 4036 (15.1) |
| Current medication, n (%) | ||||
| Antihypertensive | 7139 (23.9) | 27 068 (47.7) | 1590 (52.0) | 5549 (20.7) |
| NSAIDs | 2061 (6.9) | 3662 (6.4) | 232 (7.6) | 1829 (6.8) |
| Paracetamol | 2551 (8.6) | 7995 (14.1) | 522 (17.1) | 2029 (7.6) |
| Lipid-modifying agents | 5507 (18.5) | 20 288 (35.7) | 1132 (37.0) | 4375 (16.4) |
| Anticoagulants | 1671 (5.6) | 6643 (11.7) | 497 (16.2) | 1174 (4.4) |
| Antiplatelet agents | 2206 (7.4) | 9582 (16.9) | 608 (19.9) | 1598 (6.0) |
| Glucocorticoids | 1639 (5.5) | 3757 (6.6) | 426 (13.9) | 1213 (4.5) |
NSAIDs, non-steroidal anti-inflammatory drugs.
COVID-19 severity
Risk of hospitalization
We analysed a sample of 357 subjects taking dapagliflozin (in the 6 months previous to the hospitalization), of whom 331 subjects were in the control group and 26 subjects had a PCR+ and had been hospitalized due to COVID-19 (crude OR 1.46; 95% CI 0.98–2.18). When adjustment was made for the covariates, the results showed a neutral effect between dapagliflozin use and risk of hospitalization due to COVID-19 (aOR 0.98; 95% CI 0.65–1.48; P = 0.915) (see Table 3).
OR of hospitalization, ICU admission, mortality, susceptibility to SARS-CoV-2 and COVID-19 progression from the last 6 month exposure to dapagliflozin
| . | Cases . | Controls . | Adjusted OR CI 95% . | P value . |
|---|---|---|---|---|
| HOSPITALIZATION Risk of hospitalization due to COVID-19 versus healthy controls | 26/3060 (0.8%) | 331/56 785 (0.6%) | 0.98 (0.65–1.48) | 0.915 |
| ICU ADMISSION Risk of ICU admission due to COVID-19 versus healthy controls | 3/228 (1.3%) | 26/4398 (0.6%) | 1.21 (0.34–4.25) | 0.767 |
| IN-HOSPITAL DEATH Risk of mortality due to COVID-19 versus healthy controls | 6/413 (1.5%) | 46/7408 (0.6%) | 1.33 (0.53–3.30) | 0.543 |
| PROGRESSION Risk of hospitalization among subjects with PCR+ | 26/3060 (0.8%) | 118/26 757 (0.4%) | 0.65 (0.40–1.06) | 0.086 |
| SUSCEPTIBILITY Risk of PCR+ versus not having COVID | 144/29 817 (0.5%) | 331/56 785 (0.6%) | 1.31 (1.05–1.62) | 0.015 |
| . | Cases . | Controls . | Adjusted OR CI 95% . | P value . |
|---|---|---|---|---|
| HOSPITALIZATION Risk of hospitalization due to COVID-19 versus healthy controls | 26/3060 (0.8%) | 331/56 785 (0.6%) | 0.98 (0.65–1.48) | 0.915 |
| ICU ADMISSION Risk of ICU admission due to COVID-19 versus healthy controls | 3/228 (1.3%) | 26/4398 (0.6%) | 1.21 (0.34–4.25) | 0.767 |
| IN-HOSPITAL DEATH Risk of mortality due to COVID-19 versus healthy controls | 6/413 (1.5%) | 46/7408 (0.6%) | 1.33 (0.53–3.30) | 0.543 |
| PROGRESSION Risk of hospitalization among subjects with PCR+ | 26/3060 (0.8%) | 118/26 757 (0.4%) | 0.65 (0.40–1.06) | 0.086 |
| SUSCEPTIBILITY Risk of PCR+ versus not having COVID | 144/29 817 (0.5%) | 331/56 785 (0.6%) | 1.31 (1.05–1.62) | 0.015 |
OR adjusted for sex, age and comorbidities: hypertension, diabetes, COPD, obesity, ischaemic heart disease, cerebrovascular accident, heart failure, atrial fibrillation, chronic renal failure, cancer, asthma, current smoker, current use of other pharmacological treatment (antihypertensives, diuretics, non-steroidal anti-inflammatory drugs, hypolipidaemic agents, anticoagulants, antiplatelet agents and glucocorticoids) and chronic proxy (number of different chronic treatments).
OR of hospitalization, ICU admission, mortality, susceptibility to SARS-CoV-2 and COVID-19 progression from the last 6 month exposure to dapagliflozin
| . | Cases . | Controls . | Adjusted OR CI 95% . | P value . |
|---|---|---|---|---|
| HOSPITALIZATION Risk of hospitalization due to COVID-19 versus healthy controls | 26/3060 (0.8%) | 331/56 785 (0.6%) | 0.98 (0.65–1.48) | 0.915 |
| ICU ADMISSION Risk of ICU admission due to COVID-19 versus healthy controls | 3/228 (1.3%) | 26/4398 (0.6%) | 1.21 (0.34–4.25) | 0.767 |
| IN-HOSPITAL DEATH Risk of mortality due to COVID-19 versus healthy controls | 6/413 (1.5%) | 46/7408 (0.6%) | 1.33 (0.53–3.30) | 0.543 |
| PROGRESSION Risk of hospitalization among subjects with PCR+ | 26/3060 (0.8%) | 118/26 757 (0.4%) | 0.65 (0.40–1.06) | 0.086 |
| SUSCEPTIBILITY Risk of PCR+ versus not having COVID | 144/29 817 (0.5%) | 331/56 785 (0.6%) | 1.31 (1.05–1.62) | 0.015 |
| . | Cases . | Controls . | Adjusted OR CI 95% . | P value . |
|---|---|---|---|---|
| HOSPITALIZATION Risk of hospitalization due to COVID-19 versus healthy controls | 26/3060 (0.8%) | 331/56 785 (0.6%) | 0.98 (0.65–1.48) | 0.915 |
| ICU ADMISSION Risk of ICU admission due to COVID-19 versus healthy controls | 3/228 (1.3%) | 26/4398 (0.6%) | 1.21 (0.34–4.25) | 0.767 |
| IN-HOSPITAL DEATH Risk of mortality due to COVID-19 versus healthy controls | 6/413 (1.5%) | 46/7408 (0.6%) | 1.33 (0.53–3.30) | 0.543 |
| PROGRESSION Risk of hospitalization among subjects with PCR+ | 26/3060 (0.8%) | 118/26 757 (0.4%) | 0.65 (0.40–1.06) | 0.086 |
| SUSCEPTIBILITY Risk of PCR+ versus not having COVID | 144/29 817 (0.5%) | 331/56 785 (0.6%) | 1.31 (1.05–1.62) | 0.015 |
OR adjusted for sex, age and comorbidities: hypertension, diabetes, COPD, obesity, ischaemic heart disease, cerebrovascular accident, heart failure, atrial fibrillation, chronic renal failure, cancer, asthma, current smoker, current use of other pharmacological treatment (antihypertensives, diuretics, non-steroidal anti-inflammatory drugs, hypolipidaemic agents, anticoagulants, antiplatelet agents and glucocorticoids) and chronic proxy (number of different chronic treatments).
Risk of ICU admission
The control group comprised 26 subjects without a PCR+ and 3 subjects who were admitted to the ICU with PCR+. We found no effect between dapagliflozin use and risk of ICU admission due to COVID-19 (aOR 1.21; 95% CI 0.34–4.25; P = 0.767) (see Table 3).
Mortality
As shown Table 3, we found no effect between dapagliflozin consumption and mortality (aOR 1.33; 95% CI 0.53–3.3; P = 0.543).
Progression of COVID-19
For progression of COVID-19 analysis purposes, 144 subjects taking dapagliflozin were included; 118 of them were the control group (subjects who had COVID-19 but did not require hospitalization) and 26 patients who presented with COVID-19 and were hospitalized. Dapagliflozin showed no statistically significant difference in the risk of progression to severe COVID-19 (aOR 0.65; 95% CI 0.40–1.06; P = 0.086) (see Table 3).
Risk of susceptibility
Finally, we analysed the effects of dapagliflozin on risk of contracting COVID-19. The use of dapagliflozin appeared to increase the risk of susceptibility to infection (COVID-19+) by 30% (aOR 1.31; 95% CI 1.05–1.62; P = 0.015) (see Table 3).
Results of all analyses are shown in Figure 2.
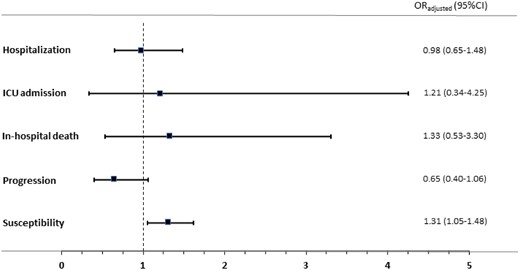
Odds ratios of the association between current use of dapagliflozin and COVID-19-related outcomes. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Discussion
In this large-scale, population-based case-control study, dapagliflozin had a neutral effect on risk of hospitalization and did not seem to influence ICU admissions or total mortality. Although the risk of progression to severe COVID-19 appeared to be reduced by 35%, it was not statistically significant. Lastly, dapagliflozin use was associated with a 30% higher risk of susceptibility to SARS-CoV-2 infection in those subjects who were taking dapagliflozin.
All in all, these results seem to indicate that dapagliflozin use is safe and due to its positive cardiovascular effect, should be maintained in patients for whom it is indicated. Further studies will be needed to assess its anti-inflammatory and antiviral effects or viral entry mechanisms in the setting of COVID-19 patients.
Outcomes
In relation to risk of hospitalization, our study is in line with a meta-analysis by Zhu et al.20 that suggests that the use of an SGLT2i before COVID-19 infection in T2D patients was associated with a lower incidence of adverse outcomes after infection. The effect was potentially linked to better blood glucose and blood pressure control, a reduction in body weight, and its metabolic effect. From our perspective, due to these potential beneficial effects on cardiovascular and renal systems, studies addressing the effect of SGLT2i agents in relation to COVID-19 are needed.
With respect to ICU admissions, to the best of our knowledge, no data have been published in this regard. Our results show no differences in the risk of being admitted to ICU. This represents an important point due to the reported ketoacidosis in people with T2D admitted to hospital with COVID-19. As a matter of fact, it is recommended to discontinue SGLT2i use in patients with COVID-19 due to the potential risk of dehydration and euglycaemic diabetic ketoacidosis.20 A multicentre cohort study from Australia showed a higher risk of ketoacidosis in SGLT2i users versus non-users, whereas in a cohort study from 40 centres in the UK, SGLT2i use demonstrated a low risk of ketoacidosis.21 Our results might support the safe use of SGLT2i in this group of patients.
Regarding mortality, dapagliflozin did not increase the risk. These results are in line with a study from Khunti et al.21 that reported that in those subjects who were prescribed an SGLT2i prior to hospital admission, dapagliflozin had a neutral effect on overall mortality. On the other hand, in a recent meta-analysis, metformin, Glucagon-like Peptide-1 Receptor Agonists and SGLT2i were associated with lower mortality rates in T2D patients with COVID-19.15 Our results support the use of dapagliflozin in this group of patients.
Regarding the progression to severe COVID-19, in 2021 a randomized, double-blind, placebo-controlled Phase 3 trial with dapagliflozin in patients with cardiometabolic risk factors hospitalized with COVID-19 (DARE-19) was published. The dapagliflozin group (n = 547 patients versus 532 controls) showed clinical status improvement, although this was not statistically significant (OR 1.09, 95% CI 0.97–1.22; P = 0.14).14 These results are in line with our study, where dapagliflozin presented a trend towards a 35% reduced risk of progression to severe COVID-19. Due to the relevance of this observation, further randomized clinical trials are needed to confirm these results.
Finally, the most relevant result from our analysis is the fact that dapagliflozin appeared to increase the risk of susceptibility to infection (COVID-19+) by 30% (aOR 1.31; 95% CI 1.05–1.62; P = 0.015). To our knowledge, this is the first time this has been reported. We find the results very relevant overall for the non-vaccinated T2D population.
Potential mechanisms
Dapagliflozin is a drug that has been shown to reduce cardiovascular and kidney events in large trials of patients with T2D, cardiovascular disease or kidney disease.4–10 Furthermore, some studies have indicated that SGLT2i drugs have a favourable effect on pathways that are dysregulated in the context of acute illness (such as COVID-19), including inflammation, oxidative stress, glycolysis, lipogenesis, endothelial function and oxygen-carrying capacity.11 The UK recently announced empagliflozin treatment in the Randomized Evaluation of COVID-19 Therapy (RECOVERY) platform trial,22 and the US NIH have added SGLT2i to the Accelerating COVID-19 Therapeutic Interventions and Vaccines 4 ACUTE (Activ4a) pragmatic trial platform, which is evaluating promising treatments in patients hospitalized with COVID-19;23 all are in hospitalized patients, a subgroup population analysed in the present study.
Our results could be due to different action mechanisms. The explanation for the reduced risk of severity, despite being statistically non-significant, could be due to some effects observed with SGLT2i, especially reno-cardiovascular protective effects. Furthermore, some anti-inflammatory activity of SGLT2i has been shown recently.11 This could explain a beneficial effect in serious COVID-19 infection by mitigating cytokine production and the inflammatory response. SGLT2i agents have also been proven to reduce some inflammatory indicators such as C-reactive protein, ferritin and IL-6. These effects can protect vital organs from the risk of severe progression and death by SARS-CoV-2 infection.11 However, we do not have a clear explanation for the increased risk of infection. A theoretical antiviral activity for SGLT2i has been recently studied in which these agents could elevate lactate levels and decrease intracellular pH, reducing the viral burden. In addition, there are some data from a South Korean cohort study that shows the benefits of SGLT2i on respiratory events regarding Dipeptidyl peptidase 4 inhibitors, which are another class of oral antidiabetic drugs.24 SGLT2i might reduce the effects of SARS-CoV-2 on chronic respiratory diseases. One mechanism of SARS-CoV-2 entry into host cells is mainly through angiotensin-converting enzyme 2 (ACE2). However, other receptors contribute to multiple organ invasion, namely KREMEN1 (Kringle-containing protein marking the eye and the nose protein 1) and ASGR1 (asialoglycoprotein receptor 1).25 The ACE2 and transmembrane serine protease 2 (TMPRSS2), and metalloproteases ADAM10 and ADAM17 that enhance the viral-ACE2 complex entry and degradation, are increased in diabetic hearts.26 The factor mainly associated with this increment of ACE2 is glycated haemoglobin more than antihypertensive treatment. There is some indication of its modulation by SGLT2i treatment because this drug normalizes glucose levels and lowers susceptibility to viral infection, as was demonstrated in diabetic patients.27 However, our study population represents patients with and without diabetes, and other mechanisms might explain the greater susceptibility to viral infection in the dapagliflozin group. Hence it is our opinion that more preclinical research, in vitro studies and randomized clinical trials are necessary in this regard.
Limitations
The conclusions of the present study need to be interpretated in view of the limitations of a non-randomized study. An important limitation of our study is that our results are adjusted for the presence or absence of pathology because we lacked data to adjust for the severity or type of pathology. To minimize this limitation, we adjusted the models for variables such as diabetes and other comorbidities, as well as for chronic treatments prescribed in the previous 6 months, which allowed us to reduce the risk of confounding. In addition, the number of different medications prescribed and dispensed for chronic diseases in the last 6 months before the index day was used as a proxy for the degree of chronicity of the patients. Moreover, in our study there is a very low risk of selection bias because all COVID-19 PCR+ patients (hospitalized and non-hospitalized) and all hospital deaths due to COVID-19 in the study area (Galicia) were included.
Importantly, it cannot be excluded that the control group with PCR−/no COVID had subclinical infection.
Conclusions
Use of dapagliflozin prior to COVID-19 infection was not associated with an increased risk of hospitalization, ICU admission, mortality or progression to severe COVID-19. However, dapagliflozin was associated with a higher risk of susceptibility to COVID-19 infection, which could be of extreme importance to the non-vaccinated T2D population, as well as the management of these types of patients in different scenarios.
Acknowledgements
We would like to thank the SERGAS General Healthcare Directorate for furnishing the data needed to conduct this study, and DXC Technology for its work in extracting the study data. Investigators received the support of the Fundación Instituto de Investigación Sanitaria de Santiago de Compostela (FIDIS); the Consortium for Biomedical Research in Epidemiology and Public Health (CIBER en Epidemiología y Salud Pública-CIBERESP), Santiago de Compostela, Spain; and the National Network for Biomedical Cardiovascular Research of Cardiovascular Disease (CIBERCV, Centro de Investigación Biomédica en Red de Enfermedades Cardiovasculares). Bonnie Dyer contributed to assistance with writing the English version.
Funding
This study was sponsored by the Carlos III Institute of Health via the ‘COV20/00470’ project (co-funded by the European Regional Development Fund, ‘A way to make Europe’). Funding for open access charge was from the Universidade de Santiago de Compostela/CISUG.
Transparency declarations
All of the authors declare no conflict of interest.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work, and have given their approval for this version to be published.
References
Author notes
Angel Salgado-Barreira and Jose Seijas-Amigo contributed equally.



