-
PDF
- Split View
-
Views
-
Cite
Cite
Paul Thoueille, Eva Choong, Matthias Cavassini, Thierry Buclin, Laurent A. Decosterd, Long-acting antiretrovirals: a new era for the management and prevention of HIV infection, Journal of Antimicrobial Chemotherapy, Volume 77, Issue 2, February 2022, Pages 290–302, https://doi.org/10.1093/jac/dkab324
Close - Share Icon Share
Abstract
The long-acting antiretroviral cabotegravir and rilpivirine combination has just received FDA, EMA and Health Canada approval. This novel drug delivery approach is about to revolutionize the therapy of people living with HIV, decreasing the 365 daily pill burden to only six intramuscular injections per year. In addition, islatravir, a first-in-class nucleoside reverse transcriptase translocation inhibitor, is intended to be formulated as an implant with a dosing interval of 1 year or more. At present, long-acting antiretroviral therapies (LA-ARTs) are given at fixed standard doses, irrespectively of the patient’s weight and BMI, and without consideration for host genetic and non-genetic factors likely influencing their systemic disposition. Despite a few remaining challenges related to administration (e.g. pain, dedicated medical procedure), the development and implementation of LA-ARTs can overcome long-term adherence issues by improving patients’ privacy and reducing social stigma associated with the daily oral intake of anti-HIV treatments. Yet, the current ‘one-size-fits-all’ approach does not account for the recognized significant inter-individual variability in LA-ART pharmacokinetics. Therapeutic drug monitoring (TDM), an important tool for precision medicine, may provide physicians with valuable information on actual drug exposure in patients, contributing to improve their management in real life. The present review aims to update the current state of knowledge on these novel promising LA-ARTs and discusses their implications, particularly from a clinical pharmacokinetics perspective, for the future management and prevention of HIV infection, issues of ongoing importance in the absence of curative treatment or an effective vaccine.
Introduction
Since the introduction of highly active ART about 20 years ago, a succession of ameliorations, including simplification from complex regimens to single fixed-dose multidrug pills, have definitely improved both the efficacy and the tolerability of HIV infection management. In the absence of curative treatment or an effective vaccine, ART remains the mainstay of HIV treatment and prevention.
Current oral triple-drug treatments of HIV infection combine a potent HIV integrase strand transfer inhibitor (INSTI) (e.g. dolutegravir, bictegravir) or an NNRTI (e.g. rilpivirine, doravirine, etravirine) plus two NRTIs (e.g. tenofovir, emtricitabine, lamivudine). Lately, simplified dual-therapy combinations have revealed the same activity as conventional triple therapy.1–3 However, non-virological outcomes remain uncertain with dual therapy, and maintaining triple therapy has recently been advised by some authors since it is associated with a more favourable long-term anti-inflammatory profile.4 Non-boosted integrase inhibitor-based regimens are currently the preferred first-line treatment. They have been shown to confer a high rate of viral suppression, a good tolerance and a high barrier to resistance.5,6
Current antiretroviral regimens have, for the most part, achieved optimal antiretroviral efficacy and tolerability, transforming HIV infection from a deadly disease into a manageable chronic condition. Still, adherence to daily oral drug intake remains an issue, as it is the most important determinant for sustained viral suppression and prevention of emergence of drug-resistant viral strains. In fact, fewer than two thirds of patients maintain the frequently reported 90% adherence level associated with optimal viral suppression.7 Notably, with the improved pharmacokinetic (PK) profiles of the recent antiretrovirals, the threshold of adherence required to achieve viral suppression might now be lowered to 80%.8,9 Long-term adherence is hampered by several factors, including treatment fatigue for multidrug regimens that need to be taken indefinitely. Additional co-medications and drug–drug interactions (DDIs) bring further complexity, notably in the ageing population of people living with HIV (PLWH).10–12 A promising approach to overcome the adherence challenge and prevent drug resistance is the development of long-acting formulations, which at present can ensure 2 month-long effective plasma concentrations.13,14 Such a dosing interval is likely to be prolonged in the near future with the development of novel antiretroviral agents. Long-acting antiretroviral therapy (LA-ART) will simultaneously improve patient privacy and reduce social stigmas associated with HIV. Eligible patients willing to start long-acting injectable ART (LAI-ART) are particularly interested (reportedly up to 70%)15 in the improved convenience, freedom, confidentiality and emotional benefits of not being constantly reminded of their HIV status through daily pill use. LAI-ART will raise an even higher rate of interest once the interval between injections has been further extended, thus decreasing the injection discomfort.16
For the prevention of HIV infection, pre-exposure prophylaxis (PrEP) by dual therapy with NRTIs, e.g. tenofovir disoproxil fumarate and emtricitabine or lamivudine, has demonstrated high efficacy in prospective trials.17,18 It has been recommended, among other prevention approaches, since 2015 by the WHO for populations with an HIV incidence above 3%. However, adherence to oral PrEP regimens is low in some populations17,19 and extended PrEP is needed in women because of the delay for the full protective effect in the vaginal tract. Detectable drug levels in blood are strongly correlated with the prophylactic effect (limit of quantification of 10 ng/mL in plasma, and 2.5 fmol/2 × 106 cells for tenofovir diphosphate and 0.1 pmol/2 × 106 cells for emtricitabine triphosphate in PBMCs).18 Indeed, there is an approximately 90% reduction in the risk of acquiring HIV-1 when drug levels in blood are detectable.19 As highly variable adherence to daily oral regimens profoundly affects the prophylactic effect, LAI-ART raises a strong interest in PrEP, in the absence of an effective vaccine against HIV.
During the past decades, blood concentration measurement has been increasingly invoked to optimize the therapeutic use of critical drugs through adjustment of concentration exposure via therapeutic drug monitoring (TDM). Candidate drugs for TDM have significant inter-subject PK variability, properly quantified by population PK studies and poorly predictable from individual patients’ characteristics, along with limited within-subject PK variability over time. Their concentration–response and/or concentration–toxicity relationships must be consistent, with defined concentration ranges associated with optimal efficacy and minimal toxicity. TDM is current practice for some antibiotics, antiepileptics, immunosuppressants, antifungals and anti-HIV drugs.20 Despite limited clinical validation, TDM of antiretrovirals is now commonly used in the case of drug interaction problems, virological failure, adverse drug reactions, special clinical conditions (pregnancy, paediatrics, liver failure, dialysis etc.)21 and for assessing short-term compliance. In the LA-ART era, where adherence will no longer be a confounding factor for inadequate clinical response, TDM might still benefit patients in clinical practice by preventing or correcting under- or overdosing (especially with respect to the dosage scheme), which increase the risk of potential treatment failure or toxicity, respectively.
The present narrative review aims to update the current state of knowledge regarding novel promising LA-ARTs, to discuss their implications for HIV management, and to focus on the clinical PK aspects, particularly on the suitability of a TDM service for optimizing ART blood exposure in patients. This article is divided into two sections covering antiretroviral agents that either have intrinsically favourable PK properties for LA-ART application, or whose half-life (t½) has been considerably increased by pharmaceutical technology means.
Methods
For this review, we searched PubMed and Embase for publications and Clinicaltrials.gov for registered studies. We used the search terms ‘long-acting antiretrovirals’ or the names of the compounds presented, in combination with specific terms such as ‘formulations’ or ‘pharmacokinetics’. The references of the identified articles were also examined and we selected those we considered relevant. In addition, we consulted, among others, reports of the Conference on Retroviruses and Opportunistic Infections (CROI) and the AIDS conference, as well as the pipeline of major HIV drug development companies. The compounds presented in the first part of the review are molecules that have reached Phase III clinical studies, or are already marketed and that are about to revolutionize the care of PLWH. On the other hand, promising formulation developments have been selected on the basis of their relevance for timely clinical implementation.
Molecules with suitable characteristics for LA-ART
Cabotegravir and rilpivirine
Cabotegravir is a potent INSTI, structurally similar to dolutegravir (Figure 1), with a high barrier to resistance and high antiviral potency.22 In individuals with HIV infection, trough concentrations (Cmin) under oral cabotegravir 30 mg once daily were roughly 25 times higher than the 90% protein-adjusted inhibitory concentration (PAIC90) for HIV of 166 ng/mL.23
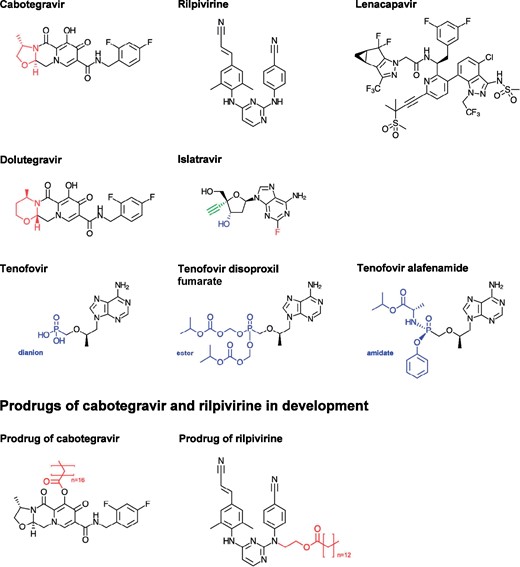
Molecular structures of the compounds presented. The structural difference between cabotegravir and dolutegravir is highlighted in red. The chemical groups in colour for islatravir are discussed in the text. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Rilpivirine is a potent NNRTI, active against NNRTI-resistant HIV, with favourable allosteric binding due to its diarylpyrimidine structure, as shown in Figure 1.10 Oral rilpivirine 25 mg, marketed as EDURANT®, is prescribed in combination with emtricitabine and tenofovir alafenamide in treatment-naive patients with a viral load below 100 000 copies/mL at baseline. The median EC50 of rilpivirine against HIV clinical isolates cultivated in PBMCs was 0.095 ng/mL, leading to a PAIC90 of 12 ng/mL.24 Yet, this in vitro target value differs from in vivo levels indicated by clinical studies,25 which concluded instead that a minimal plasma concentration of 50 ng/mL should be maintained to optimize the probability of therapeutic response.26,27
Table 1 summarizes the differences in the PK parameters between oral and intramuscular (IM) cabotegravir and rilpivirine regimens.28–35
| CAB . | Cmax (μg/mL) . | Tmax . | AUCΤ (μg·h/mL) . | Ctrough (μg/mL) . | t½ . | V (L) . | Substrate . |
|---|---|---|---|---|---|---|---|
| Oral CAB 30 mg q24h | 8.1 (7.9–8.2)a | 3 ha | 146 (143–149)a | 4.7 (4.6–4.8)a | 41 ha | 12.3b,c | UGT1A1 (UGT1A9)b,e |
| LA CAB 400 mg q4w | 4.2 (4.1–4.3)a | 7 da | 2461 (2413–2510)a | 2.9 (2.9–3.0)a | 5.6–11.5 wa | ||
| LA CAB 600 mg q8w | 4.0b | 7 da | 3764b | 1.6b | 5.6–11.5 wa | ||
| RPV | Cmax (ng/mL) | Tmax | AUCΤ (ng·h/mL) | Ctrough (ng/mL) | t½ | V (L) | Substrate |
| Oral RPV 25 mg q24h | 204 ± 76b,d | 4–5 h28 | 2589 ± 869b, d | 67 ± 30b, d | 45–50 hb | 152b | CYP3A (CYP2C19)b,e |
| LA RPV 600 mg q4w | 116 (113–119)a | 3–4 da | 65 603 (63 756–67 503)a | 82.2 (79.9–84.6)a | 13–28 wa | 132b | |
| LA RPV 900 mg q8w | 133b | 3–4 da | 127 031b | 65.6b | 13–28 wa | ||
| CAB . | Cmax (μg/mL) . | Tmax . | AUCΤ (μg·h/mL) . | Ctrough (μg/mL) . | t½ . | V (L) . | Substrate . |
|---|---|---|---|---|---|---|---|
| Oral CAB 30 mg q24h | 8.1 (7.9–8.2)a | 3 ha | 146 (143–149)a | 4.7 (4.6–4.8)a | 41 ha | 12.3b,c | UGT1A1 (UGT1A9)b,e |
| LA CAB 400 mg q4w | 4.2 (4.1–4.3)a | 7 da | 2461 (2413–2510)a | 2.9 (2.9–3.0)a | 5.6–11.5 wa | ||
| LA CAB 600 mg q8w | 4.0b | 7 da | 3764b | 1.6b | 5.6–11.5 wa | ||
| RPV | Cmax (ng/mL) | Tmax | AUCΤ (ng·h/mL) | Ctrough (ng/mL) | t½ | V (L) | Substrate |
| Oral RPV 25 mg q24h | 204 ± 76b,d | 4–5 h28 | 2589 ± 869b, d | 67 ± 30b, d | 45–50 hb | 152b | CYP3A (CYP2C19)b,e |
| LA RPV 600 mg q4w | 116 (113–119)a | 3–4 da | 65 603 (63 756–67 503)a | 82.2 (79.9–84.6)a | 13–28 wa | 132b | |
| LA RPV 900 mg q8w | 133b | 3–4 da | 127 031b | 65.6b | 13–28 wa | ||
CAB, cabotegravir; RPV, rilpivirine; Cmax, maximum concentration; Tmax, time to achieve Cmax; AUCT, AUC to trough concentration; t½, terminal half-life; q24h, once daily; q4w, every 4 weeks; q8w, every 8 weeks; d, days; w, weeks; UGT, uridine 5′-diphospho-glucuronosyltransferase; CYP, cytochrome P450.
Geometric mean (95% CI) obtained from the official product monograph.29 The values presented are based on individual a posteriori estimates for subjects in the FLAIR30 and ATLAS31 studies from separate population PK analysis models generated for cabotegravir and rilpivirine.
These parameters were obtained from the HIV Drug Interactions fact sheets of the University of Liverpool.32–35
Following oral administration.
n = 12, healthy volunteers.
In brackets, minor or potential contribution.
| CAB . | Cmax (μg/mL) . | Tmax . | AUCΤ (μg·h/mL) . | Ctrough (μg/mL) . | t½ . | V (L) . | Substrate . |
|---|---|---|---|---|---|---|---|
| Oral CAB 30 mg q24h | 8.1 (7.9–8.2)a | 3 ha | 146 (143–149)a | 4.7 (4.6–4.8)a | 41 ha | 12.3b,c | UGT1A1 (UGT1A9)b,e |
| LA CAB 400 mg q4w | 4.2 (4.1–4.3)a | 7 da | 2461 (2413–2510)a | 2.9 (2.9–3.0)a | 5.6–11.5 wa | ||
| LA CAB 600 mg q8w | 4.0b | 7 da | 3764b | 1.6b | 5.6–11.5 wa | ||
| RPV | Cmax (ng/mL) | Tmax | AUCΤ (ng·h/mL) | Ctrough (ng/mL) | t½ | V (L) | Substrate |
| Oral RPV 25 mg q24h | 204 ± 76b,d | 4–5 h28 | 2589 ± 869b, d | 67 ± 30b, d | 45–50 hb | 152b | CYP3A (CYP2C19)b,e |
| LA RPV 600 mg q4w | 116 (113–119)a | 3–4 da | 65 603 (63 756–67 503)a | 82.2 (79.9–84.6)a | 13–28 wa | 132b | |
| LA RPV 900 mg q8w | 133b | 3–4 da | 127 031b | 65.6b | 13–28 wa | ||
| CAB . | Cmax (μg/mL) . | Tmax . | AUCΤ (μg·h/mL) . | Ctrough (μg/mL) . | t½ . | V (L) . | Substrate . |
|---|---|---|---|---|---|---|---|
| Oral CAB 30 mg q24h | 8.1 (7.9–8.2)a | 3 ha | 146 (143–149)a | 4.7 (4.6–4.8)a | 41 ha | 12.3b,c | UGT1A1 (UGT1A9)b,e |
| LA CAB 400 mg q4w | 4.2 (4.1–4.3)a | 7 da | 2461 (2413–2510)a | 2.9 (2.9–3.0)a | 5.6–11.5 wa | ||
| LA CAB 600 mg q8w | 4.0b | 7 da | 3764b | 1.6b | 5.6–11.5 wa | ||
| RPV | Cmax (ng/mL) | Tmax | AUCΤ (ng·h/mL) | Ctrough (ng/mL) | t½ | V (L) | Substrate |
| Oral RPV 25 mg q24h | 204 ± 76b,d | 4–5 h28 | 2589 ± 869b, d | 67 ± 30b, d | 45–50 hb | 152b | CYP3A (CYP2C19)b,e |
| LA RPV 600 mg q4w | 116 (113–119)a | 3–4 da | 65 603 (63 756–67 503)a | 82.2 (79.9–84.6)a | 13–28 wa | 132b | |
| LA RPV 900 mg q8w | 133b | 3–4 da | 127 031b | 65.6b | 13–28 wa | ||
CAB, cabotegravir; RPV, rilpivirine; Cmax, maximum concentration; Tmax, time to achieve Cmax; AUCT, AUC to trough concentration; t½, terminal half-life; q24h, once daily; q4w, every 4 weeks; q8w, every 8 weeks; d, days; w, weeks; UGT, uridine 5′-diphospho-glucuronosyltransferase; CYP, cytochrome P450.
Geometric mean (95% CI) obtained from the official product monograph.29 The values presented are based on individual a posteriori estimates for subjects in the FLAIR30 and ATLAS31 studies from separate population PK analysis models generated for cabotegravir and rilpivirine.
These parameters were obtained from the HIV Drug Interactions fact sheets of the University of Liverpool.32–35
Following oral administration.
n = 12, healthy volunteers.
In brackets, minor or potential contribution.
So far, cabotegravir and rilpivirine have been the most extensively studied drugs for LAI-ART, both alone and in combination, due to their prolonged t½ and their high intrinsic antiretroviral potency. Cabotegravir and rilpivirine have low aqueous solubility, allowing their formulation into, respectively, 200 or 300 mg/mL wet-milled suspension.36,37 This preparation produces pure nanosized drug crystals stabilized by surfactants, a formulation suitable for IM depot injection.10,22,36 This nanosuspension technology increases the apparent t½ of cabotegravir and rilpivirine, from 41 and 45 h to approximately 8.5 and 20.5 weeks, respectively, with the LAI formulation, although with substantial interindividual variability.38 This variability in dose–exposure–response relationships could be addressed by TDM. In patients exhibiting substantially lower or higher Cmin, a personalized dosing schedule, shortening or extending the dosing intervals, could be implemented with benefits in terms of efficacy or costs, respectively. Nanocrystals form a depot at the injection site, from which drug is slowly released. Since their particle size allows their physical filtration, nanoparticles are also drained into lymphatic vessels, where they form secondary depots. They can also undergo phagocytosis by macrophages infiltrating the administration site, or incorporation into T lymphocytes. The lymphatic system then slowly releases the drug into the systemic circulation, contributing to the long-acting antiretroviral effect.39 Measurable plasma cabotegravir concentrations have been reported in individuals up to 1 year after a single injection.22,40 Thus, such observed long t½ results from a combination of both the suitable intrinsic properties of the molecules and their nanoformulation development.
Table 2 summarizes the main clinical trials carried out to date with LAI cabotegravir/rilpivirine in PLWH13,30,31,41 and with LAI cabotegravir for PrEP.42–45
| Trial . | Phase . | n . | Arms . | Results . | Ref. . |
|---|---|---|---|---|---|
| Treatment | |||||
| LATTE-2 | IIb | 286 |
|
| 41 |
| FLAIR | III | 566 |
|
| 30 |
| ATLAS | III | 616 |
|
| 31 |
| ATLAS-2M | IIIb | 1045 |
|
| 13 |
| PrEP | |||||
| HPTN083a | IIb/III | 4566 |
|
| 42,43 |
| HPTN084a | III | 3224 |
|
| 44,45 |
| Trial . | Phase . | n . | Arms . | Results . | Ref. . |
|---|---|---|---|---|---|
| Treatment | |||||
| LATTE-2 | IIb | 286 |
|
| 41 |
| FLAIR | III | 566 |
|
| 30 |
| ATLAS | III | 616 |
|
| 31 |
| ATLAS-2M | IIIb | 1045 |
|
| 13 |
| PrEP | |||||
| HPTN083a | IIb/III | 4566 |
|
| 42,43 |
| HPTN084a | III | 3224 |
|
| 44,45 |
CAB, cabotegravir; RPV, rilpivirine; ABC, abacavir; 3TC, lamivudine; DTG, dolutegravir; ATV, atazanavir; TDF, tenofovir disoproxil fumarate; FTC, emtricitabine; n, number of participants assigned to randomization; q4w, every 4 weeks; q8w, every 8 weeks; q24h, once daily.
Ongoing studies.
| Trial . | Phase . | n . | Arms . | Results . | Ref. . |
|---|---|---|---|---|---|
| Treatment | |||||
| LATTE-2 | IIb | 286 |
|
| 41 |
| FLAIR | III | 566 |
|
| 30 |
| ATLAS | III | 616 |
|
| 31 |
| ATLAS-2M | IIIb | 1045 |
|
| 13 |
| PrEP | |||||
| HPTN083a | IIb/III | 4566 |
|
| 42,43 |
| HPTN084a | III | 3224 |
|
| 44,45 |
| Trial . | Phase . | n . | Arms . | Results . | Ref. . |
|---|---|---|---|---|---|
| Treatment | |||||
| LATTE-2 | IIb | 286 |
|
| 41 |
| FLAIR | III | 566 |
|
| 30 |
| ATLAS | III | 616 |
|
| 31 |
| ATLAS-2M | IIIb | 1045 |
|
| 13 |
| PrEP | |||||
| HPTN083a | IIb/III | 4566 |
|
| 42,43 |
| HPTN084a | III | 3224 |
|
| 44,45 |
CAB, cabotegravir; RPV, rilpivirine; ABC, abacavir; 3TC, lamivudine; DTG, dolutegravir; ATV, atazanavir; TDF, tenofovir disoproxil fumarate; FTC, emtricitabine; n, number of participants assigned to randomization; q4w, every 4 weeks; q8w, every 8 weeks; q24h, once daily.
Ongoing studies.
In Phase III clinical studies, the first dual LAI-ART combination of cabotegravir and rilpivirine has been given after a preliminary 4 week oral lead-in period (cabotegravir 30 mg plus rilpivirine 25 mg, once daily), to assess drug tolerability, followed by IM injections of each drug into the gluteal muscles. The regimens best investigated so far consist of 2 or 3 mL IM injections, at 4 or 8 week dosing intervals, respectively.
This combination was studied in the landmark LATTE-2 trial,41 which compared the dosing regimens of LAI cabotegravir/rilpivirine 400/600 mg (two 2 mL injections) every 4 weeks, or LAI cabotegravir/rilpivirine 600/900 mg (two 3 mL injections) every 8 weeks, with an oral three-drug regimen of cabotegravir/lamivudine/abacavir. At Week 96, viral suppression was maintained in 84% of patients receiving oral treatment, 87% of patients in the 4 week group and 94% of patients in the 8 week group. In the 8 week group, mean cabotegravir and rilpivirine Cmin were nine and five times, respectively, above the PAIC90 against WT HIV.
The FLAIR and ATLAS Phase III studies compared the LAI cabotegravir/rilpivirine regimen with standard daily three-drug oral regimens.30,31 After the oral lead-in period, the participants received an initial loading dose of IM LAI cabotegravir/rilpivirine 600/900 mg, followed by LAI cabotegravir/rilpivirine 400/600 mg every 4 weeks through the maintenance phase. At Week 48, the FLAIR trial concluded that viral suppression was maintained in 93.6% of patients receiving the LAI therapy and in 93.3% of patients on oral triple therapy. The ATLAS study showed, for its part, that viral suppression was maintained in 92.5% of patients receiving the injections and in 95.5% of patients on oral therapy. Efficacy and safety of the long-acting therapy in these trials were similar and confirmed that the 4 week LAI cabotegravir/rilpivirine regimen was non-inferior to standard daily three-drug oral regimens.
The subsequent ATLAS-2M study, which specifically compared the 4 week regimen and the 8 week regimen, reaffirmed that the LAI cabotegravir/rilpivirine 8 week regimen was highly effective and non-inferior to the 4 week injection regimen.13 Viral suppression was maintained in 94% and 93% of patients in the 8 and 4 week regimens, respectively. The efficacy and safety of the LAI cabotegravir/rilpivirine 8 week regimen also appears to be supported by the ongoing Phase III POLAR study,46 which has included 100 treatment-naive PLWH, who had remained virologically suppressed to less than 50 copies/mL on daily oral cabotegravir plus rilpivirine in the Phase IIb LATTE trial.23
Ongoing studies are now investigating LAI cabotegravir/rilpivirine in various situations and different subpopulations. For instance, the ‘More Options for Children and Adolescents’ (MOCHA) study aims to establish the optimal dosing and assess the safety, tolerability, acceptability and PK profiles of oral cabotegravir and LAI cabotegravir/rilpivirine (alone or in combination) in virologically suppressed patients aged between 12 and 18 years. In addition, the ongoing LATITUDE Phase III trial is comparing the efficacy, safety and durability of LAI cabotegravir/rilpivirine administered every 4 weeks to a standard daily three-drug regimen in patients with a history of suboptimal adherence to oral treatment and control of their HIV infection.47
Of note, such as in the ongoing SOLAR trial,48 the decision regarding the oral lead-in period is now at the sole discretion of participating patients. Indeed, the actual necessity of the oral lead-in phase is currently debated.49
Lastly, one study examined the PK of cabotegravir and the neonatal outcomes in three women who confirmed pregnancy during clinical trials.50 Rilpivirine concentrations were not assessed. The rate of prolonged terminal decline (or ‘PK tail’, discussed later) of cabotegravir concentration after treatment discontinuation was found to be in the expected range for non-pregnant women, and no adverse effects on maternal and neonatal health were reported. After stopping treatment, all women started a standard daily oral regimen and maintained undetectable viral load during 52 weeks of follow-up. Further trials remain necessary to better inform the use of cabotegravir and rilpivirine in pregnancy.
The LAI formulations of cabotegravir and rilpivirine have also been studied for PrEP. Yet, LAI rilpivirine as a single PrEP agent appears to be of questionable relevance because of a low barrier to resistance,51,52 and storage and transportation constraints (e.g. cold chain required). Alternatively, LAI cabotegravir 800 mg injected every 12 weeks for PrEP has been studied in healthy men at high risk of HIV infection (ECLAIR study).53 Lower than expected cabotegravir plasma levels in this study led to revised LAI cabotegravir dosages, subsequently evaluated in the HIV Prevention Trials Network (HPTN). The ongoing HPTN 083 study is being conducted in 4566 HIV-uninfected MSM and transgender women. The blinded comparison was prematurely stopped in May 2020 because it had already met its specified pre-study objectives, showing that cabotegravir injected every 8 weeks was highly effective and clinically superior to daily oral tenofovir disoproxil fumarate/emtricitabine for PrEP.42 Based on these results, in November 2020 the FDA granted the breakthrough therapy designation for the single agent LAI cabotegravir as PrEP. However, despite these impressive results, the HPTN 083 trial raised some concerns.54 It has been suggested that cabotegravir may delay the detection of HIV infection in people starting PrEP because it rapidly suppresses the viral load to undetectable levels. In addition, ‘breakthrough’ infections despite adequate plasma levels were reported in four participants. Multiple hypotheses should therefore be addressed in further investigations, including how cabotegravir concentrations vary between various body compartments (e.g. diffusion into rectal, vaginal and penile mucosal tissues) and whether higher Cmin should be targeted in some individuals. In the absence of biomarkers for PrEP, TDM could be advised in specific cases for ascertaining trough levels at the start of prophylaxis or in case of comorbidities or co-medications that could affect exposure to cabotegravir and hence would impair the protection against HIV infection. Moreover, one participant who was found retrospectively to be HIV positive at baseline developed cabotegravir resistance mutations during PrEP.
In the ongoing HPTN 084 study, LAI cabotegravir is being compared with daily oral tenofovir disoproxil fumarate/emtricitabine in 3224 women, aged 18–45 years, at risk of HIV infection in sub-Saharan Africa. LAI cabotegravir was found to be significantly more effective than oral PrEP in preventing HIV acquisition among cisgender women.44,55 LAI cabotegravir is planned to be administered also to adolescent girls within the framework of the ancillary study HPTN 084/01 (safety, tolerability and acceptability of LA cabotegravir for the prevention of HIV among adolescents).56
From a regulatory perspective, the Health Canada authority was the first to approve the LAI cabotegravir/rilpivirine combination in March 2020 under the brand name CABENUVA®, which is marketed as a co-pack with two separate injectable medicines. It is indicated, after an oral lead-in period, as a complete regimen for the maintenance treatment of HIV-1 infection in adults, replacing a current oral ART regimen ensuring virological suppression (HIV-1 RNA less than 50 copies/mL).29 The EMA has recently approved LAI cabotegravir/rilpivirine under the name VOCABRIA® (cabotegravir injection and tablets), REKAMBYS® (rilpivirine injection) and EDURANT® (rilpivirine tablets). The FDA initially raised some concerns about the treatment’s manufacturing process (albeit not related to safety), which were addressed by the manufacturers, allowing approval of the LAI cabotegravir/rilpivirine combination CABENUVA® on 21 January 2021.
Islatravir
Islatravir is a first-in-class nucleoside reverse transcriptase translocation inhibitor (Figure 1) with multiple mechanisms of action. The unusual 4′-ethynyl group (Figure 1, in green) blocks primer translocation and causes chain termination during viral RNA transcription. The 3′-hydroxy group (Figure 1, in blue) contributes to the high binding affinity for the reverse transcriptase. The 2-fluoro group (Figure 1, in red) protects islatravir from metabolism by hampering its deamination by adenosine deaminase, and this contributes to its long t½.57,58
In humans, islatravir is phosphorylated intracellularly into the active metabolite islatravir triphosphate. The plasma t½ of islatravir after oral administration is 50–60 h, while the intracellular t½ of islatravir triphosphate is 130–210 h. At steady state, intracellular concentrations of islatravir triphosphate in PBMCs were 1000 times higher than concomitant islatravir levels measured in plasma.57 Islatravir has been given orally at dosages of 10, 30 and 100 mg weekly, with good viral responses and tolerance. Notably, in a Phase Ib study,58 a single dose of 0.5 mg was found to significantly suppress plasma HIV-1 RNA for at least 7 days. With higher doses, an extended period of viral suppression could be achieved, opening the way for possible regimens with longer dosing intervals. Moreover, islatravir in combination with the NNRTI doravirine 100 mg per day appeared to work at least as well as the three-drug regimen of doravirine/tenofovir disoproxil fumarate/lamivudine, such as described in a double-blind randomized dose-ranging Phase IIb trial.59 After 96 weeks, the highest response was observed in the arm of patients receiving 0.75 mg islatravir, with 90.0% having an undetectable viral load (i.e. HIV RNA less than 50 copies/mL), compared with 80.6% in the control group. This optimal daily dosing regimen of doravirine/islatravir 100/0.75 mg is now being tested in an ongoing Phase III comprehensive clinical development programme among various PLWH populations.60,61
Islatravir was also shown to have a particular potency against resistant HIV variants58 and, to date, no resistance to islatravir has been observed in PLWH treated by islatravir.57 Consequently, islatravir is currently being evaluated in a Phase IIa study as a monthly oral PrEP.62
Based on the technology successfully used for implantable contraceptives, islatravir has been formulated as a non-degradable subcutaneous implant that slowly releases drug from a biodegradable polylactic co-glycolic acid matrix. The t½ described for implants of approximately 100 days might make it amenable to LA administration with dosing intervals of 1 year or more.57,63 Indeed, the results of a Phase I study with radiopaque islatravir-eluting implants seems to support sufficient drug release for HIV prophylaxis for at least 1 year.64 Islatravir implants are also currently being investigated in combination with other LA drugs for HIV treatment.65
Lenacapavir
Lenacapavir (GS-6207) (Figure 1) is a first-in-class capsid inhibitor, which interferes with multiple capsid-dependent functions essential for viral replication, namely capsid assembly and disassembly, as well as nuclear transport and virus production.66 Lenacapavir exhibits antiviral activity at picomolar levels (mean EC50 of 0.05 ng/mL) in vitro. After a single dose of 100 mg or more, plasma concentrations at 12 weeks remained above the reported 95% protein-adjusted effective concentration (PAEC95) of 3.87 ng/mL. Moreover, it showed high synergy and no cross-resistance with currently approved antiretroviral drugs.66,67
The ongoing CAPELLA Phase II/III trial will assess the safety and efficacy of oral lenacapavir in PLWH with multiresistant viral strains, when administered as an add-on to a failing regimen.68 Preliminary results presented at CROI 2021 showed that lenacapavir was safe and well tolerated, and led to a rapid and clinically relevant decline in viral load.69 Those results support its use in treatment and prevention of HIV. In addition, the manufacturer announced that a single 900 mg dose (3 × 1 mL or 2 × 1.5 mL injection), formulated as sustained-delivery subcutaneous injection, yielded therapeutic plasma concentration coverage for at least 6 months.70 Lenacapavir has therefore the potential to be administered every 6 months by subcutaneous injections.47
Notably, the manufacturers of islatravir and lenacapavir have recently announced a joint venture for the clinical evaluation of a combination of these two long-acting drugs.71,72 This collaboration is likely to accelerate the development of a highly promising dual formulation.
Antibody-based strategies
Ibalizumab is a humanized IgG4 antibody that binds to the extracellular CD4 domain and prevents HIV entry through allosteric inhibition. It was the first monoclonal antibody approved by the FDA, specifically in combination with other antiretrovirals, for patients failing to respond to treatment due to multiresistance.5,73 Ibalizumab is injected intravenously (IV) as a single loading dose of 2000 mg, followed by maintenance doses of 800 mg injected IV every 2 weeks.73,74
Before entering into Phase III, ibalizumab received an orphan drug status from the FDA and then a breakthrough therapy status.75 Subsequently, the single-arm open-label Phase III TMB-301 trial found that ibalizumab combined with an optimized background regimen significantly reduced viral load and increased CD4 count.76 Like other monoclonal antibodies targeting cell surface molecules, ibalizumab exhibits non-linear PK. This is probably due to dose-dependent receptor-mediated saturable elimination.77
Compared with other LAI-ART, ibalizumab has the disadvantage of requiring a shorter administration interval. Moreover, if a maintenance dose is missed by 3 days or more, a loading dose has to be readministered, leading to potential discomfort for the patients.74 Despite a shorter administration schedule compared with other LAI-ARTs, ibalizumab represents a novel opportunity for heavily treatment-experienced adults with multiresistant HIV infection.
Leronlimab is a humanized IgG4 directed towards CCR5 and can therefore only be given to individuals infected with CCR5-tropic HIV, which represents the vast majority of patients. Leronlimab seems to have a synergistic effect with the CCR5 antagonist maraviroc, but also to be active against maraviroc-resistant strains.73,74
Unlike ibalizumab, leronlimab is administered by subcutaneous injection every week. It has demonstrated efficacy in maintaining viral suppression in virologically suppressed patients as monotherapy, and can be effective as well in treatment-experienced patients with MDR HIV in combination with other ART. Currently being studied in Phase IIb/III studies, it has received fast-track orphan drug status from the FDA.73,74
It should be noted that, like all therapeutic proteins, ibalizumab and leronlimab have the potential to trigger immunogenicity but, to our knowledge, no cases have yet been reported.
LA-ART agents based on novel pharmaceutical formulations
Cabotegravir and rilpivirine development
One limitation that may hamper wide acceptance of LAI cabotegravir/rilpivirine is the rather high volume of the two extended-release suspensions that, at present, need to be slowly injected IM, associated with significant pain at the injection site frequently being reported. Injecting larger volumes of the current formulation as an attempt to extend the dosing interval is likely to result in unacceptable pain and to decrease patient acceptance. This may be overcome by using more potent formulations with improved physicochemical and pharmaceutical properties, which would decrease injection-related problems and allow faster injection.78 In this regard, a novel formulation of cabotegravir, referred to as long-acting slow-release (LASER) ART, was developed by esterification of cabotegravir with stearic acid, an aliphatic fatty acid, for producing a prodrug (see Figure 1) encapsulated into a poloxamer nanoformulation, which shows improved lipophilicity, hydrophobicity, cellular entry and retention. This inactive prodrug is slowly hydrolysed by esterases in physiological conditions to yield cabotegravir. This nanoparticle formulation of a crystal prodrug could thus improve the drug delivery profile in PLWH, and therefore reduce injection-related problems. The evaluation of this formulation was shown to provide a year-long PK profile, opening the path to applications that could be considered as ‘chemical vaccines’ against HIV.79–81 Similarly, for rilpivirine, a formulation as an N-acyloxyalkyl prodrug (see Figure 1) was developed to slow down the release of the active moiety and to achieve a longer t½. Prodrug bioconversion seems to proceed via enzymatic cleavage of the methylene ester by esterases into an N-hydroxymethyl rilpivirine, which in turn is hydrolysed into the active compound. More generally, alteration of physiochemical and PK properties through prodrug modifications—particularly those enhancing lipophilicity—can improve tissue distribution, cellular uptake and retention, notably in macrophages. After a single IM injection in mice, this novel rilpivirine prodrug formulation maintained concentrations above the PAIC90 for 25 weeks in plasma and in secondary tissue deposition.82
However, as both of these prodrugs depend on esterase activity for yielding their active form, inter-individual differences in enzymatic activity could influence the active drug levels and hence the therapeutic outcomes. The clinical consequences of genetic variations of esterases, which would affect their enzymatic activity, are unclear at present,83 but deserve investigation in future studies.
Secondly, the particle size of nanocarriers seems also to play an important role in the distribution of LA formulations, notably through the lymphatic system. Currently marketed LAI cabotegravir and LAI rilpivirine formulations have an average particle size of 200 nm.10,22 Reduction in particle size below 100 nm could potentially increase the uptake by the lymphatic system, resulting in improved circulation time.39,84
Implant formulations
Tenofovir alafenamide is a prodrug of tenofovir, an NRTI, and one of the antiretroviral agents most widely used at present. Tenofovir alafenamide is 10 times more potent than tenofovir disoproxil fumarate and has the valuable advantage of being directly taken up into cells to be converted intracellularly to the parent drug (tenofovir) and then to the active intracellular diphosphate. Systemic toxicity is therefore reduced with tenofovir alafenamide, compared with tenofovir disoproxil fumarate, because it produces much lower tenofovir levels in plasma, the latter being associated with kidney tubular damage and, in the long term, with bone demineralization.5,85 The three chemical forms of tenofovir are shown in Figure 1.
Preliminary new formulations of tenofovir alafenamide have frequently been associated with local toxicity problems and further developmental efforts are therefore still necessary before clinical implementation. Recently, a promising subdermal implant that releases tenofovir alafenamide for at least 6 months of HIV protection was developed. This implant appeared safe and well tolerated in mice and sheep. Given its favourable PK and tolerability profile, it seems promising for the future development of new LA technologies.86 Other examples of implants of tenofovir alafenamide87–89 are presented in Table 3.
Types of implants of tenofovir alafenamide (TAF) for long-acting therapy currently under investigation
| Types of implant . | Drug release properties . |
|---|---|
| A subcutaneous silicone implant delivers TAF from orthogonal channels coated with polyvinyl alcohol. | It provides measurable plasma concentrations of TAF over more than 6 weeks and delivers TAF at near constant rate for up to 40 days after implantation.87 |
| A reservoir-style implant with an extruded tube of a biodegradable polymer [poly (ε-caprolactone)] membrane. | It can deliver TAF with sustained zero-order release kinetics. After subcutaneous injection, the biological fluid from the environment can enter and solubilize TAF, which is then passively transported across the membrane and released from the implant.88 |
| A subcutaneous implant formed with pressed TAF pellets and extruded polyurethane tubing. | This modular implant is tunable to adjust the rate and duration of TAF release through adjustment of geometry and membrane composition.89 |
| Types of implant . | Drug release properties . |
|---|---|
| A subcutaneous silicone implant delivers TAF from orthogonal channels coated with polyvinyl alcohol. | It provides measurable plasma concentrations of TAF over more than 6 weeks and delivers TAF at near constant rate for up to 40 days after implantation.87 |
| A reservoir-style implant with an extruded tube of a biodegradable polymer [poly (ε-caprolactone)] membrane. | It can deliver TAF with sustained zero-order release kinetics. After subcutaneous injection, the biological fluid from the environment can enter and solubilize TAF, which is then passively transported across the membrane and released from the implant.88 |
| A subcutaneous implant formed with pressed TAF pellets and extruded polyurethane tubing. | This modular implant is tunable to adjust the rate and duration of TAF release through adjustment of geometry and membrane composition.89 |
Types of implants of tenofovir alafenamide (TAF) for long-acting therapy currently under investigation
| Types of implant . | Drug release properties . |
|---|---|
| A subcutaneous silicone implant delivers TAF from orthogonal channels coated with polyvinyl alcohol. | It provides measurable plasma concentrations of TAF over more than 6 weeks and delivers TAF at near constant rate for up to 40 days after implantation.87 |
| A reservoir-style implant with an extruded tube of a biodegradable polymer [poly (ε-caprolactone)] membrane. | It can deliver TAF with sustained zero-order release kinetics. After subcutaneous injection, the biological fluid from the environment can enter and solubilize TAF, which is then passively transported across the membrane and released from the implant.88 |
| A subcutaneous implant formed with pressed TAF pellets and extruded polyurethane tubing. | This modular implant is tunable to adjust the rate and duration of TAF release through adjustment of geometry and membrane composition.89 |
| Types of implant . | Drug release properties . |
|---|---|
| A subcutaneous silicone implant delivers TAF from orthogonal channels coated with polyvinyl alcohol. | It provides measurable plasma concentrations of TAF over more than 6 weeks and delivers TAF at near constant rate for up to 40 days after implantation.87 |
| A reservoir-style implant with an extruded tube of a biodegradable polymer [poly (ε-caprolactone)] membrane. | It can deliver TAF with sustained zero-order release kinetics. After subcutaneous injection, the biological fluid from the environment can enter and solubilize TAF, which is then passively transported across the membrane and released from the implant.88 |
| A subcutaneous implant formed with pressed TAF pellets and extruded polyurethane tubing. | This modular implant is tunable to adjust the rate and duration of TAF release through adjustment of geometry and membrane composition.89 |
Dolutegravir-based regimens are one of the preferred first-line and second-line treatments, associating dolutegravir with two NRTIs.5,90 Therefore, different models of implants have been developed for dolutegravir to overcome the lack of adherence associated with daily oral pill intake. An ultra-long-acting removable drug delivery system could deliver dolutegravir for up to 9 months. Following subcutaneous drug injection, the implant solidifies in vivo within 48 h, and its subsequent biodegradation results in sustained drug release.91 This breakthrough formulation allows modulation of the kinetics of drug release through careful adjustment of polymer lactic/glycolic acid ratios and molecular weights. In addition, multiple drugs could be included, and possibly refilled in situ.92,93 Lastly, it does not need surgical removal after complete drug release.
Overall, the possibility of rapid surgical removal of such implants from patients would exempt patients from an oral lead-in period for tolerability assessment.
Microarray patches
Microarray patch (MAP) technologies, also referred to as microneedle patches, have been explored in a proof-of-concept trial for the use of LA rilpivirine to facilitate intradermal administration.94 Nanoparticles could be delivered into the systemic circulation by slow dissolution (approximately 25 min) and absorption through the skin. Concentration maintenance at four times the PAIC90 for 28 days was reported in rats.95
Overall, MAPs could expand access and adherence to HIV treatment. In particular, in low-resource settings where the number of trained medical staff is limited, potential self-administration could be of great interest.94,96
Dapivirine vaginal ring
Dapivirine, an NNRTI formulated as a monthly vaginal ring, was recently approved by the EMA for HIV prevention in women living in high HIV-burden settings. The ring is intended for adult women as a complementary approach to reduce the risk of HIV infection during vaginal sex. It should be used in addition to safer sex practices when women cannot use or do not have access to oral PrEP.97
According to a systematic review of two Phase III trials (RING study98 and ASPIRE study99), a beneficial 29% reduction in HIV risk has been demonstrated with the intravaginal dapivirine ring (relative risk of 0.71; 95% CI 0.57–0.89).100 Despite appearing to be less effective than oral PrEP, the vaginal ring represents an alternative for women who are unable to take oral PrEP according to the recommendations. Moreover, current developments are underway to include both contraception and HIV prevention in a single intravaginal ring.97
Challenges for the development of LA-ART formulations
Despite their promises, neither the approved LAI cabotegravir/rilpivirine combination, nor further LA-ARTs to come are fully devoid of limitations. The existence of the so-called ‘PK tail’ after treatment cessation is of particular concern.53,101 During this prolonged terminal decay, ART plasma concentrations are declining to reach non-suppressive levels below PAEC95, leading to a risk of viral replication rebound together with selection of drug-resistant variants.101 It is therefore currently recommended to initiate daily oral ART as soon as the LA-ART discontinuation is considered, so as to maintain therapeutic plasma levels throughout the PK tail period.85 However, according to the interim results of the HPTN 083 study, no resistance has yet emerged in people with low cabotegravir levels during this decline.54 On the other hand, it has been claimed that the overall DDI risk would be smaller for LAI-ARTs than for oral ARTs, due to the lower importance of intestinal absorption, liver first-pass, metabolism and transport processes.102,103 However, DDIs affecting drug clearance might still occur. Drug transporters (e.g. ABCC1, ABCC4, ABCC5, OATP2B1 etc.) and metabolizing enzymes (CYP450s, UGTs) are expressed in skeletal muscles and subcutaneous adipose tissue, and some of them have shown functional activity.84 The vulnerability of LAI-ART to DDIs constitutes therefore a relevant issue warranting further investigations.
Globally, treatment constraints and stigma are recognized as definite hurdles against optimal adherence to HIV therapy. LAI-ART represents therefore a promising opportunity to overcome such barriers by improving patient privacy and reducing the psychological and social burden associated with the daily intake of anti-HIV pills. Yet, such a novel mode of ART administration will certainly imply organizational constraints, including infrastructures for parenteral administration and thorough selection of patients suitable for such therapy, not to mention discomfort due to injections.40,104,105 However, there are good chances that current and next-generation LA-ARTs will ultimately benefit a large number of PLWH.
The first-generation injectable formulation of LAI cabotegravir/rilpivirine is temperature sensitive and requires a cold chain at 2°C–8°C throughout the drug shipment and storage, which probably makes it less suitable for countries with limited access to refrigeration. Thus, the second wave of LAI-ARTs will need to remain stable at the temperatures and moisture conditions of tropical resource-limited settings.
Further formulation efforts are also appropriate to alleviate the injection-induced pain, possibly using removable microneedle patches. Less harsh and painful modes of administration would be better accepted, particularly by children and adolescents.106 This would facilitate optimization of the dosing regimen to the target patient population at special risk of virological failure.107
Moreover, only potent antiretroviral drugs are likely candidates for implant formulation because the quantity that they can accommodate is small. Drug candidates must therefore demonstrate inhibition of viral replication at conveniently low concentrations.63,92 Yet, implant formulations seem so far to provide more predictable and constant drug release than LAI formulations. In the case of adverse events, DDI occurrence or treatment discontinuation, the implant may be surgically removed, while LAI cessation inevitably results in a sustained period of subtherapeutic concentrations. Indeed, once the implant is removed, the drug concentration decreases rapidly, as does the associated effect. The PK tail problem, as observed for LAI-ART, may thus not be a concern for implants. Obviously, this is not the case with molecules having a long t½, because the drug in circulation after implant removal could remain at significant levels for a considerable period. In addition, multiple implants with different dosages would be necessary to allow for dose escalation in clinical dose-finding and safety studies.91 When starting a treatment, multiple dosages would probably be necessary in order to assess tolerance. Depending on the manufacturing cost of these products, their implementation could then be largely hindered.
Furthermore, in the case of non-biodegradable polymers, the insertion and removal of the implant need surgical intervention by trained personnel. Nevertheless, the widespread use and general acceptance of contraceptive implants in low-income countries indicate that antiretroviral implants could be appropriate worldwide.63 One possible improvement might be to make them refillable, so that implantation and removal do not need to be repeated.108 For instance, a transcutaneously reloadable drug-eluting implant using nanochannels was shown to release tenofovir alafenamide and emtricitabine over an extended period of more than 2 months.109
On the other hand, even if biodegradable polymer implants may a priori appear more attractive in the case of adverse events, they can be removed only early after injection; surgical removal weeks or months after implantation will probably fail because of implant dispersion.91
Ultimately, the acceptability of these new technologies will be key to their implementation in clinical practice. As with contraceptive implants, multiple barriers will arise, such as patient worries and misconceptions, access to the treatment and the price of these new products.110,111 In fact, these new technologies may be more readily accepted by people who are already familiar with them (whether through injections or implants) as part of contraception or disease treatment.110 In this context, long-release implants would potentially be the most acceptable for PLWH, as they would require fewer clinic visits and invasive procedures.
Formulations for the co-administration of LA-ART and a contraceptive are currently being developed in Phase I studies. These combined technologies address multiple sexual and health needs for women, particularly in sub-Saharan Africa. Other drugs that prevent sexually transmitted infections could also be combined.112 Vaginal rings containing dapivirine with levonorgestrel,113 and tenofovir with levonorgestrel,114,115 are being studied. In addition, cabotegravir could also be injected every 8 weeks for PrEP, aligned with appointments for LA contraceptive injections. As previously mentioned, an implant of islatravir is currently under development for PrEP and could potentially be administered with a hormonal contraceptive in the same device.
Finally, LA oral treatment may ultimately become the best way to improve HIV management for the majority of PLWH, as there would be no inconveniences associated with an invasive procedure, little or no infrastructure requirements and no additional investments on the part of patients. The combination of the highly promising LA drugs lenacapavir and islatravir will probably represent a turning point in the management and prevention of HIV. Indeed, given the intrinsic characteristics and formulation possibilities of both these molecules, it is likely that this combination would allow for less frequent administration than, for example, the current LAI cabotegravir/rilpivirine.
Research gaps
It is noteworthy that LAI-ART is expected to make treatment adherence no longer represent a confounding factor for insufficient clinical response, as drugs will be administered parenterally under direct medical supervision. Nevertheless, in specific instances such as DDIs, issues at the injection site or special pathophysiological conditions, the monitoring of ART plasma levels will remain an important component of optimal patient follow-up in the LA-ART era. As prescription of LAI-ART represents a novel therapeutic paradigm, infectious diseases specialists may wish to obtain information on whether their patients are exposed to appropriate antiretroviral drug levels over the whole IM dosing intervals, with regard to not only efficacy but also tolerability and long-term safety. The potential impact of DDIs and pharmacogenetic traits might deserve further attention.
At present, LAI cabotegravir and LAI rilpivirine are marketed at standard dosage for all patients, while highly variable situations may occur in the real world. Marked alterations of LA-ART exposure can be postulated and might be simply unknown. We are notably concerned by the initiation of treatments for coincidental or inaugural diseases with definite risk of DDIs (TB, HCV infection or cancer etc.) in underweight or obese patients and, should it occur, in the case of pregnancy. The influence of physiological changes during pregnancy on ART exposure in women receiving LA-ART is unknown: alterations in the protein binding and volume of distribution of these new drugs could indeed lead to changes in PK and possibly to insufficient HIV coverage or adverse fetal events. Monitoring of pregnancy outcomes will require open-label extension studies. Further issues may add to the complexity of the management of PLWH on LA-ART, particularly in the ageing population who develop age-related physiological changes and frequently comorbidities. Polypharmacy is common among elderly PLWH and may cause DDIs that could affect their quality of life. The acceptability and actual benefits of LAI-ART in this population deserve thorough investigation. LA oral drugs to come might be revealed as more promising in older PLWH.
Similarly to implantable contraception exposure, where decreased etonorgestrel levels were reported in higher body weight individuals,116 BMI and gender have been identified in clinical trials to affect absorption rate51 and overall exposure to LAI-ART.51,117 A high BMI seems indeed to lead to a slower absorption rate and lower plasma concentrations of LAI-ART,22,52,117 and may be associated, among other baseline factors, with an increased risk of virological failure.118 Independently, longer periods with undetectable ART plasma concentrations were also observed in patients with higher BMI.101 This issue has been addressed in injection guideline recommendations, whereby longer needles are required for patients with a BMI of 30 or greater.101 Yet, BMI does not distinguish between adipose and muscle tissue, and therefore does not provide information on the distribution of body fat.22,117 Muscle density, accumulated scar tissue after prolonged therapy, physical activity, as well as ambient temperature, may have an impact on the PK exposure after LAI administration.84 In addition, unforeseen local problems at the injection site might also have important consequences on drug absorption.
Finally, precision medicine strategies should take into account the all-too-often neglected dimension of variability in dose–exposure–response relationships. In fact, it is likely that TDM, despite non-negligible costs, will be needed at least in selected subgroups of patients to assess the concentration exposure resulting from a given dosing regimen.
To this end, concentration monitoring of the LA-ARTs, namely LAI cabotegravir/rilpivirine in the first instance, is currently being initiated within the framework of the Swiss HIV Cohort Study (SHCS).119 It aims to verify whether standard dosage ensures appropriate antiretroviral plasma exposure in various types of real-life patients receiving LA-ART. It will bring indications on the potential suitability of altering the LA-ART dosing schedule, through shortening or extending dosing intervals, in certain patients exhibiting, respectively, lower or higher Cmin.
Conclusions
Injectable-based formulations of molecules with intrinsic high potency and long t½, such as cabotegravir and rilpivirine, are on their way to being implemented worldwide. The LAI cabotegravir/rilpivirine formulation can ensure 2 month-long effective plasma concentrations and thus could have a major impact on HIV management in the coming years. This dosing interval is due to be further extended in the near future, with the development of novel antiretroviral agents. Nevertheless, potential issues related to the long PK profile will need to be further characterized, to ensure the safe and effective use of this treatment in all patients. Alternatively, implant development represents another promising approach to improve HIV treatment and prevention. As for contraceptives, this implant approach should be rather well accepted. However, further developments remain necessary to make the use of these technologies definitely amenable to patients. Lastly, LA oral drugs or implants to come may appear best suited to implementation in elderly PLWH or any other subpopulation that may be refractory to relatively invasive treatments.
In the growing movement of precision medicine, further research efforts might improve the prescription of LA-ARTs, with regard to not only efficacy but also tolerability, long-term safety, overcoming of DDIs and possibly pharmacogenetic traits, together with patients’ choice and best convenience. In this regard, TDM is at the forefront of this trend to personalize treatment, and even prophylaxis, to best meet the needs of the patient. LA-ARTs will transform not only the treatment of HIV infection, but also its prevention. Yet, these approaches have so far been tested in the strict framework of clinical trials, which do not account for the complex real-world situation of many PLWH. The implementation and deployment of such revolutionary approaches for the treatment and prevention of HIV infection need, at present, to be accompanied by close follow-up of patients on LA-ART. This includes the monitoring of viral suppression and CD4 count, and also the measurement of antiretroviral drug levels in plasma—and possibly in body tissues and cellular compartments. Further studies are thus warranted for maximizing the remarkable therapeutic and prophylactic potential of LA-ARTs against HIV infection in the real-life situation.
Funding
This work was supported by the Swiss National Science Foundation, grant number 324730_192449 (to L.A.D.).
Transparency declarations
None to declare.
References
- hiv
- body mass index procedure
- seizures
- canada
- drug delivery systems
- intramuscular injections
- nucleosides
- pain
- privacy
- rna-directed dna polymerase
- translocation (genetics)
- united states food and drug administration
- vaccines
- infections
- arts
- genetics
- pharmacokinetics
- therapeutic drug monitoring
- anti-retroviral agents
- medical procedures
- social stigma
- rilpivirine
- dosing interval
- hiv prevention
- implants
- patient disposition
- cabotegravir
- precision medicine
- liposomal amikacin for inhalation



