-
PDF
- Split View
-
Views
-
Cite
Cite
Sara Comini, Gabriele Bianco, Matteo Boattini, Giuliana Banche, Guido Ricciardelli, Valeria Allizond, Rossana Cavallo, Cristina Costa, Evaluation of a diagnostic algorithm for rapid identification of Gram-negative species and detection of extended-spectrum β-lactamase and carbapenemase directly from blood cultures, Journal of Antimicrobial Chemotherapy, Volume 77, Issue 10, October 2022, Pages 2632–2641, https://doi.org/10.1093/jac/dkac230
Close - Share Icon Share
Abstract
To evaluate a rapid diagnostic algorithm based on MALDI-TOF MS, lateral flow immunoassays (LFIAs) and molecular testing performed directly from positive blood cultures (BCs) for Gram-negative species identification and detection of CTX-M extended-spectrum β-lactamases and main carbapenemases.
Non-duplicate BCs positive to Gram-negative bacteria at microscope examination were subjected to species identification by direct MALDI-TOF MS following recovery of bacterial pellet by Rapid MBT Sepsityper® kit. Subsequently, NG-Test® CARBA 5 and NG-Test® CTX-M MULTI LFIAs were performed according to identified microbial species. Eazyplex® SuperBug CRE molecular assay was performed in cases of NG-Test® CARBA 5 negative results in patients with documented carbapenemase-producers carriage. Results of rapid diagnostic workflow were compared with those obtained by conventional diagnostic routine.
Overall, the direct MALDI-TOF MS protocol allowed reliable identification to the species level of 92.1% of the 2133 monomicrobial BCs. Rate of matched identification was significantly higher for Enterobacterales (97.3%) in comparison to non-fermenting Gram-negative species (80.2%), obligate anaerobic bacteria (42.1%) and fastidious Gram-negative species (41.5%). The overall sensitivity of NG-Test® CARBA 5 and NG-Test® CTX-M MULTI was 92.2% and 91.6%, respectively. Integration of Easyplex® SuperBug CRE allowed the detection of blaKPC mutants associated with ceftazidime/avibactam resistance, reaching 100% sensitivity in carbapenemase detection. Both LFIAs and molecular testing showed no false-positive results.
Algorithms based on MALDI-TOF MS, LFIAs and molecular testing may represent a cost-effective tool to timely identify Gram-negative species and detect resistance markers directly from BCs. According to local epidemiology, these results may allow antimicrobial stewardship interventions including prompt use of new approved drugs.
Introduction
Bloodstream infection (BSI) is a major cause of morbidity and mortality despite the advances in both antimicrobial therapy and supportive care. Gram-negative bacteria are among the leading causes of nosocomial BSIs, and Escherichia coli, Klebsiella pneumoniae and Pseudomonas aeruginosa represent the key species covering more than 50% of BSIs episodes.1–3 Among patients suffering from Gram-negative BSI, the effectiveness of empirical antibiotic therapy is hampered by the growing threat of antimicrobial resistance. Therefore, the daily challenge in treating BSI patients is to choose the optimal antibiotic regimen according to local epidemiology of antibiotic resistance, risk factors for MDR bacteria and patient clinical features.
Blood culture (BC) remains the diagnostic reference standard for BSI, as it allows both bacterial identification and antimicrobial susceptibility pattern to be obtained. In the conventional diagnostic workflow, identification and AST of the BSI agent are usually performed on pure overnight subcultures, leading to non-negligible times to results of at least 48 h from positive BC bottle processing.
With the aim of reducing time to results, optimizing costs and limiting the use of molecular methods to the most complex cases, microbiological diagnostics has sought to develop rapid workflows that can incorporate the technologies already present in most laboratories. With these prerogatives, MALDI-TOF MS has become a well-established method for the identification of microorganisms directly from positive BCs with a turnaround time of less than 1 h.4 Moreover, given the high rate of multidrug resistance among Gram-negative bacteria and new therapeutic options, several phenotypic tests such as lateral flow immunoassays (LFIAs) have been developed and proven valuable for the detection of main resistance markers towards β-lactams.5,6 These assays can be performed on isolated bacterial colonies, but their performance has also been evaluated to detect resistance mechanisms directly from positive BCs, providing reliable results within less than 15 min. Despite this, the data available in the literature derive from studies performed on spiked BC samples or on limited numbers of clinical specimens (range 30–310).7–13 Two CE-marked LFIAs available on the market are the NG-Test® CARBA 5 and the NG-Test® CTX-M MULTI (NG Biotech, France). These tests rely on the immunochromatographic detection and differentiation of the five major carbapenemase enzymes and the most common CTX-M enzyme groups, respectively.5,13
This study was designed to evaluate the implementation of a rapid diagnostic algorithm for Gram-negative BSIs based on direct MALDI-TOF MS, NG-Test® CARBA 5 and the NG-Test® CTX-M MULTI LFIAs, and Eazyplex® SuperBug CRE molecular carbapenemase detection testing. Secondly, we evaluated the association between detected resistance markers and antimicrobial susceptibility towards the main antimicrobials used to treat Gram-negative bacteria infections, including those newly approved such as ceftolozane/tazobactam, ceftazidime/avibactam, meropenem/vaborbactam and cefiderocol.
Materials and methods
Study design
This study was performed in a 2300 bed tertiary teaching hospital in North-western Italy over a 3 year period (2019–22).
The BactAlert Virtuo instrument (Marcy l’Ètoile, France) was used to detect positivity of all BC bottles during the study. BC bottles that tested positive on microscopic examination for Gram-negative bacteria were included in the analysis. Only one BC bottle per patient/BSI event was included in the analysis and samples collected from patients with previous Gram-negative BSI within the previous 15 days were excluded from the analysis. Positive BC bottles were subjected to the rapid diagnostic workflow as follows (Figure 1):
Recovery of the bacterial pellet by Rapid MBT Sepsityper® IVD Kit (Bruker Daltonik, Bremen, Germany).
MALDI-TOF MS analysis by Bruker Microflex LT mass spectrometer.
NG-Test® CARBA 5 and NG-Test® CTX-M MULTI LFIAs on selected BC bottles using the remaining pellet. Selection was performed to optimize costs according to local epidemiology of carbapenemases and ESBL in Enterobacterales3 as follows: (a) NG-Test® CARBA 5 and NG-Test® CTX-M MULTI in case of K. pneumoniae identification; (b) NG-Test CTX-M MULTI in case of E. coli identification; and (c) NG-Test® CARBA 5 in case of identification of Enterobacterales species other than K. pneumoniae in patients with documented carriage by the same bacterial species found to be a carbapenemase producer.
Eazyplex® SuperBug CRE molecular testing (Amplex Diagnostics GmbH, Gars am Inn, Germany)14 directly from BC fluid in case of NG-Test® CARBA 5 negative result and patient with documented carriage by the same species identified in BC and found to be a carbapenemase producer.
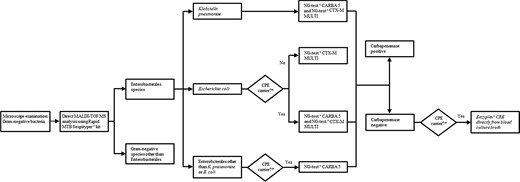
Rapid diagnostic algorithm for Gram-negative BSI during the study period (April 2019 to March 2022). *Documented carriage by the same bacterial species identified in BC found to be a carbapenemase producer.
Results obtained from rapid diagnostic workflow were compared with those of conventional diagnostic routine.
Conventional diagnostic routine
The Microbiology and Virology Unit of Città della Salute e della Scienza di Torino hospital is routinely open 7 days a week from 08:00 to 20:00 h. Outside of this time frame, biological samples reaching the laboratory are processed the next day.
In the study period, BCs were processed using BACT/ALERT® FA Plus aerobic BACT/ALERT®, FN Plus anaerobic and BACT/ALERT® PF Plus pediatric bottles (bioMérieux, Marcy l’Ètoile, France) and incubated in the BACT/ALERT® Virtuo® (bioMérieux). BC bottles flagged positive by the instrument were subjected to subcultures on agar media and slides preparation by WASPLab® instrument (Copan, Brescia, Italy). Gram staining of slides was performed using automated stainer Aerospray® (ELITechGroup, Turin, Italy). After overnight growth on solid media at 36 ± 1°C and proper atmosphere conditions, colonies were subjected to microbial identification by MALDI-TOF MS following the manufacturer’s instructions.
Non-fastidious Gram-negative isolates were tested for antimicrobial susceptibility to cefotaxime, ceftazidime, cefepime, piperacillin/tazobactam, ceftolozane/tazobactam, ceftazidime/avibactam, meropenem, imipenem, ertapenem, gentamicin, amikacin, colistin, trimethoprim/sulfamethoxazole, ciprofloxacin and levofloxacin by a commercially available microdilution assay (Panel NMDR on automated Microscan WalkAway 96 Plus System, Beckman Coulter, Switzerland) according to the manufacturer’s instructions. Once the conventional AST results become available, confirmatory tests for resistance mechanisms were performed according to EUCAST guidelines (https://www.eucast.org) to identify ESBL- and carbapenemase-producing Enterobacterales (CPE) strains. The phenotypic test included in the NMDR microdilution panel was used to detect ESBLs. This confirmatory test is based on synergy of β-lactamases inhibitor clavulanic acid on cefotaxime and ceftazidime MICs. On the other hand, the main carbapenemase genes in Enterobacterales (blaKPC, blaNDM, blaVIM, blaIMP and blaOXA-48-like) were investigated by genotypic assay Xpert Carba-R on GeneXpert platform (Cepheid, Sunnyvale, CA, USA) when meropenem and/or ertapenem MICs were >0.12 mg/L. CPE isolates were tested for meropenem/vaborbactam and cefiderocol susceptibility by gradient diffusion and disc diffusion testing (Liofilchem®, Roseto degli Abruzzi, Italy), respectively. Microbial identification and susceptibility results were promptly uploaded to the laboratory information system.
Rapid diagnostic workflow
During the study period, the rapid protocol of MBT Sepsityper® IVD kit was used to recover bacteria from BCs fluid tested positive to Gram-negative bacteria at microscope examination. The procedure recommended by the manufacturer was modified and performed as follows: (i) transfer nominal 1 mL of BC fluid to an Eppendorf tube; (ii) add nominal 200 μL of lysis buffer and mix by vortexing for 10 (±5) s; (iii) centrifuge the tube for 2 min at 13 000 rpm at room temperature; (iv) remove the supernatant by pipetting and discard; (v) add nominal 1 mL of washing buffer and resuspend the pellet by pipetting up and down; (vi) centrifuge the tube for 1 min at 13 000 rpm at room temperature; (vii) remove the supernatant from the pellet by pipetting and discard; and (viii) if the bacterial pellet is contaminated with material from the blood, add 1 mL of washing buffer, vortex for 10 (±5) s to suspend the bacterial pellet, allow corpuscular material to settle for 30 s, transfer the supernatant bacterial suspension into a new Eppendorf tube and proceed to step vi.
The bacterial pellet was then transferred onto the MALDI-TOF steel target plate using a toothpick, coated with 1 μL of HCCA matrix, allowed to dry at room temperature and subjected to MALDI-TOF MS analysis on Bruker instrument. Each sample was tested in duplicate and species identification with the highest confidence score from each pair was recorded. In cases of discordant results, the identification was considered unreliable. Confidence score value threshold ≥ 1.8 was accepted for identification to species level. MALDI-TOF MS analysis performed on pure overnight subcultures was considered as reference identification method.
NG-Test® CTX-M MULTI and/or NG-Test® CARBA 5 LFIAs were performed adding 5–10 (for one or both tests, respectively) drops of lysis buffer to the remaining bacterial pellet; after vortexing for 5 s, 100 μL of each suspension was added to the sample well of the respective test cassette, as previously described.12
Eazyplex® SuperBug CRE loop-mediated isothermal amplification assay was performed directly from BC fluid using the previously described protocol.15
Statistical analysis
Descriptive data are presented as absolute (n) and relative (%) frequencies. Sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of the rapid diagnostic workflow in detecting ESBL and carbapenemase producers with 95% CI were computed using MedCalc software. Comparison involving dichotomous variables was tested using χ2 test. Statistical significance was set at P value <0.05.
Ethics approval
This study was conducted in accordance with the Declaration of Helsinki. Formal ethics approval was obtained by our Centre’s institutional review board (Protocol No. 0029345).
Results
Over the study period, a total of 2274 BC bottles tested positive to Gram-negative bacteria at microscopic examination and thus enrolled in the study (Figure 2). Overall, direct MALDI-TOF MS achieved reliable species identification (score ≥1.8) in 2089 samples (91.9%). The NG-Test® CTX-M MULTI was performed on 756 and 468 BC bottles tested positive to E. coli and K. pneumoniae by direct MALDI-TOF MS, respectively. The NG-Test® CARBA 5 was performed on the 468 BCs tested positive to K. pneumoniae, and on 10 BCs tested positive to Enterobacterales species other than K. pneumoniae, and which had been collected from patients with documented carriage by the same bacterial species found to be a carbapenemase producer. Additionally, 19 BCs were subjected to the direct molecular carbapenemase detection testing.
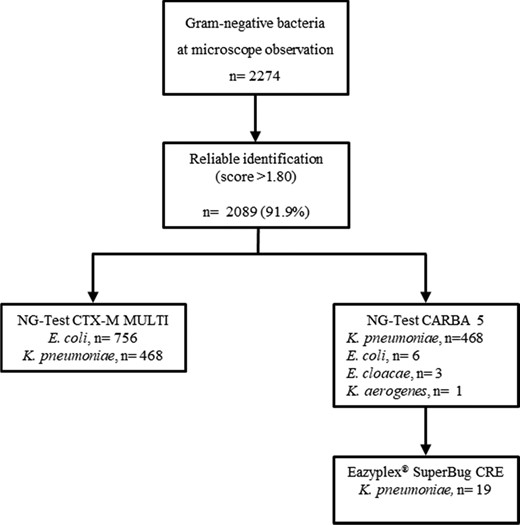
Rapid BCs workflow according to the study’s diagnostic algorithm.
Overall, 6.2% (n = 141) of BCs were found to be polymicrobial by examination of overnight cultures, thus bringing the total number of Gram-negative isolates to 2354. Overall, Enterobacterales were by far the most prevalent (80%, n = 1817), followed by non-fermenting Gram-negative species (20.1%; n = 456). Performance of direct MALDI-TOF MS for species-level identification was 92.1% (1964/2133) and 33.9% (75/221) from monomicrobial and polymicrobial BCs (P < 0.01). Considering monomicrobial BCs, identification rate ranged from 97.3% for Enterobacterales to 80.2% for non-fermenting species, 42.1% for obligate anaerobic bacteria and 41.5% for fastidious species not included in the aforementioned categories. The most frequent Enterobacterales species were E. coli, K. pneumoniae and Enterobacter cloacae complex. They accounted for 35.1% (n = 797), 21.7% (n = 493) and 7% (n = 159) of the total Gram-negative isolates, respectively, with high rate of direct MALDI-TOF MS identification (97.7%, 98.7% and 96.5% from monomicrobial BC samples, respectively). P. aeruginosa and Acinetobacter baumannii complex were the most frequent non-fermenting Gram-negative bacteria (12.5% and 4.9%, respectively). Their correct species-level identification rates were 89.3% and 73.7% from monomicrobial BCs, and 10% and 6.7% from polymicrobial BCs, respectively (P < 0.01). Bacteroides fragilis was the predominant obligate anaerobic species (28/40; 70%) and was successfully identified from 16 out of 38 (42.3%) monomicrobial BCs. Among fastidious Gram-negative species, Campylobacter spp., Moraxella spp. and Haemophilus influenzae were the most frequent isolates and were successfully identified by direct MALDI-TOF MS in 35%, 70% and 40% of samples, respectively (Table 1).
Direct MALDI-TOF MS identification of Gram-negative isolates (n = 2354) from Gram-negative positive BCs using Rapid MBT Sepsityper® protocol
| Gram-negative organism . | No. of isolates . | Monomicrobial cultures (n = 2133) . | Polymicrobial cultures (n = 141) . |
|---|---|---|---|
| . | . | Identified (%) score ≥1.8 . | Identified (%) score ≥1.8 . |
| Enterobacterales | 1817 | 1615/1660 (97.3) | 69/157 (43.9) |
| Escherichia coli | 797 | 737/754 (97.7) | 19/43 (44.2) |
| Klebsiella pneumoniae | 493 | 446/452 (98.7) | 22/41 (53.6) |
| Enterobacter cloacae complex | 159 | 137/142 (96.5) | 8/17 (47) |
| Klebsiella oxytoca | 60 | 51/52 (98.1) | 3/8 (37.5) |
| Serratia marcescens | 80 | 71/74 (95.9) | 3/6 (50) |
| Klebsiella aerogenes | 46 | 38/41 (92.6) | 2/5 (40) |
| Proteus mirabilis | 62 | 41/43 (95.3) | 6/19 (31.6) |
| Citrobacter koseri | 28 | 23/24 (95.8) | 1/4 (25) |
| Citrobacter freundii | 18 | 16/16 (100) | 1/2 (50) |
| Klebsiella variicola | 14 | 10/11 (90.9) | 1/3 (33.3) |
| Morganella morganii | 16 | 10/11 (90.9) | 2/5 (40) |
| Salmonella enterica | 7 | 7/7 (100) | |
| Pantoea agglomerans | 9 | 7/8 (87.5) | 0/1 (0) |
| Hafnia alvei | 9 | 8/8 (100) | 1/1 (100) |
| Pantoea eucrina | 1 | 1/1 (100) | |
| Pantoea septica | 3 | 2/3 (66.7) | |
| Providencia rettgeri | 1 | 1 (100) | |
| Providencia stuartii | 3 | 1 (100) | 0/2 (0) |
| Serratia liquefaciens | 3 | 2/3 (66.7) | |
| Raoultella ornithinolytica | 2 | 2/2 (100) | |
| Serratia rubidea | 1 | 1/1 (100) | |
| Yersinia enterocolitica | 1 | 1/1 (100) | |
| Proteus vulgaris | 3 | 2/3 (66.7) | |
| Klebsiella ozaenae | 1 | 0/1 (0) | |
| Non-fermenting species | 456 | 316/394 (80.2) | 6/62 (9.7) |
| Pseudomonas aeruginosa | 283 | 217/243 (89.3) | 4/40 (10) |
| Acinetobacter baumannii complex complexcomplex | 112 | 70/97 (73.7) | 1/15 (6.7) |
| Stenotrophomonas maltophilia | 18 | 9/13 (69.2) | 0/5 (0) |
| Aeromonas caviae | 4 | 2/4 (50) | |
| Burkholderia cenocepacia | 2 | 1/2 (50) | |
| Achromobacter xylosoxidans | 10 | 7/9 (77.8) | 1/1 (100) |
| Acinetobacter lwoffii | 5 | 3/5 (60) | |
| Acinetobacter radioresistens | 1 | 1 (100) | |
| Aeromonas veronii | 1 | 1 (100) | |
| Pseudochrobactrum asaccharolyticum | 1 | 0/1 (0) | |
| Pseudomonas chlororaphis | 1 | 0/1 (0) | |
| Pseudomonas pseudomallei | 1 | 0/1 (0) | |
| Pseudomonas monteilii | 1 | 0/1 (0) | |
| Pseudomonas putida | 1 | 1/1 (100) | |
| Stenotrophomonas rhizophila | 1 | 1/1 (100) | |
| Acinetobacter ursingii | 3 | 0/2 (0) | 0/1 (0) |
| Acinetobacter vivianii | 1 | 0/1 (0) | |
| Acinetobacter guillouiae | 1 | 0/1 (0) | |
| Ochrobactrum anthropi | 3 | 1/3 (33.3) | |
| Paracoccus yeei | 3 | 0/3 (0) | |
| Roseomonas mucosa | 1 | 1/1 (100) | |
| Rhizobium radiobacter | 1 | 0/1 (0) | |
| Cupriavidus basilensis | 1 | 1/1 (100) | |
| Obligate anaerobic species | 40 | 16/38 (42.1) | 0/2 (0) |
| Bacteroides fragilis | 28 | 11/26 (42.3) | 0/2 (0) |
| Bacteroides vulgatus | 2 | 1/2 (50) | |
| Leptotrichia spp. | 2 | 0/2 (0) | |
| Fusobacterium gonidiaformans | 1 | 1 (100) | |
| Parabacteroides goldsteinii | 1 | 1/1 (100) | |
| Prevotella denticola | 1 | 1/1 (100) | |
| Prevotella melaninogenica | 1 | 1/1 (100) | |
| Prevotella baroniae | 1 | 0/1 (0) | |
| Bacteroides uniformis | 1 | 0/1 (0) | |
| Bacteroides pyogenes | 1 | 0/1 (0) | |
| Bacteroides thetaiotaomicron | 1 | 0/1 (0) | |
| Other species | 41 | 17/41 (41.5) | |
| Campylobacter jejuni | 16 | 5/16 (31.2) | |
| Campylobacter coli | 1 | 1/1 (100) | |
| Campylobacter fetus | 3 | 1/3 (33.3) | |
| Eikenella corrodens | 1 | 0/1 (0) | |
| Haemophilus influenzae | 5 | 2/5 (40) | |
| Capnocytophaga canimorsus | 1 | 0/1 (0) | |
| Leptotrichia trevisanii | 2 | 0/2 (0) | |
| Neisseria meningitidis | 1 | 0/1 (0) | |
| Neisseria gonorrhoeae | 1 | 1/1 (100) | |
| Moraxella liquefaciens | 2 | 1/2 (50) | |
| Moraxella nonliquefaciens | 1 | 1/1 (100) | |
| Moraxella osloensis | 7 | 5/7 (71.4) |
| Gram-negative organism . | No. of isolates . | Monomicrobial cultures (n = 2133) . | Polymicrobial cultures (n = 141) . |
|---|---|---|---|
| . | . | Identified (%) score ≥1.8 . | Identified (%) score ≥1.8 . |
| Enterobacterales | 1817 | 1615/1660 (97.3) | 69/157 (43.9) |
| Escherichia coli | 797 | 737/754 (97.7) | 19/43 (44.2) |
| Klebsiella pneumoniae | 493 | 446/452 (98.7) | 22/41 (53.6) |
| Enterobacter cloacae complex | 159 | 137/142 (96.5) | 8/17 (47) |
| Klebsiella oxytoca | 60 | 51/52 (98.1) | 3/8 (37.5) |
| Serratia marcescens | 80 | 71/74 (95.9) | 3/6 (50) |
| Klebsiella aerogenes | 46 | 38/41 (92.6) | 2/5 (40) |
| Proteus mirabilis | 62 | 41/43 (95.3) | 6/19 (31.6) |
| Citrobacter koseri | 28 | 23/24 (95.8) | 1/4 (25) |
| Citrobacter freundii | 18 | 16/16 (100) | 1/2 (50) |
| Klebsiella variicola | 14 | 10/11 (90.9) | 1/3 (33.3) |
| Morganella morganii | 16 | 10/11 (90.9) | 2/5 (40) |
| Salmonella enterica | 7 | 7/7 (100) | |
| Pantoea agglomerans | 9 | 7/8 (87.5) | 0/1 (0) |
| Hafnia alvei | 9 | 8/8 (100) | 1/1 (100) |
| Pantoea eucrina | 1 | 1/1 (100) | |
| Pantoea septica | 3 | 2/3 (66.7) | |
| Providencia rettgeri | 1 | 1 (100) | |
| Providencia stuartii | 3 | 1 (100) | 0/2 (0) |
| Serratia liquefaciens | 3 | 2/3 (66.7) | |
| Raoultella ornithinolytica | 2 | 2/2 (100) | |
| Serratia rubidea | 1 | 1/1 (100) | |
| Yersinia enterocolitica | 1 | 1/1 (100) | |
| Proteus vulgaris | 3 | 2/3 (66.7) | |
| Klebsiella ozaenae | 1 | 0/1 (0) | |
| Non-fermenting species | 456 | 316/394 (80.2) | 6/62 (9.7) |
| Pseudomonas aeruginosa | 283 | 217/243 (89.3) | 4/40 (10) |
| Acinetobacter baumannii complex complexcomplex | 112 | 70/97 (73.7) | 1/15 (6.7) |
| Stenotrophomonas maltophilia | 18 | 9/13 (69.2) | 0/5 (0) |
| Aeromonas caviae | 4 | 2/4 (50) | |
| Burkholderia cenocepacia | 2 | 1/2 (50) | |
| Achromobacter xylosoxidans | 10 | 7/9 (77.8) | 1/1 (100) |
| Acinetobacter lwoffii | 5 | 3/5 (60) | |
| Acinetobacter radioresistens | 1 | 1 (100) | |
| Aeromonas veronii | 1 | 1 (100) | |
| Pseudochrobactrum asaccharolyticum | 1 | 0/1 (0) | |
| Pseudomonas chlororaphis | 1 | 0/1 (0) | |
| Pseudomonas pseudomallei | 1 | 0/1 (0) | |
| Pseudomonas monteilii | 1 | 0/1 (0) | |
| Pseudomonas putida | 1 | 1/1 (100) | |
| Stenotrophomonas rhizophila | 1 | 1/1 (100) | |
| Acinetobacter ursingii | 3 | 0/2 (0) | 0/1 (0) |
| Acinetobacter vivianii | 1 | 0/1 (0) | |
| Acinetobacter guillouiae | 1 | 0/1 (0) | |
| Ochrobactrum anthropi | 3 | 1/3 (33.3) | |
| Paracoccus yeei | 3 | 0/3 (0) | |
| Roseomonas mucosa | 1 | 1/1 (100) | |
| Rhizobium radiobacter | 1 | 0/1 (0) | |
| Cupriavidus basilensis | 1 | 1/1 (100) | |
| Obligate anaerobic species | 40 | 16/38 (42.1) | 0/2 (0) |
| Bacteroides fragilis | 28 | 11/26 (42.3) | 0/2 (0) |
| Bacteroides vulgatus | 2 | 1/2 (50) | |
| Leptotrichia spp. | 2 | 0/2 (0) | |
| Fusobacterium gonidiaformans | 1 | 1 (100) | |
| Parabacteroides goldsteinii | 1 | 1/1 (100) | |
| Prevotella denticola | 1 | 1/1 (100) | |
| Prevotella melaninogenica | 1 | 1/1 (100) | |
| Prevotella baroniae | 1 | 0/1 (0) | |
| Bacteroides uniformis | 1 | 0/1 (0) | |
| Bacteroides pyogenes | 1 | 0/1 (0) | |
| Bacteroides thetaiotaomicron | 1 | 0/1 (0) | |
| Other species | 41 | 17/41 (41.5) | |
| Campylobacter jejuni | 16 | 5/16 (31.2) | |
| Campylobacter coli | 1 | 1/1 (100) | |
| Campylobacter fetus | 3 | 1/3 (33.3) | |
| Eikenella corrodens | 1 | 0/1 (0) | |
| Haemophilus influenzae | 5 | 2/5 (40) | |
| Capnocytophaga canimorsus | 1 | 0/1 (0) | |
| Leptotrichia trevisanii | 2 | 0/2 (0) | |
| Neisseria meningitidis | 1 | 0/1 (0) | |
| Neisseria gonorrhoeae | 1 | 1/1 (100) | |
| Moraxella liquefaciens | 2 | 1/2 (50) | |
| Moraxella nonliquefaciens | 1 | 1/1 (100) | |
| Moraxella osloensis | 7 | 5/7 (71.4) |
Direct MALDI-TOF MS identification of Gram-negative isolates (n = 2354) from Gram-negative positive BCs using Rapid MBT Sepsityper® protocol
| Gram-negative organism . | No. of isolates . | Monomicrobial cultures (n = 2133) . | Polymicrobial cultures (n = 141) . |
|---|---|---|---|
| . | . | Identified (%) score ≥1.8 . | Identified (%) score ≥1.8 . |
| Enterobacterales | 1817 | 1615/1660 (97.3) | 69/157 (43.9) |
| Escherichia coli | 797 | 737/754 (97.7) | 19/43 (44.2) |
| Klebsiella pneumoniae | 493 | 446/452 (98.7) | 22/41 (53.6) |
| Enterobacter cloacae complex | 159 | 137/142 (96.5) | 8/17 (47) |
| Klebsiella oxytoca | 60 | 51/52 (98.1) | 3/8 (37.5) |
| Serratia marcescens | 80 | 71/74 (95.9) | 3/6 (50) |
| Klebsiella aerogenes | 46 | 38/41 (92.6) | 2/5 (40) |
| Proteus mirabilis | 62 | 41/43 (95.3) | 6/19 (31.6) |
| Citrobacter koseri | 28 | 23/24 (95.8) | 1/4 (25) |
| Citrobacter freundii | 18 | 16/16 (100) | 1/2 (50) |
| Klebsiella variicola | 14 | 10/11 (90.9) | 1/3 (33.3) |
| Morganella morganii | 16 | 10/11 (90.9) | 2/5 (40) |
| Salmonella enterica | 7 | 7/7 (100) | |
| Pantoea agglomerans | 9 | 7/8 (87.5) | 0/1 (0) |
| Hafnia alvei | 9 | 8/8 (100) | 1/1 (100) |
| Pantoea eucrina | 1 | 1/1 (100) | |
| Pantoea septica | 3 | 2/3 (66.7) | |
| Providencia rettgeri | 1 | 1 (100) | |
| Providencia stuartii | 3 | 1 (100) | 0/2 (0) |
| Serratia liquefaciens | 3 | 2/3 (66.7) | |
| Raoultella ornithinolytica | 2 | 2/2 (100) | |
| Serratia rubidea | 1 | 1/1 (100) | |
| Yersinia enterocolitica | 1 | 1/1 (100) | |
| Proteus vulgaris | 3 | 2/3 (66.7) | |
| Klebsiella ozaenae | 1 | 0/1 (0) | |
| Non-fermenting species | 456 | 316/394 (80.2) | 6/62 (9.7) |
| Pseudomonas aeruginosa | 283 | 217/243 (89.3) | 4/40 (10) |
| Acinetobacter baumannii complex complexcomplex | 112 | 70/97 (73.7) | 1/15 (6.7) |
| Stenotrophomonas maltophilia | 18 | 9/13 (69.2) | 0/5 (0) |
| Aeromonas caviae | 4 | 2/4 (50) | |
| Burkholderia cenocepacia | 2 | 1/2 (50) | |
| Achromobacter xylosoxidans | 10 | 7/9 (77.8) | 1/1 (100) |
| Acinetobacter lwoffii | 5 | 3/5 (60) | |
| Acinetobacter radioresistens | 1 | 1 (100) | |
| Aeromonas veronii | 1 | 1 (100) | |
| Pseudochrobactrum asaccharolyticum | 1 | 0/1 (0) | |
| Pseudomonas chlororaphis | 1 | 0/1 (0) | |
| Pseudomonas pseudomallei | 1 | 0/1 (0) | |
| Pseudomonas monteilii | 1 | 0/1 (0) | |
| Pseudomonas putida | 1 | 1/1 (100) | |
| Stenotrophomonas rhizophila | 1 | 1/1 (100) | |
| Acinetobacter ursingii | 3 | 0/2 (0) | 0/1 (0) |
| Acinetobacter vivianii | 1 | 0/1 (0) | |
| Acinetobacter guillouiae | 1 | 0/1 (0) | |
| Ochrobactrum anthropi | 3 | 1/3 (33.3) | |
| Paracoccus yeei | 3 | 0/3 (0) | |
| Roseomonas mucosa | 1 | 1/1 (100) | |
| Rhizobium radiobacter | 1 | 0/1 (0) | |
| Cupriavidus basilensis | 1 | 1/1 (100) | |
| Obligate anaerobic species | 40 | 16/38 (42.1) | 0/2 (0) |
| Bacteroides fragilis | 28 | 11/26 (42.3) | 0/2 (0) |
| Bacteroides vulgatus | 2 | 1/2 (50) | |
| Leptotrichia spp. | 2 | 0/2 (0) | |
| Fusobacterium gonidiaformans | 1 | 1 (100) | |
| Parabacteroides goldsteinii | 1 | 1/1 (100) | |
| Prevotella denticola | 1 | 1/1 (100) | |
| Prevotella melaninogenica | 1 | 1/1 (100) | |
| Prevotella baroniae | 1 | 0/1 (0) | |
| Bacteroides uniformis | 1 | 0/1 (0) | |
| Bacteroides pyogenes | 1 | 0/1 (0) | |
| Bacteroides thetaiotaomicron | 1 | 0/1 (0) | |
| Other species | 41 | 17/41 (41.5) | |
| Campylobacter jejuni | 16 | 5/16 (31.2) | |
| Campylobacter coli | 1 | 1/1 (100) | |
| Campylobacter fetus | 3 | 1/3 (33.3) | |
| Eikenella corrodens | 1 | 0/1 (0) | |
| Haemophilus influenzae | 5 | 2/5 (40) | |
| Capnocytophaga canimorsus | 1 | 0/1 (0) | |
| Leptotrichia trevisanii | 2 | 0/2 (0) | |
| Neisseria meningitidis | 1 | 0/1 (0) | |
| Neisseria gonorrhoeae | 1 | 1/1 (100) | |
| Moraxella liquefaciens | 2 | 1/2 (50) | |
| Moraxella nonliquefaciens | 1 | 1/1 (100) | |
| Moraxella osloensis | 7 | 5/7 (71.4) |
| Gram-negative organism . | No. of isolates . | Monomicrobial cultures (n = 2133) . | Polymicrobial cultures (n = 141) . |
|---|---|---|---|
| . | . | Identified (%) score ≥1.8 . | Identified (%) score ≥1.8 . |
| Enterobacterales | 1817 | 1615/1660 (97.3) | 69/157 (43.9) |
| Escherichia coli | 797 | 737/754 (97.7) | 19/43 (44.2) |
| Klebsiella pneumoniae | 493 | 446/452 (98.7) | 22/41 (53.6) |
| Enterobacter cloacae complex | 159 | 137/142 (96.5) | 8/17 (47) |
| Klebsiella oxytoca | 60 | 51/52 (98.1) | 3/8 (37.5) |
| Serratia marcescens | 80 | 71/74 (95.9) | 3/6 (50) |
| Klebsiella aerogenes | 46 | 38/41 (92.6) | 2/5 (40) |
| Proteus mirabilis | 62 | 41/43 (95.3) | 6/19 (31.6) |
| Citrobacter koseri | 28 | 23/24 (95.8) | 1/4 (25) |
| Citrobacter freundii | 18 | 16/16 (100) | 1/2 (50) |
| Klebsiella variicola | 14 | 10/11 (90.9) | 1/3 (33.3) |
| Morganella morganii | 16 | 10/11 (90.9) | 2/5 (40) |
| Salmonella enterica | 7 | 7/7 (100) | |
| Pantoea agglomerans | 9 | 7/8 (87.5) | 0/1 (0) |
| Hafnia alvei | 9 | 8/8 (100) | 1/1 (100) |
| Pantoea eucrina | 1 | 1/1 (100) | |
| Pantoea septica | 3 | 2/3 (66.7) | |
| Providencia rettgeri | 1 | 1 (100) | |
| Providencia stuartii | 3 | 1 (100) | 0/2 (0) |
| Serratia liquefaciens | 3 | 2/3 (66.7) | |
| Raoultella ornithinolytica | 2 | 2/2 (100) | |
| Serratia rubidea | 1 | 1/1 (100) | |
| Yersinia enterocolitica | 1 | 1/1 (100) | |
| Proteus vulgaris | 3 | 2/3 (66.7) | |
| Klebsiella ozaenae | 1 | 0/1 (0) | |
| Non-fermenting species | 456 | 316/394 (80.2) | 6/62 (9.7) |
| Pseudomonas aeruginosa | 283 | 217/243 (89.3) | 4/40 (10) |
| Acinetobacter baumannii complex complexcomplex | 112 | 70/97 (73.7) | 1/15 (6.7) |
| Stenotrophomonas maltophilia | 18 | 9/13 (69.2) | 0/5 (0) |
| Aeromonas caviae | 4 | 2/4 (50) | |
| Burkholderia cenocepacia | 2 | 1/2 (50) | |
| Achromobacter xylosoxidans | 10 | 7/9 (77.8) | 1/1 (100) |
| Acinetobacter lwoffii | 5 | 3/5 (60) | |
| Acinetobacter radioresistens | 1 | 1 (100) | |
| Aeromonas veronii | 1 | 1 (100) | |
| Pseudochrobactrum asaccharolyticum | 1 | 0/1 (0) | |
| Pseudomonas chlororaphis | 1 | 0/1 (0) | |
| Pseudomonas pseudomallei | 1 | 0/1 (0) | |
| Pseudomonas monteilii | 1 | 0/1 (0) | |
| Pseudomonas putida | 1 | 1/1 (100) | |
| Stenotrophomonas rhizophila | 1 | 1/1 (100) | |
| Acinetobacter ursingii | 3 | 0/2 (0) | 0/1 (0) |
| Acinetobacter vivianii | 1 | 0/1 (0) | |
| Acinetobacter guillouiae | 1 | 0/1 (0) | |
| Ochrobactrum anthropi | 3 | 1/3 (33.3) | |
| Paracoccus yeei | 3 | 0/3 (0) | |
| Roseomonas mucosa | 1 | 1/1 (100) | |
| Rhizobium radiobacter | 1 | 0/1 (0) | |
| Cupriavidus basilensis | 1 | 1/1 (100) | |
| Obligate anaerobic species | 40 | 16/38 (42.1) | 0/2 (0) |
| Bacteroides fragilis | 28 | 11/26 (42.3) | 0/2 (0) |
| Bacteroides vulgatus | 2 | 1/2 (50) | |
| Leptotrichia spp. | 2 | 0/2 (0) | |
| Fusobacterium gonidiaformans | 1 | 1 (100) | |
| Parabacteroides goldsteinii | 1 | 1/1 (100) | |
| Prevotella denticola | 1 | 1/1 (100) | |
| Prevotella melaninogenica | 1 | 1/1 (100) | |
| Prevotella baroniae | 1 | 0/1 (0) | |
| Bacteroides uniformis | 1 | 0/1 (0) | |
| Bacteroides pyogenes | 1 | 0/1 (0) | |
| Bacteroides thetaiotaomicron | 1 | 0/1 (0) | |
| Other species | 41 | 17/41 (41.5) | |
| Campylobacter jejuni | 16 | 5/16 (31.2) | |
| Campylobacter coli | 1 | 1/1 (100) | |
| Campylobacter fetus | 3 | 1/3 (33.3) | |
| Eikenella corrodens | 1 | 0/1 (0) | |
| Haemophilus influenzae | 5 | 2/5 (40) | |
| Capnocytophaga canimorsus | 1 | 0/1 (0) | |
| Leptotrichia trevisanii | 2 | 0/2 (0) | |
| Neisseria meningitidis | 1 | 0/1 (0) | |
| Neisseria gonorrhoeae | 1 | 1/1 (100) | |
| Moraxella liquefaciens | 2 | 1/2 (50) | |
| Moraxella nonliquefaciens | 1 | 1/1 (100) | |
| Moraxella osloensis | 7 | 5/7 (71.4) |
Considering the total of Enterobacterales isolates, the rate of carbapenemase carriage was 10% (181/1817) according to the reference diagnostic workflow. The main carbapenemases detected were KPC (88.9%, n = 161), followed by VIM (4.4%; n = 8), NDM (2.8%; n = 5), OXA-48 (2.2%; n = 4) and KPC + VIM (1.6%, n = 3). K. pneumoniae was the main species carrying carbapenemase genes (169/181, 93.4%) (P < 0.01). Diagnostic performance of NG-Test® CARBA 5 according to bacterial species and carbapenemase type is shown in Table 2 and Table 3, respectively. The overall sensitivity, specificity, PPV and NPV were 92.2% [95% CI 87.2%–95.7%], 100% [95% CI 98.8%–100%], 100% and 95.5% [95% CI 92.8%–97.2%], respectively. The 14 false-negative NG-Test® CARBA 5 results were achieved for BCs tested positive to K. pneumoniae isolates and collected by 14 distinct patients with documented carriage of KPC-producing K. pneumoniae. On these samples, the Easyplex® SuperBug CRE molecular test was performed directly from BC fluid and showed positivity to blaKPC. No false-positive results were observed among the 19 BCs samples investigated by Easyplex® SuperBug CRE. Therefore, combination of NG-Test® CARBA 5 and Easyplex® SuperBug CRE showed 100% of sensitivity and specificity compared with conventional diagnostic routine (Table 3). Overall, the proposed carbapenemases detection algorithm allowed the identification of 179 out of 181 CPE BC isolates. The two isolates that escaped the carbapenemase detection algorithm were carbapenemase-producing E. coli (KPC, n = 1; NDM, n = 1) isolated from patients without documented CPE carriage.
Performance of NG-Test® CARBA 5 and NG-Test® CTX-M MULTI for rapid detection of carbapenemase and ESBL CTX-M directly from Enterobacterales positive BCs in comparison to reference methods
| . | . | Positive . | Negative . | Sensitivity [95% CI] . | Specificity [95% CI] . | PPV [95% CI] . | NPV [95% CI] . |
|---|---|---|---|---|---|---|---|
| Carbapenemase detection according to reference method . | NG-Test® CARBA 5 . | ||||||
| K. pneumoniae (n = 468) | Positive | 155 | 14 | 91.7 [86.5–95.4] | 100 [98.8–100] | 100 | 95.5 [92.8–97.2] |
| Negative | 0 | 299 | |||||
| E. coli (n = 6) | Positive | 6 | 0 | 100 [54.1–100] | 100 | ||
| Negative | 0 | 0 | |||||
| E. cloacae (n = 3) | Positive | 3 | 0 | 100 [29.2–100] | 100 | ||
| Negative | 0 | 0 | |||||
| K. aerogenes (n = 1) | Positive | 1 | 0 | 100 [2.5–100] | 100 | ||
| Negative | 0 | 0 | |||||
| Total (n = 478) | Positive | 165 | 14 | 92.2 [87.2–95.7] | 100 [98.8–100] | 100 | 95.5 [92.8–97.2] |
| Negative | 0 | 299 | |||||
| ESBL detection according to reference method | NG-Test® CTX-M MULTI | ||||||
| E. coli (n = 756) | Positive | 173 | 17 | 91 [86.1–94.7] | 100 [99.3–100] | 100 | 97.1[95.5–98.1] |
| Negative | 0 | 566 | |||||
| K. pneumoniae (n = 299)a | Positive | 100 | 8 | 92.6 [85.9–96.7] | 100 [98.1–100] | 100 | 96 [92.6–97.9] |
| Negative | 0 | 191 | |||||
| Total (n = 1055) | Positive | 273 | 25 | 91.6 [87.9–94.5] | 100 [99.5–100] | 100 | 96.8 [95.4–97.8] |
| Negative | 0 | 757 | |||||
| . | . | Positive . | Negative . | Sensitivity [95% CI] . | Specificity [95% CI] . | PPV [95% CI] . | NPV [95% CI] . |
|---|---|---|---|---|---|---|---|
| Carbapenemase detection according to reference method . | NG-Test® CARBA 5 . | ||||||
| K. pneumoniae (n = 468) | Positive | 155 | 14 | 91.7 [86.5–95.4] | 100 [98.8–100] | 100 | 95.5 [92.8–97.2] |
| Negative | 0 | 299 | |||||
| E. coli (n = 6) | Positive | 6 | 0 | 100 [54.1–100] | 100 | ||
| Negative | 0 | 0 | |||||
| E. cloacae (n = 3) | Positive | 3 | 0 | 100 [29.2–100] | 100 | ||
| Negative | 0 | 0 | |||||
| K. aerogenes (n = 1) | Positive | 1 | 0 | 100 [2.5–100] | 100 | ||
| Negative | 0 | 0 | |||||
| Total (n = 478) | Positive | 165 | 14 | 92.2 [87.2–95.7] | 100 [98.8–100] | 100 | 95.5 [92.8–97.2] |
| Negative | 0 | 299 | |||||
| ESBL detection according to reference method | NG-Test® CTX-M MULTI | ||||||
| E. coli (n = 756) | Positive | 173 | 17 | 91 [86.1–94.7] | 100 [99.3–100] | 100 | 97.1[95.5–98.1] |
| Negative | 0 | 566 | |||||
| K. pneumoniae (n = 299)a | Positive | 100 | 8 | 92.6 [85.9–96.7] | 100 [98.1–100] | 100 | 96 [92.6–97.9] |
| Negative | 0 | 191 | |||||
| Total (n = 1055) | Positive | 273 | 25 | 91.6 [87.9–94.5] | 100 [99.5–100] | 100 | 96.8 [95.4–97.8] |
| Negative | 0 | 757 | |||||
Carbapenemase-producing isolates were excluded.
Performance of NG-Test® CARBA 5 and NG-Test® CTX-M MULTI for rapid detection of carbapenemase and ESBL CTX-M directly from Enterobacterales positive BCs in comparison to reference methods
| . | . | Positive . | Negative . | Sensitivity [95% CI] . | Specificity [95% CI] . | PPV [95% CI] . | NPV [95% CI] . |
|---|---|---|---|---|---|---|---|
| Carbapenemase detection according to reference method . | NG-Test® CARBA 5 . | ||||||
| K. pneumoniae (n = 468) | Positive | 155 | 14 | 91.7 [86.5–95.4] | 100 [98.8–100] | 100 | 95.5 [92.8–97.2] |
| Negative | 0 | 299 | |||||
| E. coli (n = 6) | Positive | 6 | 0 | 100 [54.1–100] | 100 | ||
| Negative | 0 | 0 | |||||
| E. cloacae (n = 3) | Positive | 3 | 0 | 100 [29.2–100] | 100 | ||
| Negative | 0 | 0 | |||||
| K. aerogenes (n = 1) | Positive | 1 | 0 | 100 [2.5–100] | 100 | ||
| Negative | 0 | 0 | |||||
| Total (n = 478) | Positive | 165 | 14 | 92.2 [87.2–95.7] | 100 [98.8–100] | 100 | 95.5 [92.8–97.2] |
| Negative | 0 | 299 | |||||
| ESBL detection according to reference method | NG-Test® CTX-M MULTI | ||||||
| E. coli (n = 756) | Positive | 173 | 17 | 91 [86.1–94.7] | 100 [99.3–100] | 100 | 97.1[95.5–98.1] |
| Negative | 0 | 566 | |||||
| K. pneumoniae (n = 299)a | Positive | 100 | 8 | 92.6 [85.9–96.7] | 100 [98.1–100] | 100 | 96 [92.6–97.9] |
| Negative | 0 | 191 | |||||
| Total (n = 1055) | Positive | 273 | 25 | 91.6 [87.9–94.5] | 100 [99.5–100] | 100 | 96.8 [95.4–97.8] |
| Negative | 0 | 757 | |||||
| . | . | Positive . | Negative . | Sensitivity [95% CI] . | Specificity [95% CI] . | PPV [95% CI] . | NPV [95% CI] . |
|---|---|---|---|---|---|---|---|
| Carbapenemase detection according to reference method . | NG-Test® CARBA 5 . | ||||||
| K. pneumoniae (n = 468) | Positive | 155 | 14 | 91.7 [86.5–95.4] | 100 [98.8–100] | 100 | 95.5 [92.8–97.2] |
| Negative | 0 | 299 | |||||
| E. coli (n = 6) | Positive | 6 | 0 | 100 [54.1–100] | 100 | ||
| Negative | 0 | 0 | |||||
| E. cloacae (n = 3) | Positive | 3 | 0 | 100 [29.2–100] | 100 | ||
| Negative | 0 | 0 | |||||
| K. aerogenes (n = 1) | Positive | 1 | 0 | 100 [2.5–100] | 100 | ||
| Negative | 0 | 0 | |||||
| Total (n = 478) | Positive | 165 | 14 | 92.2 [87.2–95.7] | 100 [98.8–100] | 100 | 95.5 [92.8–97.2] |
| Negative | 0 | 299 | |||||
| ESBL detection according to reference method | NG-Test® CTX-M MULTI | ||||||
| E. coli (n = 756) | Positive | 173 | 17 | 91 [86.1–94.7] | 100 [99.3–100] | 100 | 97.1[95.5–98.1] |
| Negative | 0 | 566 | |||||
| K. pneumoniae (n = 299)a | Positive | 100 | 8 | 92.6 [85.9–96.7] | 100 [98.1–100] | 100 | 96 [92.6–97.9] |
| Negative | 0 | 191 | |||||
| Total (n = 1055) | Positive | 273 | 25 | 91.6 [87.9–94.5] | 100 [99.5–100] | 100 | 96.8 [95.4–97.8] |
| Negative | 0 | 757 | |||||
Carbapenemase-producing isolates were excluded.
Detection of main carbapenemases by NG-Test® CARBA 5 and Eazyplex® SuperBug CRE molecular testing
| Carbapenemase genotype . | Bacterial species . | NG-Test® CARBA 5 . | NG-Test® CARBA 5 plus Eazyplex® SuperBug CRE molecular testing . | ||
|---|---|---|---|---|---|
| . | . | Sensitivity . | Specificity . | Sensitivity . | Specificity . |
| blaKPC | K. pneumoniae (n = 160) | 91.2 (146/160) | 100 | 100 | 100 |
| blaNDM | K. pneumoniae (n = 2), E. coli (n = 2) | 100 | 100 | 100 | 100 |
| blaVIM | E. cloacae (n = 2), K. pneumoniae (n = 4), E. coli (n = 2) | 100 | 100 | ||
| blaOXA-48 | K. pneumoniae (n = 1), E. coli (n = 2), K. aerogenes (n = 1) | 100 | 100 | ||
| blaKPC + blaVIM | K. pneumoniae (n = 2), E. cloacae (n = 1) | 100 | 100 | ||
| Carbapenemase genotype . | Bacterial species . | NG-Test® CARBA 5 . | NG-Test® CARBA 5 plus Eazyplex® SuperBug CRE molecular testing . | ||
|---|---|---|---|---|---|
| . | . | Sensitivity . | Specificity . | Sensitivity . | Specificity . |
| blaKPC | K. pneumoniae (n = 160) | 91.2 (146/160) | 100 | 100 | 100 |
| blaNDM | K. pneumoniae (n = 2), E. coli (n = 2) | 100 | 100 | 100 | 100 |
| blaVIM | E. cloacae (n = 2), K. pneumoniae (n = 4), E. coli (n = 2) | 100 | 100 | ||
| blaOXA-48 | K. pneumoniae (n = 1), E. coli (n = 2), K. aerogenes (n = 1) | 100 | 100 | ||
| blaKPC + blaVIM | K. pneumoniae (n = 2), E. cloacae (n = 1) | 100 | 100 | ||
Detection of main carbapenemases by NG-Test® CARBA 5 and Eazyplex® SuperBug CRE molecular testing
| Carbapenemase genotype . | Bacterial species . | NG-Test® CARBA 5 . | NG-Test® CARBA 5 plus Eazyplex® SuperBug CRE molecular testing . | ||
|---|---|---|---|---|---|
| . | . | Sensitivity . | Specificity . | Sensitivity . | Specificity . |
| blaKPC | K. pneumoniae (n = 160) | 91.2 (146/160) | 100 | 100 | 100 |
| blaNDM | K. pneumoniae (n = 2), E. coli (n = 2) | 100 | 100 | 100 | 100 |
| blaVIM | E. cloacae (n = 2), K. pneumoniae (n = 4), E. coli (n = 2) | 100 | 100 | ||
| blaOXA-48 | K. pneumoniae (n = 1), E. coli (n = 2), K. aerogenes (n = 1) | 100 | 100 | ||
| blaKPC + blaVIM | K. pneumoniae (n = 2), E. cloacae (n = 1) | 100 | 100 | ||
| Carbapenemase genotype . | Bacterial species . | NG-Test® CARBA 5 . | NG-Test® CARBA 5 plus Eazyplex® SuperBug CRE molecular testing . | ||
|---|---|---|---|---|---|
| . | . | Sensitivity . | Specificity . | Sensitivity . | Specificity . |
| blaKPC | K. pneumoniae (n = 160) | 91.2 (146/160) | 100 | 100 | 100 |
| blaNDM | K. pneumoniae (n = 2), E. coli (n = 2) | 100 | 100 | 100 | 100 |
| blaVIM | E. cloacae (n = 2), K. pneumoniae (n = 4), E. coli (n = 2) | 100 | 100 | ||
| blaOXA-48 | K. pneumoniae (n = 1), E. coli (n = 2), K. aerogenes (n = 1) | 100 | 100 | ||
| blaKPC + blaVIM | K. pneumoniae (n = 2), E. cloacae (n = 1) | 100 | 100 | ||
As shown in Table 2, the NG-Test® CTX-M MULTI detected 273 K. pneumoniae or E. coli expressing ESBL phenotype, showing 91.6% sensitivity [95% CI, 87.9%–94.5%], 100% specificity [95% CI 99.5%–100%], 100% PPV and 96.8% NPV [95% CI, 95.4%–97.8%] compared with conventional diagnostic results. Furthermore, 29.7% (n = 32) of carbapenemase-producing K. pneumoniae were also found to be CTX-M producers by NG-Test® CTX-M MULTI assay (data not shown).
In vitro susceptibility towards the main antimicrobials including those recently approved, according to resistance markers detected, are shown in Table 4. CTX-M enzyme detection among non-carbapenemase-producing E. coli and K. pneumoniae isolates was associated with increased resistance to third- and fourth-generation cephalosporins, piperacillin/tazobactam, ceftolozane/tazobactam, ertapenem, and non-β-lactam antimicrobials such as aminoglycosides, fluoroquinolones, colistin and cotrimoxazole.
Cumulative resistance rates (%) of Enterobacterales BC isolates according to antimicrobial resistance markers detected by rapid diagnostic algorithm
| . | E. coli . | . | K. pneumoniae . | . | Enterobacterales . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antimicrobial . | Carbapenemase negative/CTX-M positive (n = 173) . | Carbapenemase negative/CTX-M negative (n = 566) . | P value . | Carbapenemase negative/CTX-M positive (n = 100) . | Carbapenemase negative/CTX-M negative (n = 191) . | P value . | KPC positive by NG-Test CARBA 5 (n = 146) . | KPC negative by NG-Test CARBA 5/blaKPC positive by Eazyplex® SuperBug CRE (n = 14) . | P value . | NDM (n = 4) . | VIM (n = 8) . | OXA-48 (n = 4) . | KPC + VIM (n = 3) . |
| Ceftazidime/avibactam | 0 | 0 | 1 | 0 | 0 | 1 | 3.4 | 92.8 | <0.01 | 100 | 100 | 0 | 100 |
| Ceftolozane/tazobactam | 4.6 | 0 | <0.01 | 14.4 | 0 | <0.01 | 100 | 100 | 1 | 100 | 100 | 25 | 100 |
| Meropenem/vaborbactam | – | – | – | – | – | – | 6.2 | 0 | 1 | 100 | 100 | 25 | 100 |
| Cefiderocol | – | – | – | – | – | – | 5.5 | 85.7 | <0.01 | 75 | 0 | 0 | 0 |
| Piperacillin/tazobactam | 13.9 | 7.8 | 0.01 | 44 | 20.9 | 0.003 | 100 | 85.7 | 0.005 | 100 | 100 | 100 | 100 |
| Imipenem | 0 | 0 | 1 | 0 | 0 | 1 | 90.4 | 0 | <0.01 | 100 | 62.5 | 25 | 100 |
| Meropenem | 0 | 0 | 1 | 0 | 0 | 1 | 91.8 | 0 | <0.01 | 100 | 37.5 | 25 | 100 |
| Ertapenem | 3.5 | 0 | <0.01 | 11 | 0 | <0.01 | 100 | 92.8 | 0.07 | 100 | 75 | 50 | 100 |
| Cefotaxime | 97.7 | 3.2 | <0.01 | 98 | 4.2 | <0.01 | 100 | 100 | 1 | 100 | 100 | 25 | 100 |
| Ceftazidime | 74 | 1.8 | <0.01 | 91 | 4.7 | <0.01 | 100 | 100 | 1 | 100 | 100 | 75 | 100 |
| Cefepime | 85 | 1.4 | <0.01 | 92 | 4.2 | <0.01 | 100 | 100 | 1 | 100 | 87.5 | 0 | 100 |
| Gentamicin | 29.5 | 6.9 | <0.01 | 36 | 2.1 | <0.01 | 60.9 | 42.8 | 0.07 | 25 | 50 | 25 | 0 |
| Amikacin | 13.9 | 0.5 | <0.01 | 36 | 1 | <0.01 | 53.4 | 85.7 | 0.06 | 25 | 12.5 | 0 | 0 |
| Colistin | 4 | 0.2 | <0.01 | 19 | 2.1 | <0.01 | 13.7 | 50 | 0.05 | 0 | 0 | 0 | 0 |
| Ciprofloxacin | 75.1 | 19.1 | <0.01 | 62 | 7.8 | <0.01 | 99.3 | 92.8 | 0.14 | 100 | 62.5 | 75 | 100 |
| Levofloxacin | 71.7 | 17 | <0.01 | 49 | 5.7 | <0.01 | 98.6 | 92.8 | 0.21 | 100 | 50 | 75 | 100 |
| Cotrimoxazole | 57.8 | 34.3 | <0.01 | 74 | 12.6 | <0.01 | 60.9 | 92.8 | 0.06 | 75 | 62.5 | 50 | 66.6 |
| . | E. coli . | . | K. pneumoniae . | . | Enterobacterales . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antimicrobial . | Carbapenemase negative/CTX-M positive (n = 173) . | Carbapenemase negative/CTX-M negative (n = 566) . | P value . | Carbapenemase negative/CTX-M positive (n = 100) . | Carbapenemase negative/CTX-M negative (n = 191) . | P value . | KPC positive by NG-Test CARBA 5 (n = 146) . | KPC negative by NG-Test CARBA 5/blaKPC positive by Eazyplex® SuperBug CRE (n = 14) . | P value . | NDM (n = 4) . | VIM (n = 8) . | OXA-48 (n = 4) . | KPC + VIM (n = 3) . |
| Ceftazidime/avibactam | 0 | 0 | 1 | 0 | 0 | 1 | 3.4 | 92.8 | <0.01 | 100 | 100 | 0 | 100 |
| Ceftolozane/tazobactam | 4.6 | 0 | <0.01 | 14.4 | 0 | <0.01 | 100 | 100 | 1 | 100 | 100 | 25 | 100 |
| Meropenem/vaborbactam | – | – | – | – | – | – | 6.2 | 0 | 1 | 100 | 100 | 25 | 100 |
| Cefiderocol | – | – | – | – | – | – | 5.5 | 85.7 | <0.01 | 75 | 0 | 0 | 0 |
| Piperacillin/tazobactam | 13.9 | 7.8 | 0.01 | 44 | 20.9 | 0.003 | 100 | 85.7 | 0.005 | 100 | 100 | 100 | 100 |
| Imipenem | 0 | 0 | 1 | 0 | 0 | 1 | 90.4 | 0 | <0.01 | 100 | 62.5 | 25 | 100 |
| Meropenem | 0 | 0 | 1 | 0 | 0 | 1 | 91.8 | 0 | <0.01 | 100 | 37.5 | 25 | 100 |
| Ertapenem | 3.5 | 0 | <0.01 | 11 | 0 | <0.01 | 100 | 92.8 | 0.07 | 100 | 75 | 50 | 100 |
| Cefotaxime | 97.7 | 3.2 | <0.01 | 98 | 4.2 | <0.01 | 100 | 100 | 1 | 100 | 100 | 25 | 100 |
| Ceftazidime | 74 | 1.8 | <0.01 | 91 | 4.7 | <0.01 | 100 | 100 | 1 | 100 | 100 | 75 | 100 |
| Cefepime | 85 | 1.4 | <0.01 | 92 | 4.2 | <0.01 | 100 | 100 | 1 | 100 | 87.5 | 0 | 100 |
| Gentamicin | 29.5 | 6.9 | <0.01 | 36 | 2.1 | <0.01 | 60.9 | 42.8 | 0.07 | 25 | 50 | 25 | 0 |
| Amikacin | 13.9 | 0.5 | <0.01 | 36 | 1 | <0.01 | 53.4 | 85.7 | 0.06 | 25 | 12.5 | 0 | 0 |
| Colistin | 4 | 0.2 | <0.01 | 19 | 2.1 | <0.01 | 13.7 | 50 | 0.05 | 0 | 0 | 0 | 0 |
| Ciprofloxacin | 75.1 | 19.1 | <0.01 | 62 | 7.8 | <0.01 | 99.3 | 92.8 | 0.14 | 100 | 62.5 | 75 | 100 |
| Levofloxacin | 71.7 | 17 | <0.01 | 49 | 5.7 | <0.01 | 98.6 | 92.8 | 0.21 | 100 | 50 | 75 | 100 |
| Cotrimoxazole | 57.8 | 34.3 | <0.01 | 74 | 12.6 | <0.01 | 60.9 | 92.8 | 0.06 | 75 | 62.5 | 50 | 66.6 |
Cumulative resistance rates (%) of Enterobacterales BC isolates according to antimicrobial resistance markers detected by rapid diagnostic algorithm
| . | E. coli . | . | K. pneumoniae . | . | Enterobacterales . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antimicrobial . | Carbapenemase negative/CTX-M positive (n = 173) . | Carbapenemase negative/CTX-M negative (n = 566) . | P value . | Carbapenemase negative/CTX-M positive (n = 100) . | Carbapenemase negative/CTX-M negative (n = 191) . | P value . | KPC positive by NG-Test CARBA 5 (n = 146) . | KPC negative by NG-Test CARBA 5/blaKPC positive by Eazyplex® SuperBug CRE (n = 14) . | P value . | NDM (n = 4) . | VIM (n = 8) . | OXA-48 (n = 4) . | KPC + VIM (n = 3) . |
| Ceftazidime/avibactam | 0 | 0 | 1 | 0 | 0 | 1 | 3.4 | 92.8 | <0.01 | 100 | 100 | 0 | 100 |
| Ceftolozane/tazobactam | 4.6 | 0 | <0.01 | 14.4 | 0 | <0.01 | 100 | 100 | 1 | 100 | 100 | 25 | 100 |
| Meropenem/vaborbactam | – | – | – | – | – | – | 6.2 | 0 | 1 | 100 | 100 | 25 | 100 |
| Cefiderocol | – | – | – | – | – | – | 5.5 | 85.7 | <0.01 | 75 | 0 | 0 | 0 |
| Piperacillin/tazobactam | 13.9 | 7.8 | 0.01 | 44 | 20.9 | 0.003 | 100 | 85.7 | 0.005 | 100 | 100 | 100 | 100 |
| Imipenem | 0 | 0 | 1 | 0 | 0 | 1 | 90.4 | 0 | <0.01 | 100 | 62.5 | 25 | 100 |
| Meropenem | 0 | 0 | 1 | 0 | 0 | 1 | 91.8 | 0 | <0.01 | 100 | 37.5 | 25 | 100 |
| Ertapenem | 3.5 | 0 | <0.01 | 11 | 0 | <0.01 | 100 | 92.8 | 0.07 | 100 | 75 | 50 | 100 |
| Cefotaxime | 97.7 | 3.2 | <0.01 | 98 | 4.2 | <0.01 | 100 | 100 | 1 | 100 | 100 | 25 | 100 |
| Ceftazidime | 74 | 1.8 | <0.01 | 91 | 4.7 | <0.01 | 100 | 100 | 1 | 100 | 100 | 75 | 100 |
| Cefepime | 85 | 1.4 | <0.01 | 92 | 4.2 | <0.01 | 100 | 100 | 1 | 100 | 87.5 | 0 | 100 |
| Gentamicin | 29.5 | 6.9 | <0.01 | 36 | 2.1 | <0.01 | 60.9 | 42.8 | 0.07 | 25 | 50 | 25 | 0 |
| Amikacin | 13.9 | 0.5 | <0.01 | 36 | 1 | <0.01 | 53.4 | 85.7 | 0.06 | 25 | 12.5 | 0 | 0 |
| Colistin | 4 | 0.2 | <0.01 | 19 | 2.1 | <0.01 | 13.7 | 50 | 0.05 | 0 | 0 | 0 | 0 |
| Ciprofloxacin | 75.1 | 19.1 | <0.01 | 62 | 7.8 | <0.01 | 99.3 | 92.8 | 0.14 | 100 | 62.5 | 75 | 100 |
| Levofloxacin | 71.7 | 17 | <0.01 | 49 | 5.7 | <0.01 | 98.6 | 92.8 | 0.21 | 100 | 50 | 75 | 100 |
| Cotrimoxazole | 57.8 | 34.3 | <0.01 | 74 | 12.6 | <0.01 | 60.9 | 92.8 | 0.06 | 75 | 62.5 | 50 | 66.6 |
| . | E. coli . | . | K. pneumoniae . | . | Enterobacterales . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antimicrobial . | Carbapenemase negative/CTX-M positive (n = 173) . | Carbapenemase negative/CTX-M negative (n = 566) . | P value . | Carbapenemase negative/CTX-M positive (n = 100) . | Carbapenemase negative/CTX-M negative (n = 191) . | P value . | KPC positive by NG-Test CARBA 5 (n = 146) . | KPC negative by NG-Test CARBA 5/blaKPC positive by Eazyplex® SuperBug CRE (n = 14) . | P value . | NDM (n = 4) . | VIM (n = 8) . | OXA-48 (n = 4) . | KPC + VIM (n = 3) . |
| Ceftazidime/avibactam | 0 | 0 | 1 | 0 | 0 | 1 | 3.4 | 92.8 | <0.01 | 100 | 100 | 0 | 100 |
| Ceftolozane/tazobactam | 4.6 | 0 | <0.01 | 14.4 | 0 | <0.01 | 100 | 100 | 1 | 100 | 100 | 25 | 100 |
| Meropenem/vaborbactam | – | – | – | – | – | – | 6.2 | 0 | 1 | 100 | 100 | 25 | 100 |
| Cefiderocol | – | – | – | – | – | – | 5.5 | 85.7 | <0.01 | 75 | 0 | 0 | 0 |
| Piperacillin/tazobactam | 13.9 | 7.8 | 0.01 | 44 | 20.9 | 0.003 | 100 | 85.7 | 0.005 | 100 | 100 | 100 | 100 |
| Imipenem | 0 | 0 | 1 | 0 | 0 | 1 | 90.4 | 0 | <0.01 | 100 | 62.5 | 25 | 100 |
| Meropenem | 0 | 0 | 1 | 0 | 0 | 1 | 91.8 | 0 | <0.01 | 100 | 37.5 | 25 | 100 |
| Ertapenem | 3.5 | 0 | <0.01 | 11 | 0 | <0.01 | 100 | 92.8 | 0.07 | 100 | 75 | 50 | 100 |
| Cefotaxime | 97.7 | 3.2 | <0.01 | 98 | 4.2 | <0.01 | 100 | 100 | 1 | 100 | 100 | 25 | 100 |
| Ceftazidime | 74 | 1.8 | <0.01 | 91 | 4.7 | <0.01 | 100 | 100 | 1 | 100 | 100 | 75 | 100 |
| Cefepime | 85 | 1.4 | <0.01 | 92 | 4.2 | <0.01 | 100 | 100 | 1 | 100 | 87.5 | 0 | 100 |
| Gentamicin | 29.5 | 6.9 | <0.01 | 36 | 2.1 | <0.01 | 60.9 | 42.8 | 0.07 | 25 | 50 | 25 | 0 |
| Amikacin | 13.9 | 0.5 | <0.01 | 36 | 1 | <0.01 | 53.4 | 85.7 | 0.06 | 25 | 12.5 | 0 | 0 |
| Colistin | 4 | 0.2 | <0.01 | 19 | 2.1 | <0.01 | 13.7 | 50 | 0.05 | 0 | 0 | 0 | 0 |
| Ciprofloxacin | 75.1 | 19.1 | <0.01 | 62 | 7.8 | <0.01 | 99.3 | 92.8 | 0.14 | 100 | 62.5 | 75 | 100 |
| Levofloxacin | 71.7 | 17 | <0.01 | 49 | 5.7 | <0.01 | 98.6 | 92.8 | 0.21 | 100 | 50 | 75 | 100 |
| Cotrimoxazole | 57.8 | 34.3 | <0.01 | 74 | 12.6 | <0.01 | 60.9 | 92.8 | 0.06 | 75 | 62.5 | 50 | 66.6 |
KPC enzyme detection was associated with high rates of resistance (>60%) to all antimicrobials tested except ceftazidime/avibactam, meropenem/vaborbactam, cefiderocol and colistin (3.4%, 6.2%, 5.5% and 13.7%, respectively). Conversely, negative NG-Test® CARBA 5 result and blaKPC positivity by Easyplex® SuperBug CRE molecular testing were associated with high resistance rates to ceftazidime/avibactam and cefiderocol (P < 0.01), and full susceptibility to meropenem and imipenem (P < 0.01).
MBL-producing Enterobacterales showed resistance to ceftazidime/avibactam and meropenem/vaborbactam regardless of carbapenemase type. Three out of four NDM producers were resistant to cefiderocol whereas all VIM producers were susceptible. All OXA-48-producing Enterobacterales were susceptible to ceftazidime/avibactam, cefiderocol, cefepime, amikacin and colistin.
Discussion
Several molecular-based systems have been developed to rapidly detect microbial species commonly causing BSI and specific markers of antimicrobial resistance directly from positive BCs.16,17 However, although simple to implement, these systems have high costs and are capable of identifying a limited number of species. In this study, we evaluated a cost-effective diagnostic algorithm based on MALDI-TOF MS, LFIA and molecular tests on Gram-negative positive BCs, capable of providing important information to support timely transition to effective therapy including the appropriate use of newly approved drugs in less than 2 h and thus 34–40 h earlier than conventional results (Figure 3).
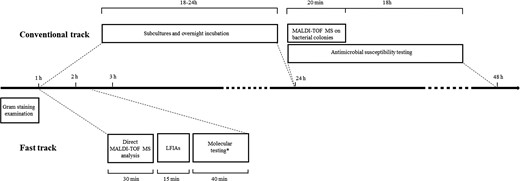
Timeline of conventional and fast-track BCs diagnostics starting from positive BC bottle. Conventional track (∼36–42 h): positive BCs are subjected to Gram staining examination (∼1 h), subcultures on solid media and overnight incubation for ∼18–24 h. Species identification and antimicrobial susceptibility testing are performed on overnight colonies by MALDI-TOF MS (∼20 min) and automated microdilution system (18 h), respectively. Fast track (∼105–145 min): BC positive to Gram-negative rods are subjected to direct MALDI-TOF MS analysis (∼30 min). LFIAs are performed according to species identification (15 min). *Molecular testing for carbapenemase genes (∼40 min) is performed in case of NG-Test CARBA 5 negative result and patient with documented carriage by the same bacterial species identified in BC and found to be a carbapenemase producer.
As microorganisms in BC broth are not readily available for identification, an earlier step of extraction remains mandatory. In-house extraction protocols have a lack of standardization, often use modified cut-offs not validated by the MALDI-TOF MS manufacture and require many steps with a time to results of up to 40 min.18 The Sepsityper® kit is a CE-IVD commercial kit. According to a review and meta-analysis on its performance, it allows 79.8% of bacterial identification to the species level (76.1% and 89.6% for monomicrobial Gram-positive and Gram-negative BCs, respectively) in 30 min.19 However, its important technical turnaround time, due to at least five centrifugation steps, limits its integration in routine procedures. In this study, Rapid MTB Sepsityper® protocol reduced hands-on time and identification delay to 10 min. Overall, the rapid MALDI-TOF MS protocol allowed reliable identification to the species level of 92.1% of monomicrobial BCs. These findings are consistent with literature data, which showed correct identification rates generally above 90% and often higher than 95%.19,20 Rates of correct identification among monomicrobial BCs involving anaerobe obligate and fastidious Gram-negative species were lower (42.1% and 41.5%, respectively). Among polymicrobial BCs, identification of one of the species involved was achieved in 81 out of the 141 (57.4%) samples with identification rates ranging from 43.9% for Enterobacterales to 9.7% for non-fermenting Gram-negatives, while no obligate anaerobe species were identified. Implementation of the Bruker® MBT Sepsityper IVD module, which can detect a combination of two single spectra discriminating different species from the same sample, could represent a viable solution to improve performance of direct MALDI-TOF MS on polymicrobial BCs.21
Although mechanisms associated with antimicrobial resistance in Gram-negative bacteria are multiple, CTX-M-type ESBLs and KPC-, NDM-, VIM-, IMP- and OXA-48-like carbapenemase genes in Enterobacterales, especially in K. pneumoniae and E. coli, represent the most widespread.22–24 Thus, combination of MALDI-TOF MS and LFIAs represents a novel and cost-effective approach to provide accurate and rapid results. In accordance with local epidemiology, the selection of LFIAs based on the species identification represents an additional cost saving. Herein, the NG-Test® CTX-M MULTI assay identified 91.6% of K. pneumoniae or E. coli expressing an ESBL phenotype. The ESBL-producing isolates tested negative may suggest the expression of other ESBL enzymes or CTX-M variants that remain undetected by the NG-Test® CTX-M MULTI.11,12 Therefore, in countries where the prevalence of other-than-CTX-M types ESBL enzymes is remarkable, hydrolysis-based assays could be more appropriate.10 On the other hand, the NG-Test® CARBA 5 identified 92.1% of CPEs tested. The assay showed discordant results with molecular testing in 14 cases, most involving ceftazidime/avibactam-resistant KPC-producing K. pneumoniae isolates. Seven of these isolates were previously investigated by DNA sequencing revealing D179Y mutation in blaKPC.25 The D179Y mutation in the omega loop domain of the KPC enzyme is the most frequently encountered mechanism causing ceftazidime/avibactam resistance in KPC producers and is associated with relevant issues regarding carbapenemase phenotypic detection. As other phenotypic methods, LFIAs are unable to detect these KPC mutants, probably due to changes in the antigenic set-up of the enzyme.13,25 Although KPCD179Y and many other recently discovered KPC variants associated with ceftazidime/avibactam resistance are characterized by impaired carbapenemase activity, their rapid detection is of paramount importance for antimicrobial management and infection control purpose. In this 3 year study, integration of Eazyplex® SuperBug CRE assay in case of NG-Test CARBA 5 negative result for BCs collected from patients with documented CPE carriage allowed to obtain 100% sensitivity in detection of Enterobacterales carrying carbapenemase genes, including blaKPC mutants. Only two BCs positive to CPE were not investigated by rapid diagnostics because patients had no documented CPE carriage. This finding highlights the importance of surveillance cultures, both for infection control purposes and identification of a relevant risk factor for CPE infection in hospitalized patients.13 Furthermore, since Enterobacterales harbouring KPC mutants undetectable by phenotypic testing and associated with ceftazidime/avibactam resistance are increasing worldwide with hospital outbreaks also documented,26 adoption of diagnostic protocols in surveillance cultures capable of identifying patients colonized by these strains is essential to ensure maximum effectiveness of the proposed rapid BC diagnostic algorithm.26 However, the development of rapid diagnostic tests (e.g. immunochromatographic assays) that can differentiate major KPC variants of clinical relevance is desirable.
The goal of rapid diagnostics in BCs workflow is to rule out susceptibility to certain antibiotics and escalate therapy if a specific resistance marker is detected. Herein, we showed that CTX-M detection is associated with in vitro inactivity of third- and fourth-generation cephalosporins and increased resistance to non-β-lactams antimicrobial classes, such as aminoglycosides and fluoroquinolones, in comparison to CTX-M-negative isolates. All CTX-M-positive isolates showed susceptibility towards meropenem, imipenem and ceftazidime/avibactam. Among CTX-M producers, higher resistance rates towards piperacillin/tazobactam, ceftolozane/tazobactam and ertapenem in K. pneumoniae than E. coli were observed, highlighting the importance of species identification.
According to our data, detection of KPC and OXA-48 carbapenemases allows the deduction of the probable activity of ceftazidime/avibactam, meropenem/vaborbactam and cefiderocol. Conversely, detection of MBLs should exclude meropenem/vaborbactam and ceftazidime/avibactam use. Among MBLs producers, cefiderocol susceptibility is predictable only for VIM producers. In the context of KPC producers, a picture characterized by negativity to immunochromatographic test together with blaKPC positivity suggests resistance to ceftazidime/avibactam for the expression of KPC mutants above described. In these cases, treatment with meropenem/vaborbactam should be considered since cross-resistance to cefiderocol has been observed. This finding is supported by literature that showed a relationship between KPC mutants and co-resistance to ceftazidime/avibactam and cefiderocol.27,28
This study has some limitations. The rapid diagnostic BC workflow presented was set according to local epidemiology and it represents the main limitation for its generalizability. Moreover, our rapid workflow is not capable of detecting all resistance mechanisms in a comprehensive manner. For example, ESBL other than CTX-M or plasmid-acquired AmpC enzymes are not detectable. Furthermore, resistance markers were investigated on Enterobacterales species (especially E. coli and K. pneumoniae) and not on non-fermenting Gram-negative species. However, because non-fermenting species, such as A. baumannii and P. aeruginosa, can express β-lactams resistance mechanisms undetectable from the assays presented here, additional rapid diagnostic tests should be incorporated to extend the potential impact of BC rapid diagnostics.15 In conclusion, there was considerable agreement between the results provided by the rapid workflow and conventional diagnostic workflow. Although the conventional BC workflow remains essential, combination of direct MALDI-TOF MS and LFIAs was shown to timely provide Gram-negative species identification and detection of most ESBL- and carbapenemase-producing Enterobacterales. Integration of molecular testing was shown to be essential to avoid non-detection of the emerging KPC mutants associated with ceftazidime/avibactam resistance.
Implementation of the proposed diagnostic algorithm in BC routine together with analysis of cumulative antimicrobial susceptibility results may represent a key tool for early optimization of antibiotic therapy, including the reasoned use of new approved drugs. Further studies are needed to analyse the impact of the results obtained by the proposed diagnostic algorithm on antimicrobial management and outcomes of patients suffering from Gram-negative bacteria BSI.
Funding
This study was supported by Fondazione Ricerca Molinette, Turin, Italy.
Transparency declarations
None to declare.



