-
PDF
- Split View
-
Views
-
Cite
Cite
Yue Zhu, Yuanmei Huang, Chenli Zheng, Jie Tang, Guang Zeng, Wei Xie, Hui Wang, Lukun Zhang, Shaochu Liu, Yan Zhang, Wei Tan, Jingguang Tan, Lijuan Jiang, Yun He, Liumei Xu, Zhengrong Yang, Jin Zhao, Primary resistance to integrase inhibitors in Shenzhen, Journal of Antimicrobial Chemotherapy, Volume 78, Issue 2, February 2023, Pages 546–549, https://doi.org/10.1093/jac/dkac442
Close - Share Icon Share
Abstract
In recent years, integrase strand transfer inhibitor (INSTI)-containing regimens have been increasingly adopted in treatment for HIV/AIDS and promoted as non-occupational post-exposure prophylaxis in China. This study aims to describe the prevalence of resistance to integrase and drug resistance mutations (DRMs) among ART-naive patients in Shenzhen, China.
Serum samples and demographic information were collected from newly reported ART-naive patients in Shenzhen in 2020. The study sequenced the coding sequence of the HIV-1 integrase gene and determined the DRMs.
In this study, 1682 newly reported cases were included and 1071 of them were successfully sequenced finally. The prevalence of primary drug resistance was 1.77%, with 19 samples showing varying degrees of resistance to INSTIs. The study detected six major DRMs in 16 individuals and eight accessory DRMs in 24 individuals. The prevalence of transmitted drug resistance (TDR) mutations was 1.21%, with five transmitted mutations detected in 13 individuals. The prevalence of drug resistance to raltegravir and elvitegravir was statistically higher than to bictegravir, cabotegravir and dolutegravir.
The prevalence of INSTI resistance in Shenzhen in 2020 was relatively high. Continued surveillance for resistance to INSTIs is recommended and treatment regimens should be adopted based on the pattern of resistance to INSTIs. Dolutegravir or bictegravir is first recommended when considering INSTIs as treatment regimens.
Introduction
Combination ART (cART) can decrease the morbidity and mortality of people living with HIV.1 Integrase strand transfer inhibitors (INSTIs) have become an essential part of cART, with a high genetic barrier to resistance, and have proven to be highly effective for treatment.2 The five INSTIs, raltegravir and elvitegravir (the first-generation INSTIs) and dolutegravir, bictegravir and cabotegravir (the second-generation INSTIs) have been approved by the US FDA for the treatment of HIV/AIDS.3 Raltegravir was approved by the Chinese Food and Drug Administration (CFDA) for HIV/AIDS treatment in 2009, while elvitegravir, bictegravir and dolutegravir were approved by the CFDA in 2018, 2019 and 2021, respectively.4–6 According to the Chinese Guidelines for Diagnosis and Treatment of HIV/AIDS, INSTI-containing regimens have been recommended as first-line treatment for HIV/AIDS since 2018.6 In recent years, INSTIs have been gradually promoted as non-occupational post-exposure prophylaxis (nPEP) in China among high-risk groups, especially MSM, which may lead to the development of drug resistance. Limited studies in China showed that the prevalence of resistance to INSTIs among ART-naive patients was relatively low in Beijing and Henan.4,7 The previous study showed that no major mutations were detected in Guangdong,8 the province where Shenzhen is located. Shenzhen was one of the pilot cities in China to include elvitegravir in the National Health Insurance System. The proportion of elvitegravir use among ART-treated patients in 2020 was up to 15% (unpublished data), due to its low price. Meanwhile, Shenzhen also was one of the pioneer cities to open an nPEP clinic in China, and the rate of nPEP use among MSM was relatively high in 2020.9 The epidemic trends of INSTI resistance and resistance-associated mutations in Shenzhen may predict the overall epidemic of INSTI resistance in China. Therefore, it is essential to conduct research on INSTI resistance in Shenzhen. This study analysed HIV-1 drug resistance mutations (DRMs) in the integrase gene and the prevalence of resistance to INSTIs among ART-naive patients in Shenzhen in 2020, to provide a basis for the clinical treatment of HIV/AIDS.
Methods
Ethics
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of Shenzhen Center for Disease Control and Prevention (No. QS2021070037, approval date: 4 November 2021). All included patients provided written informed consent.
Study design
The inclusion criteria of the subjects were HIV-1 ART-naive patients who were newly reported in Shenzhen in 2020. Five millilitres of blood with EDTA were drawn before cART. The demographic and epidemiological information of the patients was collected.
Drug resistance analysis and HIV-1 subtype identification
After RNA extraction and RT–PCR amplification, the obtained sequences were assembled and edited using DNA sequence analysis software V5.4.6. We used the Stanford HIV-1 Drug Resistance Database to determine DRMs and analyse sensitivity to INSTIs. Sequences were divided into five levels: sensitive, potential resistance, low-level resistance, intermediate resistance, and high-level resistance, of which low-level, intermediate and high-level resistance were defined as INSTI resistance. The Calibrated Population Resistance Tool was used to identify transmitted drug resistance (TDR) mutations. HIV-1 subtype of the study sequences was determined using the HIVDB subtyping program.
Results
Characteristics of the study population
In total, 1682 newly reported cases were included, and 1071 of them were successfully sequenced. The majority (91.6%) of newly reported cases were male, the average age was 34.8 ± 11.6 years, and the dominant transmission route was homosexual contact (62.4%) (Table S1, available as Supplementary data at JAC Online). The study subjects, patients who were successfully sequenced, were also primarily male (90.8%), the average age was 34.9 ± 12.3 years, and the main transmission route was homosexual contact (59.9%) (Table S1). There was no statistically significant difference between the newly reported cases and the study subjects concerning age, gender and other demographic characteristics (P > 0.05).
DRMs
There were 39 samples (3.64%) that harboured INSTI DRMs among 1071 study samples, of which 16 (1.49%) and 24 (2.24%) harboured major and accessory mutations, respectively, while one sample had dual mutations. Detailed information is shown in Table 1. The most common major mutations were E138K and R263K (4/16; 25%). E138K was a non-polymorphic mutation associated with resistance to raltegravir and elvitegravir, and R263K was the main mutation responsible for resistance to dolutegravir and bictegravir.3,10–12 Major mutations, T66A/I, N155H/S/D, S147G and G140R, were also detected. T66A/I, N155H and S147G were associated with high-level resistance to elvitegravir, and G140R was related to cabotegravir resistance.12,13 The most common accessory mutation was E157Q (9/24; 37.5%), which had little effect on susceptibility to INSTIs.14 Five TDR mutations, R263K (n = 4), E138K (n = 4), T66A/I (n = 3), N155H (n = 1) and S127G (n = 1), occurred in 13 samples with a prevalence of 1.21%.
Drug resistance-associated mutations and INSTI resistance of the study population
| Resistance mutation . | Mutant cases . | Mutant rate (%) . | Drug resistance level . | ||||
|---|---|---|---|---|---|---|---|
| BIC . | CAB . | DTG . | EVG . | RAL . | |||
| Major mutations | |||||||
| ȃT66A | 2 | 0.19 | S | S | S | H | L |
| ȃT66I | 1 | 0.09 | S | P | S | H | L |
| ȃE138K | 4 | 0.37 | P | P | P | L | L |
| ȃR263K | 4 | 0.37 | I | I | I | I | L |
| ȃN155H | 1 | 0.09 | P | L | P | H | H |
| ȃN155S | 1 | 0.09 | S | L | S | I | I |
| ȃN155D | 1 | 0.09 | S | S | S | S | S |
| ȃS147G | 1 | 0.09 | P | P | P | H | P |
| ȃG140Ra | 1 | 0.09 | P | H | P | I | I |
| Accessory mutations | |||||||
| ȃG163K/Ra | 4 | 0.37 | S | S | S | L | L |
| ȃH51Y | 1 | 0.09 | P | L | P | L | L |
| ȃE157Q | 9 | 0.84 | S | S | S | P | P |
| ȃT97A | 2 | 0.19 | S | S | S | P | P |
| ȃD232N | 1 | 0.09 | S | S | S | P | P |
| ȃG140D/E | 2 | 0.19 | S | S | S | S | S |
| ȃA128T | 4 | 0.37 | S | S | S | S | S |
| ȃA49G | 1 | 0.09 | S | S | S | S | S |
| Resistance mutation . | Mutant cases . | Mutant rate (%) . | Drug resistance level . | ||||
|---|---|---|---|---|---|---|---|
| BIC . | CAB . | DTG . | EVG . | RAL . | |||
| Major mutations | |||||||
| ȃT66A | 2 | 0.19 | S | S | S | H | L |
| ȃT66I | 1 | 0.09 | S | P | S | H | L |
| ȃE138K | 4 | 0.37 | P | P | P | L | L |
| ȃR263K | 4 | 0.37 | I | I | I | I | L |
| ȃN155H | 1 | 0.09 | P | L | P | H | H |
| ȃN155S | 1 | 0.09 | S | L | S | I | I |
| ȃN155D | 1 | 0.09 | S | S | S | S | S |
| ȃS147G | 1 | 0.09 | P | P | P | H | P |
| ȃG140Ra | 1 | 0.09 | P | H | P | I | I |
| Accessory mutations | |||||||
| ȃG163K/Ra | 4 | 0.37 | S | S | S | L | L |
| ȃH51Y | 1 | 0.09 | P | L | P | L | L |
| ȃE157Q | 9 | 0.84 | S | S | S | P | P |
| ȃT97A | 2 | 0.19 | S | S | S | P | P |
| ȃD232N | 1 | 0.09 | S | S | S | P | P |
| ȃG140D/E | 2 | 0.19 | S | S | S | S | S |
| ȃA128T | 4 | 0.37 | S | S | S | S | S |
| ȃA49G | 1 | 0.09 | S | S | S | S | S |
BIC, bictegravir; CAB, cabotegravir; DTG, dolutegravir; EVG, elvitegravir; RAL, raltegravir; S, sensitive, P, potentially resistant, L, low resistant, I, intermediate resistant; H, high resistant.
One patient had both G140R and G163R mutations.
Drug resistance-associated mutations and INSTI resistance of the study population
| Resistance mutation . | Mutant cases . | Mutant rate (%) . | Drug resistance level . | ||||
|---|---|---|---|---|---|---|---|
| BIC . | CAB . | DTG . | EVG . | RAL . | |||
| Major mutations | |||||||
| ȃT66A | 2 | 0.19 | S | S | S | H | L |
| ȃT66I | 1 | 0.09 | S | P | S | H | L |
| ȃE138K | 4 | 0.37 | P | P | P | L | L |
| ȃR263K | 4 | 0.37 | I | I | I | I | L |
| ȃN155H | 1 | 0.09 | P | L | P | H | H |
| ȃN155S | 1 | 0.09 | S | L | S | I | I |
| ȃN155D | 1 | 0.09 | S | S | S | S | S |
| ȃS147G | 1 | 0.09 | P | P | P | H | P |
| ȃG140Ra | 1 | 0.09 | P | H | P | I | I |
| Accessory mutations | |||||||
| ȃG163K/Ra | 4 | 0.37 | S | S | S | L | L |
| ȃH51Y | 1 | 0.09 | P | L | P | L | L |
| ȃE157Q | 9 | 0.84 | S | S | S | P | P |
| ȃT97A | 2 | 0.19 | S | S | S | P | P |
| ȃD232N | 1 | 0.09 | S | S | S | P | P |
| ȃG140D/E | 2 | 0.19 | S | S | S | S | S |
| ȃA128T | 4 | 0.37 | S | S | S | S | S |
| ȃA49G | 1 | 0.09 | S | S | S | S | S |
| Resistance mutation . | Mutant cases . | Mutant rate (%) . | Drug resistance level . | ||||
|---|---|---|---|---|---|---|---|
| BIC . | CAB . | DTG . | EVG . | RAL . | |||
| Major mutations | |||||||
| ȃT66A | 2 | 0.19 | S | S | S | H | L |
| ȃT66I | 1 | 0.09 | S | P | S | H | L |
| ȃE138K | 4 | 0.37 | P | P | P | L | L |
| ȃR263K | 4 | 0.37 | I | I | I | I | L |
| ȃN155H | 1 | 0.09 | P | L | P | H | H |
| ȃN155S | 1 | 0.09 | S | L | S | I | I |
| ȃN155D | 1 | 0.09 | S | S | S | S | S |
| ȃS147G | 1 | 0.09 | P | P | P | H | P |
| ȃG140Ra | 1 | 0.09 | P | H | P | I | I |
| Accessory mutations | |||||||
| ȃG163K/Ra | 4 | 0.37 | S | S | S | L | L |
| ȃH51Y | 1 | 0.09 | P | L | P | L | L |
| ȃE157Q | 9 | 0.84 | S | S | S | P | P |
| ȃT97A | 2 | 0.19 | S | S | S | P | P |
| ȃD232N | 1 | 0.09 | S | S | S | P | P |
| ȃG140D/E | 2 | 0.19 | S | S | S | S | S |
| ȃA128T | 4 | 0.37 | S | S | S | S | S |
| ȃA49G | 1 | 0.09 | S | S | S | S | S |
BIC, bictegravir; CAB, cabotegravir; DTG, dolutegravir; EVG, elvitegravir; RAL, raltegravir; S, sensitive, P, potentially resistant, L, low resistant, I, intermediate resistant; H, high resistant.
One patient had both G140R and G163R mutations.
Resistance to INSTIs
Nineteen samples were resistant to the five INSTIs, of which all 19 samples developed resistance to elvitegravir with a resistance rate of 1.77%, 18 samples were resistant to raltegravir (1.68%), 8 samples to cabotegravir (0.75%), and 4 samples to bictegravir and dolutegravir (0.37%). There were statistically significant differences in resistance to different INSTIs (Q = 49.818, P < 0.001). The results of pairwise comparisons showed that the drug resistance rates of raltegravir and elvitegravir were higher than the rates of bictegravir, cabotegravir and dolutegravir (P < 0.001), and no significant difference in other pair-based comparisons (Figure 1).
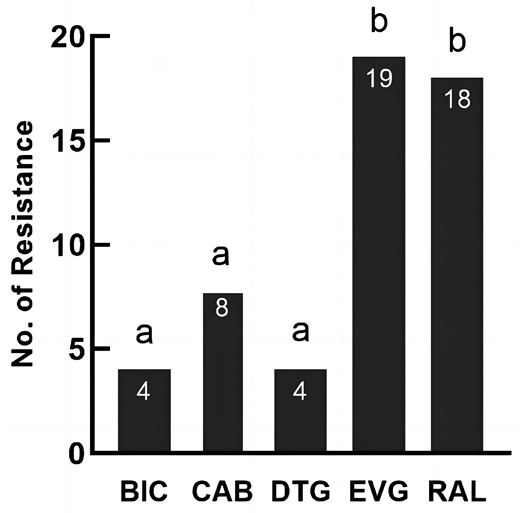
Pairwise comparisons of drug resistance among five integrase inhibitors. BIC, bictegravir; CAB, cabotegravir; DTG, dolutegravir; EVG, elvitegravir; RAL, raltegravir. Inconsistent letters indicate that the difference in drug resistance between the two integrase inhibitors is statistically significant (P < 0.05). Same letter indicates no statistically difference.
HIV-1 subtype of INSTI-resistant samples
The HIV-1 subtypes of the 19 INSTI-resistant samples were successively CRF07_BC (47.4%), CRF55_01B (26.3%), CRF01_AE (15.8%), CRF08_BC (5.3%) and subtype B (5.3%). There were no statistical differences in resistance to INSTIs between different HIV-1 subtypes.
Discussion
Most studies about INSTI resistance have shown that no major mutations were detected in ART-naive patients. However, various major mutations were detected in this study and the proportion of major mutations associated with raltegravir and elvitegravir was relatively high. The resistance rate to INSTIs among ART-naive patients in Shenzhen in 2020 was 1.77%, which was similar to the results of Yunnan Province (1.80%),15 but higher than the results of Beijing (0.53%) and Henan (0.63%).4,7
The study found that the drug resistance to elvitegravir was relatively high. Nineteen resistant samples all developed resistance to elvitegravir, and four of the five highly INSTI-resistant strains were associated with elvitegravir. Elvitegravir, to which there are lower genetic resistance barriers and high levels of cross-resistance, showing widespread resistance after long-term use, was earlier approved for sale as a first-generation INSTI.3,10 In 2020, the mainly used INSTI for cART in Shenzhen was elvitegravir, while the proportion of raltegravir used among ART-treated patients was extremely low. However, according to our unpublished data, the drug resistance rate to INSTIs among ART-treated patients in Shenzhen in 2020 was extremely low (less than 0.2%), suggesting that the high resistance to elvitegravir cannot be attributed exclusively to the treatment. China implemented the nPEP policy relatively late, and it was not until 2019 that the nPEP clinic was opened officially in Shenzhen.9 The main prescription regimens of the nPEP clinic in Shenzhen included raltegravir or dolutegravir during the study time. However, according to our previous study in 2020, 8.67% of the MSM population used nPEP ever, but few of them went to the clinic, and only 31% of them were tested for HIV before starting nPEP.9 A lot of MSM might obtain INSTIs for nPEP use from other routes, including smuggled medicine, generic medicine, or treatment medicine from ART-treated patients. Therefore, the resistance to INSTIs in Shenzhen in 2020 may be probably affected by the irregular use of nPEP without HIV testing. The health departments should standardize nPEP use and focus on surveillance of INSTI resistance in nPEP users. Dolutegravir, bictegravir and cabotegravir are the second-generation INSTIs with relatively high genetic resistance.3 In the present study, one sample was highly resistant to cabotegravir, and resistance to dolutegravir or bictegravir was uncommon. Cabotegravir, which was proved to be more effective in preventing HIV-1 infection in clinical trials,16 may increasingly be used for treatment and pre-exposure prophylaxis.3 So it is necessary to be vigilant against the occurrence of resistance when cabotegravir is promoted in China in the future.
Among the 1071 study cases, the proportion of HIV-1 subtypes were CRF07_BC (49.6%), CRF01_AE (24.6%), CRF55_01B (10.1%), subtype B (4.0%), CRF08_BC (3.0%) and other subtypes (8.8%). The rate of INSTI resistance among CRF55_01B was the highest (4.63%), followed by CRF08_BC (3.13%), subtype B (2.33%), CRF07_BC (1.69%) and CRF01_AE (1.14%). The difference in INSTI resistance between the subtypes was not statistically significant, probably due to the small sample size. In our future study, we will increase the sample size to further investigate the relationship between HIV-1 subtypes and INSTI resistance, especially CRF55_01B.
In summary, the prevalence of INSTI resistance in Shenzhen in 2020 is relatively high. It is necessary to strengthen the surveillance of INSTI resistance among ART-naive patients in Shenzhen, and the treatment regimens should be adopted based on the pattern of resistance to INSTIs. Dolutegravir or bictegravir is first recommended when INSTIs are considered as treatment regimens for HIV/AIDS.
Acknowledgements
We would like to thank all participants, peer researchers and the staff of the HIV Prevention and Control Department of Shenzhen Center for Disease Control and Prevention.
Funding
This work was supported by Guangdong Basic and Applied Basic Research Foundation (2019B1515120003), the National Natural Science Foundation of China (81573211), San-Ming Project of Medicine in Shenzhen (SZSM201811071) and Shenzhen Key Medical Discipline Construction Fund (SZXK064).
Transparency declarations
All authors have no conflict of interest to declare.
Supplementary data
Table S1 is available as Supplementary data at JAC Online.
References
Author notes
Yue Zhu, Yuanmei Huang and Chenli Zheng contributed equally to this work.



