-
PDF
- Split View
-
Views
-
Cite
Cite
Bernadette Jakeman, Alexandra Scherrer, Manuel Battegay, Huldrych F. Gunthard, Anna Hachfeld, Alexandra Calmy, Patrick Schmid, Enos Bernasconi, Matthias Cavassini, Catia Marzolini, on behalf of the Swiss HIV Cohort Study, Anticholinergic medication use in elderly people living with HIV and self-reported neurocognitive impairment: a prospective cohort study, Journal of Antimicrobial Chemotherapy, Volume 77, Issue 2, February 2022, Pages 492–499, https://doi.org/10.1093/jac/dkab386
Close - Share Icon Share
Abstract
Anticholinergic (ACH) medications have been associated with neurocognitive impairment, particularly in the elderly. This study determined prospectively the prevalence of prescribed ACH medications and their association with self-reported neurocognitive impairment (SRNI) in elderly people living with HIV (PLWH) of the Swiss HIV Cohort Study (SHCS).
A literature review was performed to identify ACH medications, which were scored 0 to 3 (higher score indicating more ACH burden). Prescriptions were reviewed in July 2019 for all SHCS participants ≥65 years old to assess the prevalence of ACH medications. Association between ACH burden and neurocognitive impairment was evaluated using the SHCS SRNI questions addressing memory loss, attention difficulties and slowing in reasoning.
One thousand and nineteen PLWH (82% male) with a median age of 70 (IQR = 67–74) years were included. Most participants were on ART (99%). The average number of non-HIV drugs was 5.1 ± 3.6, representing a polypharmacy prevalence of 50%. Two hundred participants (20%) were on ≥1 ACH medication, with an average ACH score of 1.7 ± 1.3. SRNI, adjusted for age, sex, CD4, nadir CD4, viral load, efavirenz use and polypharmacy, was associated with depression (OR = 4.60; 95% CI = 2.62–8.09) and a trend was observed with being on ≥1 ACH medication (OR = 1.69; 95% CI = 0.97–2.95). In a subgroup analysis of participants without depression (n = 911), SRNI was associated with the use of ≥1 ACH medication (OR = 2.51; 95% CI = 1.31–4.80).
ACH medication use is common in elderly PLWH and contributes to SRNI. The effect of ACH medications on neurocognitive impairment warrants further evaluation using neurocognitive tests.
Introduction
The use of medications with anticholinergic (ACH) activity has been associated with neurocognitive impairment, including dementia.1,2 Elderly patients are at increased risk for both peripheral and CNS side effects of ACH medications due to a decrease in cholinergic neurons or receptors in the brain, decreased ACH drug metabolism and elimination, and increased permeability of the blood–brain barrier.3 In addition, the number of prescribed medications and rate of polypharmacy (≥5 medications) increases with age, leading to a higher risk of inappropriate prescribing and ACH medication exposure.4
Many commonly used medications have ACH activity (e.g. antidepressants, antipsychotics and antihistamines). Concomitant use of ACH medications may result in increased cumulative ACH burden and medication side effects. There is a lack of consensus, however, regarding the most appropriate method for identifying ACH drugs and calculating ACH burden.5–16 Scales differ in medication inclusion and burden classification. For example, quetiapine and paroxetine receive an ACH burden score of 3 on the Anticholinergic Cognitive Burden Scale,11 but both are scored as a 1 on the Anticholinergic Risk Scale7 and a 0 and 1, respectively, on the Anticholinergic Drug Scale.8 In addition, scales may not include medications due to date of development or country of origin, potentially underestimating ACH burden and limiting generalizability. Conversely, some scales may include medications for which there is no evidence for ACH activity.
In people living with HIV (PLWH) rates of neurocognitive impairment remain high despite advances in HIV therapy.17–19 Risk factors for neurocognitive impairment identified in PLWH include duration of HIV infection, CD4 cell count, chronic inflammation, toxicity associated with ART, increased cardiovascular risk and depression, older age, unemployment and history of CNS infections.17,19–22 In addition, it was recently reported that use of ACH medication is higher in PLWH compared with HIV-uninfected individuals.23
The aims of this study were to: (i) establish a medication list based on medications available in Switzerland and Europe with ACH activity that may contribute to ACH burden and neurocognitive impairment; (ii) determine the prevalence of prescribed ACH medications for patients ≥65 years of age within the Swiss HIV Cohort Study (SHCS); and (iii) evaluate the effect of ACH medication use on self-reported neurocognitive impairment (SRNI).
Methods
Establishment of the ACH medication list
A literature review was performed using PubMed/MEDLINE® to identify commonly used ACH medication lists, scales and prescribing tools for the elderly (e.g. Beers criteria).15 ACH medications were then excluded if they were available only in otic, ophthalmic or topical formulations or if they were not listed in the Electronic Medicines Compendium (EMC).24 The EMC is reviewed by the UK Medicines and Healthcare Products Regulatory Agency (MHRA) and the EMA, which are medication licensing agencies. Excluded ACH medications licensed by the FDA, but not listed in the EMC, included benztropine, brompheniramine, carbinoxamine, cyclobenzaprine, cyproheptadine, desipramine, dicyclomine, hyoscyamine, molindone, perphenazine and thiothixene. Exceptions were made for ACH medications that were available in Switzerland, but not listed in the EMC (e.g. clorazepate and chlorprothixene). These medications were not removed from the list of ACH medications. Further review of the literature and drug monographs was performed by two study investigators with expertise in pharmacology to identify ACH medications, considering ACH activity, supporting side effect profile and CNS penetration. Medications without supporting evidence were removed from the final ACH list. ACH medications on the final list were scored as having an ACH burden score of 1 to 3 based on evidence of ACH activity and potential cognitive side effects (i.e. score 1 = low ACH burden and score 3 = high ACH burden).
Study population and ACH medication use
The SHCS is a nationwide ongoing prospective multicentre cohort of individuals with HIV aged older than 16 years.25 The cohort collects information on sociodemographic characteristics, clinical course, laboratory data and medications, including both HIV and non-HIV medications.
Patients in the SHCS were included in the study if they were ≥65 years of age and were actively followed in the SHCS (≥2 visits within 12 months of review). A cross-sectional review of their medications was performed in July 2019 to identify ACH medication use using the ACH medication list developed by the study investigators. Additional data reviewed included demographics, non-ACH medications, clinical chemistry laboratory data and depression (diagnosed by SHCS physician, psychiatrist or other physician).
SRNI
Responses to three cognitive screening questions have been collected every year since 2013 during follow-up visits for patients enrolled in the SHCS.19,26 The screening questions address memory loss, attention difficulties and the slowing of reasoning ability. Responses to screening questions include ‘never’, ‘hardly ever’, ‘yes, definitely’ and ‘does not understand question’. SRNI was defined as a positive response (‘yes, definitely’) to ≥1 of the three screening questions.19
Statistical analysis
Patient characteristics were compared between patients with ACH medication use and patients with no ACH medication use. Differences were assessed using χ2 tests for categorical variables and Student’s t-test for continuous variables. Multivariable logistic regression was used to evaluate the impact of ACH burden on SRNI. The model was adjusted with the following variables: age, sex, CD4, nadir CD4, HIV viral load, polypharmacy, depression, efavirenz use and ACH medication use. P values <0.05 were considered statistically significant. Statistical analysis was conducted using SPSS version 27 2020.
Results
ACH medications
The review of the literature and the standardized evaluation of potential ACH activity data allowed us to identify 93 medications with ACH activity, as listed in Table 1. Of those, 51 medications have an ACH burden score of 1, 11 medications have an ACH burden score of 2 and 31 medications have an ACH burden score of 3. Medications identified as having ACH activity were mostly represented by anticholinergics (17%), antidepressants (17%), antipsychotics (16%), antihistamines (14%), benzodiazepines (6%), opioids (6%) and cardiovascular medications (5%). Seventy-nine of the ACH medications in the final ACH medication list were available for use in Switzerland.
| ACH score 3 | ACH score 1 |
| amitriptyline | alprazolam |
| atropine | alimemazinea |
| chlorpheniramine/chlorphenamine | asenapine |
| chlorpromazinea | bupropion |
| chlorprothixeneb | celecoxib |
| clemastine | cetirizine |
| clomipramine | chlorthalidone |
| clozapine | cimetidinea |
| diphenhydramine | citalopram |
| doxepin | clidiniumb |
| doxylamine | clorazepateb |
| flavoxate | clothiapineb |
| hydroxyzine | codeine |
| imipraminea | desloratadine |
| meclizineb | diamorphine |
| methotrimeprazine/levomepromazine | diazepam |
| nortriptyline | digoxin |
| olanzapine | dihydrocodeine |
| orphenadrinea | disopyramidea |
| oxybutynin | duloxetine |
| paroxetine | escitalopram |
| procyclidine | fentanyl |
| promethazinea | fluoxetine |
| propanthelinea | fluphenazinea |
| scopolamine/hyoscine | fluvoxamine |
| solifenacin | furosemide |
| tolterodine | glycopyrronium |
| trihexyphenidyl | haloperidol |
| trimipramine | hydrocortisone |
| triprolidine | ipratropium |
| trospium | isosorbide |
| levocetirizine | |
| ACH score 2 | lithium |
| amantadine | loratadine |
| carbamazepine | methocarbamola |
| darifenacin | midazolam |
| fesoterodine | mirtazapine |
| meperidine/pethidine | morphine |
| opipramolb | nifedipine |
| oxcarbazepine | paliperidone |
| pimozidea | prednisolone |
| prochlorperazinea | prednisoneb |
| quetiapine | propiverinea |
| trifluoperazinea | ranitidine |
| risperidone | |
| sertraline | |
| temazepam | |
| theophyllineb | |
| triazolamb | |
| valproic acid (divalproex sodium) | |
| warfarin |
| ACH score 3 | ACH score 1 |
| amitriptyline | alprazolam |
| atropine | alimemazinea |
| chlorpheniramine/chlorphenamine | asenapine |
| chlorpromazinea | bupropion |
| chlorprothixeneb | celecoxib |
| clemastine | cetirizine |
| clomipramine | chlorthalidone |
| clozapine | cimetidinea |
| diphenhydramine | citalopram |
| doxepin | clidiniumb |
| doxylamine | clorazepateb |
| flavoxate | clothiapineb |
| hydroxyzine | codeine |
| imipraminea | desloratadine |
| meclizineb | diamorphine |
| methotrimeprazine/levomepromazine | diazepam |
| nortriptyline | digoxin |
| olanzapine | dihydrocodeine |
| orphenadrinea | disopyramidea |
| oxybutynin | duloxetine |
| paroxetine | escitalopram |
| procyclidine | fentanyl |
| promethazinea | fluoxetine |
| propanthelinea | fluphenazinea |
| scopolamine/hyoscine | fluvoxamine |
| solifenacin | furosemide |
| tolterodine | glycopyrronium |
| trihexyphenidyl | haloperidol |
| trimipramine | hydrocortisone |
| triprolidine | ipratropium |
| trospium | isosorbide |
| levocetirizine | |
| ACH score 2 | lithium |
| amantadine | loratadine |
| carbamazepine | methocarbamola |
| darifenacin | midazolam |
| fesoterodine | mirtazapine |
| meperidine/pethidine | morphine |
| opipramolb | nifedipine |
| oxcarbazepine | paliperidone |
| pimozidea | prednisolone |
| prochlorperazinea | prednisoneb |
| quetiapine | propiverinea |
| trifluoperazinea | ranitidine |
| risperidone | |
| sertraline | |
| temazepam | |
| theophyllineb | |
| triazolamb | |
| valproic acid (divalproex sodium) | |
| warfarin |
ACH score 3, greatest ACH burden; ACH score 1, least ACH burden.
Medications included in the EMC, but not available in Switzerland.
Medications omitted from the EMC, but available in Switzerland.
| ACH score 3 | ACH score 1 |
| amitriptyline | alprazolam |
| atropine | alimemazinea |
| chlorpheniramine/chlorphenamine | asenapine |
| chlorpromazinea | bupropion |
| chlorprothixeneb | celecoxib |
| clemastine | cetirizine |
| clomipramine | chlorthalidone |
| clozapine | cimetidinea |
| diphenhydramine | citalopram |
| doxepin | clidiniumb |
| doxylamine | clorazepateb |
| flavoxate | clothiapineb |
| hydroxyzine | codeine |
| imipraminea | desloratadine |
| meclizineb | diamorphine |
| methotrimeprazine/levomepromazine | diazepam |
| nortriptyline | digoxin |
| olanzapine | dihydrocodeine |
| orphenadrinea | disopyramidea |
| oxybutynin | duloxetine |
| paroxetine | escitalopram |
| procyclidine | fentanyl |
| promethazinea | fluoxetine |
| propanthelinea | fluphenazinea |
| scopolamine/hyoscine | fluvoxamine |
| solifenacin | furosemide |
| tolterodine | glycopyrronium |
| trihexyphenidyl | haloperidol |
| trimipramine | hydrocortisone |
| triprolidine | ipratropium |
| trospium | isosorbide |
| levocetirizine | |
| ACH score 2 | lithium |
| amantadine | loratadine |
| carbamazepine | methocarbamola |
| darifenacin | midazolam |
| fesoterodine | mirtazapine |
| meperidine/pethidine | morphine |
| opipramolb | nifedipine |
| oxcarbazepine | paliperidone |
| pimozidea | prednisolone |
| prochlorperazinea | prednisoneb |
| quetiapine | propiverinea |
| trifluoperazinea | ranitidine |
| risperidone | |
| sertraline | |
| temazepam | |
| theophyllineb | |
| triazolamb | |
| valproic acid (divalproex sodium) | |
| warfarin |
| ACH score 3 | ACH score 1 |
| amitriptyline | alprazolam |
| atropine | alimemazinea |
| chlorpheniramine/chlorphenamine | asenapine |
| chlorpromazinea | bupropion |
| chlorprothixeneb | celecoxib |
| clemastine | cetirizine |
| clomipramine | chlorthalidone |
| clozapine | cimetidinea |
| diphenhydramine | citalopram |
| doxepin | clidiniumb |
| doxylamine | clorazepateb |
| flavoxate | clothiapineb |
| hydroxyzine | codeine |
| imipraminea | desloratadine |
| meclizineb | diamorphine |
| methotrimeprazine/levomepromazine | diazepam |
| nortriptyline | digoxin |
| olanzapine | dihydrocodeine |
| orphenadrinea | disopyramidea |
| oxybutynin | duloxetine |
| paroxetine | escitalopram |
| procyclidine | fentanyl |
| promethazinea | fluoxetine |
| propanthelinea | fluphenazinea |
| scopolamine/hyoscine | fluvoxamine |
| solifenacin | furosemide |
| tolterodine | glycopyrronium |
| trihexyphenidyl | haloperidol |
| trimipramine | hydrocortisone |
| triprolidine | ipratropium |
| trospium | isosorbide |
| levocetirizine | |
| ACH score 2 | lithium |
| amantadine | loratadine |
| carbamazepine | methocarbamola |
| darifenacin | midazolam |
| fesoterodine | mirtazapine |
| meperidine/pethidine | morphine |
| opipramolb | nifedipine |
| oxcarbazepine | paliperidone |
| pimozidea | prednisolone |
| prochlorperazinea | prednisoneb |
| quetiapine | propiverinea |
| trifluoperazinea | ranitidine |
| risperidone | |
| sertraline | |
| temazepam | |
| theophyllineb | |
| triazolamb | |
| valproic acid (divalproex sodium) | |
| warfarin |
ACH score 3, greatest ACH burden; ACH score 1, least ACH burden.
Medications included in the EMC, but not available in Switzerland.
Medications omitted from the EMC, but available in Switzerland.
Study population
A total of 1019 PLWH were included in the study. Table 2 describes patient characteristics. The majority of patients were male (n = 836, 82%) with a median age of 70 (IQR = 67–74) years and were virologically suppressed (HIV RNA <20 copies/mL) (n = 927, 91%). Most study participants were receiving ART (n = 1007, 99%), predominantly with integrase strand transfer inhibitor (INSTI)-based regimens (n = 512, 50%). A total of 512 participants (50%) met the criterion for polypharmacy (≥5 non-HIV medications); the average number of non-HIV drugs was 5.1 ± 3.6. A diagnosis of depression was made in 108 (11%) participants.
| Characteristic . | All patients (N = 1019) . | Patients with ACH drug (N = 200) . | Patients with no ACH drug (N = 819) . | Pa . |
|---|---|---|---|---|
| Age (years), median (IQR) | 70 (67-74) | 70 (67-75) | 70 (68-74) | 0.58 |
| Male, n (%) | 836 (82) | 157 (79) | 679 (83) | 0.15 |
| CD4 cell count <200 cells/mm3, n (%) | 34 (3) | 11 (6) | 23 (3) | 0.06 |
| Nadir CD4 cell count <200 cells/mm3, n (%) | 611 (60) | 119 (60) | 492 (60) | 0.88 |
| HIV-1 RNA <20 copies/mL, n (%) | 927 (91) | 180 (90) | 747 (91) | 0.56 |
| Diagnosis of depression, n (%) | 108 (11) | 66 (33) | 42 (5) | <0.001 |
| Antiretroviral drug classb, n (%) | ||||
| NNRTI based | 226 (22) | 37 (19) | 189 (23) | 0.47 |
| PI based | 48 (5) | 7 (4) | 41 (5) | |
| INSTI based | 512 (50) | 104 (52) | 408 (50) | |
| other | 221 (22) | 49 (25) | 172 (21) | |
| no antiretroviral | 12 (1) | 3 (2) | 9 (1) | |
| Efavirenz use, n (%) | 76 (7) | 11 (6) | 65 (8) | 0.24 |
| Dolutegravir use, n (%) | 428 (42) | 88 (44) | 340 (42) | 0.52 |
| Number of non-HIV medications, mean ± SD | 5.1 ± 3.6 | 8.0 ± 4.1 | 4.4 ± 3.1 | <0.001 |
| Polypharmacy (≥5 non-HIV medications), n (%) | 512 (50) | 162 (81) | 350 (43) | <0.001 |
| Characteristic . | All patients (N = 1019) . | Patients with ACH drug (N = 200) . | Patients with no ACH drug (N = 819) . | Pa . |
|---|---|---|---|---|
| Age (years), median (IQR) | 70 (67-74) | 70 (67-75) | 70 (68-74) | 0.58 |
| Male, n (%) | 836 (82) | 157 (79) | 679 (83) | 0.15 |
| CD4 cell count <200 cells/mm3, n (%) | 34 (3) | 11 (6) | 23 (3) | 0.06 |
| Nadir CD4 cell count <200 cells/mm3, n (%) | 611 (60) | 119 (60) | 492 (60) | 0.88 |
| HIV-1 RNA <20 copies/mL, n (%) | 927 (91) | 180 (90) | 747 (91) | 0.56 |
| Diagnosis of depression, n (%) | 108 (11) | 66 (33) | 42 (5) | <0.001 |
| Antiretroviral drug classb, n (%) | ||||
| NNRTI based | 226 (22) | 37 (19) | 189 (23) | 0.47 |
| PI based | 48 (5) | 7 (4) | 41 (5) | |
| INSTI based | 512 (50) | 104 (52) | 408 (50) | |
| other | 221 (22) | 49 (25) | 172 (21) | |
| no antiretroviral | 12 (1) | 3 (2) | 9 (1) | |
| Efavirenz use, n (%) | 76 (7) | 11 (6) | 65 (8) | 0.24 |
| Dolutegravir use, n (%) | 428 (42) | 88 (44) | 340 (42) | 0.52 |
| Number of non-HIV medications, mean ± SD | 5.1 ± 3.6 | 8.0 ± 4.1 | 4.4 ± 3.1 | <0.001 |
| Polypharmacy (≥5 non-HIV medications), n (%) | 512 (50) | 162 (81) | 350 (43) | <0.001 |
Determined using independent samples median test to compare medians, χ2 tests for categorical variables and Student’s t-test for continuous variables.
NNRTI-, PI- and INSTI-based treatments include only combinations of these anchor drug classes with 2 NRTIs. The group ‘other’ includes other drug class combinations, such as: PI + INSTI without or with 2 NRTIs or 1 NRTI; PI + INSTI + NNRTI without or with 2 NRTIs; PI + NNRTI with 1 or 2 NRTI(s); PI + 1 NRTI; and INSTI + 1 NRTI.
| Characteristic . | All patients (N = 1019) . | Patients with ACH drug (N = 200) . | Patients with no ACH drug (N = 819) . | Pa . |
|---|---|---|---|---|
| Age (years), median (IQR) | 70 (67-74) | 70 (67-75) | 70 (68-74) | 0.58 |
| Male, n (%) | 836 (82) | 157 (79) | 679 (83) | 0.15 |
| CD4 cell count <200 cells/mm3, n (%) | 34 (3) | 11 (6) | 23 (3) | 0.06 |
| Nadir CD4 cell count <200 cells/mm3, n (%) | 611 (60) | 119 (60) | 492 (60) | 0.88 |
| HIV-1 RNA <20 copies/mL, n (%) | 927 (91) | 180 (90) | 747 (91) | 0.56 |
| Diagnosis of depression, n (%) | 108 (11) | 66 (33) | 42 (5) | <0.001 |
| Antiretroviral drug classb, n (%) | ||||
| NNRTI based | 226 (22) | 37 (19) | 189 (23) | 0.47 |
| PI based | 48 (5) | 7 (4) | 41 (5) | |
| INSTI based | 512 (50) | 104 (52) | 408 (50) | |
| other | 221 (22) | 49 (25) | 172 (21) | |
| no antiretroviral | 12 (1) | 3 (2) | 9 (1) | |
| Efavirenz use, n (%) | 76 (7) | 11 (6) | 65 (8) | 0.24 |
| Dolutegravir use, n (%) | 428 (42) | 88 (44) | 340 (42) | 0.52 |
| Number of non-HIV medications, mean ± SD | 5.1 ± 3.6 | 8.0 ± 4.1 | 4.4 ± 3.1 | <0.001 |
| Polypharmacy (≥5 non-HIV medications), n (%) | 512 (50) | 162 (81) | 350 (43) | <0.001 |
| Characteristic . | All patients (N = 1019) . | Patients with ACH drug (N = 200) . | Patients with no ACH drug (N = 819) . | Pa . |
|---|---|---|---|---|
| Age (years), median (IQR) | 70 (67-74) | 70 (67-75) | 70 (68-74) | 0.58 |
| Male, n (%) | 836 (82) | 157 (79) | 679 (83) | 0.15 |
| CD4 cell count <200 cells/mm3, n (%) | 34 (3) | 11 (6) | 23 (3) | 0.06 |
| Nadir CD4 cell count <200 cells/mm3, n (%) | 611 (60) | 119 (60) | 492 (60) | 0.88 |
| HIV-1 RNA <20 copies/mL, n (%) | 927 (91) | 180 (90) | 747 (91) | 0.56 |
| Diagnosis of depression, n (%) | 108 (11) | 66 (33) | 42 (5) | <0.001 |
| Antiretroviral drug classb, n (%) | ||||
| NNRTI based | 226 (22) | 37 (19) | 189 (23) | 0.47 |
| PI based | 48 (5) | 7 (4) | 41 (5) | |
| INSTI based | 512 (50) | 104 (52) | 408 (50) | |
| other | 221 (22) | 49 (25) | 172 (21) | |
| no antiretroviral | 12 (1) | 3 (2) | 9 (1) | |
| Efavirenz use, n (%) | 76 (7) | 11 (6) | 65 (8) | 0.24 |
| Dolutegravir use, n (%) | 428 (42) | 88 (44) | 340 (42) | 0.52 |
| Number of non-HIV medications, mean ± SD | 5.1 ± 3.6 | 8.0 ± 4.1 | 4.4 ± 3.1 | <0.001 |
| Polypharmacy (≥5 non-HIV medications), n (%) | 512 (50) | 162 (81) | 350 (43) | <0.001 |
Determined using independent samples median test to compare medians, χ2 tests for categorical variables and Student’s t-test for continuous variables.
NNRTI-, PI- and INSTI-based treatments include only combinations of these anchor drug classes with 2 NRTIs. The group ‘other’ includes other drug class combinations, such as: PI + INSTI without or with 2 NRTIs or 1 NRTI; PI + INSTI + NNRTI without or with 2 NRTIs; PI + NNRTI with 1 or 2 NRTI(s); PI + 1 NRTI; and INSTI + 1 NRTI.
ACH medication use
ACH medication use was identified in 200 PLWH (20%). In this group, there was a total of 257 prescriptions for ACH medications; average of 1.3 ± 0.7 ACH medications. The majority of prescriptions (81%) were for medications with an ACH burden score of 1 (Table 1). Overall, 132, 22 and 46 participants had a total ACH burden score of 1, 2 and ≥3, respectively, with an average ACH score of 1.7 ± 1.3. Antidepressants, including tricyclic antidepressants, were the most commonly prescribed medications with ACH activity (n = 127, 49%), followed by antipsychotics (n = 18, 7%), benzodiazepines (n = 16, 6%), antihistamines (n = 15, 6%), urinary antispasmodic anticholinergics (n = 12, 5%), opioids (n = 12, 5%) and corticosteroids (n = 12, 5%). Table 3 lists the most commonly prescribed ACH medications during the evaluation period. Among the 200 PLWH on at least one ACH medication, 49 (25%) were treated with a boosted antiretroviral agent. Of those, only 15 (8%) had a potential drug–drug interaction that could have caused a significant increase in the exposure of the ACH medication. Not surprisingly, individuals receiving ACH drugs tended to report depression more often than those not receiving an ACH medication. Gender and age were not associated with ACH medication use. Polypharmacy, however, was associated with ACH medication use after adjustment for age, sex, CD4, nadir CD4, HIV viral load and depression (OR = 5.84; 95% CI = 3.98–8.55; P < 0.001). ACH medication use increased with increasing number of non-HIV medications (Figure 1).
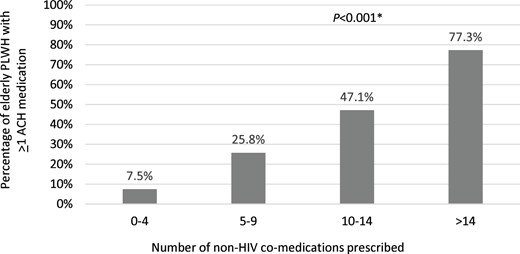
ACH medication use according to the number of prescribed non-HIV co-medications. *χ2.
| . | n (%) . |
|---|---|
| ACH score 1 | |
| mirtazapine | 25 (10) |
| citalopram | 22 (9) |
| escitalopram | 19 (7) |
| duloxetine | 17 (7) |
| sertraline | 14 (5) |
| glycopyrronium | 9 (4) |
| ranitidine | 7 (3) |
| fluoxetine | 7 (3) |
| ACH score 2 | |
| quetiapine | 7 (3) |
| ACH score 3 | |
| trimipramine | 10 (4) |
| paroxetine | 8 (3) |
| . | n (%) . |
|---|---|
| ACH score 1 | |
| mirtazapine | 25 (10) |
| citalopram | 22 (9) |
| escitalopram | 19 (7) |
| duloxetine | 17 (7) |
| sertraline | 14 (5) |
| glycopyrronium | 9 (4) |
| ranitidine | 7 (3) |
| fluoxetine | 7 (3) |
| ACH score 2 | |
| quetiapine | 7 (3) |
| ACH score 3 | |
| trimipramine | 10 (4) |
| paroxetine | 8 (3) |
ACH score 1, low ACH burden; ACH score 2, moderate ACH burden; ACH score 3, high ACH burden.
| . | n (%) . |
|---|---|
| ACH score 1 | |
| mirtazapine | 25 (10) |
| citalopram | 22 (9) |
| escitalopram | 19 (7) |
| duloxetine | 17 (7) |
| sertraline | 14 (5) |
| glycopyrronium | 9 (4) |
| ranitidine | 7 (3) |
| fluoxetine | 7 (3) |
| ACH score 2 | |
| quetiapine | 7 (3) |
| ACH score 3 | |
| trimipramine | 10 (4) |
| paroxetine | 8 (3) |
| . | n (%) . |
|---|---|
| ACH score 1 | |
| mirtazapine | 25 (10) |
| citalopram | 22 (9) |
| escitalopram | 19 (7) |
| duloxetine | 17 (7) |
| sertraline | 14 (5) |
| glycopyrronium | 9 (4) |
| ranitidine | 7 (3) |
| fluoxetine | 7 (3) |
| ACH score 2 | |
| quetiapine | 7 (3) |
| ACH score 3 | |
| trimipramine | 10 (4) |
| paroxetine | 8 (3) |
ACH score 1, low ACH burden; ACH score 2, moderate ACH burden; ACH score 3, high ACH burden.
SRNI
Four patients were excluded from the analysis because a response of ‘does not understand question’ was recorded for all three European AIDS Clinical Society (EACS) screening questions. A total of 87 out of 1015 patients (9%) had SRNI; 75 (7%) had complaints of memory loss, 49 (5%) had complaints of concentration difficulties and 40 (4%) had complaints of mental slowing. The prevalence of participants with SRNI was 36%, 34% and 37% when considering the participants with a total ACH score of 1, 2 and ≥3, respectively. ACH medication use was higher (39% versus 18%; P < 0.001) in patients with SRNI compared with patients without SRNI (Figure 2). PLWH receiving efavirenz or dolutegravir did not have a higher likelihood of SRNI (Table 2). High rates of SRNI were reported in patients with depression (n = 108) compared with patients without depression (n = 907) (28% versus 6%; P < 0.001). In the adjusted multivariable analysis, a detectable HIV viral load and depression were independently associated with SRNI, as indicated in Figure 3. The use of an ACH medication had an OR of 1.69 (95% CI = 0.97–2.95; P = 0.06). Depression, a known risk factor for neurocognitive impairment, appeared to have the strongest association, with an OR of 4.60 (95% CI = 2.62–8.09; P < 0.001). In a subgroup analysis of participants without depressive symptoms (n = 911), only use of an ACH drug (OR = 2.51; 95% CI = 1.31–4.80; P = 0.006) and a detectable viral load were associated with SRNI after adjustment.
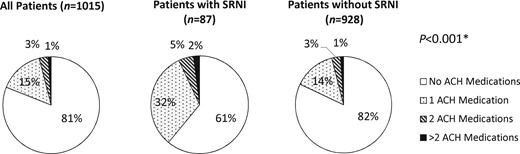
ACH medication use in patients with and without SRNI. *For ACH use versus no ACH use in patients with and without SRNI; χ2.
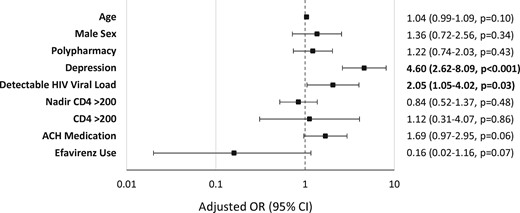
Predictors of SRNI in all patients. Detectable viral load = HIV RNA ≥20 copies/mL.
Discussion
This study shows that ACH medication use (even drugs with a low ACH burden) is associated with SRNI in PLWH ≥65 years of age. This finding is not unexpected, considering that medications with ACH properties have been shown to increase the risk of cognitive decline in HIV-uninfected elderly individuals1,2 and to reduce brain volumes, as well as alter white matter integrity.27 There is robust literature supporting worsening cognitive performance, particularly in memory and executive functioning, associated with ACH medication use in the general elderly population.2 The potentially deleterious effect of ACH medications on cognition has been indirectly demonstrated in virologically suppressed PLWH with HIV-associated neurocognitive disorders (HAND). The administration of rivastigmine, a cholinesterase inhibitor that acts by reducing the breakdown of acetylcholine (i.e. opposite effect compared with ACH medications), has indeed been shown to improve psychomotor speed in these patients.28 Specific cognitive effects appear to depend on the selectivity of the medication for one of the five muscarinic receptor subtypes. In addition, cumulative ACH burden has been associated with greater cognitive impairment in HIV-uninfected elderly individuals.29
Despite known risks associated with ACH medication use in the elderly, ACH use was common (∼20%) in patients ≥65 years of age in the SHCS. The rate of ACH medication use described in this study was similar to rates seen previously (15%–26%) in younger cohorts of PLWH.30,31 The majority (∼81%) of the ACH medications prescribed in our study population were low-burden ACH medications (i.e. score of 1). More frequent prescribing of low- versus high-burden ACH medications has also been reported in HIV-uninfected elderly individuals.18 Many of these low-burden ACH medications are prescribed to treat chronic conditions (e.g. depression) and prescribers may not easily identify low-burden ACH medications or recognize the potential risks associated with the use of these medications.
Although the risk of neurocognitive impairment has generally been attributed to drugs with a high ACH burden (i.e. score of ≥3), our study shows, interestingly, that even low-burden ACH medication use is associated with SRNI. This observation may be a result of higher sensitivity to ACH drugs in PLWH compared with HIV-uninfected individuals. Rubin et al.32 showed that female PLWH have increased cognitive vulnerabilities to ACH medications compared with HIV-uninfected females. The authors hypothesized that neurotoxicity resulting from HIV viral proteins may have additive or interactive effects with ACH medications. In addition, the types of ACH medications used by the different groups may have also contributed to the differences seen. Differences have been observed among drug classes with respect to their ACH effect on cognitive performance. For instance, antipsychotic drugs were shown to have a large effect on executive function, whereas small and moderate effects were observed for drugs targeting the gastrointestinal tract or metabolism, opioids and anxiolytics.29
Considering that PLWH may be more sensitive to ACH drugs and that higher ACH medication use and burden has been documented in PLWH compared with HIV-uninfected individuals,23,32 efforts should be made to avoid ACH drugs in elderly PLWH. Polypharmacy, itself, was not a predictor of SRNI. However, particular attention is warranted in patients with polypharmacy, as we found that polypharmacy was associated with an increased risk of ACH medication use and may also serve as an indicator or criterion for medication safety evaluation. The evaluation of ACH burden should be considered systematically when reviewing medications. The incorporation of ACH calculators into electronic prescribing could facilitate the evaluation of ACH burden in daily practice, as prescribers may not recognize the risk associated with the use of medications with low ACH burden or the potential for cumulative burden. However, avoidance of ACH drugs may be challenging, particularly when there are limited alternatives. Our systematic review of the literature and of the ACH medication lists, scales and criteria indicates that the antidepressants venlafaxine and vortioxetine do not have significant ACH properties or effects and therefore could be suitable alternatives for elderly PLWH needing antidepressants.
Detectable HIV viral load was associated with SRNI. This finding is consistent with Kusejko et al.21 who found that SHCS PLWH with persisting SRNI more often had self-reported imperfect adherence to ART, which has previously been shown to correlate well with virological failure.33
In this population of well-controlled PLWH (91% with HIV viral loads <20 copies/mL), SRNI was documented in 8.6% of patients. This is less than the previously reported SRNI prevalence of 25.1% by Metral et al.17 in PLWH with a mean age of 54.5 years enrolled between 2013 and 2016 (79.7% were male with a similar rate of HIV control, when considering the threshold of <50 copies/mL). In our present study, SRNI was also defined as a ‘yes, definitely’ response to at least one of the EACS screening questions. A possible explanation for the lower prevalence of SRNI in our study may be related to the fact that older individuals may minimize issues of memory loss, attention deficits and slow reasoning, attributing changes to the normal course of ageing. In contrast, younger adults may find these changes more acute and concerning. Prior studies in HIV-uninfected patients have indeed shown a tendency for patients to overestimate cognitive function with age34 or mild cognitive impairment.35 Additionally, a recent analysis of the SHCS showed that the overall percentage of PLWH with SRNI decreased from 19.6% in 2013 to 10.7% in 2017.21 Thus, another explanation could relate to differences in ART, given that our analysis was performed in 2019 and INSTI-based regimens were the most commonly prescribed ART regimens. Lastly, when considering all active SHCS participants in 2019, the prevalence of depression was lower in participants aged ≥65 years compared with <65 years (9.7% versus 13.8%) (unpublished data, A. Scherrer). Thus, the lower prevalence of SRNI observed in our study could also be explained by the lower depression rate in elderly compared with younger PLWH.
In our study, the most frequently reported complaint was memory loss, followed by concentration difficulties and complaints of mental slowing. It should be highlighted that the sensitivity of the three cognitive screening questions has been reported to be low in individuals with symptomatic forms of HAND,36,37 as well as in individuals with asymptomatic forms of HAND.19 For instance, the positive and negative predictive values of the three questions to predict cognitive impairment, using the Frascati criteria, were shown to be poor (0.35 and 0.7, respectively) in middle-aged PLWH mostly virologically suppressed.17
Consistent with previous studies,17,21,38 depression was a main driver of SRNI in our study. This association is not surprising, considering that, in depressed individuals, executive function, speed of information processing, attention and working memory and verbal episodic memory have been reported to be impaired.39 Higher depression burden has been associated with steeper neurocognitive decline compared with low depression.40 It has been suggested that the relationship between cumulative depression burden and neurocognitive decline may possibly relate to sustained depressive symptoms and stress, which can lead to chronic neuro-inflammation and subsequent neuronal damage.41 Paolillo et al.40 found that the steepest cognitive decline was seen in their group of patients with a high depression burden who were also on antidepressants. This observation would tend to support a contributing role of antidepressants, per se, in cognitive decline and would be consistent with our observation that ACH medications (mostly antidepressants in our study) were associated with SRNI, even after removing patients with depression. Altogether, our findings tend to suggest that factors other than HIV infection (i.e. depression and ACH medications) play an important role in the occurrence of neurocognitive impairment. The use of efavirenz, an antiretroviral drug known to cause CNS adverse effects, was not associated with an increased risk of SNRI in our study.
This study has several strengths. A large number of elderly PLWH were included. Since 2015, an online drug entry system for the SHCS allows the prospective systematic documentation of all medications, thereby allowing a comprehensive collection of medications. Furthermore, only medications with documented evidence of ACH activity (based on a systematic drug review) were retained, thus providing a more specific analysis of the effect of ACH medications on SRNI. There are several limitations to this study. First, the diagnosis of depression was based on a medical evaluation. Self-filled depression rating scales were not available and thus the diagnosis of depression, particularly mild depression, may have been missed in some participants. However, it should be noted that a Centre for Epidemiological Studies Depression (CES-D) score of 16 (indicative of a mild depression) was shown to have a low sensitivity and specificity to predict neurocognitive impairment, thus highlighting the complexity of the association between depression and neurocognitive impairment.38 Adherence is only assessed for HIV drugs and therefore we could not determine if patients prescribed ACH medications were indeed taking these medications. A previous study of the SHCS has shown that PLWH tend to prioritize (and better adhere to) ART compared with their co-medications.42 In addition, we did not evaluate ACH medication dose or duration, which may impact neurocognitive function. We also did not evaluate for history of CNS infection. Lastly, we used SRNI to assess neurocognitive impairment. The predictive value of SRNI to detect NCI has not been evaluated in elderly PLWH. Thus, the effect of ACH medications should be further evaluated using more comprehensive neurocognitive testing. Future studies will also need to evaluate whether the reduction of ACH burden can decrease SRNI and improve neurocognitive function. Cooley et al.23 performed a longitudinal analysis in a cohort of 50-year-old PLWH and found that a decrease in ACH score was associated with improved executive function and cognition. It is currently unknown whether this holds true for elderly PLWH.
In conclusion, ACH drug use is common in elderly PLWH and contributes to SRNI, including drugs with a low ACH burden. The effect of ACH medications on neurocognitive impairment warrants further evaluation using neurocognitive tests. Furthermore, studies will need to address whether reducing ACH burden can improve neurocognitive function.
Acknowledgements
Members of the Swiss HIV Cohort Study
Abela I, Aebi-Popp K, Anagnostopoulos A, Battegay M, Bernasconi E, Böni J, Braun DL, Bucher HC, Calmy A, Cavassini M, Ciuffi A, Dollenmaier G, Egger M, Elzi L, Fehr J, Fellay J, Furrer H, Fux CA, Günthard HF (President of the SHCS), Hachfeld A, Haerry D (Deputy of ‘Positive Council’), Hasse B, Hirsch HH, Hoffmann M, Hösli I, Huber M, Kahlert CR (Chairman of the Mother & Child Substudy), Kaiser L, Keiser O, Klimkait T, Kouyos RD, Kovari H, Kusejko K (Head of Data Centre), Martinetti G, Martinez de Tejada B, Marzolini C, Metzner KJ, Müller N, Nemeth J, Nicca D, Paioni P, Pantaleo G, Perreau M, Rauch A (Chairman of the Scientific Board), Schmid P, Speck R, Stöckle M (Chairman of the Clinical and Laboratory Committee), Tarr P, Trkola A, Wandeler G, Yerly S.
Funding
This study was financed within the framework of the Swiss HIV Cohort Study (SCHS), supported by the Swiss National Science Foundation (grant #177499), by SHCS Project #700 and by the SHCS Research Foundation. C.M. was supported by a grant of the Swiss National Science Foundation (grant #166204).
Transparency declarations
B.J. serves as an infectious diseases drug information clinical consultant for Wolters Kluwer. H.F.G. has received unrestricted research grants from Gilead Sciences and Roche, and fees for data and safety monitoring board membership and for advisory board and consulting activities from Gilead Sciences, Merck, ViiV, Sandoz and Mepha. A.H. has received travel grants from ViiV, Gilead and MSD, has participated in advisory boards of MSD and is a coinvestigator of a clinical trial of MSD unrelated to the submitted work. The institution of E.B. has received travel grants and payments for his participation in advisory boards of Gilead Sciences, MSD, ViiV Healthcare, Pfizer and AbbVie. The institution of M.C. has received research grants from Gilead, MSD and ViiV. C.M. has received a research grant from Gilead for a project unrelated to the present study. All other authors: none to declare.
The data were gathered by five Swiss university hospitals, two Cantonal hospitals, 15 affiliated hospitals and 36 private physicians (listed in www.shcs.ch/180-health-care-providers).
References
Author notes
Members are listed in the Acknowledgements section.



