-
PDF
- Split View
-
Views
-
Cite
Cite
Min-Ge Wang, Chang Fang, Kai-Di Liu, Lin-Lin Wang, Ruan-Yang Sun, Rong-Min Zhang, Liang-Xing Fang, Jian Sun, Ya-Hong Liu, Xiao-Ping Liao, Transmission and molecular characteristics of blaNDM-producing Escherichia coli between companion animals and their healthcare providers in Guangzhou, China, Journal of Antimicrobial Chemotherapy, Volume 77, Issue 2, February 2022, Pages 351–355, https://doi.org/10.1093/jac/dkab382
Close - Share Icon Share
Abstract
To determine the transmission and molecular characteristics of blaNDM-producing Escherichia coli between companion animals and their healthcare providers at veterinary clinics in Guangzhou, China.
A total of 359 samples from companion animals and their healthcare providers were collected at 14 veterinary clinics in Guangzhou, China. Genomic characteristics and clonal relationships for blaNDM-positive E. coli and complete plasmid sequences were characterized based on WGS data from combined Illumina and MinION platform reads.
Forty-five blaNDM-positive bacteria were recovered from companion animals (n = 43) and their healthcare providers (n = 2) at 10 veterinary clinics. Overall, E. coli (73.3%, 33/45) and Klebsiella pneumoniae (13.3%, 6/45) were the most prevalent species among the seven species of blaNDM-positive bacteria. Four blaNDM variants (blaNDM-1, blaNDM-4, blaNDM-5 and blaNDM-7) were identified in 45 blaNDM-positive bacteria and blaNDM-5 was the most prevalent (77.8%, 35/45). WGS indicated that the most prevalent STs were ST405 (8/33), ST453 (6/33), ST457 (6/33) and ST410 (5/33) among the 33 blaNDM-positive E. coli isolates. Phylogenomics and PFGE analysis revealed that clonal spread of blaNDM-positive ST453 E. coli isolates between companion animals and their healthcare providers was evident. In addition, two novel IncFIB plasmids carrying blaNDM-4 (pF765_FIB and pG908_FIB) were found in this study and indicated that IS26 may promote the horizontal transmission of blaNDM between different plasmid types.
In this study we conducted a large-scale investigation on the prevalence of blaNDM-positive E. coli isolates from companion animals and their healthcare providers and revealed the clonal spread of blaNDM-positive E. coli isolates between these two groups.
Introduction
Carbapenems are an important antimicrobial class for the treatment of human clinical infections caused by MDR Gram-negative bacteria.1 The current worldwide emergence of carbapenem resistance in Enterobacteriaceae therefore constitutes a growing public health threat.2 Carbapenem antibiotics have never been licensed for veterinary use in any country, so the emergence of blaNDM-positive bacteria in companion animals is a serious concern.3 Recent studies showed that lower levels of blaNDM-positive Enterobacteriaceae have been reported for Beijing and Guangzhou in China, although these studies were not comprehensive.4,5 Therefore, a large-scale survey of the blaNDM-positive Escherichia coli isolates from companion animals was necessary for China, since veterinary clinics can be a source for human infections. For instance, there was an epidemic outbreak of carbapenemase-producing E. coli in employees at Swiss veterinary clinics and transmission of blaNDM-5-positive ST167 E. coli between dogs and family members in Finland in 2015.6,7 This prompted us to explore the relationships between blaNDM-positive E. coli isolates from companion animals and their healthcare providers.
The aim of this study was to investigate the prevalence and transmission of blaNDM-positive E. coli in multiple veterinary clinics and to assess whether there was clonal spread of blaNDM-positive E. coli between companion animals and healthcare providers.
Materials and methods
Sample collection
A total of 359 samples (332 rectal swab samples and 27 faecal samples) were collected from companion animals (146 dogs, 185 cats and 1 tortoise) and their associated healthcare providers (n = 27) in 14 veterinary clinics in downtown Guangzhou that were distributed in Haizhu [the four clinics were named A (n = 60), B (n = 32), C (n = 63) and O (n = 16)], Liwan [the three clinics were named I (n = 24), L (n = 16) and P (n = 19)], Tianhe [the four clinics were named E (n = 8), F (n = 49), K (n = 17) and M (n = 14)] and Yuexiu [the three clinics were named D (n = 23), G (n = 8) and Q (n = 10)]. The number of collected samples from different hosts in each veterinary clinic is shown in Table S1 (available as Supplementary data at JAC Online). In addition, information was provided by the animal owners and employees that included animal age, sex, disease diagnosis and antimicrobials used for the 30 days prior to treatment. Animal samples were collected with approval of the animal owners and employee samples were collected on a voluntary basis.
Methods
The detection of five major carbapenemase-encoding genes (blaKPC, blaNDM, blaIMP, blaOXA-48 and blaVIM), bacterial species identification, antimicrobial susceptibility testing, the relationship of isolates, the transferability of blaNDM and gene localization were carried out as previously reported.8 WGS and phylogenetic analysis were carried out as previously described.9
Statistical analysis
We used unconditional logistic regression models to estimate ORs and 95% CIs for univariable analysis of antibiotic risk factors associated with blaNDM-positive E. coli isolates.
Results and discussion
Species diversity and prevalence of blaNDM-positive isolates
We recovered 104 meropenem-resistant isolates from 359 collected samples in 14 veterinary clinics. These resistant isolates included 45 (12.5%, 45/359) that possessed the blaNDM gene and included E. coli (33/45), Klebsiella pneumoniae (6/45), Enterobacter cloacae (2/45), Klebsiella aerogenes (1/45), Pseudomonas putida (1/45), Pseudomonas aeruginosa (1/45) and Stenotrophomonas maltophilia (1/45). Interestingly, a single dog rectal swab was the source of two blaNDM-positive strains [an E. cloacae isolate (PA16-1) and a K. aerogenes isolate (PA16-2)]. No other carbapenemase-encoding genes were detected among these carbapenem-resistant isolates (Table S2). These results demonstrated that the blaNDM gene was carried by a broad range of bacterial hosts and this could facilitate transfer between different species in the intestinal environment.
Our group of 45 blaNDM-positive isolates was recovered from dogs (17.8%, 26/146), cats (8.6%, 16/185), their healthcare providers (7.4%, 2/27) and a tortoise (Table S2). In addition, the prevalence of blaNDM-positive isolates by district was as follows: Yuexiu, 41.5% (17/41); Tianhe, 12.5% (11/88); Haizhu, 7.6% (13/171); and Liwan, 6.8% (4/59) (Figure S1). The prevalence of blaNDM-positive isolates in Yuexiu was significantly higher than in the other three districts (P < 0.005) (Table S3). We also identified four blaNDM variants in our blaNDM-positive collection and blaNDM-5 was the most prevalent, followed by blaNDM-4, blaNDM-7 and blaNDM-1 (Table S2). These results were consistent with a previous report demonstrating that blaNDM-5 was the most prevalent blaNDM variant in humans and backyard animals, including pigs, chickens, dogs, cattle and flies.10
Antibiotic usage and disease diagnosis of animal hosts
The 44 hosts carrying blaNDM-positive isolates included 27 that had taken antimicrobials within the 30 days prior to examination and these included β-lactams (n = 11), aminoglycosides (n = 2), tetracyclines (n = 2), fluoroquinolones (n = 1), colistin (n = 1), bacitracin (n = 1), methicillin (n = 1), erythromycin (n = 1), florfenicol (n = 1) and metronidazole (n = 1) (Table S2). We used unconditional logistic regression models to estimate ORs and 95% CIs for univariable analysis of antibiotic risk factors associated with blaNDM-positive isolates. Results showed that isolates from hosts treated with antibiotics were more likely to be blaNDM-positive isolates than isolates from hosts not treated with antibiotics. β-Lactams had the highest risk among different classes of antibiotics (Table S4).
Molecular characteristics of blaNDM-positive E. coli
Our WGS results indicated that 33 of the blaNDM-positive E. coli isolates had 11 distinct STs and ST405 was the most prevalent (24.2%, 8/33), followed by ST453 (18.2%, 6/33), ST457 (18.2%, 6/33), ST410 (15.2%, 5/33) and ST224 (6.1%, 2/33). The remaining seven STs were represented by single isolates (Figure 1). NDM-positive ST405 E. coli isolates have been linked to intestinal persistence and identified as a high-risk clone found in humans, animals and environmental samples.11
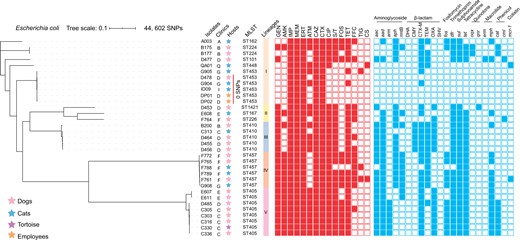
Phylogenetic structures, MLST and antibiotic resistance phenotypes and genotypes of blaNDM-positive Enterobacteriaceae from companion animals and employees in veterinary clinics. Relationships of blaNDM-positive E. coli isolates are indicated using a maximum likelihood tree. The red and blue squares represent positivity for resistance phenotypes and genotypes, respectively. GEN, gentamicin; AMK, amikacin; IMP, imipenem; MEM, meropenem; ERT, ertapenem; ATM, aztreonam; CAZ, ceftazidime; CTX, cefotaxime; CIP, ciprofloxacin; S/T, trimethoprim/sulfamethoxazole; FOS, fosfomycin; TET, tetracycline; FFC, florfenicol; TIG, tigecycline; CS, colistin. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Phylogenomic analysis revealed that all the E. coli isolates were classified into five lineages, sharing a total of 44 602 SNPs. Importantly, three blaNDM-positive ST453 E. coli isolates were derived from a dog (D478) and its healthcare providers (DP01 and DP02) in the same veterinary clinic. These three strains displayed identical core-genome sequences and the number of pairwise SNPs was zero (Figure 1). The three blaNDM-positive ST453 E. coli isolates were also the same PFGE genotype (Figure S2). These findings indicated the transmission of blaNDM-positive ST453 E. coli isolates between a companion animal and its healthcare providers in a veterinary clinic. In addition, our phylogenomic analysis revealed that a majority of the blaNDM-positive E. coli isolates showed a high degree of core-genome sequence similarity (Figure 1). This suggested that there is clonal dissemination of blaNDM-positive E. coli isolates in different veterinary clinics.
Antibiotic resistance phenotypes and genotypes
The group of 33 blaNDM-positive E. coli isolates all displayed reduced susceptibilities to meropenem and were highly resistant to ceftazidime, cefotaxime, imipenem and ertapenem (Table S5). Moreover, the majority were resistant to gentamicin, amikacin, aztreonam, ciprofloxacin, trimethoprim/sulfamethoxazole, fosfomycin, tetracycline and florfenicol. However, a few of the isolates showed resistance to tigecycline and colistin (Figure 1). The E. coli isolates also possessed numerous antimicrobial resistance genes (ARGs), including aac, aad, aph, arm, rmtB, blaCMY, blaCTX-M, blaTEM, blaOXA, fosA3, dfr, sul, tet and mph (Figure 1). Overall, the resistance phenotypes of the blaNDM-positive E. coli isolates were consistent with their genotypes.
Plasmid analysis
Conjugation experiments suggested that all the blaNDM-carrying plasmids could be successfully transferred to E. coli C600str among blaNDM-positive E. coli. S1-PFGE and hybridization analyses confirmed that blaNDM genes from 19 isolates were located on ∼50 kb plasmids and from 14 isolates on ∼80 to ∼170 kb plasmids (Figure S3). The ∼50 kb plasmids were confirmed to be IncX3 plasmids through alignment with the reference plasmid (Figure S4). This provides evidence for the horizontal transfer of blaNDM-carrying IncX3 plasmids in companion animals. The ∼80 to ∼170 kb plasmids were confirmed to be IncF types by simultaneously combining short-read and long-read data.
We also identified two novel IncFIB plasmids carrying blaNDM-4 (pF765_FIB and pG908_FIB), which contained a conjugative transfer region and an MDR region (Figure 2). The pF765_FIB plasmid was 148 088 bp and was a hybrid of plasmid pT28R_2 (accession no. NZ_CP049355) that had captured a 21.2 kb MDR region that contained blaNDM-4 flanked by two copies of IS26. The pG908_FIB plasmid was 125 463 bp and highly similar to the pF765_FIB plasmid except for the deletion of a 36.1 kb MDR region. The pG908_FIB plasmid also contained several mobile elements and ARGs, including blaCTX-M-14, fosA3, aac(3)-Iva, blaTEM, mph(A) and aph(3’)-Ia (Figure 2). The carbapenemase-encoding gene blaNDM-4 has been previously associated with IncFII, IncHI2 and IncX3 plasmid types and rarely with IncFIB.12,13 In addition, the MDR region that contained blaNDM-4 was bracketed by two copies of IS26 that can potentially mobilize this gene to different plasmids.14 These results indicated that IS26 probably mediated the transfer of the blaNDM-4 gene into IncFIB plasmids.
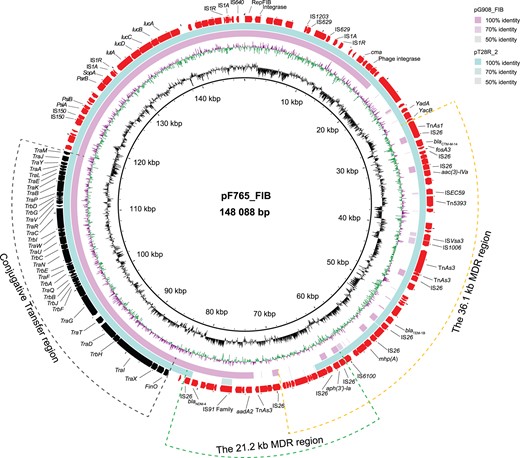
Circular sequence alignments of blaNDM-4-carrying plasmids pF765_FIB and pG908_FIB. Genes depicted in the outer circle belong to plasmid pF765_FIB that was included as a reference and the middle circle represents a structurally similar plasmid (pT28R_2) obtained from the GenBank database. The inner circle depicts plasmid pG908_FIB. The green and orange dashed lines denote MDR regions that possessed and lacked blaNDM-4, respectively. The black dashed line denotes a conjugative transfer region. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Conclusions
We identified a total of 45 blaNDM-positive bacteria isolates from companion animals and their healthcare providers in samples from 14 veterinary clinics in Guangzhou, China. The most prevalent STs among blaNDM-positive E. coli isolates from companion animals were ST405, ST453 and ST457. In addition, transmission of blaNDM-positive ST453 E. coli isolates most likely occurred between two healthcare providers and a dog. We identified two novel IncFIB plasmids carrying blaNDM-4 (pF765_FIB and pG908_FIB) and suggested that IS26 may promote the horizontal transmission of blaNDM between different types of plasmids. These results demonstrated that continuous monitoring of blaNDM-positive isolates in companion animals and their healthcare providers in veterinary clinics is necessary to ensure public health and safety.
Funding
This work was jointly supported by the National Natural Science Foundation of China (31772793, 31730097 and 31802243), the Guangdong Special Support Program Innovation Team (2019BT02N054) and the Program for Changjiang Scholars and Innovative Research Team at the University of Ministry of Education of China (Grant No. IRT_17R39).
Transparency declarations
None to declare.
Supplementary data
Tables S1 to S5 and Figures S1 to S4 are available as Supplementary data at JAC Online.



