-
PDF
- Split View
-
Views
-
Cite
Cite
Adrián Martínez-Serra, Elisa De Lazzari, Leire Berrocal, Alberto Foncillas, Lorena De La Mora, Alexy Inciarte, Iván Chivite, Ana González-Cordón, María Martínez-Rebollar, Berta Torres, Montserrat Laguno, José Luis Blanco, Esteban Martínez, Josep Mallolas, Juan Ambrosioni, Clinical use and effectiveness of dolutegravir and lamivudine: a long-term, real-world, retrospective study, Journal of Antimicrobial Chemotherapy, Volume 78, Issue 8, August 2023, Pages 1955–1962, https://doi.org/10.1093/jac/dkad189
Close - Share Icon Share
Abstract
The use of dolutegravir/lamivudine is based on solid clinical trials; however, real-world data remain limited.
To provide data on the clinical use and effectiveness of dolutegravir/lamivudine in persons with HIV in a real-world scenario.
Retrospective, single-centre and observational study. We included all adults starting dolutegravir/lamivudine since November 2014. We reported all demographic, virological and immunological variables at baseline and assessed effectiveness [on treatment (OT), modified ITT (mITT) and ITT in those persons who reached 6 and 12 month follow-ups (M6 and M12).
Of the 1058 persons, 9 were treatment-naive; the final analysis included 1049 treatment-experienced people with HIV. Median (IQR) follow-up was 1 (0.3–1.6) years, with 81% and 63% persons reaching M6 and M12, respectively. The longest use of dolutegravir/lamivudine was 7.4 years. Per OT, mITT and ITT, HIV-RNA < 50 copies/mL was 97%, 92% and 81% (M6) and 98%, 90% and 80% (M12), respectively. Females [adjusted risk ratio, aRR (95% CI): 1.69 (1.19–2.40)]; immediate, previous PI-based regimen [aRR (95% CI): 1.67 (1.09–2.56)]; and viral load (VL) ≥ 50 copies/mL at dolutegravir/lamivudine initiation [aRR (95% CI): 3.36 (2.32–4.88)] were independently associated with lack of effectiveness at M12; other demographic, immunological and virological variables like previous M184V/I substitutions or virological failure, were unrelated. Of the total, 944 (90%) continued dolutegravir/lamivudine. The most frequent known reason for discontinuation was toxicity [48 (46%) cases].
In our real-world experience, virological suppression rates were high for treatment-experienced persons on dolutegravir/lamivudine; however, we identified subgroups with a higher risk of lack of effectiveness at M12, who may benefit from closer follow-ups.
Introduction
Current ART regimens recommended for both treatment-naive (TN) and treatment-experienced (TE) people with HIV include the combination of two or more drugs from the main yet different drug families [integrase strand-transfer inhibitors (InSTIs), NRTIs, NNRTIs and PIs].1–3 Given the higher efficacy and favourable safety profile compared with other families, InSTI-based regimens currently represent the preferred combinations for ART initiation and simplification.1–3 The association of dolutegravir (an InSTI) plus lamivudine (an NRTI) in treating persons with HIV has shown non-inferiority compared with three-drug regimens in clinical trials4–6 with a follow-up reaching 3 years in both TN7 and TE persons.8
Different real-world studies have also tested dolutegravir/lamivudine.9–14 Real-world studies complement information provided by clinical trials and provide a more accurate view of the clinical use and effectiveness of the different treatments prescribed. This varies far from the controlled conditions of clinical trials, in which adherence to therapy is promoted throughout the study. Moreover, given these favourable results in clinical trials, real-world use of dolutegravir/lamivudine has expanded beyond the initially approved indications. In a recent meta-analysis of real-world studies, high virological suppression was achieved and maintained at 48 weeks in many different persons with HIV, with good tolerance of dolutegravir/lamivudine, small impact on comorbidities and a low dropout rate due to side effects.15 Studies with long-term follow-up are, however, lacking.
In addition, the benefits of simplifying treatment regimens not only lie in maintaining the same efficacy with fewer drugs, which reduces the possibility of adverse effects; such a change could also result in economic savings.16 Considering the near-normal life expectancy of persons with HIV following long-term ART compared with the general population17 and the current requirement of lifelong ART, providing ART with the highest possible efficacy and the lowest possible toxicity may represent important cost reductions.
The aim of this study is to determine the real clinical use and effectiveness of dolutegravir/lamivudine in persons with HIV in a tertiary referral centre for HIV/AIDS over a long period (since 2014 to current date).
Patients and methods
Hospital Clinic is a community hospital that provides health and care services for a population of 600 000 inhabitants in the city of Barcelona (Spain). At the same time, the institution operates as a reference care facility for specific diseases, such as HIV infections for all of Catalonia (https://www.clinicbarcelona.org/en). The hospital currently provides ambulatory care, ART and hospitalization, if necessary, for more than 6000 adults with HIV. Indeed, Hospital Clinic is the largest HIV care centre in Spain. It has been also providing post-exposure prophylaxis for HIV since 2003, and pre-exposure prophylaxis (PrEP) for HIV since its approval by the Spanish National Health System in November 2019.
This was a single-centre, observational and retrospective study. The study population included all people living with HIV who had received a dolutegravir/lamivudine regimen (either separately as dolutegravir + lamivudine or co-formulated dolutegravir/lamivudine) since November 2014—initiation date of clinical use of these combined drugs—until 30 June 2022 (corresponding to closing of the dataset). Furthermore, TN individuals were very few (1%) and excluded from the study. We provide information on demographics, HIV-related characteristics and comorbidities, as well as previous ART regimens and reasons for discontinuation of the last previous ART. We performed the whole study with information collected for routine clinical work and entered in our database.
The main endpoint of this study was to determine the proportion of persons with HIV with an undetectable viral load (VL, defined as VL < 50 RNA copies/mL) at 6 and 12 month follow-ups (M6 and M12, respectively, with a 3 month window), if reached. Additional secondary objectives were to evaluate both safety of this ART regimen and suppression rates in those individuals with known resistance substitutions (such as M184V/I for lamivudine and InSTI substitutions).
We assessed effectiveness (VL < 50 RNA copies/mL) on the basis of on treatment (OT; discontinuation/missing = excluded), modified ITT (mITT; discontinuation = failure, missing = excluded) or ITT (discontinuation/missing = failure) for all subjects receiving at least one dose of dolutegravir/lamivudine.
We performed resistance testing until May 2015, using Sanger population sequencing. After that date onwards, we employed ultra-deep sequencing (UDS) using a 1% frequency threshold for variant detection. For UDS, we reported the proportion of sequences in which we detected substitutions.
Statistical analyses
Data were retrieved from the HIV Unit [Hospital Clinic Barcelona (HCB)] electronic health record systems. Summary statistics were based on frequency and percentage for qualitative variables, while mean (SD) or median (IQR) were used for quantitative characteristics. The discontinuation rate was presented as the number of events per 100 person-years and reported throughout the CI.
The association between baseline variables and detectable VL (VL ≥ 50 copies/mL) was estimated as risk ratio (RR) using either the Poisson regression model with robust standard errors or, in the case of sparse data (genotypic resistance and previous M184V/I substitution), penalized logistic regression via data augmentation. In the latter, we set the prior RR at 1. For the regression model, we performed a backward stepwise selection of variables, setting the P value for removal from the model equal to 0.1. Tests were two-tailed, and the significance level was set at 5%.
We performed statistical analyses using Stata 17 software (StataCorp LLC, College Station, TX, USA).
Ethics
The Institutional Ethics Committee approved this study (HCB.2022.012).
Results
Baseline cohort
Between 20 November 2014 and 30 June 2022, 1058 persons with HIV received at least one dose of dolutegravir/lamivudine, either separately (dolutegravir + lamivudine) or co-formulated (dolutegravir/lamivudine). There was a limited number of TN individuals (n = 9); therefore, we focused the analysis on TE persons. The baseline cohort included 1049 (18% of the total cohort of people on ART) TE persons (Figure 1).
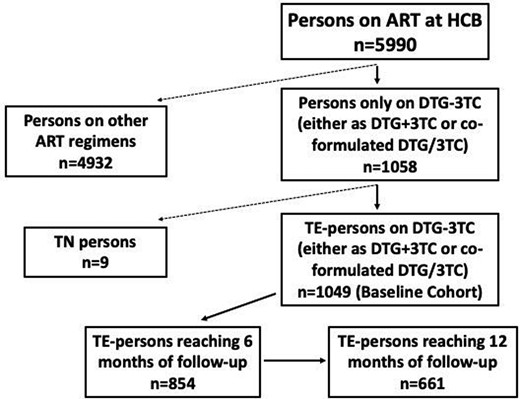
Flow chart of the persons with HIV cohort who received at least one dose of dolutegravir/lamivudine (DTG/3TC).
The median (IQR) follow-up time on dolutegravir/lamivudine was 1 (0.3–1.6) year. The median (IQR) time for VL determination after dolutegravir/lamivudine switch was 22 weeks (11–24); the median (IQR) number of VL determinations from switch to M6 was 0 (0–1) and from switch to M12 was 1 (1–2). The longer follow-up was 7.4 years in a person who started dolutegravir + lamivudine in November 2014. Afterwards, between 20 and 100 subjects started this combination per year until 2020. There was then a noteworthy increase to approximately 400 initiations per year, coinciding with the availability of co-formulated dolutegravir/lamivudine in February 2020. Figure S1 (available as Supplementary data at JAC Online) shows the number of dolutegravir/lamivudine (dolutegravir + lamivudine and dolutegravir/lamivudine) initiations per year and semester. The first person who started dolutegravir + lamivudine was a 63-year-old man who developed renal failure due to tenofovir disoproxil fumarate use and was positive for HLA-B5701, contraindicating the use of abacavir. Tenofovir alafenamide was not available at that time, and the subject started dolutegravir + lamivudine in November 2014 (more than 5 years before the publication of 48 week (48W) results of the TANGO clinical trial). The subject continues with dolutegravir + lamivudine, which was simplified to co-formulated dolutegravir/lamivudine in 2020, after it became available. At the moment of closing the dataset, he was on dolutegravir/lamivudine (7.4 years with this regimen).
Of the 1049 persons included in the baseline cohort, 85% were male and the median (IQR) age was 47 (35–59) years. Furthermore, 58% of the patients were younger than 50 years old, and 70% were infected via sexual contact among MSM. The median (IQR) years since diagnosis to dolutegravir/lamivudine initiation was 11.9 (6.7–19.5) years. Additionally, 97% had undetectable VL at dolutegravir/lamivudine initiation with a median (IQR) CD4 count of 728 (546–959) cells/mm3. Only 2% had a CD4 of less than 200 cells/mm3; 15% had positive HCV serology; 7% had estimated glomerular filtration rates by Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) at 30–59 mL/min (12 subjects, 1% were <30 mL/min), whilst the remaining were above 60 mL/min; and 14% of those who underwent a DXA scan presented osteoporosis. Baseline characteristics did not differ according to sex, although females had a slightly lower proportion of undetectable VL at dolutegravir/lamivudine initiation (95%). Table 1 shows the baseline characteristics of the entire cohort.
Baseline characteristics of 1049 persons with HIV who received at least one dose of dolutegravir/lamivudine (DTG/3TC)
| Characteristic . | N=1049 . |
|---|---|
| DTG/3TC initial formulation | |
| DTG/3TC (co-formulated), n (%) | 881 (84) |
| DTG + 3TC, n (%)a | 168 (16) |
| Sex at birth male, n (%) | 894 (85) |
| Age (years), mean (SD) [n] | 47 (12) [1049] |
| <50, n (%) | 607 (58) |
| ≥50, n (%) | 442 (42) |
| Mode of infection, n (%) | |
| MSM/bisexual | 735 (70) |
| Heterosexual intercourse | 216 (21) |
| Injection drug use | 48 (5) |
| Other/unknown | 50 (5) |
| HIV-related characteristics | |
| Years since diagnosis, median (IQR) [n] | 11.9 (6.7–19.5) [1022] |
| Years on ART previous to DTG/3TC, median (IQR) | 9.1 (4.7–15.9) |
| ART regimens previous to DTG/3TC, median (IQR) | 3 (2–5)b |
| HIV-1 RNA <50 copies/mL, n (%) | 1015 (97) |
| HIV-1 RNA 50–199 copies/mL, n (%) | 15 (1) |
| HIV-1 RNA ≥200 copies/mL | 19 (1) |
| CD4 count, cells/mm3, median (IQR) [n] | 728 (546–959) [1049] |
| CD4 count <200 cells/mm3, n(%) | 16 (2) |
| CD4 count 200–499 cells/mm3, n(%) | 193 (18) |
| CD4 count ≥500 cells/mm3, n(%) | 840 (80) |
| Comorbidities and coinfections | |
| HBsAg negative, N = 708, n (%) | 707 (100) |
| IgG HCV negative, N = 1043, n (%) | 888 (85) |
| CKD-EPI median (IQR) [n] | 87 (74–90) [1047] |
| CKD-EPI ≥90 mL/min, n (%) | 463 (44) |
| CKD-EPI 60–89 mL/min, n (%) | 501 (48) |
| CKD-EPI 30–59 mL/min, n (%) | 71 (7) |
| CKD-EPI <30 mL/min, n (%) | 12 (7) |
| DXA scan available data, n (%) | 728 (69) |
| DXA scan-evidenced osteopenia | 367 (35) |
| DXA scan-evidenced osteoporosis | 100 (10) |
| Characteristic . | N=1049 . |
|---|---|
| DTG/3TC initial formulation | |
| DTG/3TC (co-formulated), n (%) | 881 (84) |
| DTG + 3TC, n (%)a | 168 (16) |
| Sex at birth male, n (%) | 894 (85) |
| Age (years), mean (SD) [n] | 47 (12) [1049] |
| <50, n (%) | 607 (58) |
| ≥50, n (%) | 442 (42) |
| Mode of infection, n (%) | |
| MSM/bisexual | 735 (70) |
| Heterosexual intercourse | 216 (21) |
| Injection drug use | 48 (5) |
| Other/unknown | 50 (5) |
| HIV-related characteristics | |
| Years since diagnosis, median (IQR) [n] | 11.9 (6.7–19.5) [1022] |
| Years on ART previous to DTG/3TC, median (IQR) | 9.1 (4.7–15.9) |
| ART regimens previous to DTG/3TC, median (IQR) | 3 (2–5)b |
| HIV-1 RNA <50 copies/mL, n (%) | 1015 (97) |
| HIV-1 RNA 50–199 copies/mL, n (%) | 15 (1) |
| HIV-1 RNA ≥200 copies/mL | 19 (1) |
| CD4 count, cells/mm3, median (IQR) [n] | 728 (546–959) [1049] |
| CD4 count <200 cells/mm3, n(%) | 16 (2) |
| CD4 count 200–499 cells/mm3, n(%) | 193 (18) |
| CD4 count ≥500 cells/mm3, n(%) | 840 (80) |
| Comorbidities and coinfections | |
| HBsAg negative, N = 708, n (%) | 707 (100) |
| IgG HCV negative, N = 1043, n (%) | 888 (85) |
| CKD-EPI median (IQR) [n] | 87 (74–90) [1047] |
| CKD-EPI ≥90 mL/min, n (%) | 463 (44) |
| CKD-EPI 60–89 mL/min, n (%) | 501 (48) |
| CKD-EPI 30–59 mL/min, n (%) | 71 (7) |
| CKD-EPI <30 mL/min, n (%) | 12 (7) |
| DXA scan available data, n (%) | 728 (69) |
| DXA scan-evidenced osteopenia | 367 (35) |
| DXA scan-evidenced osteoporosis | 100 (10) |
One hundred and five (63%) individuals switched to co-formulated DTG/3TC when it became available, and on 30 June 2022 (when database was locked) 10 patients were receiving 3TC + DTG and 53 had discontinued it.
There were 38 events of virological failure (VF) in 32 persons (27 persons had a single VF, 4 persons had two previous VFs and 1 person had 3).
Baseline characteristics of 1049 persons with HIV who received at least one dose of dolutegravir/lamivudine (DTG/3TC)
| Characteristic . | N=1049 . |
|---|---|
| DTG/3TC initial formulation | |
| DTG/3TC (co-formulated), n (%) | 881 (84) |
| DTG + 3TC, n (%)a | 168 (16) |
| Sex at birth male, n (%) | 894 (85) |
| Age (years), mean (SD) [n] | 47 (12) [1049] |
| <50, n (%) | 607 (58) |
| ≥50, n (%) | 442 (42) |
| Mode of infection, n (%) | |
| MSM/bisexual | 735 (70) |
| Heterosexual intercourse | 216 (21) |
| Injection drug use | 48 (5) |
| Other/unknown | 50 (5) |
| HIV-related characteristics | |
| Years since diagnosis, median (IQR) [n] | 11.9 (6.7–19.5) [1022] |
| Years on ART previous to DTG/3TC, median (IQR) | 9.1 (4.7–15.9) |
| ART regimens previous to DTG/3TC, median (IQR) | 3 (2–5)b |
| HIV-1 RNA <50 copies/mL, n (%) | 1015 (97) |
| HIV-1 RNA 50–199 copies/mL, n (%) | 15 (1) |
| HIV-1 RNA ≥200 copies/mL | 19 (1) |
| CD4 count, cells/mm3, median (IQR) [n] | 728 (546–959) [1049] |
| CD4 count <200 cells/mm3, n(%) | 16 (2) |
| CD4 count 200–499 cells/mm3, n(%) | 193 (18) |
| CD4 count ≥500 cells/mm3, n(%) | 840 (80) |
| Comorbidities and coinfections | |
| HBsAg negative, N = 708, n (%) | 707 (100) |
| IgG HCV negative, N = 1043, n (%) | 888 (85) |
| CKD-EPI median (IQR) [n] | 87 (74–90) [1047] |
| CKD-EPI ≥90 mL/min, n (%) | 463 (44) |
| CKD-EPI 60–89 mL/min, n (%) | 501 (48) |
| CKD-EPI 30–59 mL/min, n (%) | 71 (7) |
| CKD-EPI <30 mL/min, n (%) | 12 (7) |
| DXA scan available data, n (%) | 728 (69) |
| DXA scan-evidenced osteopenia | 367 (35) |
| DXA scan-evidenced osteoporosis | 100 (10) |
| Characteristic . | N=1049 . |
|---|---|
| DTG/3TC initial formulation | |
| DTG/3TC (co-formulated), n (%) | 881 (84) |
| DTG + 3TC, n (%)a | 168 (16) |
| Sex at birth male, n (%) | 894 (85) |
| Age (years), mean (SD) [n] | 47 (12) [1049] |
| <50, n (%) | 607 (58) |
| ≥50, n (%) | 442 (42) |
| Mode of infection, n (%) | |
| MSM/bisexual | 735 (70) |
| Heterosexual intercourse | 216 (21) |
| Injection drug use | 48 (5) |
| Other/unknown | 50 (5) |
| HIV-related characteristics | |
| Years since diagnosis, median (IQR) [n] | 11.9 (6.7–19.5) [1022] |
| Years on ART previous to DTG/3TC, median (IQR) | 9.1 (4.7–15.9) |
| ART regimens previous to DTG/3TC, median (IQR) | 3 (2–5)b |
| HIV-1 RNA <50 copies/mL, n (%) | 1015 (97) |
| HIV-1 RNA 50–199 copies/mL, n (%) | 15 (1) |
| HIV-1 RNA ≥200 copies/mL | 19 (1) |
| CD4 count, cells/mm3, median (IQR) [n] | 728 (546–959) [1049] |
| CD4 count <200 cells/mm3, n(%) | 16 (2) |
| CD4 count 200–499 cells/mm3, n(%) | 193 (18) |
| CD4 count ≥500 cells/mm3, n(%) | 840 (80) |
| Comorbidities and coinfections | |
| HBsAg negative, N = 708, n (%) | 707 (100) |
| IgG HCV negative, N = 1043, n (%) | 888 (85) |
| CKD-EPI median (IQR) [n] | 87 (74–90) [1047] |
| CKD-EPI ≥90 mL/min, n (%) | 463 (44) |
| CKD-EPI 60–89 mL/min, n (%) | 501 (48) |
| CKD-EPI 30–59 mL/min, n (%) | 71 (7) |
| CKD-EPI <30 mL/min, n (%) | 12 (7) |
| DXA scan available data, n (%) | 728 (69) |
| DXA scan-evidenced osteopenia | 367 (35) |
| DXA scan-evidenced osteoporosis | 100 (10) |
One hundred and five (63%) individuals switched to co-formulated DTG/3TC when it became available, and on 30 June 2022 (when database was locked) 10 patients were receiving 3TC + DTG and 53 had discontinued it.
There were 38 events of virological failure (VF) in 32 persons (27 persons had a single VF, 4 persons had two previous VFs and 1 person had 3).
The reasons for discontinuation of the last previous ART and subsequent dolutegravir/lamivudine prescription were known in 945 (90%) cases, with the most common reason being simplification (72%) followed by toxicity (9%). Table 2 details the reasons for discontinuation of the last previous ART. Data about previous ART regimens were available in 968 (92%) cases; the median (IQR) number of previous ART regimens before dolutegravir/lamivudine initiation was 3 (2–5). Most cases started dolutegravir/lamivudine after a last regimen based on InSTI triple therapy (69%), and the single most frequent previous regimen was co-formulated dolutegravir/lamivudine/abacavir (n = 485). However, 50% of cases (n = 484) came from other triple-, double- or single-drug ART regimens. Table 3 reports data about the last previous ART regimen.
Reasons for discontinuation of last previous ART regimen in 945 persons with HIV starting dolutegravir/lamivudine (DTG/3TC)
| Reasons for discontinuation . | n (%) . |
|---|---|
| Simplification | 679 (72) |
| Toxicity | 86 (9) |
| Avoidance of drug–drug interactions | 29 (3) |
| Patient’s preference | 9 (1) |
| ART discontinuation without medical indication | 6 (1) |
| Other causes | 136 (14)a |
| Reasons for discontinuation . | n (%) . |
|---|---|
| Simplification | 679 (72) |
| Toxicity | 86 (9) |
| Avoidance of drug–drug interactions | 29 (3) |
| Patient’s preference | 9 (1) |
| ART discontinuation without medical indication | 6 (1) |
| Other causes | 136 (14)a |
Including 50 cases included in the open-label DOLAM trial (EudraCT 201500027435).
Reasons for discontinuation of last previous ART regimen in 945 persons with HIV starting dolutegravir/lamivudine (DTG/3TC)
| Reasons for discontinuation . | n (%) . |
|---|---|
| Simplification | 679 (72) |
| Toxicity | 86 (9) |
| Avoidance of drug–drug interactions | 29 (3) |
| Patient’s preference | 9 (1) |
| ART discontinuation without medical indication | 6 (1) |
| Other causes | 136 (14)a |
| Reasons for discontinuation . | n (%) . |
|---|---|
| Simplification | 679 (72) |
| Toxicity | 86 (9) |
| Avoidance of drug–drug interactions | 29 (3) |
| Patient’s preference | 9 (1) |
| ART discontinuation without medical indication | 6 (1) |
| Other causes | 136 (14)a |
Including 50 cases included in the open-label DOLAM trial (EudraCT 201500027435).
| Previous ART . | . |
|---|---|
| Triple ART, n (%) | 844 (87) |
| InSTI-based, n (%) | 669 (69) |
| Dolutegravir-based, n | 513a |
| Elvitegravir/cobicistat-based, n | 83 |
| Raltegravir-based, n | 47 |
| Bictegravir-based, n | 26 |
| NNRTI-based, n (%) | 137 (14) |
| Rilpivirine-based, n | 45 |
| Efavirenz-based, n | 44 |
| Nevirapine-based, n | 40 |
| Etravirine-based, n | 6 |
| Doravirine-based, n | 2 |
| PI-based, n (%) | 38 (4%) |
| Darunavir-based, n | 19 |
| Atazanavir-based, n | 18 |
| Lopinavir-based, n | 1 |
| Other triple combinations, n (%) | 2 (0) |
| Double ART, n (%) | 75 (8) |
| INSTI-basedb | 40 (4) |
| PI-basedc | 30 (3) |
| PI + InSTI | 5 (1) |
| Other ART, n (%) | 49 (5) |
| PI monotherapy | 27 (3) |
| InSTI monotherapy | 19 (2) |
| Other combinations | 3 (0) |
| Previous ART . | . |
|---|---|
| Triple ART, n (%) | 844 (87) |
| InSTI-based, n (%) | 669 (69) |
| Dolutegravir-based, n | 513a |
| Elvitegravir/cobicistat-based, n | 83 |
| Raltegravir-based, n | 47 |
| Bictegravir-based, n | 26 |
| NNRTI-based, n (%) | 137 (14) |
| Rilpivirine-based, n | 45 |
| Efavirenz-based, n | 44 |
| Nevirapine-based, n | 40 |
| Etravirine-based, n | 6 |
| Doravirine-based, n | 2 |
| PI-based, n (%) | 38 (4%) |
| Darunavir-based, n | 19 |
| Atazanavir-based, n | 18 |
| Lopinavir-based, n | 1 |
| Other triple combinations, n (%) | 2 (0) |
| Double ART, n (%) | 75 (8) |
| INSTI-basedb | 40 (4) |
| PI-basedc | 30 (3) |
| PI + InSTI | 5 (1) |
| Other ART, n (%) | 49 (5) |
| PI monotherapy | 27 (3) |
| InSTI monotherapy | 19 (2) |
| Other combinations | 3 (0) |
Four hundred and eighty-five cases corresponded to co-formulated dolutegravir/lamivudine/abacavir.
Including 29 cases of raltegravir/lamivudine and 1 case of dolutegravir/tenofovir disoproxil fumarate.
Including 19 cases of boosted darunavir/lamivudine and 11 cases of other boosted PIs/lamivudine.
| Previous ART . | . |
|---|---|
| Triple ART, n (%) | 844 (87) |
| InSTI-based, n (%) | 669 (69) |
| Dolutegravir-based, n | 513a |
| Elvitegravir/cobicistat-based, n | 83 |
| Raltegravir-based, n | 47 |
| Bictegravir-based, n | 26 |
| NNRTI-based, n (%) | 137 (14) |
| Rilpivirine-based, n | 45 |
| Efavirenz-based, n | 44 |
| Nevirapine-based, n | 40 |
| Etravirine-based, n | 6 |
| Doravirine-based, n | 2 |
| PI-based, n (%) | 38 (4%) |
| Darunavir-based, n | 19 |
| Atazanavir-based, n | 18 |
| Lopinavir-based, n | 1 |
| Other triple combinations, n (%) | 2 (0) |
| Double ART, n (%) | 75 (8) |
| INSTI-basedb | 40 (4) |
| PI-basedc | 30 (3) |
| PI + InSTI | 5 (1) |
| Other ART, n (%) | 49 (5) |
| PI monotherapy | 27 (3) |
| InSTI monotherapy | 19 (2) |
| Other combinations | 3 (0) |
| Previous ART . | . |
|---|---|
| Triple ART, n (%) | 844 (87) |
| InSTI-based, n (%) | 669 (69) |
| Dolutegravir-based, n | 513a |
| Elvitegravir/cobicistat-based, n | 83 |
| Raltegravir-based, n | 47 |
| Bictegravir-based, n | 26 |
| NNRTI-based, n (%) | 137 (14) |
| Rilpivirine-based, n | 45 |
| Efavirenz-based, n | 44 |
| Nevirapine-based, n | 40 |
| Etravirine-based, n | 6 |
| Doravirine-based, n | 2 |
| PI-based, n (%) | 38 (4%) |
| Darunavir-based, n | 19 |
| Atazanavir-based, n | 18 |
| Lopinavir-based, n | 1 |
| Other triple combinations, n (%) | 2 (0) |
| Double ART, n (%) | 75 (8) |
| INSTI-basedb | 40 (4) |
| PI-basedc | 30 (3) |
| PI + InSTI | 5 (1) |
| Other ART, n (%) | 49 (5) |
| PI monotherapy | 27 (3) |
| InSTI monotherapy | 19 (2) |
| Other combinations | 3 (0) |
Four hundred and eighty-five cases corresponded to co-formulated dolutegravir/lamivudine/abacavir.
Including 29 cases of raltegravir/lamivudine and 1 case of dolutegravir/tenofovir disoproxil fumarate.
Including 19 cases of boosted darunavir/lamivudine and 11 cases of other boosted PIs/lamivudine.
Follow-up of cohort at M6 and M12
Figure 1 shows the flow chart and distribution of the cohorts. Of the 1049 persons included in the TE baseline cohort, 854 and 661 reached M6 and M12 of follow-up, respectively. At M6, there were 40 discontinuations and 98 cases with missing data; at M12 there were 47 (39 of them with detectable VL) and 72, respectively. A hundred and ninety-five subjects (19%) started a dolutegravir/lamivudine regimen shortly before the database lock and had less than 6 months of follow-up (Figure 1). The baseline cohort of 1049 subjects provided a total of 1305.9 person-years of follow-up. Only 105 discontinued dolutegravir/lamivudine throughout the follow-up period, with the rate of discontinuation being 8.0 discontinuations per 100 person-years (95% CI 6.6–9.7). The most common reason for discontinuation was toxicity, in 46% of cases; of these, 44% were neuropsychiatric side effects, 23% gastrointestinal, 12.5% weight gain, 20% other types and 17% experienced more than one type of toxicity. Other discontinuation reasons included transfer to another institution (19%), death (10%, all unrelated to dolutegravir/lamivudine), personal preference (8%), simplification and loss of efficacy (4% each), to avoid drug–drug interactions (3%) and pregnancy (1%); in 5% of cases, the reason for discontinuation was unknown. Rates of dolutegravir/lamivudine discontinuation did not differ between sexes, although there was a trend of higher discontinuation among females (17% versus 10%, P = 0.07).
Suppression rates at M6 and M12
Suppression rates were reported according to the three predefined definitions, as shown in Figure 2. Suppression rates were remarkably high at both M6 and M12 (97% and 98% respectively, OT). Suppression rates remained similar after we removed DOLAM trial participants (97% and 97% OT at M6 and M12, respectively).
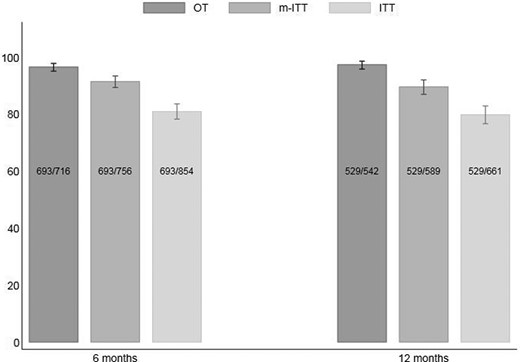
Suppression rates (effectiveness) of dolutegravir/lamivudine at M6 and M12.
Resistance analysis and suppression rates in patients carrying antiretroviral (ARV)-resistant substitutions
At least one set of genotypic resistance test (GRT) data was available from 551 cases. We found at least one relevant substitution in 292 (53%) cases in the cumulative resistance profile. Of these persons, 29 (10%) had either M184V or M184I substitution. We detected M184V/I as the only substitution in one case; in the other 28, there were substitutions accompanying M184V/I in RT (n = 28; 97%), protease (n = 18; 62%) and integrase (n = 3; 10%) (Table S1). In all but two recent cases, M184V was either detected in populational genotypes or in UDS at >20% of sequences. The median time that had elapsed between M184V/I detection and initiation of dolutegravir/lamivudine was 13.7 (IQR 7.7–17.1) years. The VL at dolutegravir/lamivudine initiation was undetectable in 24 (82%) persons carrying the M184V/I substitution. Due to a short follow-up since dolutegravir/lamivudine initiation in most cases, VL was available in only 11 cases at M6 (8 with VL < 50 copies/mL) and in 7 cases at M12 (6 with VL < 50 copies/mL).
Prognostic factors of lack of effectiveness
As detailed in Table 4 of the unadjusted models, factors associated with an increased risk of lack of effectiveness at M12 were: female (at birth) subjects; years since HIV diagnosis; previous virological failure; number of previous ART regimens; and a previous PI-based regimen. In the adjusted model, female sex [adjusted RR, aRR = 1.69 (95% CI = 1.19–2.40)], those with the last previous PI-based regimen use [aRR = 1.67 (95% CI = 1.09–2.56)] and those starting dolutegravir/lamivudine with VL > 50 copies/mL [aRR = 3.36 (95% CI = 2.32–4.88)] were at a higher risk of lack of effectiveness at M12. For all other demographic and virological variables (including no GRT before switching to dolutegravir/lamivudine or the presence of M184V/I substitution in historical GRT), there was no evidence of a statistical association with lack of effectiveness. The results from the model did not change (the same variables were identified) after we removed DOLAM participants.
Analysis of variables associated with lack of effectiveness at M12 (ITT) (n = 597)a.
| Variable . | uRR . | (95% CI) . | P value . | aRR . | (95% CI) . | P value . |
|---|---|---|---|---|---|---|
| Female sex at birth (versus male) | 1.77 | (1.24–2.52) | <0.01 | 1.69 | (1.19–2.40) | 0.0033 |
| Age (per 5 year increase) | 1.02 | (0.96–1.09) | 0.51 | |||
| MSM or bisexual (versus other) | 0.63 | (0.45–0.87) | <0.01 | |||
| Years since HIV diagnosis (per 5 year increase) | 1.14 | (1.04–1.25) | <0.01 | |||
| PHI patient (versus chronic) | 0.97 | (0.59–1.60) | 0.89 | |||
| Previous VF (versus no) | 2.34 | (1.36–4.03) | <0.01 | |||
| Number of previous ART (per 1 treatment increase) | 1.07 | (1.04–1.11) | < 0.01 | |||
| Previous last regimen InSTI-based (versus no) | 0.83 | (0.58–1.19) | 0.32 | |||
| Previous last regimen PI-based (versus no) | 1.67 | (1.10–2.55) | 0.02 | 1.67 | (1.09–2.56) | 0.0183 |
| Previous last regimen NNRTI-based (versus no) | 0.94 | (0.59–1.49) | 0.78 | |||
| CD4 count at DTG/3TC start (≥500 versus <500 cells/mm3) | 0.75 | (0.52–1.07) | 0.11 | |||
| CD4:CD8 ratio at DTG/3TC start (≥1 versus <1) | 1.09 | (0.79–1.51) | 0.59 | |||
| VL at DTG/3TC start (≥50 versus <50 copies/mL) | 3.48 | (2.44–4.96) | < 0.01 | 3.36 | (2.32–4.88) | < 0.0001 |
| Previous GRT at DTG/3TC start (done versus not done) | 1.26 | (0.90–1.76) | 0.18 |
| Variable . | uRR . | (95% CI) . | P value . | aRR . | (95% CI) . | P value . |
|---|---|---|---|---|---|---|
| Female sex at birth (versus male) | 1.77 | (1.24–2.52) | <0.01 | 1.69 | (1.19–2.40) | 0.0033 |
| Age (per 5 year increase) | 1.02 | (0.96–1.09) | 0.51 | |||
| MSM or bisexual (versus other) | 0.63 | (0.45–0.87) | <0.01 | |||
| Years since HIV diagnosis (per 5 year increase) | 1.14 | (1.04–1.25) | <0.01 | |||
| PHI patient (versus chronic) | 0.97 | (0.59–1.60) | 0.89 | |||
| Previous VF (versus no) | 2.34 | (1.36–4.03) | <0.01 | |||
| Number of previous ART (per 1 treatment increase) | 1.07 | (1.04–1.11) | < 0.01 | |||
| Previous last regimen InSTI-based (versus no) | 0.83 | (0.58–1.19) | 0.32 | |||
| Previous last regimen PI-based (versus no) | 1.67 | (1.10–2.55) | 0.02 | 1.67 | (1.09–2.56) | 0.0183 |
| Previous last regimen NNRTI-based (versus no) | 0.94 | (0.59–1.49) | 0.78 | |||
| CD4 count at DTG/3TC start (≥500 versus <500 cells/mm3) | 0.75 | (0.52–1.07) | 0.11 | |||
| CD4:CD8 ratio at DTG/3TC start (≥1 versus <1) | 1.09 | (0.79–1.51) | 0.59 | |||
| VL at DTG/3TC start (≥50 versus <50 copies/mL) | 3.48 | (2.44–4.96) | < 0.01 | 3.36 | (2.32–4.88) | < 0.0001 |
| Previous GRT at DTG/3TC start (done versus not done) | 1.26 | (0.90–1.76) | 0.18 |
Poisson regression model with robust standard errors. DTG, dolutegravir; 3TC, lamivudine; uRR, unadjusted RR; PHI, primary HIV infection; VF, virological failure.
Cases where any of the covariables analysed had missing values were excluded.
Analysis of variables associated with lack of effectiveness at M12 (ITT) (n = 597)a.
| Variable . | uRR . | (95% CI) . | P value . | aRR . | (95% CI) . | P value . |
|---|---|---|---|---|---|---|
| Female sex at birth (versus male) | 1.77 | (1.24–2.52) | <0.01 | 1.69 | (1.19–2.40) | 0.0033 |
| Age (per 5 year increase) | 1.02 | (0.96–1.09) | 0.51 | |||
| MSM or bisexual (versus other) | 0.63 | (0.45–0.87) | <0.01 | |||
| Years since HIV diagnosis (per 5 year increase) | 1.14 | (1.04–1.25) | <0.01 | |||
| PHI patient (versus chronic) | 0.97 | (0.59–1.60) | 0.89 | |||
| Previous VF (versus no) | 2.34 | (1.36–4.03) | <0.01 | |||
| Number of previous ART (per 1 treatment increase) | 1.07 | (1.04–1.11) | < 0.01 | |||
| Previous last regimen InSTI-based (versus no) | 0.83 | (0.58–1.19) | 0.32 | |||
| Previous last regimen PI-based (versus no) | 1.67 | (1.10–2.55) | 0.02 | 1.67 | (1.09–2.56) | 0.0183 |
| Previous last regimen NNRTI-based (versus no) | 0.94 | (0.59–1.49) | 0.78 | |||
| CD4 count at DTG/3TC start (≥500 versus <500 cells/mm3) | 0.75 | (0.52–1.07) | 0.11 | |||
| CD4:CD8 ratio at DTG/3TC start (≥1 versus <1) | 1.09 | (0.79–1.51) | 0.59 | |||
| VL at DTG/3TC start (≥50 versus <50 copies/mL) | 3.48 | (2.44–4.96) | < 0.01 | 3.36 | (2.32–4.88) | < 0.0001 |
| Previous GRT at DTG/3TC start (done versus not done) | 1.26 | (0.90–1.76) | 0.18 |
| Variable . | uRR . | (95% CI) . | P value . | aRR . | (95% CI) . | P value . |
|---|---|---|---|---|---|---|
| Female sex at birth (versus male) | 1.77 | (1.24–2.52) | <0.01 | 1.69 | (1.19–2.40) | 0.0033 |
| Age (per 5 year increase) | 1.02 | (0.96–1.09) | 0.51 | |||
| MSM or bisexual (versus other) | 0.63 | (0.45–0.87) | <0.01 | |||
| Years since HIV diagnosis (per 5 year increase) | 1.14 | (1.04–1.25) | <0.01 | |||
| PHI patient (versus chronic) | 0.97 | (0.59–1.60) | 0.89 | |||
| Previous VF (versus no) | 2.34 | (1.36–4.03) | <0.01 | |||
| Number of previous ART (per 1 treatment increase) | 1.07 | (1.04–1.11) | < 0.01 | |||
| Previous last regimen InSTI-based (versus no) | 0.83 | (0.58–1.19) | 0.32 | |||
| Previous last regimen PI-based (versus no) | 1.67 | (1.10–2.55) | 0.02 | 1.67 | (1.09–2.56) | 0.0183 |
| Previous last regimen NNRTI-based (versus no) | 0.94 | (0.59–1.49) | 0.78 | |||
| CD4 count at DTG/3TC start (≥500 versus <500 cells/mm3) | 0.75 | (0.52–1.07) | 0.11 | |||
| CD4:CD8 ratio at DTG/3TC start (≥1 versus <1) | 1.09 | (0.79–1.51) | 0.59 | |||
| VL at DTG/3TC start (≥50 versus <50 copies/mL) | 3.48 | (2.44–4.96) | < 0.01 | 3.36 | (2.32–4.88) | < 0.0001 |
| Previous GRT at DTG/3TC start (done versus not done) | 1.26 | (0.90–1.76) | 0.18 |
Poisson regression model with robust standard errors. DTG, dolutegravir; 3TC, lamivudine; uRR, unadjusted RR; PHI, primary HIV infection; VF, virological failure.
Cases where any of the covariables analysed had missing values were excluded.
Discussion
Based on randomized controlled trial (RCT) results, dolutegravir/lamivudine is a preferred regimen for TN and TE persons, with other studies8,12,18–20 also strengthening real-world data on its use in both settings.9,10,13–15,21,22 Our study provides additional and robust data to support the effectiveness of dolutegravir/lamivudine, with virological suppression rates reaching 97% and 98% at M6 and M12 (OT), respectively.
Our cohort is representative of the current HIV epidemiology in Western/Central Europe, consisting of mainly MSM, median/older age (42% patients were older than 50 years) and a very low prevalence of injecting drug users (5%). Given the low number of TN persons, the entire cohort analysed here is TE. This low number of TN is explainable for several reasons. First, both new diagnoses and infections have been decreasing in the last few years, given the expansion of ART and PrEP. Second, most TN individuals receive priority for clinical trials, especially in most tertiary reference centres. Third, most of the included study subjects started dolutegravir/lamivudine during the COVID-19 pandemic, and other combinations recommended for rapid ART initiation (active against HBV and with no need to wait for laboratory results) were given priority in these periods.23 Moreover, the PrEP scope has largely expanded in our setting; individuals previously engaged in PrEP or in contact with PrEP settings may have a higher prevalence of M184V substitution,24 a situation where dolutegravir/lamivudine is currently not recommended. Finally, clinicians’ perception of the excellent results obtained with simplification using dolutegravir/lamivudine in several clinical scenarios may lead to much more frequent use of this line of therapy in this situation. Our cohort presented a good immunological status (80% had >500 CD4/cells/mm3) and had a median of three previous ART regimens over a median course of 11.9 years since diagnosis.
The longest follow-up was 7.4 years. The first person who started dolutegravir + lamivudine was a 63-year-old man on a tenofovir disoproxil fumarate-based regimen (tenofovir disoproxil fumarate/lamivudine/rilpivirine) who developed renal failure in 2014, and was positive for HLA-B5701. This subject began taking dolutegravir/lamivudine in November 2014 (more than 5 years before the publication of the 48W results of the TANGO clinical trial). The early use of this combination highlights the appealing profile of dolutegravir/lamivudine among our centre’s clinicians, even before the publication of results from pivotal switch trials (such as 48W in the TANGO trial).25 However, as expected, the number of individuals receiving dolutegravir/lamivudine largely increased in recent times, following both the TANGO RCT 48W results and availability of co-formulation.
The main reason for dolutegravir/lamivudine discontinuation in our study was toxicity; however, given that only 5% of our patients had to change their ART for this particular reason, we can assume this regimen’s overall safety.
Our real-world data also show the clinical use of the dolutegravir/lamivudine regimen in situations beyond those approved by regulatory agencies, such as use in individuals with known resistance substitutions or a non-undetectable status at the moment of switch. Even though we detected the M184V/I mutation in 29 persons, the statistical analysis proved that it was not independently associated with lower odds of virological suppression. These findings are in line with recent studies that found that 3 years after the switch to dolutegravir/lamivudine in patients carrying the M184V/I mutation, the probability of virological failure and blips was very low (6.9%).26 This was particularly so if the person’s VL remained undetectable for long periods since the substitution was identified.27
However, several points need to be addressed for these multivariable analysis findings. The median time elapsed for dolutegravir/lamivudine initiation following M184V/I was extremely long (13.7 years). This highlights the decreased relevance given by clinicians when substitutions were detected in old samples and individuals’ VL remained undetectable for long periods. However, there were a few cases reaching M6 and M12 of follow-up. That stated, statistical power was very low to conclude that M184V/I was unrelated to lack of effectiveness at M12, and these results should be interpreted with caution to avoid type II statistical error. Finally, all but one case had other substitutions detected in historical GRT, indicating a long history of exposure to ART. Indeed, previous PI use was associated with lack of effectiveness in the adjusted model—perhaps illustrating this fact—with higher potency than the history of M184V/I substitution itself. Females and a detectable VL at dolutegravir/lamivudine initiation were also factors associated with lack of effectiveness at M12. Females are frequently reported as having lower virological success compared with males, especially the MSM population. This may be related to different barriers for adherence to follow-up and ART,28,29 and not necessarily regimen-specific. Finally, detectable VL at dolutegravir/lamivudine switch (a non-approved indication) was also an independent factor for lack of effectiveness at M12. This may highlight the importance of switching to this regimen only in virologically suppressed persons; it may also indicate a lower adherence, irrespective of the ART combination used. These results may suggest that these subgroups of people could benefit from closer follow-up and a reinforcement of adherence when switching to dolutegravir/lamivudine.
Virological suppression was assessed through different methods (OT, ITT and mITT), showing overall high suppression rates. Considering this is a real-world study, OT analysis is probably the best approach in defining effectiveness since the data provided better reflect the everyday clinical reality—that is, it is common that patients are lost to follow-up. However, the proportion of individuals without virological data on window was not particularly high in our study.
We recently reported our cohort of people with HIV on bictegravir/tenofovir alafenamide/emtricitabine (BIC/TAF/FTC).30 Some interesting differences with the cohort of those receiving dolutegravir/lamivudine, which also shows the perception of regimens by clinicians, should be mentioned. In the BIC/TAF/FTC study, only 82% of those included (in the switch group) had undetectable VL at BIC/TAF/FTC initiation, compared with 97% starting dolutegravir/lamivudine. Although both combinations are recommended for switch strategies, this varying proportion highlights a different use of such regimens: BIC/TAF/FTC is reserved for those perceived as more difficult to treat, less adherent to therapy, or even in need of viraemia resuppression or salvage regimen. In contrast, dolutegravir/lamivudine seems to be used more frequently for those perceived as highly adherent, where an improvement of the combination is desired in terms of long-term safety, without losing virological efficacy.
Our paper has several strengths. It represents a large cohort from a single centre with a typical, current people with HIV population. The number of study subjects is comparable to that of multicentre cohorts. It also provides evidence of this regimen’s efficacy, supported by multiple analyses. The present wide cohort also allows for a relevant number of patients carrying mutations to be represented; moreover, the follow-up period for the first cases was extremely large (compared with previously published real-world studies). However, the paper is not exempt from some major limitations, such as the absence of data in our database regarding some relevant comorbidities (e.g. cardiovascular risk). Due to the ageing of the population of persons with HIV, such data are becoming increasingly relevant. For other comorbidities like bone disease, data were only available for a percentage of the patients. This type of information would have proven interesting in illustrating the profile of persons who were prescribed dolutegravir/lamivudine in our cohort. It also provides data only from a single hospital. Although the centre is the reference unit for the area, it may not represent some characteristics of persons with HIV residing in other regions. As this was a real-world study, a non-depreciable number of persons had missing data. Finally, many cases started this regimen recently, including those carrying ARV-resistant substitutions, so the cohort experienced significant attrition at M6 and M12.
In conclusion, in our cohort of persons with HIV, a significant proportion started dolutegravir/lamivudine as a switch strategy. It was exponentially used in recent years, although initially prescribed in selected individuals and a small subset as part of an open clinical trial. Effectiveness (OT) was remarkably high and with low levels of discontinuation. Individuals with immediate previous PI use, females and those starting dolutegravir/lamivudine with detectable VL more frequently had a lack of virological effectiveness on dolutegravir/lamivudine at M12 since initiation. They may benefit from close follow-up after simplification.
Acknowledgements
This study was part of the MD degree project of A.M.-S. and PhD degree project of E.d.L., Faculty of Medicine, University of Barcelona.
We would like to thank all of the participants, as well as Anthony Armenta for providing editing assistance with respect to language, syntax and style.
Funding
The project was done with internal funding from the HIV Unit, Hospital Clinic-IDIBAPS.
Transparency declarations
J.A. has received research funding from ViiV and Gilead, has received personal fees from ViiV, Gilead, Janssen and MSD, has participated in Advisory Boards for ViiV, Gilead, Janssen and MSD, has participated in Data Safety Monitoring Boards for HIPRA and Grifols, all these activities outside of the current work. For all authors, no conflict of interest to declare related to this work.
Supplementary data
Table S1 and Figure S1 are available as Supplementary data at JAC Online.
References
Author notes
Contributed equally as first authors.
Contributed equally as last authors.



