-
PDF
- Split View
-
Views
-
Cite
Cite
Yangfan Li, Kai Peng, Qiaojun Wang, Xia Xiao, Ruichao Li, Zhiqiang Wang, Characterization of a cfr-bearing integrative and conjugative element in Proteus cibarius coharbouring tet(X6) on the chromosome, Journal of Antimicrobial Chemotherapy, Volume 78, Issue 10, October 2023, Pages 2597–2599, https://doi.org/10.1093/jac/dkad220
Close - Share Icon Share
Plasmid- and chromosome-mediated multiresistance gene cfr and tigecycline resistance gene tet(X6) have been reported in Proteus spp. isolated from food-producing animals.1 Recently, two chromosome-mediated tet(X6) genes in Proteus cibarius were found in chicken faeces.2 In addition, integrative and conjugative elements (ICEs) carrying the cfr were identified in Proteus mirabilis isolated from swine-derived faeces and cloacae and could be transferred into Escherichia coli by conjugation, providing for the dissemination of antibiotic resistance genes (ARGs).3,4 Despite the high mobility of the tet(X6) and cfr genes, coexistence of two such genes on one chromosome has not been reported. In this study, we identified a strain CZP50 encoding the tet(X6) gene on the chromosome and the cfr gene on the ICEPciChnCZP50.
P. cibarius isolate CZP50 was isolated from a layer chicken faecal sample in March 2022 in Jiangsu, China. MICs were determined by broth microdilution and interpreted in accordance with the CLSI standards, using E. coli ATCC 25922 as the control. CZP50 was found to be resistant to tigecycline (MIC = 64 mg/L) in addition to intrinsic resistance to colistin (Table S1, available as Supplementary data at JAC Online), and also resistant to ciprofloxacin, ceftiofur, streptomycin and enrofloxacin.
To investigate the genomic characteristics of CZP50, the genomic DNA was extracted using the FastPure Bacteria DNA Isolation Mini Kit (Vazyme, China), and the complete genome sequence of CZP50 was obtained with a hybrid assembly strategy combining short-read Illumina sequencing and long-read Oxford nanopore sequencing.5,6 One chromosome, 4 074 672 bp in size and 38.4% in GC content was identified, and no plasmid existed in CZP50. The tet(X6) gene was found on a chromosomal MDR region (Figure 1a), which showed 96% coverage and 99% identity to the corresponding MDR region of isolates P. cibarius HNCF43W2 and ZJ19PC7 and showed about 75% coverage and 97% identity to that of many other isolates including P. cibarius G11 (Figure 1a). It is likely that this MDR region evolved from the G11 chromosome backbone structure. The phylogenetic analysis shows that CZP50 is closely related to the strains HNCF44W, HNCF43W and ZJ19PC isolated from chicken farms, but they were isolated from different cities in China (Figure S1). The tet(X6) gene, cfr gene and ICEs are likely to have undergone genetic recombination during transmission, resulting in the formation of CZP50. It is of great significance to focus on P. cibarius as an intermediate host for transmission of drug resistance genes in the future. Apart from the tet(X6)-bearing MDR region, one SXT/R391 ICE with a size of 118 536 bp and GC content of 48.0% was detected in the genome of CZP50, which was designated as ICEPciChnCZP50. BLASTn analysis showed ICEPciChnCZP50 shared 97% coverage and 99.99% identity to ICEPmiChnSTP3, indicating they might have a common ancestor (Figure 1b). ICEPmiChnSTP3 carried floR, strAB and sul2 resistance genes in VRIII.4 Compared with a typical ICEPmiChn1, ICEPciChnCZP50 lacked the tet(G)-tetR region and carried the floR, strAB and sul2 resistance genes in VRIII. Notably, an ISPpu12-mediated composite transposon with 35 651 bp was found in HS4 of ICEPciChnCZP50, which contained 14 ARGs, including the clinically important rRNA methyltransferase gene cfr (Figure 1b). As far as we know, this is the first report in P. cibarius that cfr and tet(X6) coexisted on one chromosome.
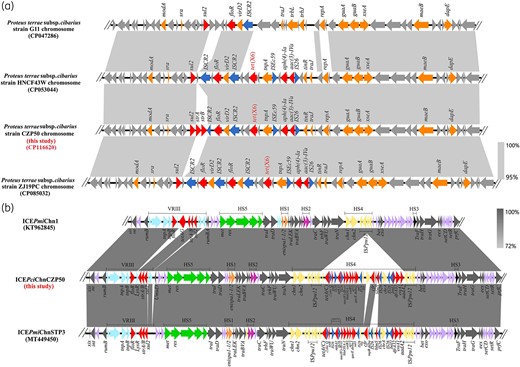
(a) Genetic environments of the tet(X6) gene in CZP50 (CP116620) chromosome and other similar P. cibarius chromosomal MDR regions. (b) Genetic structure comparison of cfr-bearing ICEPciChnCZP50 with ICEPmiChn1 (KT962845) and ICEPmiChnSTP3 (MT449450). The arrows indicate the direction of gene transcription. The hotspots (HS1 to HS5) and variable region (VRIII) are shown with different colours: HS1, orange; HS2, purple; HS3, black; HS4, yellow; HS5, green; and VRIII, light blue. Resistance genes are in red, and transposase or integrase genes within the ISPpu12-mediated composite transposon are in blue. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
A conjugation assay was conducted using rifampicin-resistant E. coli C600 as the recipient to investigate the transferability of tet(X6) and the cfr-bearing ICE. The donor and recipient strains were cultured into the logarithmic growth phase with an OD600 value of 0.4 in LB broth and then mixed at a ratio of 1:4, followed by culturing overnight on LB agar plates at 37°C. Afterwards, the transconjugants were screened on LB plates containing tigecycline (4 mg/L) or trimethoprim/sulfamethoxazole (4/76 mg/L) with rifampicin (300 mg/L), and confirmed by PCR (Table S2). The conjugation experiment showed that ICEPciChnCZP50 could be successfully transferred to E. coli C600 at a frequency of 9.25 × 10−6 ± 0.28 × 10−6 transconjugants per donor (average of three independent determinations) (Figure S2), but failed to recover transconjugants positive for tet(X6). The acquisition of the mobile genetic element was considered to have a direct biological cost.8 The fitness cost of ICEPciChnCZP50 was investigated by growth curves, in vitro competition, biofilm formation capacity, motility and stability assays as previously reported.9,10 The in vitro competition assay, motility assay and stability assay showed no significant fitness cost when the recipient strain acquired ICEPciChnCZP50 (Figure S3a, b and c). Interestingly, the transconjugants differed significantly in biofilm formation capacity compared with the recipient strain (Figure S3d), but was not slowed down in in vitro growth assays (Figure S3e). We hypothesized that the cause of this phenomenon might be that ICEs had the adaptive advantage to C600 to enable it to exist stably.
To conclude, this study identified the emergence of critical resistance genes tet(X6) and cfr converged on the same chromosome by different MDR regions in Proteus. As an important reservoir of ARGs, it is necessary to continue monitoring Proteus harbouring important ARGs and the role of SXT/R391 ICEs in the transmission and evolution of resistance genes.
Funding
This work was supported by the National Natural Science Foundation of China (32161133005), the Sichuan Science and Technology Program (no. 22ZDZX0011) and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Transparency declarations
The authors declare that there are no conflicts of interest.
Data availability
The complete genome sequence of CZP50 was submitted in the figshare database (https://dx.doi.org/10.6084/m9.figshare.21921936) and the NCBI database with accession number (CP116620).
Supplementary data
Figures S1 to S3 and Tables S1 and S2 are available as Supplementary data at JAC Online.
References
Author notes
Ruichao Li and Zhiqiang Wang supervised this work equally.



