-
PDF
- Split View
-
Views
-
Cite
Cite
Alice-Andrée Mariaggi, Rebecca Bauer, Caroline Charre, Elise Gardiennet, Vincent Meiffredy, Faiza Ajana, Karine Lacombe, Gilles Pialoux, Eric Cua, Christine Rouzioux, Laurence Meyer, Antoine Cheret, Véronique Avettand-Fenoel, HIV-1-RNA and total HIV-1-DNA loads in the genital compartment in men receiving dolutegravir- versus darunavir-based combined ART (cART) regimens during primary HIV infection, Journal of Antimicrobial Chemotherapy, Volume 77, Issue 3, March 2022, Pages 735–739, https://doi.org/10.1093/jac/dkab427
Close - Share Icon Share
Abstract
Dolutegravir is a widespread integrase strand-transfer inhibitor (INSTI) recommended for treatment of primary HIV infection (PHI). PHI is a high-risk stage for sexual transmission because of the high viral load in semen. Yet dolutegravir concentrations in semen are lower than in blood during chronic treatment.
To compare the kinetics of HIV-RNA and total HIV-DNA in the genital compartment in subjects receiving either tenofovir/emtricitabine/dolutegravir or tenofovir/emtricitabine/darunavir/cobicistat as a first-line combined ART (cART) at the time of PHI.
Eighteen subjects receiving tenofovir/emtricitabine/dolutegravir and 19 receiving tenofovir/emtricitabine/darunavir/cobicistat enrolled in the ANRS169 OPTIPRIM-2 trial participated in the genital substudy.
Between week (W) 0 and W2 HIV-RNA in seminal plasma (SP) decreased by 1 log10 copies/mL. Undetectable SP HIV-RNA was achieved in similar proportions between the two regimens at each timepoint. Overall, eight patients still presented detectable HIV-RNA or HIV-DNA in semen at W48; 15.4% and 28.6% presented detectable HIV-RNA and 9.1% and 14.3% presented detectable HIV-DNA in dolutegravir- and darunavir-based cART groups, respectively, with no significant difference.
For the first time, to the best of our knowledge, we showed that a dolutegravir-based regimen initiated as soon as PHI reduces HIV-RNA and HIV-DNA with no difference compared with a control group receiving a darunavir-based regimen. Although the viral purge in semen seems longer after treatment in PHI than CHI, due to high viral loads, early dolutegravir-based treatment initiation permits a major decay of both viral particles and infected cells in semen, efficiently reducing the high risk of transmission during PHI.
Introduction
Treatment of subjects infected with HIV as soon as the time of primary HIV infection (PHI) is essential to preserve immune function, to limit the viral reservoir and to prevent transmission. The main risk of HIV transmission remains sexual contact.1 High HIV-RNA load in semen during the acute phase of infection is associated with an increased risk of sexual transmission.2,3
Dolutegravir is a widespread integrase strand-transfer inhibitor (INSTI) with a high genetic barrier. Considering the benefit of early treatment, dolutegravir has recently been recommended for the treatment of PHI.4,5 As dolutegravir is highly bound to plasma proteins and its concentration in the genital tract is lower than in blood plasma (BP),6 its impact on the genital compartment has to be explored. Most studies have focused on the impact of dolutegravir initiation in subjects with chronic HIV infection (CHI)7,8 or in switch therapy.9 These studies have shown that dolutegravir is reliable to suppress or maintain the suppression of HIV replication in the genital tract.
Herein, we aimed to compare the kinetics of semen HIV-RNA and HIV-DNA in subjects of a substudy of the ANRS169 OPTIPRIM-2 trial, receiving either tenofovir/emtricitabine/dolutegravir or tenofovir/emtricitabine/darunavir/cobicistat as a first-line combined antiretroviral therapy (cART) since PHI.
Patients and methods
ANRS169 OPTIPRIM-2 is a randomized multicentre trial including subjects diagnosed during PHI (https://clinicaltrials.gov/ct2/show/NCT02987530?term=optiprim&draw=2&rank=2).10 One hundred and one participants were enrolled to evaluate the impact of tenofovir/emtricitabine/dolutegravir (n = 51) versus tenofovir/emtricitabine/darunavir/cobicistat (n = 50) at the time of early PHI on the HIV reservoir during 48 weeks. The objective of the genital compartment substudy was to include 20 subjects from each group. Participants were proposed to participate in the substudy if they met additional requirements. Eighteen subjects receiving dolutegravir-based cART and 19 subjects receiving darunavir-based cART agreed to participate in this genital substudy. Semen and blood samples were collected at inclusion and/or week (W) 2, W4, W12, W24 and W48. A collection container (‘Clinisperm’) was delivered to participants at W0. The participants could either collect and return a semen sample at W0 or at the next visit (W2). One participant of each group unexpectedly collected semen samples both at W0 and W2. After baseline, containers were delivered for the next timepoints W4, W12, W24 and W48.
All samples collected in each participating centre were centralized in the Necker Hospital virology laboratory (Paris, France) where cells and seminal plasma (SP) were separated by Ficoll-Hypaque and frozen at −80°C. HIV-RNA was quantified in SP with the Cobas Ampliprep Cobas Taqman v2 assay (Roche, France). The median limit of quantification (LOQ) was 57 copies/mL (1.76 log10 copies/mL) (range: from 12 to 408 copies/mL according to the SP available). Total DNA was extracted from semen cells with the QIAamp DNA Microkit (Qiagen, Courtaboeuf, France). Total HIV-DNA was quantified with an ultrasensitive real-time PCR method (two to four replicates) (Generic HIV-DNA Assay, Biocentric, Bandol, France) with a median LOQ of 141 copies/106 cells (range: from 7 to 14 301 copies/106 cells depending on the number of cells available).11,12 Only HIV-RNA was quantified for semen samples received in insufficient quantities.
For statistical analysis, positive data below the LOQ were arbitrarily set at a value equal to half the LOQ and a value of 0.5 copies/mL or 0.5 copies/106 cells was attributed to data below the limit of detection (LOD). To compare the distributions of the two groups, we used the Wilcoxon test. Proportions were compared using a Fisher test (Prism 8.0, GraphPad). Viral loads are expressed as median (IQR).
Results
Partition of subjects according to Fiebig stage13 and regimen type was as follows: Fiebig II, zero and two receiving dolutegravir- and darunavir-based cART, respectively; Fiebig III, one in each group; Fiebig IV, six in each group; Fiebig V, eight and nine receiving dolutegravir- and darunavir-based cART, respectively; and Fiebig VI, two in each group. BP HIV-RNA loads of subjects enrolled in the substudy were comparable between the two groups at W0 [5.33 (4.64–6.41) and 5.71 (4.94–6.80) log10 copies/mL for subjects receiving dolutegravir- and darunavir-based regimens, respectively, P = 0.42]. The median HIV-DNA level in blood at W0 was 3.45 (3.06–3.99) and 3.96 (3.37–4.21) log10 copies/106 PBMCs for subjects receiving dolutegravir- and darunavir-based regimens, respectively.
Semen samples were collected at inclusion for 14 subjects, W2 for 14 subjects, W4 for 32 subjects, W12 for 26 subjects, W24 for 28 subjects and W48 for 29 subjects, as detailed in Table S1 (available as Supplementary data at JAC Online). At W0, SP HIV-RNA loads were 5.22 (3.45–6.21) and 4.96 (4.49–5.82) log10 copies/mL for subjects receiving dolutegravir- and darunavir-based regimens, respectively (Figure 1a). Between W0 and W2, we observed a decrease in SP HIV-RNA of 1.38 and 1.45 log10 copies/mL in the groups receiving dolutegravir- and darunavir-based cART, respectively (unpaired data). For two patients with semen samples available at W0 and W2, we observed a decrease in SP HIV-RNA of 0.93 log10 copies/mL for the one receiving dolutegravir-based cART and 0.97 log10 copies/mL for the one receiving darunavir-based cART. Considering the decrease in HIV-RNA, we did not merge W0 and W2 as baseline results and analysed these timepoints separately. Undetectable HIV-RNA was achieved in similar proportions between the two groups at each timepoint (Figure 1a). Overall, SP HIV-RNA remained positive in 30% of samples at W24. At W48, SP HIV-RNA remained positive in six subjects; two receiving dolutegravir (1.79 and 2.94 log10 copies/mL) and four receiving darunavir (from 1.50 to 3.28 log10 copies/mL) (Table 1).
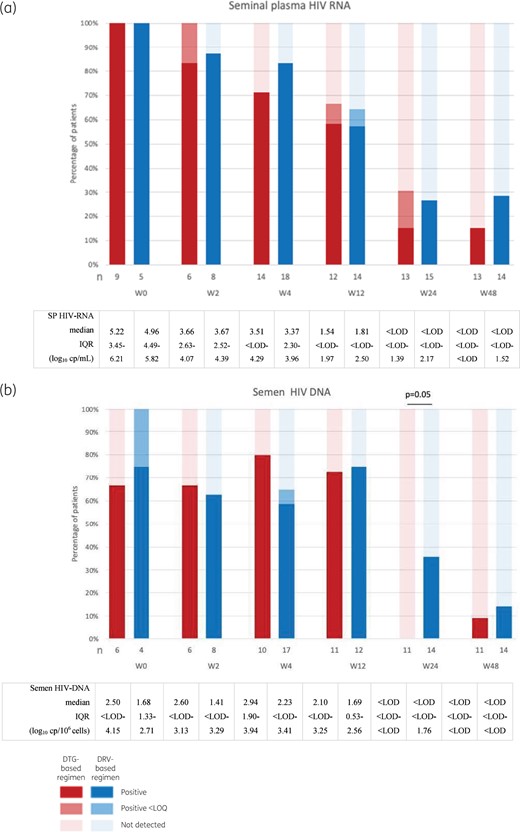
Percentage of patients with positive, positive <LOQ and negative HIV-RNA (a) and total HIV-DNA (b) in semen (from dark to light red and blue). Significant differences in percentages of subjects with undetectable viral load between the two groups are specified in the figure. DTG, dolutegravir; DRV, darunavir. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
HIV-RNA and total HIV-DNA loads in plasma and semen in patients with detectable HIV-RNA or HIV-DNA in semen at W48
| Subject ID . | Regimen type . | Semen HIV-1- RNA (log10 copies/mL) . | Semen total HIV-1-DNA (log10 copies/106 cells) . | Plasma HIV-1- RNA (log10 copies/mL) . | Blood total HIV-1-DNA (log10 copies/106 PBMCs) . | Observations . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| W0 . | W48 . | W0 . | W48 . | W0 . | W48 . | W0 . | W48 . | |||
| #1 | DTG | (W2) 2.75 | 1.79 | (W2) 2.84 | <3.20 | 4.16 | <0.52 | 3.22 | 2.76 | self-reported correct treatment adherence until W24; no response for W48 |
| #2 | DTG | 6.48 | 2.94 | NA | NA | 6.39 | 1.69 | 3.72 | 2.83 | self-reported adherence failures at W4 and W48 |
| #3 | DRV | NA | 2.40 | NA | 1.88 | 6.45 | detected <0.52 | 4.21 | 2.74 | self-reported adherence failures at W4 and W24 |
| #4 | DRV | 6.04 | 1.57 | 1.59 | <1.50 | 5.74 | 1.00 | 3.96 | 2.80 | self-reported adherence failures at W8 and W24 |
| #5 | DRV | NA | 3.28 | NA | <2.11 | 6.89 | 2.20 | 4.26 | 3.56 | self-reported correct treatment adherence until W12; no responses for W24 and W48; STI W24, W36 and W48 |
| #6 | DRV | NA | 1.50 | NA | <1.99 | 5.85 | 1.75 | 4.05 | 3.15 | self-reported adherence failures at W8, W24, W36 and W48; STI W24 |
| #7 | DRV | (W2) <1.71 | <2.13 | (W2) <1.71 | 2.36 | 4.94 | 0.48 | 3.18 | 2.39 | self-reported adherence failures at W4, W8, W36 and W48; STI W36 |
| #8 | DTG | NA | <2.01 | NA | 2.57 | 5.31 | 1.18 | 3.76 | 2.76 | self-reported adherence failures at each timepoint |
| Subject ID . | Regimen type . | Semen HIV-1- RNA (log10 copies/mL) . | Semen total HIV-1-DNA (log10 copies/106 cells) . | Plasma HIV-1- RNA (log10 copies/mL) . | Blood total HIV-1-DNA (log10 copies/106 PBMCs) . | Observations . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| W0 . | W48 . | W0 . | W48 . | W0 . | W48 . | W0 . | W48 . | |||
| #1 | DTG | (W2) 2.75 | 1.79 | (W2) 2.84 | <3.20 | 4.16 | <0.52 | 3.22 | 2.76 | self-reported correct treatment adherence until W24; no response for W48 |
| #2 | DTG | 6.48 | 2.94 | NA | NA | 6.39 | 1.69 | 3.72 | 2.83 | self-reported adherence failures at W4 and W48 |
| #3 | DRV | NA | 2.40 | NA | 1.88 | 6.45 | detected <0.52 | 4.21 | 2.74 | self-reported adherence failures at W4 and W24 |
| #4 | DRV | 6.04 | 1.57 | 1.59 | <1.50 | 5.74 | 1.00 | 3.96 | 2.80 | self-reported adherence failures at W8 and W24 |
| #5 | DRV | NA | 3.28 | NA | <2.11 | 6.89 | 2.20 | 4.26 | 3.56 | self-reported correct treatment adherence until W12; no responses for W24 and W48; STI W24, W36 and W48 |
| #6 | DRV | NA | 1.50 | NA | <1.99 | 5.85 | 1.75 | 4.05 | 3.15 | self-reported adherence failures at W8, W24, W36 and W48; STI W24 |
| #7 | DRV | (W2) <1.71 | <2.13 | (W2) <1.71 | 2.36 | 4.94 | 0.48 | 3.18 | 2.39 | self-reported adherence failures at W4, W8, W36 and W48; STI W36 |
| #8 | DTG | NA | <2.01 | NA | 2.57 | 5.31 | 1.18 | 3.76 | 2.76 | self-reported adherence failures at each timepoint |
NA, not available; DTG, dolutegravir-based regimen; DRV, darunavir-based regimen; STI, sexually transmitted infection.
HIV-RNA and total HIV-DNA loads in plasma and semen in patients with detectable HIV-RNA or HIV-DNA in semen at W48
| Subject ID . | Regimen type . | Semen HIV-1- RNA (log10 copies/mL) . | Semen total HIV-1-DNA (log10 copies/106 cells) . | Plasma HIV-1- RNA (log10 copies/mL) . | Blood total HIV-1-DNA (log10 copies/106 PBMCs) . | Observations . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| W0 . | W48 . | W0 . | W48 . | W0 . | W48 . | W0 . | W48 . | |||
| #1 | DTG | (W2) 2.75 | 1.79 | (W2) 2.84 | <3.20 | 4.16 | <0.52 | 3.22 | 2.76 | self-reported correct treatment adherence until W24; no response for W48 |
| #2 | DTG | 6.48 | 2.94 | NA | NA | 6.39 | 1.69 | 3.72 | 2.83 | self-reported adherence failures at W4 and W48 |
| #3 | DRV | NA | 2.40 | NA | 1.88 | 6.45 | detected <0.52 | 4.21 | 2.74 | self-reported adherence failures at W4 and W24 |
| #4 | DRV | 6.04 | 1.57 | 1.59 | <1.50 | 5.74 | 1.00 | 3.96 | 2.80 | self-reported adherence failures at W8 and W24 |
| #5 | DRV | NA | 3.28 | NA | <2.11 | 6.89 | 2.20 | 4.26 | 3.56 | self-reported correct treatment adherence until W12; no responses for W24 and W48; STI W24, W36 and W48 |
| #6 | DRV | NA | 1.50 | NA | <1.99 | 5.85 | 1.75 | 4.05 | 3.15 | self-reported adherence failures at W8, W24, W36 and W48; STI W24 |
| #7 | DRV | (W2) <1.71 | <2.13 | (W2) <1.71 | 2.36 | 4.94 | 0.48 | 3.18 | 2.39 | self-reported adherence failures at W4, W8, W36 and W48; STI W36 |
| #8 | DTG | NA | <2.01 | NA | 2.57 | 5.31 | 1.18 | 3.76 | 2.76 | self-reported adherence failures at each timepoint |
| Subject ID . | Regimen type . | Semen HIV-1- RNA (log10 copies/mL) . | Semen total HIV-1-DNA (log10 copies/106 cells) . | Plasma HIV-1- RNA (log10 copies/mL) . | Blood total HIV-1-DNA (log10 copies/106 PBMCs) . | Observations . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| W0 . | W48 . | W0 . | W48 . | W0 . | W48 . | W0 . | W48 . | |||
| #1 | DTG | (W2) 2.75 | 1.79 | (W2) 2.84 | <3.20 | 4.16 | <0.52 | 3.22 | 2.76 | self-reported correct treatment adherence until W24; no response for W48 |
| #2 | DTG | 6.48 | 2.94 | NA | NA | 6.39 | 1.69 | 3.72 | 2.83 | self-reported adherence failures at W4 and W48 |
| #3 | DRV | NA | 2.40 | NA | 1.88 | 6.45 | detected <0.52 | 4.21 | 2.74 | self-reported adherence failures at W4 and W24 |
| #4 | DRV | 6.04 | 1.57 | 1.59 | <1.50 | 5.74 | 1.00 | 3.96 | 2.80 | self-reported adherence failures at W8 and W24 |
| #5 | DRV | NA | 3.28 | NA | <2.11 | 6.89 | 2.20 | 4.26 | 3.56 | self-reported correct treatment adherence until W12; no responses for W24 and W48; STI W24, W36 and W48 |
| #6 | DRV | NA | 1.50 | NA | <1.99 | 5.85 | 1.75 | 4.05 | 3.15 | self-reported adherence failures at W8, W24, W36 and W48; STI W24 |
| #7 | DRV | (W2) <1.71 | <2.13 | (W2) <1.71 | 2.36 | 4.94 | 0.48 | 3.18 | 2.39 | self-reported adherence failures at W4, W8, W36 and W48; STI W36 |
| #8 | DTG | NA | <2.01 | NA | 2.57 | 5.31 | 1.18 | 3.76 | 2.76 | self-reported adherence failures at each timepoint |
NA, not available; DTG, dolutegravir-based regimen; DRV, darunavir-based regimen; STI, sexually transmitted infection.
Median semen total HIV-DNA loads were 2.50 (<LOD–4.15) and 1.68 (1.33–2.71) log10 copies/106 cells for subjects receiving dolutegravir- and darunavir-based regimens at W0, respectively (Figure 1b). Undetectable HIV-DNA was achieved in a similar proportion at W4 (P = 0.67) and W12 (P > 0.99) for subjects receiving dolutegravir- and darunavir-based cART, respectively (Figure 1b). Nevertheless, undetectable semen total HIV-DNA was achieved in a higher proportion of subjects receiving dolutegravir-based cART compared with darunavir-based cART at W24 (P = 0.05) (Figure 1b). At W48, similar proportions of undetectable HIV-DNA were achieved in semen in the two groups; HIV-DNA was detected in one subject receiving dolutegravir-based cART (HIV-DNA was unfortunately not available for previous timepoints) and in two subjects receiving darunavir-based cART (Table 1).
Overall, for the eight participants presenting detectable HIV-RNA and/or HIV-DNA in semen at W48, blood HIV-RNA or HIV-DNA at W0 was not significantly higher than that observed for participants with undetectable HIV-RNA and HIV-DNA in semen at W48 (5.79 versus 5.35 log10 copies/mL, P = 0.53, and 3.87 versus 3.52 log10 copies/106 PBMCs, P = 0.46, respectively). Semen HIV-RNA at W0, quantified for two out of these eight participants, was higher than that observed for participants with undetectable HIV-RNA or HIV-DNA in semen at W48 (6.31 versus 4.88 log10 copies/mL, P = 0.04). At W48, HIV-DNA in blood was higher for these eight participants (2.78 versus 2.23 log10 copies/mL, P < 0.01) compared with other participants, while BP HIV-RNA was not (1.09 versus 0.84 log10 copies/106 PBMCs, P = 0.39) (Table 1). Lack of adherence to treatment was reported for six out of eight participants in the self-reporting questionnaires. To note, patients 5, 6 and 7 presented sexually transmitted infection during the study.
Discussion
This study compared the impact of a dolutegravir- versus darunavir-based regimen initiated during PHI on virological parameters in semen. The risk of HIV transmission related to SP HIV-RNA seems correlated with BP HIV-RNA;2,3,14–16 however, BP and SP decays after treatment do not completely overlap.7 Considering the high BP and SP HIV-RNA loads during PHI, initiation of a treatment leading to a rapid decrease in HIV-RNA is challenging to reduce HIV transmission. Cheret et al.15 showed the interest of an early treatment to reduce the risk of transmission and limit the establishment of HIV reservoir cells in semen. Interestingly, we observed an early major decay of SP HIV-RNA between W0 and W2 with a second-generation INSTI-based triple therapy and a protease inhibitor (PI)-based triple therapy. For the first time, to the best of our knowledge, we showed a similar percentage of patients with residual HIV-RNA or HIV-DNA in semen at W48 in the two regimens initiated since PHI.
Nevertheless, the proportion of undetectable SP HIV-RNA after treatment in PHI seemed lower than that observed after a dolutegravir-based regimen initiated during CHI at W12; 33.3% and 35.7%, respectively, for subjects receiving dolutegravir- and darunavir-based cART in PHI versus 66.7% with a dolutegravir-based regimen during CHI.8 High baseline viral loads observed during PHI might be responsible for this difference, as suggested by Fernandez-Gonzalez et al.17 Our results are in line with those of studies with a similar size. SP HIV-RNA was still detectable in 15.4% (95% CI 1.9%–45.4%) and 28.6% (95% CI 8.4%–58.1%) after 48 weeks of a dolutegravir-based regimen versus a darunavir-based regimen, respectively. Recently, Ghosn et al.18 observed semen HIV-RNA over their LOQ of 60 copies/mL for 10.5% of subjects at the end of follow-up (36 to 48 weeks), after initiating dolutegravir-based cART in PHI. The relatively small size sample in both studies associated with interindividual variability could explained the differences between percentages of detectable HIV-RNA.
At W48, some participants still had positive HIV-DNA in semen. HIV-DNA load reflects the presence of HIV-infected cells in semen. The risk of transmission may increase by cell–cell contact19 and virion production (reflected by HIV-RNA load). Overall, compliance, but also factors modulating local activation, such as sexually transmitted infections, could have negatively impacted the viral clearance in semen. A treatment duration of 48 weeks is probably too short to suppress HIV replication and infected cells in semen for some subjects initiating treatment in PHI, as previously suggested.15 These data may suggest that de-escalation treatment should not be considered too soon after PHI.
Collection of pretreatment semen samples was challenging at the PHI diagnosis. Although the sample size of our study is small, it is part of a randomized study with a group control. Nevertheless, we successfully assessed 14 samples at W0 and 14 samples at W2. Moreover, two patients collected a semen sample at both W0 and W2, allowing specification of the rapid HIV-RNA decay.
For the first time, to the best of our knowledge, we showed that a dolutegravir-based regimen reduces HIV-RNA as well as HIV-DNA present in the male genital compartment at the time of PHI with no difference compared with a control group receiving a darunavir-based regimen in a randomized study. Although the viral purge in semen could be longer after treatment in PHI than CHI, due to high viral loads observed during PHI, early dolutegravir-based treatment initiation permits a major decay of both viral particles and infected cells in semen, reducing the high risk of sexual transmission at the onset of infection.
Funding
The ANRS OPTIPRIM2 trial is sponsored by the French National Agency for Research on AIDS and Viral Hepatitis (ANRS). This work was funded by the ANRS. The funder of the study had no role in study design, data collection, data analysis, data interpretation, report writing or the decision to submit for publication.
Transparency declarations
C.C. has received honoraria and travel grants from ViiV Healthcare, MSD, Gilead Sciences and Janssen-Cilag for participation in educational programmes and conferences. K.L. has received honoraria and travel grants from Gilead, MSD, AbbVie, ViiV and Janssen-Cilag for participation in educational programmes and conferences. G.P. has received honoraria and travel grants from Gilead, ViiV Healthcare, AbbVie, MSD, Janssen-Cilag and AAZ for participation in educational programmes and conferences. A.C. has received grants (to her institution) from the ANRS, Gilead Sciences, ViiV Healthcare and Janssen, and honoraria and travel grants from ViiV Healthcare, Gilead Sciences and Janssen-Cilag for participation in educational programmes and conferences. V.A.-F. has received grants (to her institution) from the ANRS and the MSD AVENIR Foundation, and honoraria and travel grants from ViiV Healthcare, Gilead Sciences and Janssen-Cilag for participation in educational programmes and conferences. All other authors: none to declare.
Supplementary data
Table S1 is available as Supplementary data at JAC Online.
References
Author notes
Antoine Cheret and Véronique Avettand-Fenoel contributed equally to the work.



