-
PDF
- Split View
-
Views
-
Cite
Cite
Pascale Perfezou, Nolwenn Hall, Jean-Charles Duthe, Basma Abdi, Sophie Seang, Cédric Arvieux, Isabelle Lamaury, Amélie Menard, Anne-Geneviève Marcelin, Christine Katlama, Romain Palich, the Dat’AIDS study group , Doravirine plus lamivudine two-drug regimen as maintenance antiretroviral therapy in people living with HIV: a French observational study, Journal of Antimicrobial Chemotherapy, Volume 78, Issue 8, August 2023, Pages 1929–1933, https://doi.org/10.1093/jac/dkad185
Close - Share Icon Share
Abstract
Two-drug regimens based on integrase strand transfer inhibitors (INSTIs) and boosted PIs have entered recommended ART. However, INSTIs and boosted PIs may not be suitable for all patients. We aimed to report our experience with doravirine/lamivudine as maintenance therapy in people living with HIV (PLWH) followed in French HIV settings.
This observational study enrolled all adults who initiated doravirine/lamivudine between 1 September 2019 and 31 October 2021, in French HIV centres participating in the Dat’AIDS cohort. The primary outcome was the rate of virological success (plasma HIV-RNA < 50 copies/mL) at Week (W)48. Secondary outcomes included: rate of treatment discontinuation for non-virological reasons, evolution of CD4 count and CD4/CD8 ratio over follow-up.
Fifty patients were included, with 34 (68%) men; median age: 58 years (IQR 51–62), ART duration: 20 years (13–23), duration of virological suppression: 14 years (8–19), CD4 count: 784 cells/mm3 (636–889). Prior to switching, all had plasma HIV-RNA < 50 copies/mL. All but three were naive to doravirine, and 36 (72%) came from a three-drug regimen. Median follow-up was 79 weeks (IQR 60–96). Virological success rate at W48 was 98.0% (95% CI 89.4–99.9). One virological failure occurred at W18 (HIV-RNA = 101 copies/mL) in a patient who briefly discontinued doravirine/lamivudine due to intense nightmares; there was no resistance at baseline and no resistance emergence. There were three strategy discontinuations for adverse events (digestive disorders: n = 2; insomnia: n = 1). There was no significant change in CD4/CD8 ratio, while CD4 T cell count significantly increased.
These preliminary findings suggest that doravirine/lamivudine regimens can maintain high levels of viral suppression in highly ART-experienced PLWH with long-term viral suppression, and good CD4+ T cell count.
Introduction
Although current ARTs have improved in terms of efficacy, robustness, genetic barrier to resistance and tolerability, people living with HIV (PLWH) remain exposed to drug toxicity for several decades. Two-drug regimens (2DRs) are now recommended in international guidelines as maintenance regimens for virally suppressed PLWH to limit long-term cumulated toxicity of certain drugs.1,2 Recommended 2DRs include oral boosted-PI/lamivudine, dolutegravir/lamivudine and dolutegravir/rilpivirine, and injectable cabotegravir/rilpivirine. However, not all PLWH are able to receive integrase strand transfer inhibitor- (INSTI-) or boosted PI-based 2DRs, in the event of past intolerance, resistance or drug–drug interaction.
Two-drug regimens not based on INSTIs or boosted PIs could be a suitable treatment option in these patients, but data to support this are almost non-existent. Until now, only one small pilot study has evaluated the virological efficacy of a 2DR based on an NNRTI plus an NRTI. In 2020, Kahlert et al.3 demonstrated that nevirapine/lamivudine was able to maintain control of viral replication (plasma HIV-RNA < 50 copies/mL) in 19 patients over 144 weeks.
Doravirine is a new-generation NNRTI with very few drug interactions and a better genetic barrier than older NNRTIs. It has been approved in Europe for treatment-naive and treatment-experienced PLWH, following large trials that demonstrated its virological efficacy and good safety profile.4–6 Its combination with lamivudine, as a 2DR, has never been investigated.
Although not a recommended strategy, some patients have received doravirine/lamivudine because of their virological and therapeutic history. Herein, we aimed to report the reasons for switching, as well as the ability of doravirine/lamivudine to maintain virological control in PLWH followed in five French centres.
Methods
Information was collected from centres participating in the French Dat’AIDS cohort (ClinicalTrials.gov reference: NCT02898987) after approval by the Nantes Institutional Review Board and the French National Committee on Informatics and Human Rights (CNIL number: 1357652). The Dat’AIDS cohort is an ongoing cohort, started in 2000, including at that time all PLWH with their previous medical history, and all new PLWH in care since then have become part of the cohort, after they were fully informed and provided written consent.7
This observational, non-interventional study included all adults who switched to doravirine/lamivudine between 1 September 2019 and 31 January 2022, with at least 24 weeks of follow-up at time of analysis, in five HIV centres (i.e. centres with at least one patient meeting the inclusion criteria): Quimper, Pitié-Salpêtrière (Paris), Rennes, Pointe-à-Pitre and Marseille. ART prescriptions were made during routine follow-up by HIV physicians, and were not part of any protocol. However, all these prescriptions were discussed in local ART committees, independently of this analysis. There were no additional biological samples or questionnaires used for this study. Past HIV-RNA and HIV-DNA resistance genotypes were collected, and the cumulative genotype combined all past genotypic tests with an updated interpretation using the latest version of the ANRS algorithm (www.hivfrenchresistance.org). We also collected the reasons associated with the switch to doravirine/lamivudine.
The primary outcome was maintenance of virological suppression (HIV-RNA < 50 copies/mL) at W48. Virological failure (VF) was defined as a confirmed HIV-RNA ≥ 50 copies/mL, or a single HIV-RNA ≥ 200 copies/mL, or ≥50 copies/mL with ART change. Secondary outcomes included: strategy success rate (no VF and no ART change for non-virological reasons), number of ART discontinuations, emergence of genotypic resistance in the event of VF, evolution of CD4 T cell counts and CD4/CD8 ratio over follow-up.
Changes in body weight, CD4 T cell counts and CD4/CD8 ratio were assessed using the Wilcoxon test. Statistical tests were processed using Stata v.14.
Results
Fifty patients switched to doravirine/lamivudine, including 41 who reached 48 weeks of follow-up. Median follow-up was 79 weeks (IQR 60–96). Patients’ characteristics are detailed in Table 1. Thirty-four of them (68%) were men. Median age was 58 years (IQR 51–62), median ART duration was 20 years (IQR 13–23), median duration of virological suppression was 14 years (IQR 8–19) and median CD4 T cell count was 784 cells/mm3 (IQR 636–889). All had plasma HIV-RNA < 50 copies/mL at study entry. Prior to switching, all except three were naive to doravirine, and 36 (72%) were receiving a three-drug regimen (3DR). Genotypic data (past RNA and DNA resistance genotypes) were available for 20 patients, with no resistance to either doravirine or lamivudine in 18 patients, and a past M184V mutation in two patients, conferring resistance to lamivudine. The M184V mutations were found on an RNA resistance genotype performed 9 and 11 years prior to switch, in two patients with prolonged durations of viral suppression before switching (8 and 11 years, respectively).
| Age, years, median (IQR) | 58 (51–62) |
| Gender, n (%) | |
| Male | 34 (68) |
| Female | 16 (32) |
| Birth country, n (%) | |
| France | 44 (88) |
| Other | 6 (12) |
| Transmission group, n (%) | |
| Heterosexual | 23 (46) |
| MSM | 21 (42) |
| Other | 6 (12) |
| Comorbidities, n (%) | |
| Treated diabetes | 6 (12) |
| Treated high blood pressure | 17 (34) |
| Dyslipidaemia | 22 (44) |
| Kidney disease | 5 (10) |
| Osteoporosis | 7 (14) |
| Body weight, kg, median (IQR) | 73.9 (66.3–85.0) |
| CDC stage C, n (%) | 11 (22) |
| CD4 T cell nadir, cells/mm3, median (IQR) | 258 (145–385) |
| HIV-RNA zenith, log10 copies/mL, median (IQR) | 4.79 (3.67–5.32) |
| Time from HIV diagnosis, years, median (IQR) | 24 (16–29) |
| Time from ART initiation, years, median (IQR) | 20 (13–23) |
| Previous virological failure on NNRTI, n (%) | 0 (0) |
| Previous virological failure on 3TC or FTC, n (%) | 3 (6) |
| Genotypic sensitivity score to DOR + 3TCa, n (%) | |
| 1 | 2b/20 (10) |
| 2 | 18/20 (90) |
| Duration of viral suppression, years, median (IQR) | 14 (8–19) |
| CD4 T cell count, cells/mm3, median (IQR) | 784 (636–889) |
| CD4/CD8 ratio, median (IQR) | 1.16 (0.96–1.50) |
| Antiretroviral strategy prior to DOR + 3TC, n (%) | |
| NNRTI-based 3DR | 24 (48) |
| INSTI-based 3DRc | 12 (24) |
| Dolutegravir/lamivudine | 6 (12) |
| Darunavir/ritonavir/lamivudine | 3 (6) |
| Other 2DR | 3 (6) |
| Boosted PI monotherapy | 2 (4) |
| Age, years, median (IQR) | 58 (51–62) |
| Gender, n (%) | |
| Male | 34 (68) |
| Female | 16 (32) |
| Birth country, n (%) | |
| France | 44 (88) |
| Other | 6 (12) |
| Transmission group, n (%) | |
| Heterosexual | 23 (46) |
| MSM | 21 (42) |
| Other | 6 (12) |
| Comorbidities, n (%) | |
| Treated diabetes | 6 (12) |
| Treated high blood pressure | 17 (34) |
| Dyslipidaemia | 22 (44) |
| Kidney disease | 5 (10) |
| Osteoporosis | 7 (14) |
| Body weight, kg, median (IQR) | 73.9 (66.3–85.0) |
| CDC stage C, n (%) | 11 (22) |
| CD4 T cell nadir, cells/mm3, median (IQR) | 258 (145–385) |
| HIV-RNA zenith, log10 copies/mL, median (IQR) | 4.79 (3.67–5.32) |
| Time from HIV diagnosis, years, median (IQR) | 24 (16–29) |
| Time from ART initiation, years, median (IQR) | 20 (13–23) |
| Previous virological failure on NNRTI, n (%) | 0 (0) |
| Previous virological failure on 3TC or FTC, n (%) | 3 (6) |
| Genotypic sensitivity score to DOR + 3TCa, n (%) | |
| 1 | 2b/20 (10) |
| 2 | 18/20 (90) |
| Duration of viral suppression, years, median (IQR) | 14 (8–19) |
| CD4 T cell count, cells/mm3, median (IQR) | 784 (636–889) |
| CD4/CD8 ratio, median (IQR) | 1.16 (0.96–1.50) |
| Antiretroviral strategy prior to DOR + 3TC, n (%) | |
| NNRTI-based 3DR | 24 (48) |
| INSTI-based 3DRc | 12 (24) |
| Dolutegravir/lamivudine | 6 (12) |
| Darunavir/ritonavir/lamivudine | 3 (6) |
| Other 2DR | 3 (6) |
| Boosted PI monotherapy | 2 (4) |
3TC lamivudine; FTC, emtricitabine; DOR, doravirine.
Calculated from cumulative historical HIV-RNA and HIV-DNA genotypes with RT available sequences (N = 20).
These two patients had a documented M184V mutation in past RNA genotypes, in 2010 and 2011.
These NNRTI-based 3DRs included rilpivirine (n = 11), nevirapine (n = 9), doravirine (n = 3) and efavirenz (n = 1).
| Age, years, median (IQR) | 58 (51–62) |
| Gender, n (%) | |
| Male | 34 (68) |
| Female | 16 (32) |
| Birth country, n (%) | |
| France | 44 (88) |
| Other | 6 (12) |
| Transmission group, n (%) | |
| Heterosexual | 23 (46) |
| MSM | 21 (42) |
| Other | 6 (12) |
| Comorbidities, n (%) | |
| Treated diabetes | 6 (12) |
| Treated high blood pressure | 17 (34) |
| Dyslipidaemia | 22 (44) |
| Kidney disease | 5 (10) |
| Osteoporosis | 7 (14) |
| Body weight, kg, median (IQR) | 73.9 (66.3–85.0) |
| CDC stage C, n (%) | 11 (22) |
| CD4 T cell nadir, cells/mm3, median (IQR) | 258 (145–385) |
| HIV-RNA zenith, log10 copies/mL, median (IQR) | 4.79 (3.67–5.32) |
| Time from HIV diagnosis, years, median (IQR) | 24 (16–29) |
| Time from ART initiation, years, median (IQR) | 20 (13–23) |
| Previous virological failure on NNRTI, n (%) | 0 (0) |
| Previous virological failure on 3TC or FTC, n (%) | 3 (6) |
| Genotypic sensitivity score to DOR + 3TCa, n (%) | |
| 1 | 2b/20 (10) |
| 2 | 18/20 (90) |
| Duration of viral suppression, years, median (IQR) | 14 (8–19) |
| CD4 T cell count, cells/mm3, median (IQR) | 784 (636–889) |
| CD4/CD8 ratio, median (IQR) | 1.16 (0.96–1.50) |
| Antiretroviral strategy prior to DOR + 3TC, n (%) | |
| NNRTI-based 3DR | 24 (48) |
| INSTI-based 3DRc | 12 (24) |
| Dolutegravir/lamivudine | 6 (12) |
| Darunavir/ritonavir/lamivudine | 3 (6) |
| Other 2DR | 3 (6) |
| Boosted PI monotherapy | 2 (4) |
| Age, years, median (IQR) | 58 (51–62) |
| Gender, n (%) | |
| Male | 34 (68) |
| Female | 16 (32) |
| Birth country, n (%) | |
| France | 44 (88) |
| Other | 6 (12) |
| Transmission group, n (%) | |
| Heterosexual | 23 (46) |
| MSM | 21 (42) |
| Other | 6 (12) |
| Comorbidities, n (%) | |
| Treated diabetes | 6 (12) |
| Treated high blood pressure | 17 (34) |
| Dyslipidaemia | 22 (44) |
| Kidney disease | 5 (10) |
| Osteoporosis | 7 (14) |
| Body weight, kg, median (IQR) | 73.9 (66.3–85.0) |
| CDC stage C, n (%) | 11 (22) |
| CD4 T cell nadir, cells/mm3, median (IQR) | 258 (145–385) |
| HIV-RNA zenith, log10 copies/mL, median (IQR) | 4.79 (3.67–5.32) |
| Time from HIV diagnosis, years, median (IQR) | 24 (16–29) |
| Time from ART initiation, years, median (IQR) | 20 (13–23) |
| Previous virological failure on NNRTI, n (%) | 0 (0) |
| Previous virological failure on 3TC or FTC, n (%) | 3 (6) |
| Genotypic sensitivity score to DOR + 3TCa, n (%) | |
| 1 | 2b/20 (10) |
| 2 | 18/20 (90) |
| Duration of viral suppression, years, median (IQR) | 14 (8–19) |
| CD4 T cell count, cells/mm3, median (IQR) | 784 (636–889) |
| CD4/CD8 ratio, median (IQR) | 1.16 (0.96–1.50) |
| Antiretroviral strategy prior to DOR + 3TC, n (%) | |
| NNRTI-based 3DR | 24 (48) |
| INSTI-based 3DRc | 12 (24) |
| Dolutegravir/lamivudine | 6 (12) |
| Darunavir/ritonavir/lamivudine | 3 (6) |
| Other 2DR | 3 (6) |
| Boosted PI monotherapy | 2 (4) |
3TC lamivudine; FTC, emtricitabine; DOR, doravirine.
Calculated from cumulative historical HIV-RNA and HIV-DNA genotypes with RT available sequences (N = 20).
These two patients had a documented M184V mutation in past RNA genotypes, in 2010 and 2011.
These NNRTI-based 3DRs included rilpivirine (n = 11), nevirapine (n = 9), doravirine (n = 3) and efavirenz (n = 1).
The main reason for physicians to switch ART to doravirine/lamivudine was to reduce the drug burden, and to prevent long-term toxicities and drug interactions, in 32 patients with long-term viral suppression (>10–15 years) on a stable 3DR. Six patients had adverse events related to ongoing ART, motivating an immediate switch to doravirine/lamivudine: osteoporotic bone fracture on tenofovir disoproxil fumarate (n = 2), fast weight gain on an INSTI (n = 2), digestive discomfort on rilpivirine (n = 1) and myalgia with increased creatine phosphokinase (CPK) levels on bictegravir (n = 1). Twelve presented a significant drug interaction, motivating an immediate switch to doravirine/lamivudine: rilpivirine and proton pump inhibitor (n = 7), and boosted PI and co-medications (n = 5). In addition to these reasons, the use of certain antiretroviral classes needed to be avoided, due to past side effects, restricting treatment options: weight gain under INSTI with or without tenofovir alafenamide (n = 10), weight gain under tenofovir alafenamide with no associated INSTI (n = 1), neuropsychic intolerance under INSTI (n = 7) and marked lipodystrophy attributable to past NRTIs (n = 3).
One VF occurred at W18 (plasma HIV-RNA = 101 copies/mL), leading to a virological success rate of 98.0% (95% CI 89.4–99.9) at W48 (Figure 1a). This VF occurred in a patient who briefly stopped his treatment due to intense nightmares. There was no resistance at baseline, and no resistance emerged at the time of VF (HIV-RNA successfully amplified). The patient achieved a plasma HIV-RNA of <50 copies/mL after resumption of boosted PI monotherapy. There were three other strategy discontinuations over the entire study period for adverse event (digestive disorder: n = 2, insomnia: n = 1), leading to a strategy success rate of 92.0% (95%CI 80.8–97.8) at W48 (Figure 1b).
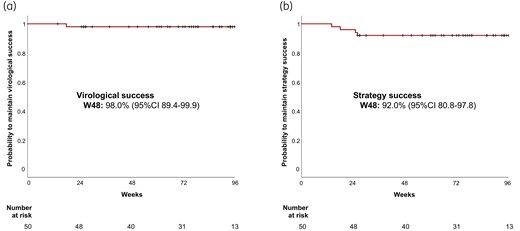
Virological success rate (a), and strategy success rate (b) under doravirine/lamivudine over time. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
There was no significant change in the body weight (−0.1 kg, P = 0.83) or the CD4/CD8 ratio (+0.01, P = 72), and we observed a significant increase in the CD4 T cell counts (+51 cells/mm3, P = 0.031) over the study period.
Discussion
We report a series of 50 PLWH with a long duration of viral suppression, who were switched by their HIV physician to doravirine/lamivudine, with a high level of virological success (98%) at W48. Only one VF occurred, in a patient who briefly discontinued doravirine/lamivudine, as attested by low level of plasma HIV-RNA. Importantly, there was no emergence of resistance at the time of virological rebound. All other PLWH remained virally controlled. There was no decrease in CD4 T cell counts or CD4/CD8 ratio on doravirine/lamivudine, as previously shown in larger studies evaluating other 2DRs as maintenance regimens.8–11 The doravirine/lamivudine combination was well tolerated, particularly with regard to weight, with no significant variation over the study period.
All prescriptions were made in real-life conditions, outside of any protocolized trial. This explains why the characteristics of the patients are fairly different from those of the participants usually included in clinical trials. Although they had high current CD4 T cell counts (>700 cells/mm3), associated with a long duration of viral suppression (14 years), 22% of them had an AIDS-related event in their history. We assume that a significant number of them received toxic and ineffective antiretroviral combinations at the start of treatment. The main reasons for choosing doravirine/lamivudine mentioned by clinicians were past or present side effects and drug interactions. These are indeed two major concerns in the daily practice of managing ageing PLWH.
Two-drug suppressive therapies are increasingly important in a context of lifelong-required ART.12 The benefit of 2DRs, such as dolutegravir/lamivudine, dolutegravir/rilpivirine or etravirine/raltegravir, has been demonstrated through improved metabolic, renal and bone biomarkers.8,9,13 Prevention of cardiovascular and renal function impairment, as well as bone fracture risk attributable to ART, is a key concern for PLWH who are all ageing. In addition, these strategies avoid most of the drug interactions. However, all PLWH are not able to receive INSTI-based 2DRs, as evidenced in the sample of patients presented here, e.g. in cases of neuropsychological intolerance or weight gain from previous INSTI exposure. Previous studies have shown the robustness and the good tolerance of doravirine, which did not appear to cause excessive weight gain, unlike INSTIs and tenofovir alafenamide. Real-life data are starting to confirm that doravirine-based combinations are safe in terms of metabolic and weight issues.14,15 Finally, the cost of the doravirine/lamivudine combination, which does not exist as a fixed dose, is lower than for other usual 2DRs.
Our study has some limitations, as it is a retrospective observational study including a limited number of patients. The lack of a comparative arm clearly limits the significance of this analysis. Many resistance genotypes were missing, as is often the case for patients with a long history of HIV infection. However, our preliminary findings suggest that a doravirine/lamivudine regimen is able to maintain high levels of viral suppression in selected highly ART-experienced PLWH with long-term viral suppression, and restored CD4+ T cell count. Given the interesting virological and pharmacological profile of both doravirine and lamivudine, we argue for a larger comparative maintenance trial evaluating the efficacy of the doravirine/lamivudine combination.
Acknowledgements
This work was presented as a poster at the HIV Drug Therapy Conference, 2022, Glasgow, UK (Abstract P094).
Members of the Dat’AIDS study group
Besançon: C. Chirouze, K. Bouiller, F. Bozon, A. S. Brunel, L. Hustache-Mathieu, J. Lagoutte, Q. Lepiller, S. Marty-Quinternet, L. Pépin-Puget, B. Rosolen, N. Tissot. Brest: S. Jaffuel, S. Ansart, Y. Quintric, S. Rezig, L. Quaesaet, P. Gazeau. Clermont-Ferrand: C. Jacomet, N. Mrozek, C. Theis, M. Vidal, C. Richaud, F. Anglade, L. Sauvat, V. Corbin, C. Aumeran, O. Baud, E. Goncalvez, D. Mazzocolin, A. Mirand, A. Brebion, C. Henquell. Guadeloupe: I. Lamaury, E. Breugnon, A. Chéret, E. Curlier, E. Duvallon, I. Fabre, C. Herrmann-Storck, S. Markowicz, M. Marquet, R. Ouissa, L. Pradat-Paz, K. Samar, B. Tressieres. La Roche sur Yon: D. Merrien, O. Bollangier, D. Boucher, T. Guimard, L. Laine, S. Leautez, M. Morrier, P. Perré, P. Point. La Rochelle: M. Roncato-Saberan, X. Pouget-Abadie, C. Chapuzet, L. Faba. Limoges: J. F. Faucher, A. Cypierre, S. Ducroix-Roubertou, H. Durox, C. Genet-Villeger, J. Pascual, P. Pinet, C. Codde, S. Rogez, J. B. Woillard, C. Benoist. Lyon: D. Alfaiate, A. Becker, L. Cotte, F. Ader, C. Brochier, F. Brunel-Dalmas, O. Cannesson, A. Conrad, S. Degroodt, T. Ferry, M. Godinot, V. Icard, J. M. Livrozet, D. Makhloufi, T. Perpoint, S. Roux, M. A. Trabaud, C. Triffault-Fillit, F. Valour, A. S. Batalla, H. Lardot, M. Simon, C. Javaux. Marseille IHU Méditerrannée: I. Ravaux, A. Ménard, Y. Belkhir, P. Colson, C. Dhiver, M. Martin-Degioanni, L. Meddeb, M. Mokhtari, A. Motte, H. Tissot-Dupont, C. Toméi. Marseille Ste Marguerite: S. Brégigeon, O. Zaegel-Faucher, H. Laroche, M. Dos Santos, M. J. Ducassou, S. Galie, A. Ivanova, I. Jaquet, V. Obry-Roguet, M. Orticoni, E. Ressiot, A. S. Ritleng, S. Benkouiten. Martinique: A. Cabié, S. Abel, B. Bigeard, C. Bidelogne, O. Cabras, L. Carnino, L. Cuzin, L. Fagour, A. Gros-Dubois, K. Guitteaud, C. Lahuna, E. Louis-Michel, A. Métais, F Quenard, S. Pierre-François. Metz: C. Robert, Z. Cavalli, L. Bucy, R. Genet, C. Schneifer, P. Perez. Montpellier: J. Reynes, M. Bistoquet, E Delaporte, V. Le Moing, J. Lejeune, N. Meftah, C. Merle de Boever, B. Montes, A. Montoya Ferrer, N. Pansu, J. Reynes, E. Tuaillon. Nancy: B. Lefèvre, M. André, G. Baronnet, S. Bevilacqua, L. Boyer, M. P. Bouillon, A. Charmillon, M. Delestan, C. Emilie, E. Frentiu, F. Goehringer, S. Hénard, E. Jeanmaire, C. Rabaud, A. Radjabaly-Mandjee. Nantes: F. Raffi, C. Allavena, E. André-Garnier, A. Asquier-Khati, E. Billaud, C. Biron, B. Bonnet, S. Bouchez, D. Boutoille, C. Brunet-Cartier, M. Cavellec, C. Deschanvres, T. Drumel, B. J. Gaborit, M. Grégoire, T. Jovelin, M. Lefebvre, R. Lecomte, R. Mahot, P. Morineau, E. Paredes, V. Reliquet, A. Soria. Nice: P. Pugliese, S. Bréaud, M. Buscot, M. Carles, D. Chirio, E. Cua, P. Dellamonica, E. Demonchy, A. De Monte, J. Durant, S. Ferrando, A. Naqvi, I. Perbost, C. Pradier, B. Prouvost-Keller, K. Risso, I. Touitou, A. Viot, S. Wehrlen-Pugliese. Niort: S. Sunder, K. Schepers, V. Goudet, A. Dos Santos, V. Rzepecki. Orléans: L. Hocqueloux, G. Béraud, C. Gubavu, V. Legros, C. Mille, F. Peira, T. Prazuck, A. Sève. Paris APHP Bicètre: C. Goujard, A. Castro-Gordon, P. David-Chevallier, V. Godard, Y. Quertainmont, E. Teicher, S. Jaureguiberry. Paris APHP Bichat: V. Joly, C. Charpentier, D. Descamps, M. Digumber, A. Gervais, J. Ghosn, Z. Julia, R. Landman, S. Lariven, S. Le Gac, F. Louni, N. Peiffer-Smadja, G. Peytavin, C. Rioux, Y. Yazdanpanah. Paris APHP Necker, Institut Pasteur: C. Duvivier, K. Amazzough, G. Benabdelmoumen, P. Bossi, G. Cessot, C. Charlier, PH. Consigny, C. De La Porte Des Vaux, M. Garzaro, E. Gomes-Pires, P. Hochedez, K. Jidar, E. Lafont, F. Lanternier, O. Lortholary, C. Louisin, J. Lourenco, C. Melenotte, O. Pacoud, P. Parize, F. Ruyno, C. Rouzaud, F. Taieb. Paris APHP Pitié Salpetrière: R. Palich, M. A. Valantin, C. Katlama, A. Faycal, R. Agher, Y. Dudoit, N. Hamani, N. Qatib, I. Qzaibri, L. Lenclume, L. Schneider, S. Seang, R. Tubiana. Quimper: N. Hall, P. Perfezou, J. C. Duthe, F. B. Drevillon, J. P. Talarmin, L. Khatchatourian. Reims: F. Bani-Sadr, J. L. Berger, V. Brodard, M. Hentzien, I. Kmiec, D. Lambert, H. Marty, Y. N’Guyen. Rennes: C. Arvieux, M. Baldeyrou, F. Benezit, J. M. Chapplain, M. Dupont, J. C. Duthé, S. Ismaël, T. Jovelin, A. Lebot, F. Lemaitre, D. Luque-Paz, A. Maillard, C. Morlat, S. Patrat-Delon, L. Picard, M. Poisson-Vannier, C. Pronier M. Revest, P. Tattevin, J. Vivent. St Etienne: A. Gagneux-Brunon, E. Botelho-Nevers, A. Frésard, A. Pouvaret, V. Ronat. Strasbourg: D. Rey, C. Cheneau, C. Bernard-Henry, E. De Mautort, S. Fafi-Kremer, P. Fischer, P. Gantner, C. Mélounou, A. Ursenbach, P. Klee, Y. Hansmann, N. Lefebvre, Y. Ruch, F. Danion, B. Hoellinger, T. Lemmet, V. Gerber, M. Bourne-Watrin. Toulouse: P. Delobel, M. Alvarez, N. Biezunski, X. Boumaza, A. Debard, C. Delpierre, C. Garnier, L. Lelièvre, G. Martin-Blondel, M. Piffaut, C. Rastoll, K. Saune. Tourcoing: O. Robineau, E. Aïssi, I. Alcaraz, E. Alidjinou, V. Baclet, L. Bocket, A. Boucher, V. Derdour, B. Lafon-Desmurs, A. Meybeck, M. Pradier, M. Tetart, M. Valette, N. Viget, A. Diarra. Vannes: G. Corvaisier, M. Brière, M. De La Chapelle, M. Gousseff, M. Le Goff, M. Thierry.
Funding
Internal funding was used to carry out this work (data management, Dat’AIDS cohort). Data collection was part of the routine work in the different centres associated with the Dat’AIDS cohort.
Transparency declarations
S.S., A.G.M., C.K. and R.P. have received travel grants and advisory fees from Gilead, ViiV Healthcare and Merck. A.G.M. have received fees from TheraTec. C.A. has received advisory fees from Gilead and ViiV. P.P., N.H., J.C.D., B.A., I.L. and A.M. have no conflicts of interest to declare.
References
Author notes
Members are listed in the Acknowledgements section.



