-
PDF
- Split View
-
Views
-
Cite
Cite
Fiona Wing Yu Lo, Fabian Yuh Shiong Kong, Jane S Hocking, Treatment efficacy for rectal Neisseria gonorrhoeae: a systematic review and meta-analysis of randomized controlled trials, Journal of Antimicrobial Chemotherapy, Volume 76, Issue 12, December 2021, Pages 3111–3124, https://doi.org/10.1093/jac/dkab315
Close - Share Icon Share
Abstract
Rectal gonorrhoea is a common sexually transmitted infection with increasing antimicrobial resistance requiring optimization of available treatments.
This systematic review aimed to assess the efficacy of current treatments, previously trialled treatments and new emerging treatments for rectal Neisseria gonorrhoeae (NG).
Online bibliographic databases were search from 1 January 1946 to 14 August 2020. All randomized controlled trials (RCTs) with rectal NG data among participants aged 15 years or above and published in English were included. Random effects meta-analyses were used to estimate overall treatment efficacy, defined as microbiological cure. Sub-group analyses included stratifying by diagnostic assay, by dual versus monotherapy, and by currently recommended treatments (e.g. ceftriaxone ± azithromycin) versus previously trialled but not recommended treatments (e.g. amoxicillin) versus emerging treatments (e.g. zoliflodacin). The study protocol was registered on PROSPERO (CRD42020202998).
54 studies including 1813 participants and 44 treatment regimens were identified. The overall summary treatment efficacy for rectal NG was 100.0% (95% CI: 99.9%–100.0%; I2 = 0.0%; P = 0.86). Efficacy estimates for monotherapies (100.0%; 95% CI: 99.88%–100.0%; I2 = 0.00%; P = 0.97) and dual therapies (100.0%; 95% CI: 97.65%–100.0%; I2 = 56.24%; P = 0.03) were similar. Efficacy was highest for current treatments (100.00%; 95% CI: 99.96%–100.00%; I2 = 0.00%; P = 0.98) versus emerging treatments (97.16%; 95% CI: 86.79%–100.00%; I2 = 0.00%; P = 0.84). There were no trials exclusively investigating rectal NG and small sample size was a limitation in most trials.
Currently recommended treatments containing ceftriaxone, as mono or dual therapy, are effective. Emerging drugs such as zoliflodacin may be potentially useful for rectal NG but further data are needed.
Introduction
Gonorrhoea is a common bacterial sexually transmitted infection (STI) worldwide with the rectum being a frequent site of infection, particularly among MSM.1 In addition, there are also concerns about rectal gonorrhoea in women, with a recent review finding that a considerable proportion of urogenital infections in women have a concurrent rectal infection raising the possibility of auto-inoculation between the two sites.2 Prevalence estimates of rectal Neisseria gonorrhoeae (NG) range from: 0.6%–35.8% (median 1.9%) in women, 0.0%–5.7% (median 3.4%) in men who have sex with women, and from 0.2%–24.0% (median 5.9%) in MSM.3
Antimicrobial-resistant NG is an increasing global concern with resistant NG strains observed for multiple classes of antimicrobials including penicillin, tetracyclines, macrolides, quinolones and cephalosporins, with a significant proportion developing extended-spectrum drug resistance.4 Currently, recommended first line therapy for NG, in settings without local resistance data, include ceftriaxone 250–1000 mg with or without azithromycin 1–2 g,5–10 but there is considerable variability in regimens used between Australia, UK, Europe, Canada, and the USA. For example, UK7 and USA8 have recently moved to monotherapy with ceftriaxone 1 g or 500 mg, respectively, but dual therapy is still recommended elsewhere. The WHO and Canada recommends ceftriaxone 250 mg plus azithromycin 1 g,5,9 Australia recommends 500 mg ceftriaxone plus 1 g azithromycin.6 The European guidelines recommend dual therapy with ceftriaxone 1 g plus azithromycin 2 g.10
Resistance to ceftriaxone has been reported and there is the very real threat that NG will become untreatable.4 Therefore, there is an urgent need to identify potential NG treatments. However, any new treatments must meet the CDC’s criteria of ≥95% efficacy with a lower CI of ≥90%.11 In light of this ongoing concern and the pressing need to have efficacious treatments available in the marketplace, identifying effective drugs that can be optimized remains an important strategy. We therefore conducted a review of published randomized controlled trials (RCTs) to assess the efficacy of treatments for rectal NG. We also aimed to investigate how efficacy varies between different regimens and identify potential candidates for optimization, including currently recommended treatments, historically trialled treatments, and emerging treatments currently being investigated in Phase 2 or 3 clinical trials.
Methods
The study protocol was registered on PROSPERO (CRD42020202998) and the results are reported according to PRISMA guidelines.12
Search strategy
We searched Ovid Medline (1946 to 12 August 2020), Ovid Embase (1947 to 12 August 2020), PubMed and Cochrane Central Register of Controlled Trials on 14 August 2020. The search terms and their associated MeSH terms for each database were the same. These were (Neisseria gonorrhoeae OR gonorrhoea OR gonorrhea) AND (randomised controlled trial OR randomised clinical trial OR randomised trial OR trial OR randomised OR randomised). No time limitation was applied to the search. This strategy captured all RCTs that studied the efficacy of treatment for NG at any site. The decision was made to not include rectal-related terms as it may have missed important results.
Eligibility criteria
Studies were included if they were RCTs that included data on the efficacy of treatment for uncomplicated rectal NG in humans. The trial did not have to exclusively investigate rectal NG but did need to include rectal NG results. Other inclusion criteria included reporting NG results based on a nucleic acid amplification test (NAAT) or culture, participants aged 15 years or older, and being published in English. Unless they were emerging treatments currently under investigation or recommended by the WHO, studies were excluded if the treatment was no longer registered with the FDA.13 Other exclusion criteria were studies where participants did not have a confirmed diagnosis of rectal NG on culture or NAAT.
Data extraction and management
Microsoft Excel was used to extract the following data from each study: author, year of publication, treatments registered with the FDA or recommended by WHO, dosage, whether dual or monotherapy, study setting (STI clinic or hospital), gender, diagnostic method (NAAT or culture), test of cure method (NAAT or culture), duration of follow up, number of people with diagnosed rectal NG, number of people cured of rectal NG at follow up, microbiological cure as a percentage, the number of people reporting side effects (nausea, vomiting, or diarrhoea) within a treatment group, and total percentage of people experiencing side effects for a particular treatment group. One author (F.W.Y.L.) independently extracted data from the included studies and a second author (F.Y.S.K) independently checked over extracted data. Any discrepancies were discussed with J.S.H until consensus was achieved. Data analysis was undertaken by F.W.Y.L. and F.Y.S.K. Disagreements were resolved in consultation with J.S.H. These decisions were recorded on the Covidence online platform.
Outcome
The primary outcome was treatment efficacy measured as microbiological cure at follow-up. This was defined as a proportion, with the numerator being the number of participants treated and testing NG NAAT and/or culture negative at follow-up, and the denominator being the total number treated and tested for rectal NG at follow-up.
The secondary outcome was the proportion of all treated participants (including those with non-rectal infections) who reported side effects (nausea, diarrhoea and vomiting) following treatment.
Analysis
Meta-analysis was used to calculate summary estimates of treatment efficacy, with efficacy based on the percentage with microbiological cure at follow up. The I2 test was used to estimate heterogeneity and random effects model was used due to small sample sizes in some studies.
The primary analysis estimated an overall treatment efficacy for all treatment regimes. Where a study used both culture and NAAT, culture results were used over NAAT results because of greater specificity.14
In subgroup analyses, we assessed overall treatment efficacy for the following groups: diagnostic method (culture versus NAAT), type of treatment (dual versus monotherapy), previously trialled treatments (amoxicillin, ampicillin, benzylpenicillin, cefuroxime, cefoxitin, cefotaxime, gatifloxacin, gemifloxacin, ofloxacin, piperacillin, procaine penicillin, tetracycline),15,16 currently recommended treatments (azithromycin, ceftriaxone, cefixime, ciprofloxacin, gentamicin, spectinomycin),5–8,10 and emerging treatments undergoing evaluation in clinical trials (aztreonam, delafloxacin, gepotidacin, solithromycin, zoliflodacin).17–22
To assess side effects, the percentages reporting nausea, vomiting or diarrhoea at follow up were estimated using a random effects meta-analysis.
Data were analysed using Stata 16 (StataCorp, College Station, TX, USA) applying the metaprop syntax using the Freeman–Tukey double arcsine transformation.
Assessment of bias and quality
The Cochrane Collaboration Risk of Bias tool version 2.0 was used to assess for risk of bias.23 The following characteristics of the studies were assessed: randomization process, deviations from the intended interventions (effect of assignment to intervention), missing outcome data, measurement of outcome, the selection of the reported result, sample size, and an overall assessment of the risk of bias. Each characteristic was assessed by two reviewers (F.W.Y.L. and F.Y.S.K) and any discrepancies were resolved by discussion with a third reviewer (J.S.H).
Results
Study selection
Overall, 5258 papers were identified through the database search and 2527 duplicates were removed, leaving 2731 unique papers to be screened by title and abstract. The initial screen excluded 2517 papers leaving 214 studies for a full-text review. Of these, 160 were discarded as follows: 93 had no rectal data, 35 were non-randomized controlled trials, 10 had outcomes that did not meet inclusion criteria, nine were reviews only, five were study supplements, three were ongoing studies without results, two were correspondences with no results, one was a news article, one was a non- English paper, and one had an intervention that did not meet inclusion criteria (Figure 1). The final number of papers for inclusion is 54, summarized in Table 1.
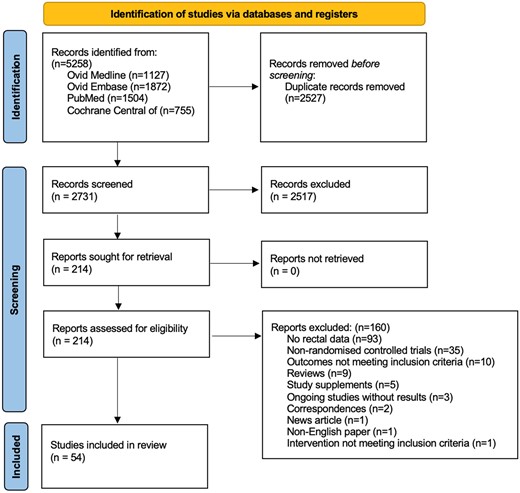
PRISMA 2020 flow diagram for new systematic reviews which included searches of databases and registers only.12 This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
| First author . | Publication year . | Treatment . | Percentage with rectal NG cured (no. cured/ no. tested) . | Setting and country . | Gender of participantsa . | Diagnostic assay . | Median follow up (days) . | Side effects % (no./total)b . |
|---|---|---|---|---|---|---|---|---|
| Baddour25 | 1989 | CFX 1 g AMX 3 g+PROB 1 g | 98.1 (53/54) 97.6 (41/42) | STI Clinic, USA | F | Culture | 5.5 | CFX 1 g N: 3.8% (9/235) V: 3.4% (8/235) D: 1.3% (3/235) AMX 3 g+PROB 1 g N: 2.2% (5/231) V: 0.9% (2/231) D: 3.5% (8/231) |
| Baddour24 | 1992 | AMP 1 g+SUL 0.5 g+PROB 1 g CRO 250 mg | 100.0 (8/8) 100.0 (7/7) | STI Clinic, USA | Both | Culture | 5.5 | AMP 1 g+SUL 0.5 g+PROB 1 g, NA CRO 250 mg N: NA V: NA D: 3.1% (3/97) |
| Batteiger26 | 1985 | AMP 3.5 g+PROB 1 g | 100.0 (12/12) | STI Clinic, USA | F | Culture | 10.5 | |
| Black27 | 1989 | OFX 400 mg AMX 3 g+PROB 1 g | 100.0 (13/13) 100.0 (17/17) | STI Clinic, USA | F | Culture | 7.5 | |
| Cavenee28 | 1993 | CRO 250 mg SPT 2 g AMX 3 g+PROB 1 g | 95.5 (21/22) 100.0 (19/19) 85.2 (23/27) | Hospital, USA | F (pregnant females only) | Culture | 7 | CRO 250 mg, NA SPT 2 g, NA AMX 3 g+PROB 1 g N: NA V: 1.2% (1/84) D: NA |
| Chen19 | 2019 | CRO 500 mg+AZM 1000 mg SOL 1000 mg | 100.0 (12/12) 83.3 (5/6) | STI Clinic, USA and Australia | Both | Culture+NAAT | 7 | CRO 500 mg+AZM 1000 mg N: 11.5% (15/131) V: 0.0% (0/131) D: 15.3% (20/131) SOL 1000 mg N: 20.8% (27/130) V: 2.3% (3/130) D: 23.8% (31/130) |
| Collier29 | 1984 | CRO 125 mg SPT 2 g | 100.0 (23/23) 100.0 (12/12) | STI Clinic, USA | F | Culture | 6 | |
| Covino30 | 1990 | OFX 400 mg CRO 250 mg | 100.0 (2/2) 83.3 (5/6) | STI Clinic, USA | F | Culture | 6 | |
| Covino31 | 1993 | CRO 250 mg | 100.0 (5/5) | STI Clinic, USA | Both | Culture | 7 | |
| Das68 | 1989 | CFX 1 g AMP 3 g+PROB 1 g | 100.0 (7/7) 100.0 (4/4) | Setting and country not stated | Both | Culture | 7 | |
| Dixon59 | 1986 | CRO 500 mg PPEN 1.5 g+BPEN 300 mg | 100.0 (1/1) 80.0 (4/5) | STI Clinic, UK | Both | Culture | 16 | |
| Dubois69 | 1990 | AMX 3 g+PROB 1 g | 100.0 (2/2) | Setting and country not stated | F | Culture | 5.5 | AMX 3 g+PROB 1 g N: 3.9% (3/77) V: 2.6% (2/77) D: 1.3% (1/77) |
| Edwards32 | 1984 | AMX 3 g+PROB 1 g | 100.0 (7/7) | STI Clinic, USA | Both | Culture | 7 | |
| Forstrom64 | 1972 | PPEN 2.4 MU | 100.0 (14/14) | Hospital, Finland | F | Culture | 7 | |
| Forstrom63 | 1974 | AMP 2000 mg+PROB 1 g | 92.9 (13/14) | Hospital, Finland | F | Culture | 7 | |
| Gottlieb33 | 1985 | ATM 1 g SPT 2 g | 100.0 (3/3) 100.0 (6/6) | STI Clinic, USA | Both | Culture | 6 | |
| Gottlieb34 | 1986 | CFX 1 g+PROB 1 g CFX 1 g AMX 3 g+PROB 1 g | 100.0 (6/6) 66.7 (4/6) 66.7 (2/3) | STI Clinic, USA | M | Culture | 6 | |
| Greaves35 | 1983 | FOX 2 g+PROB 1 g PPEN 4.8 MU+PROB 1 g | 95.7 (45/47) 97.4 (38/39) | STI Clinic, USA | Both | Culture | 8 | |
| Handsfield71 | 1981 | CRO 125 mg CRO 250 mg CRO 500 mg | 100.0 (5/5) 100.0 (3/3) 100.0 (2/2) | STI Clinic, country not stated | M | Culture | 5.5 | |
| Handsfield70 | 1983 | CRO 125 mg CRO 250 mg SPT 2 g | 100.0 (8/8) 100.0 (6/6) 95.5 (21/22) | STI Clinic, country not stated | M | Culture | 5.5 | |
| Handsfield37 | 1991 | CFM 400 mg CFM 800 mg CRO 250 mg | 100.0 (10/10) 100.0 (6/6) 100.0 (6/6) | STI Clinic, USA | F | Culture | 6.5 | CFM 400 mg N: 0.9% (1/107) V: NA D: 7.5% (8/107) CFM 800 mg N: 4.4% (4/91) V: NA D: 13.2% (12/91) CRO 250 mg N: 0.0% (0/104) V: NA D: 3.8% (4/104) |
| Handsfield36 | 1994 | AZM 2 g CRO 250 mg | 96.3 (26/27) 100.0 (17/17) | STI Clinic, USA | Both | Culture | 7 | AZM 2 g N: 19.5% (84/431) V: 7.0% (30/431) D: 13.7% (59/431) CRO 250 mg, NA |
| Hook39 | 1986 | SPT 2 g | 100.0 (33/33) | STI Clinic, USA | M | Culture | 5.5 | |
| Hook38 | 1993 | CIP 250 mg CRO 250 mg | 100.0 (20/20) 100.0 (21/21) | STI Clinic, USA | F | Culture | 7 | |
| Hook72 | 1997 | CFM 400 mg | 100.0 (3/3) | Hospital, country not stated | M | Culture | 7.5 | |
| Hook17 | 2019 | CRO 250 mg DEL 900 mg | 100.0 (13/13) 82.6 (19/23) | Setting not stated, USA | Both | Culture+NAAT | 7 | CRO 250 mg N: 1.3% (2/154) V: 0.6% (1/154) D: 7.1% (11/154) DEL 900 mg N: 7.9% (24/304) V: 2.6% (8/304) D: 31.9% (97/304) |
| Jones40 | 1991 | CTX 500 mg CRO 250 mg | 100.0 (5/5) 100.0 (4/4) | STI Clinic, USA | Both | Culture | 5.5 | CTX 500 mg, NA CRO 250 mg N: 1.7% (1/58) V: NA D: 3.4% (2/58) |
| Judson73 | 1983 | CRO 250 mg PPEN 4.8 MU (×2 doses)+PROB 1 g | 100.0 (10/10) 100.0 (11/11) | Setting and country not stated | F | Culture | 5.5 | |
| Judson41 | 1985 | CRO 125 mg SPT 2 g | 100.0 (52/52) 100.0 (9/9) | STI Clinic, USA | M | Culture | 6 | |
| Kaplowitz42 | 1987 | AMP 3.5 g+PROB 1 g | 87.5 (7/8) | STI Clinic, USA | Both | Culture | 5 | AMP 3.5 g+PROB 1 g N: 2.7% (2/75) V: 1.3% (1/75) D: NA |
| Kirkcaldy43 | 2014 | GEN 240 mg+AZM 2 g GEM 320 mg+AZM 2 g | 100.0 (1/1) 100.0 (5/5) | STI Clinic, USA | Both | Culture | 13.5 | GEN 240 mg+AZM 2 g N: 27.7% (56/202) V: 7.4% (15/202) D: 19.3% (39/202) GEM 320 mg+AZM 2 g N: 37.2% (74/199) V: 5.0% (10/199) D: 23.1% (46/199) |
| Lossick44 | 1982 | CFX 1.5 g+PROB 1 g PPEN 4.8 MU+PROB 1 g | 97.1 (34/35) 85.2 (23/27) | STI Clinic, USA | F | Culture | 5 | |
| McCormack45 | 1993 | CTX 500 mg CRO 250 mg | 100.0 (16/16) 100.0 (19/19) | Setting not stated, USA | Both | Culture | 5.5 | |
| Mogabgab46 | 1994 | CTX 500 mg CRO 250 mg | 100.0 (3/3) 100.0 (3/3) | STI Clinic, USA | Both | Culture | 5.5 | |
| Mohanty60 | 1988 | ATM 1 g | 100.0 (8/8) | Hospital, UK | Both | Culture | 14 | |
| Mroczkowski47 | 1997 | CFM 400 mg | 97.4 (37/38) | STI Clinic, USA | F | Culture | 7.5 | CFM 400 mg N: 2.1% (4/189) V: 1.1% (2/189) D: 0.5% (1/189) |
| Obaid48 | 1983 | PPEN 4.8 MU (×2 doses)+PROB 1 g | 100.0 (4/4) | STI Clinic, USA | M | Culture | 5 | |
| Pabst49 | 1989 | CRO 250 mg | 100.0 (5/5) | STI Clinic, USA | Both | Culture | 8.5 | |
| Raad58 | 1988 | PPEN 4.8 MU+PROB 1 g | 100.0 (1/1) | STI Clinic, USA and Peru | Both | Culture | 5 | |
| Ramus50 | 2001 | CRO 125 mg CFM 400 mg | 100.0 (23/23) 100.0 (16/16) | Hospital, USA | F (pregnant females only) | Culture | 7 | |
| Rob66 | 2019 | GEN 240 mg+AZM 2 g CRO 500 mg+AZM 2 g | 100.0 (40/40) 100.0 (38/38) | Hospital, Czech Republic | Both | NAAT | 10 | GEN 240 mg+AZM 2 g N: 19.4% (14/72) V: 0.0% (0/72) D: 23.6% (17/72) CRO 500 mg+AZM 2 g N: 26.8% (19/71) V: 1.4% (1/71) D: 26.8% (19/71) |
| Romanowski65 | 1984 | AMP 3.5 g+PROB 1 g | 100.0 (23/23) | Setting not stated, Canada | Both | Culture | 5.5 | |
| Ross61 | 2019 | GEN 240 mg+AZM 1 g CRO 500 mg+AZM 1 g | 89.9 (107/119) 97.8 (134/137) | STI Clinic, UK | Both | Culture+NAAT | 14 | GEN 240 mg+AZM 1 g N: 13.8% (41/298) V: 4.0% (12/298) D: NA CRO 500 mg+AZM 1 g N: 11.9% (38/320) V: 0.9% (3/320) D: NA |
| Scott62 | 1987 | CIP 250 mg AMP 500 mg q6h for 5/7+PROB 1 g | 100.0 (3/3) 100.0 (3/3) | Hospital, UK | M | Culture | 14 | |
| Simpson51 | 1981 | PPEN 4.8 MU+PROB 1 g CTX 1 g | 100.0 (9/9) 100.0 (9/9) | STI Clinic, USA | Both | Culture | 5 | |
| Simpson52 | 1982 | PIP 2 g+PROB 1 g PPEN 4.8 MU+PROB 1 g | 100.0 (1/1) 100.0 (4/4) | STI Clinic, USA | M | Culture | 5 | |
| Sinanian53 | 1973 | SPT 4 g TET 9 g | 98.2 (54/55) 93.0 (53/57) | STI Clinic, USA | F | Culture | 7 | SPT 4 g, NA TET 9 g N: 3.0% (6/198) V: NA D: NA |
| Slutkin54 | 1982 | PPEN 4.8 MU+PROB 1 g | 87.5 (14/16) | STI Clinic, USA | Both | Culture | 6 | |
| Smith55 | 1993 | CRO 250 mg | 100.0 (24/24) | STI Clinic, USA | F | Culture | 7 | CRO 250 mg N: 0.3% (1/380) V: NA D: 0.5% (2/380) |
| Stolz67 | 1984 | CTX 1 g CFX 1.5 g | 100.0 (103/103) 92.2 (71/77) | Setting not stated, The Netherlands | Both | Culture | 10.5 | CTX 1 g N: 0.3% (2/590) V: NA D: NA CFX 1.5 g N: 0.3% (2/647) V: NA D: NA |
| Stoner56 | 2001 | GAT 400 mg GAT 600 mg OFX 400 mg | 100.0 (20/20) 100.0 (16/16) 100.0 (7/7) | Setting not stated, USA | F | Culture | 7 | GAT 400 mg N: 8.1% (24/295) V: 0.7% (2/295) D: 2.0% (6/295) GAT 600 mg N: 10.7% (31/291) V: 1.7% (5/291) D: 3.4% (10/291) OFX 400 mg N: 4.2% (6/142) V: 1.4% (2/142) D: 2.8% (4/142) |
| Taylor18 | 2018 | GEP 1500 mg GEP 3000 mg | 100.0 (1/1) 100.0 (2/2) | Setting not stated, USA and UK | M | Culture+NAAT | 6 | GEP 1500 mg N: 5.8% (3/52) V: NA D: 17.3% (9/52) GEP 3000 mg N: 20.8% (11/53) V: NA D: 35.8% (19/53) |
| Taylor20 | 2018 | ZOL 2 g ZOL 3 g CRO 500 mg | 100.0 (4/4) 100.0 (6/6) 100.0 (3/3) | STI Clinic, USA | Both | Culture+NAAT | 6 | |
| Thorpe57 | 1996 | CFX 1000 mg CIP 500 mg | 96.8 (30/31) 100.0 (26/26) | STI Clinic, USA and Puerto Rico | Both | Culture | 6 |
| First author . | Publication year . | Treatment . | Percentage with rectal NG cured (no. cured/ no. tested) . | Setting and country . | Gender of participantsa . | Diagnostic assay . | Median follow up (days) . | Side effects % (no./total)b . |
|---|---|---|---|---|---|---|---|---|
| Baddour25 | 1989 | CFX 1 g AMX 3 g+PROB 1 g | 98.1 (53/54) 97.6 (41/42) | STI Clinic, USA | F | Culture | 5.5 | CFX 1 g N: 3.8% (9/235) V: 3.4% (8/235) D: 1.3% (3/235) AMX 3 g+PROB 1 g N: 2.2% (5/231) V: 0.9% (2/231) D: 3.5% (8/231) |
| Baddour24 | 1992 | AMP 1 g+SUL 0.5 g+PROB 1 g CRO 250 mg | 100.0 (8/8) 100.0 (7/7) | STI Clinic, USA | Both | Culture | 5.5 | AMP 1 g+SUL 0.5 g+PROB 1 g, NA CRO 250 mg N: NA V: NA D: 3.1% (3/97) |
| Batteiger26 | 1985 | AMP 3.5 g+PROB 1 g | 100.0 (12/12) | STI Clinic, USA | F | Culture | 10.5 | |
| Black27 | 1989 | OFX 400 mg AMX 3 g+PROB 1 g | 100.0 (13/13) 100.0 (17/17) | STI Clinic, USA | F | Culture | 7.5 | |
| Cavenee28 | 1993 | CRO 250 mg SPT 2 g AMX 3 g+PROB 1 g | 95.5 (21/22) 100.0 (19/19) 85.2 (23/27) | Hospital, USA | F (pregnant females only) | Culture | 7 | CRO 250 mg, NA SPT 2 g, NA AMX 3 g+PROB 1 g N: NA V: 1.2% (1/84) D: NA |
| Chen19 | 2019 | CRO 500 mg+AZM 1000 mg SOL 1000 mg | 100.0 (12/12) 83.3 (5/6) | STI Clinic, USA and Australia | Both | Culture+NAAT | 7 | CRO 500 mg+AZM 1000 mg N: 11.5% (15/131) V: 0.0% (0/131) D: 15.3% (20/131) SOL 1000 mg N: 20.8% (27/130) V: 2.3% (3/130) D: 23.8% (31/130) |
| Collier29 | 1984 | CRO 125 mg SPT 2 g | 100.0 (23/23) 100.0 (12/12) | STI Clinic, USA | F | Culture | 6 | |
| Covino30 | 1990 | OFX 400 mg CRO 250 mg | 100.0 (2/2) 83.3 (5/6) | STI Clinic, USA | F | Culture | 6 | |
| Covino31 | 1993 | CRO 250 mg | 100.0 (5/5) | STI Clinic, USA | Both | Culture | 7 | |
| Das68 | 1989 | CFX 1 g AMP 3 g+PROB 1 g | 100.0 (7/7) 100.0 (4/4) | Setting and country not stated | Both | Culture | 7 | |
| Dixon59 | 1986 | CRO 500 mg PPEN 1.5 g+BPEN 300 mg | 100.0 (1/1) 80.0 (4/5) | STI Clinic, UK | Both | Culture | 16 | |
| Dubois69 | 1990 | AMX 3 g+PROB 1 g | 100.0 (2/2) | Setting and country not stated | F | Culture | 5.5 | AMX 3 g+PROB 1 g N: 3.9% (3/77) V: 2.6% (2/77) D: 1.3% (1/77) |
| Edwards32 | 1984 | AMX 3 g+PROB 1 g | 100.0 (7/7) | STI Clinic, USA | Both | Culture | 7 | |
| Forstrom64 | 1972 | PPEN 2.4 MU | 100.0 (14/14) | Hospital, Finland | F | Culture | 7 | |
| Forstrom63 | 1974 | AMP 2000 mg+PROB 1 g | 92.9 (13/14) | Hospital, Finland | F | Culture | 7 | |
| Gottlieb33 | 1985 | ATM 1 g SPT 2 g | 100.0 (3/3) 100.0 (6/6) | STI Clinic, USA | Both | Culture | 6 | |
| Gottlieb34 | 1986 | CFX 1 g+PROB 1 g CFX 1 g AMX 3 g+PROB 1 g | 100.0 (6/6) 66.7 (4/6) 66.7 (2/3) | STI Clinic, USA | M | Culture | 6 | |
| Greaves35 | 1983 | FOX 2 g+PROB 1 g PPEN 4.8 MU+PROB 1 g | 95.7 (45/47) 97.4 (38/39) | STI Clinic, USA | Both | Culture | 8 | |
| Handsfield71 | 1981 | CRO 125 mg CRO 250 mg CRO 500 mg | 100.0 (5/5) 100.0 (3/3) 100.0 (2/2) | STI Clinic, country not stated | M | Culture | 5.5 | |
| Handsfield70 | 1983 | CRO 125 mg CRO 250 mg SPT 2 g | 100.0 (8/8) 100.0 (6/6) 95.5 (21/22) | STI Clinic, country not stated | M | Culture | 5.5 | |
| Handsfield37 | 1991 | CFM 400 mg CFM 800 mg CRO 250 mg | 100.0 (10/10) 100.0 (6/6) 100.0 (6/6) | STI Clinic, USA | F | Culture | 6.5 | CFM 400 mg N: 0.9% (1/107) V: NA D: 7.5% (8/107) CFM 800 mg N: 4.4% (4/91) V: NA D: 13.2% (12/91) CRO 250 mg N: 0.0% (0/104) V: NA D: 3.8% (4/104) |
| Handsfield36 | 1994 | AZM 2 g CRO 250 mg | 96.3 (26/27) 100.0 (17/17) | STI Clinic, USA | Both | Culture | 7 | AZM 2 g N: 19.5% (84/431) V: 7.0% (30/431) D: 13.7% (59/431) CRO 250 mg, NA |
| Hook39 | 1986 | SPT 2 g | 100.0 (33/33) | STI Clinic, USA | M | Culture | 5.5 | |
| Hook38 | 1993 | CIP 250 mg CRO 250 mg | 100.0 (20/20) 100.0 (21/21) | STI Clinic, USA | F | Culture | 7 | |
| Hook72 | 1997 | CFM 400 mg | 100.0 (3/3) | Hospital, country not stated | M | Culture | 7.5 | |
| Hook17 | 2019 | CRO 250 mg DEL 900 mg | 100.0 (13/13) 82.6 (19/23) | Setting not stated, USA | Both | Culture+NAAT | 7 | CRO 250 mg N: 1.3% (2/154) V: 0.6% (1/154) D: 7.1% (11/154) DEL 900 mg N: 7.9% (24/304) V: 2.6% (8/304) D: 31.9% (97/304) |
| Jones40 | 1991 | CTX 500 mg CRO 250 mg | 100.0 (5/5) 100.0 (4/4) | STI Clinic, USA | Both | Culture | 5.5 | CTX 500 mg, NA CRO 250 mg N: 1.7% (1/58) V: NA D: 3.4% (2/58) |
| Judson73 | 1983 | CRO 250 mg PPEN 4.8 MU (×2 doses)+PROB 1 g | 100.0 (10/10) 100.0 (11/11) | Setting and country not stated | F | Culture | 5.5 | |
| Judson41 | 1985 | CRO 125 mg SPT 2 g | 100.0 (52/52) 100.0 (9/9) | STI Clinic, USA | M | Culture | 6 | |
| Kaplowitz42 | 1987 | AMP 3.5 g+PROB 1 g | 87.5 (7/8) | STI Clinic, USA | Both | Culture | 5 | AMP 3.5 g+PROB 1 g N: 2.7% (2/75) V: 1.3% (1/75) D: NA |
| Kirkcaldy43 | 2014 | GEN 240 mg+AZM 2 g GEM 320 mg+AZM 2 g | 100.0 (1/1) 100.0 (5/5) | STI Clinic, USA | Both | Culture | 13.5 | GEN 240 mg+AZM 2 g N: 27.7% (56/202) V: 7.4% (15/202) D: 19.3% (39/202) GEM 320 mg+AZM 2 g N: 37.2% (74/199) V: 5.0% (10/199) D: 23.1% (46/199) |
| Lossick44 | 1982 | CFX 1.5 g+PROB 1 g PPEN 4.8 MU+PROB 1 g | 97.1 (34/35) 85.2 (23/27) | STI Clinic, USA | F | Culture | 5 | |
| McCormack45 | 1993 | CTX 500 mg CRO 250 mg | 100.0 (16/16) 100.0 (19/19) | Setting not stated, USA | Both | Culture | 5.5 | |
| Mogabgab46 | 1994 | CTX 500 mg CRO 250 mg | 100.0 (3/3) 100.0 (3/3) | STI Clinic, USA | Both | Culture | 5.5 | |
| Mohanty60 | 1988 | ATM 1 g | 100.0 (8/8) | Hospital, UK | Both | Culture | 14 | |
| Mroczkowski47 | 1997 | CFM 400 mg | 97.4 (37/38) | STI Clinic, USA | F | Culture | 7.5 | CFM 400 mg N: 2.1% (4/189) V: 1.1% (2/189) D: 0.5% (1/189) |
| Obaid48 | 1983 | PPEN 4.8 MU (×2 doses)+PROB 1 g | 100.0 (4/4) | STI Clinic, USA | M | Culture | 5 | |
| Pabst49 | 1989 | CRO 250 mg | 100.0 (5/5) | STI Clinic, USA | Both | Culture | 8.5 | |
| Raad58 | 1988 | PPEN 4.8 MU+PROB 1 g | 100.0 (1/1) | STI Clinic, USA and Peru | Both | Culture | 5 | |
| Ramus50 | 2001 | CRO 125 mg CFM 400 mg | 100.0 (23/23) 100.0 (16/16) | Hospital, USA | F (pregnant females only) | Culture | 7 | |
| Rob66 | 2019 | GEN 240 mg+AZM 2 g CRO 500 mg+AZM 2 g | 100.0 (40/40) 100.0 (38/38) | Hospital, Czech Republic | Both | NAAT | 10 | GEN 240 mg+AZM 2 g N: 19.4% (14/72) V: 0.0% (0/72) D: 23.6% (17/72) CRO 500 mg+AZM 2 g N: 26.8% (19/71) V: 1.4% (1/71) D: 26.8% (19/71) |
| Romanowski65 | 1984 | AMP 3.5 g+PROB 1 g | 100.0 (23/23) | Setting not stated, Canada | Both | Culture | 5.5 | |
| Ross61 | 2019 | GEN 240 mg+AZM 1 g CRO 500 mg+AZM 1 g | 89.9 (107/119) 97.8 (134/137) | STI Clinic, UK | Both | Culture+NAAT | 14 | GEN 240 mg+AZM 1 g N: 13.8% (41/298) V: 4.0% (12/298) D: NA CRO 500 mg+AZM 1 g N: 11.9% (38/320) V: 0.9% (3/320) D: NA |
| Scott62 | 1987 | CIP 250 mg AMP 500 mg q6h for 5/7+PROB 1 g | 100.0 (3/3) 100.0 (3/3) | Hospital, UK | M | Culture | 14 | |
| Simpson51 | 1981 | PPEN 4.8 MU+PROB 1 g CTX 1 g | 100.0 (9/9) 100.0 (9/9) | STI Clinic, USA | Both | Culture | 5 | |
| Simpson52 | 1982 | PIP 2 g+PROB 1 g PPEN 4.8 MU+PROB 1 g | 100.0 (1/1) 100.0 (4/4) | STI Clinic, USA | M | Culture | 5 | |
| Sinanian53 | 1973 | SPT 4 g TET 9 g | 98.2 (54/55) 93.0 (53/57) | STI Clinic, USA | F | Culture | 7 | SPT 4 g, NA TET 9 g N: 3.0% (6/198) V: NA D: NA |
| Slutkin54 | 1982 | PPEN 4.8 MU+PROB 1 g | 87.5 (14/16) | STI Clinic, USA | Both | Culture | 6 | |
| Smith55 | 1993 | CRO 250 mg | 100.0 (24/24) | STI Clinic, USA | F | Culture | 7 | CRO 250 mg N: 0.3% (1/380) V: NA D: 0.5% (2/380) |
| Stolz67 | 1984 | CTX 1 g CFX 1.5 g | 100.0 (103/103) 92.2 (71/77) | Setting not stated, The Netherlands | Both | Culture | 10.5 | CTX 1 g N: 0.3% (2/590) V: NA D: NA CFX 1.5 g N: 0.3% (2/647) V: NA D: NA |
| Stoner56 | 2001 | GAT 400 mg GAT 600 mg OFX 400 mg | 100.0 (20/20) 100.0 (16/16) 100.0 (7/7) | Setting not stated, USA | F | Culture | 7 | GAT 400 mg N: 8.1% (24/295) V: 0.7% (2/295) D: 2.0% (6/295) GAT 600 mg N: 10.7% (31/291) V: 1.7% (5/291) D: 3.4% (10/291) OFX 400 mg N: 4.2% (6/142) V: 1.4% (2/142) D: 2.8% (4/142) |
| Taylor18 | 2018 | GEP 1500 mg GEP 3000 mg | 100.0 (1/1) 100.0 (2/2) | Setting not stated, USA and UK | M | Culture+NAAT | 6 | GEP 1500 mg N: 5.8% (3/52) V: NA D: 17.3% (9/52) GEP 3000 mg N: 20.8% (11/53) V: NA D: 35.8% (19/53) |
| Taylor20 | 2018 | ZOL 2 g ZOL 3 g CRO 500 mg | 100.0 (4/4) 100.0 (6/6) 100.0 (3/3) | STI Clinic, USA | Both | Culture+NAAT | 6 | |
| Thorpe57 | 1996 | CFX 1000 mg CIP 500 mg | 96.8 (30/31) 100.0 (26/26) | STI Clinic, USA and Puerto Rico | Both | Culture | 6 |
AMX, amoxicillin; AMP, ampicillin; AZM, azithromycin; ATM, aztreonam; BPEN, benzylpenicillin; CRO, ceftriaxone; CFX, cefuroxime axetil/sodium; CFM, cefixime; FOX, cefoxitin; CIP, ciprofloxacin; CTX, cefotaxime; DEL, delafloxacin; GAT, gatifloxacin; GEM, Gemifloxacin; GEN, gentamicin; GEP, gepotidacin; OFX, ofloxacin; PIP, piperacillin; PPEN, procaine penicillin; PROB, probenecid; SOL, solithromycin; SPT, spectinomycin; SUL, sulbactam; TET, tetracycline; ZOL, zoliflodacin; NA, not available.
Gender of participants in the trial who contributed to the rectal endpoint.
Side effects are nausea (N), vomiting (V) or diarrhoea (D) among all participants and not specifically among those with rectal infection.
| First author . | Publication year . | Treatment . | Percentage with rectal NG cured (no. cured/ no. tested) . | Setting and country . | Gender of participantsa . | Diagnostic assay . | Median follow up (days) . | Side effects % (no./total)b . |
|---|---|---|---|---|---|---|---|---|
| Baddour25 | 1989 | CFX 1 g AMX 3 g+PROB 1 g | 98.1 (53/54) 97.6 (41/42) | STI Clinic, USA | F | Culture | 5.5 | CFX 1 g N: 3.8% (9/235) V: 3.4% (8/235) D: 1.3% (3/235) AMX 3 g+PROB 1 g N: 2.2% (5/231) V: 0.9% (2/231) D: 3.5% (8/231) |
| Baddour24 | 1992 | AMP 1 g+SUL 0.5 g+PROB 1 g CRO 250 mg | 100.0 (8/8) 100.0 (7/7) | STI Clinic, USA | Both | Culture | 5.5 | AMP 1 g+SUL 0.5 g+PROB 1 g, NA CRO 250 mg N: NA V: NA D: 3.1% (3/97) |
| Batteiger26 | 1985 | AMP 3.5 g+PROB 1 g | 100.0 (12/12) | STI Clinic, USA | F | Culture | 10.5 | |
| Black27 | 1989 | OFX 400 mg AMX 3 g+PROB 1 g | 100.0 (13/13) 100.0 (17/17) | STI Clinic, USA | F | Culture | 7.5 | |
| Cavenee28 | 1993 | CRO 250 mg SPT 2 g AMX 3 g+PROB 1 g | 95.5 (21/22) 100.0 (19/19) 85.2 (23/27) | Hospital, USA | F (pregnant females only) | Culture | 7 | CRO 250 mg, NA SPT 2 g, NA AMX 3 g+PROB 1 g N: NA V: 1.2% (1/84) D: NA |
| Chen19 | 2019 | CRO 500 mg+AZM 1000 mg SOL 1000 mg | 100.0 (12/12) 83.3 (5/6) | STI Clinic, USA and Australia | Both | Culture+NAAT | 7 | CRO 500 mg+AZM 1000 mg N: 11.5% (15/131) V: 0.0% (0/131) D: 15.3% (20/131) SOL 1000 mg N: 20.8% (27/130) V: 2.3% (3/130) D: 23.8% (31/130) |
| Collier29 | 1984 | CRO 125 mg SPT 2 g | 100.0 (23/23) 100.0 (12/12) | STI Clinic, USA | F | Culture | 6 | |
| Covino30 | 1990 | OFX 400 mg CRO 250 mg | 100.0 (2/2) 83.3 (5/6) | STI Clinic, USA | F | Culture | 6 | |
| Covino31 | 1993 | CRO 250 mg | 100.0 (5/5) | STI Clinic, USA | Both | Culture | 7 | |
| Das68 | 1989 | CFX 1 g AMP 3 g+PROB 1 g | 100.0 (7/7) 100.0 (4/4) | Setting and country not stated | Both | Culture | 7 | |
| Dixon59 | 1986 | CRO 500 mg PPEN 1.5 g+BPEN 300 mg | 100.0 (1/1) 80.0 (4/5) | STI Clinic, UK | Both | Culture | 16 | |
| Dubois69 | 1990 | AMX 3 g+PROB 1 g | 100.0 (2/2) | Setting and country not stated | F | Culture | 5.5 | AMX 3 g+PROB 1 g N: 3.9% (3/77) V: 2.6% (2/77) D: 1.3% (1/77) |
| Edwards32 | 1984 | AMX 3 g+PROB 1 g | 100.0 (7/7) | STI Clinic, USA | Both | Culture | 7 | |
| Forstrom64 | 1972 | PPEN 2.4 MU | 100.0 (14/14) | Hospital, Finland | F | Culture | 7 | |
| Forstrom63 | 1974 | AMP 2000 mg+PROB 1 g | 92.9 (13/14) | Hospital, Finland | F | Culture | 7 | |
| Gottlieb33 | 1985 | ATM 1 g SPT 2 g | 100.0 (3/3) 100.0 (6/6) | STI Clinic, USA | Both | Culture | 6 | |
| Gottlieb34 | 1986 | CFX 1 g+PROB 1 g CFX 1 g AMX 3 g+PROB 1 g | 100.0 (6/6) 66.7 (4/6) 66.7 (2/3) | STI Clinic, USA | M | Culture | 6 | |
| Greaves35 | 1983 | FOX 2 g+PROB 1 g PPEN 4.8 MU+PROB 1 g | 95.7 (45/47) 97.4 (38/39) | STI Clinic, USA | Both | Culture | 8 | |
| Handsfield71 | 1981 | CRO 125 mg CRO 250 mg CRO 500 mg | 100.0 (5/5) 100.0 (3/3) 100.0 (2/2) | STI Clinic, country not stated | M | Culture | 5.5 | |
| Handsfield70 | 1983 | CRO 125 mg CRO 250 mg SPT 2 g | 100.0 (8/8) 100.0 (6/6) 95.5 (21/22) | STI Clinic, country not stated | M | Culture | 5.5 | |
| Handsfield37 | 1991 | CFM 400 mg CFM 800 mg CRO 250 mg | 100.0 (10/10) 100.0 (6/6) 100.0 (6/6) | STI Clinic, USA | F | Culture | 6.5 | CFM 400 mg N: 0.9% (1/107) V: NA D: 7.5% (8/107) CFM 800 mg N: 4.4% (4/91) V: NA D: 13.2% (12/91) CRO 250 mg N: 0.0% (0/104) V: NA D: 3.8% (4/104) |
| Handsfield36 | 1994 | AZM 2 g CRO 250 mg | 96.3 (26/27) 100.0 (17/17) | STI Clinic, USA | Both | Culture | 7 | AZM 2 g N: 19.5% (84/431) V: 7.0% (30/431) D: 13.7% (59/431) CRO 250 mg, NA |
| Hook39 | 1986 | SPT 2 g | 100.0 (33/33) | STI Clinic, USA | M | Culture | 5.5 | |
| Hook38 | 1993 | CIP 250 mg CRO 250 mg | 100.0 (20/20) 100.0 (21/21) | STI Clinic, USA | F | Culture | 7 | |
| Hook72 | 1997 | CFM 400 mg | 100.0 (3/3) | Hospital, country not stated | M | Culture | 7.5 | |
| Hook17 | 2019 | CRO 250 mg DEL 900 mg | 100.0 (13/13) 82.6 (19/23) | Setting not stated, USA | Both | Culture+NAAT | 7 | CRO 250 mg N: 1.3% (2/154) V: 0.6% (1/154) D: 7.1% (11/154) DEL 900 mg N: 7.9% (24/304) V: 2.6% (8/304) D: 31.9% (97/304) |
| Jones40 | 1991 | CTX 500 mg CRO 250 mg | 100.0 (5/5) 100.0 (4/4) | STI Clinic, USA | Both | Culture | 5.5 | CTX 500 mg, NA CRO 250 mg N: 1.7% (1/58) V: NA D: 3.4% (2/58) |
| Judson73 | 1983 | CRO 250 mg PPEN 4.8 MU (×2 doses)+PROB 1 g | 100.0 (10/10) 100.0 (11/11) | Setting and country not stated | F | Culture | 5.5 | |
| Judson41 | 1985 | CRO 125 mg SPT 2 g | 100.0 (52/52) 100.0 (9/9) | STI Clinic, USA | M | Culture | 6 | |
| Kaplowitz42 | 1987 | AMP 3.5 g+PROB 1 g | 87.5 (7/8) | STI Clinic, USA | Both | Culture | 5 | AMP 3.5 g+PROB 1 g N: 2.7% (2/75) V: 1.3% (1/75) D: NA |
| Kirkcaldy43 | 2014 | GEN 240 mg+AZM 2 g GEM 320 mg+AZM 2 g | 100.0 (1/1) 100.0 (5/5) | STI Clinic, USA | Both | Culture | 13.5 | GEN 240 mg+AZM 2 g N: 27.7% (56/202) V: 7.4% (15/202) D: 19.3% (39/202) GEM 320 mg+AZM 2 g N: 37.2% (74/199) V: 5.0% (10/199) D: 23.1% (46/199) |
| Lossick44 | 1982 | CFX 1.5 g+PROB 1 g PPEN 4.8 MU+PROB 1 g | 97.1 (34/35) 85.2 (23/27) | STI Clinic, USA | F | Culture | 5 | |
| McCormack45 | 1993 | CTX 500 mg CRO 250 mg | 100.0 (16/16) 100.0 (19/19) | Setting not stated, USA | Both | Culture | 5.5 | |
| Mogabgab46 | 1994 | CTX 500 mg CRO 250 mg | 100.0 (3/3) 100.0 (3/3) | STI Clinic, USA | Both | Culture | 5.5 | |
| Mohanty60 | 1988 | ATM 1 g | 100.0 (8/8) | Hospital, UK | Both | Culture | 14 | |
| Mroczkowski47 | 1997 | CFM 400 mg | 97.4 (37/38) | STI Clinic, USA | F | Culture | 7.5 | CFM 400 mg N: 2.1% (4/189) V: 1.1% (2/189) D: 0.5% (1/189) |
| Obaid48 | 1983 | PPEN 4.8 MU (×2 doses)+PROB 1 g | 100.0 (4/4) | STI Clinic, USA | M | Culture | 5 | |
| Pabst49 | 1989 | CRO 250 mg | 100.0 (5/5) | STI Clinic, USA | Both | Culture | 8.5 | |
| Raad58 | 1988 | PPEN 4.8 MU+PROB 1 g | 100.0 (1/1) | STI Clinic, USA and Peru | Both | Culture | 5 | |
| Ramus50 | 2001 | CRO 125 mg CFM 400 mg | 100.0 (23/23) 100.0 (16/16) | Hospital, USA | F (pregnant females only) | Culture | 7 | |
| Rob66 | 2019 | GEN 240 mg+AZM 2 g CRO 500 mg+AZM 2 g | 100.0 (40/40) 100.0 (38/38) | Hospital, Czech Republic | Both | NAAT | 10 | GEN 240 mg+AZM 2 g N: 19.4% (14/72) V: 0.0% (0/72) D: 23.6% (17/72) CRO 500 mg+AZM 2 g N: 26.8% (19/71) V: 1.4% (1/71) D: 26.8% (19/71) |
| Romanowski65 | 1984 | AMP 3.5 g+PROB 1 g | 100.0 (23/23) | Setting not stated, Canada | Both | Culture | 5.5 | |
| Ross61 | 2019 | GEN 240 mg+AZM 1 g CRO 500 mg+AZM 1 g | 89.9 (107/119) 97.8 (134/137) | STI Clinic, UK | Both | Culture+NAAT | 14 | GEN 240 mg+AZM 1 g N: 13.8% (41/298) V: 4.0% (12/298) D: NA CRO 500 mg+AZM 1 g N: 11.9% (38/320) V: 0.9% (3/320) D: NA |
| Scott62 | 1987 | CIP 250 mg AMP 500 mg q6h for 5/7+PROB 1 g | 100.0 (3/3) 100.0 (3/3) | Hospital, UK | M | Culture | 14 | |
| Simpson51 | 1981 | PPEN 4.8 MU+PROB 1 g CTX 1 g | 100.0 (9/9) 100.0 (9/9) | STI Clinic, USA | Both | Culture | 5 | |
| Simpson52 | 1982 | PIP 2 g+PROB 1 g PPEN 4.8 MU+PROB 1 g | 100.0 (1/1) 100.0 (4/4) | STI Clinic, USA | M | Culture | 5 | |
| Sinanian53 | 1973 | SPT 4 g TET 9 g | 98.2 (54/55) 93.0 (53/57) | STI Clinic, USA | F | Culture | 7 | SPT 4 g, NA TET 9 g N: 3.0% (6/198) V: NA D: NA |
| Slutkin54 | 1982 | PPEN 4.8 MU+PROB 1 g | 87.5 (14/16) | STI Clinic, USA | Both | Culture | 6 | |
| Smith55 | 1993 | CRO 250 mg | 100.0 (24/24) | STI Clinic, USA | F | Culture | 7 | CRO 250 mg N: 0.3% (1/380) V: NA D: 0.5% (2/380) |
| Stolz67 | 1984 | CTX 1 g CFX 1.5 g | 100.0 (103/103) 92.2 (71/77) | Setting not stated, The Netherlands | Both | Culture | 10.5 | CTX 1 g N: 0.3% (2/590) V: NA D: NA CFX 1.5 g N: 0.3% (2/647) V: NA D: NA |
| Stoner56 | 2001 | GAT 400 mg GAT 600 mg OFX 400 mg | 100.0 (20/20) 100.0 (16/16) 100.0 (7/7) | Setting not stated, USA | F | Culture | 7 | GAT 400 mg N: 8.1% (24/295) V: 0.7% (2/295) D: 2.0% (6/295) GAT 600 mg N: 10.7% (31/291) V: 1.7% (5/291) D: 3.4% (10/291) OFX 400 mg N: 4.2% (6/142) V: 1.4% (2/142) D: 2.8% (4/142) |
| Taylor18 | 2018 | GEP 1500 mg GEP 3000 mg | 100.0 (1/1) 100.0 (2/2) | Setting not stated, USA and UK | M | Culture+NAAT | 6 | GEP 1500 mg N: 5.8% (3/52) V: NA D: 17.3% (9/52) GEP 3000 mg N: 20.8% (11/53) V: NA D: 35.8% (19/53) |
| Taylor20 | 2018 | ZOL 2 g ZOL 3 g CRO 500 mg | 100.0 (4/4) 100.0 (6/6) 100.0 (3/3) | STI Clinic, USA | Both | Culture+NAAT | 6 | |
| Thorpe57 | 1996 | CFX 1000 mg CIP 500 mg | 96.8 (30/31) 100.0 (26/26) | STI Clinic, USA and Puerto Rico | Both | Culture | 6 |
| First author . | Publication year . | Treatment . | Percentage with rectal NG cured (no. cured/ no. tested) . | Setting and country . | Gender of participantsa . | Diagnostic assay . | Median follow up (days) . | Side effects % (no./total)b . |
|---|---|---|---|---|---|---|---|---|
| Baddour25 | 1989 | CFX 1 g AMX 3 g+PROB 1 g | 98.1 (53/54) 97.6 (41/42) | STI Clinic, USA | F | Culture | 5.5 | CFX 1 g N: 3.8% (9/235) V: 3.4% (8/235) D: 1.3% (3/235) AMX 3 g+PROB 1 g N: 2.2% (5/231) V: 0.9% (2/231) D: 3.5% (8/231) |
| Baddour24 | 1992 | AMP 1 g+SUL 0.5 g+PROB 1 g CRO 250 mg | 100.0 (8/8) 100.0 (7/7) | STI Clinic, USA | Both | Culture | 5.5 | AMP 1 g+SUL 0.5 g+PROB 1 g, NA CRO 250 mg N: NA V: NA D: 3.1% (3/97) |
| Batteiger26 | 1985 | AMP 3.5 g+PROB 1 g | 100.0 (12/12) | STI Clinic, USA | F | Culture | 10.5 | |
| Black27 | 1989 | OFX 400 mg AMX 3 g+PROB 1 g | 100.0 (13/13) 100.0 (17/17) | STI Clinic, USA | F | Culture | 7.5 | |
| Cavenee28 | 1993 | CRO 250 mg SPT 2 g AMX 3 g+PROB 1 g | 95.5 (21/22) 100.0 (19/19) 85.2 (23/27) | Hospital, USA | F (pregnant females only) | Culture | 7 | CRO 250 mg, NA SPT 2 g, NA AMX 3 g+PROB 1 g N: NA V: 1.2% (1/84) D: NA |
| Chen19 | 2019 | CRO 500 mg+AZM 1000 mg SOL 1000 mg | 100.0 (12/12) 83.3 (5/6) | STI Clinic, USA and Australia | Both | Culture+NAAT | 7 | CRO 500 mg+AZM 1000 mg N: 11.5% (15/131) V: 0.0% (0/131) D: 15.3% (20/131) SOL 1000 mg N: 20.8% (27/130) V: 2.3% (3/130) D: 23.8% (31/130) |
| Collier29 | 1984 | CRO 125 mg SPT 2 g | 100.0 (23/23) 100.0 (12/12) | STI Clinic, USA | F | Culture | 6 | |
| Covino30 | 1990 | OFX 400 mg CRO 250 mg | 100.0 (2/2) 83.3 (5/6) | STI Clinic, USA | F | Culture | 6 | |
| Covino31 | 1993 | CRO 250 mg | 100.0 (5/5) | STI Clinic, USA | Both | Culture | 7 | |
| Das68 | 1989 | CFX 1 g AMP 3 g+PROB 1 g | 100.0 (7/7) 100.0 (4/4) | Setting and country not stated | Both | Culture | 7 | |
| Dixon59 | 1986 | CRO 500 mg PPEN 1.5 g+BPEN 300 mg | 100.0 (1/1) 80.0 (4/5) | STI Clinic, UK | Both | Culture | 16 | |
| Dubois69 | 1990 | AMX 3 g+PROB 1 g | 100.0 (2/2) | Setting and country not stated | F | Culture | 5.5 | AMX 3 g+PROB 1 g N: 3.9% (3/77) V: 2.6% (2/77) D: 1.3% (1/77) |
| Edwards32 | 1984 | AMX 3 g+PROB 1 g | 100.0 (7/7) | STI Clinic, USA | Both | Culture | 7 | |
| Forstrom64 | 1972 | PPEN 2.4 MU | 100.0 (14/14) | Hospital, Finland | F | Culture | 7 | |
| Forstrom63 | 1974 | AMP 2000 mg+PROB 1 g | 92.9 (13/14) | Hospital, Finland | F | Culture | 7 | |
| Gottlieb33 | 1985 | ATM 1 g SPT 2 g | 100.0 (3/3) 100.0 (6/6) | STI Clinic, USA | Both | Culture | 6 | |
| Gottlieb34 | 1986 | CFX 1 g+PROB 1 g CFX 1 g AMX 3 g+PROB 1 g | 100.0 (6/6) 66.7 (4/6) 66.7 (2/3) | STI Clinic, USA | M | Culture | 6 | |
| Greaves35 | 1983 | FOX 2 g+PROB 1 g PPEN 4.8 MU+PROB 1 g | 95.7 (45/47) 97.4 (38/39) | STI Clinic, USA | Both | Culture | 8 | |
| Handsfield71 | 1981 | CRO 125 mg CRO 250 mg CRO 500 mg | 100.0 (5/5) 100.0 (3/3) 100.0 (2/2) | STI Clinic, country not stated | M | Culture | 5.5 | |
| Handsfield70 | 1983 | CRO 125 mg CRO 250 mg SPT 2 g | 100.0 (8/8) 100.0 (6/6) 95.5 (21/22) | STI Clinic, country not stated | M | Culture | 5.5 | |
| Handsfield37 | 1991 | CFM 400 mg CFM 800 mg CRO 250 mg | 100.0 (10/10) 100.0 (6/6) 100.0 (6/6) | STI Clinic, USA | F | Culture | 6.5 | CFM 400 mg N: 0.9% (1/107) V: NA D: 7.5% (8/107) CFM 800 mg N: 4.4% (4/91) V: NA D: 13.2% (12/91) CRO 250 mg N: 0.0% (0/104) V: NA D: 3.8% (4/104) |
| Handsfield36 | 1994 | AZM 2 g CRO 250 mg | 96.3 (26/27) 100.0 (17/17) | STI Clinic, USA | Both | Culture | 7 | AZM 2 g N: 19.5% (84/431) V: 7.0% (30/431) D: 13.7% (59/431) CRO 250 mg, NA |
| Hook39 | 1986 | SPT 2 g | 100.0 (33/33) | STI Clinic, USA | M | Culture | 5.5 | |
| Hook38 | 1993 | CIP 250 mg CRO 250 mg | 100.0 (20/20) 100.0 (21/21) | STI Clinic, USA | F | Culture | 7 | |
| Hook72 | 1997 | CFM 400 mg | 100.0 (3/3) | Hospital, country not stated | M | Culture | 7.5 | |
| Hook17 | 2019 | CRO 250 mg DEL 900 mg | 100.0 (13/13) 82.6 (19/23) | Setting not stated, USA | Both | Culture+NAAT | 7 | CRO 250 mg N: 1.3% (2/154) V: 0.6% (1/154) D: 7.1% (11/154) DEL 900 mg N: 7.9% (24/304) V: 2.6% (8/304) D: 31.9% (97/304) |
| Jones40 | 1991 | CTX 500 mg CRO 250 mg | 100.0 (5/5) 100.0 (4/4) | STI Clinic, USA | Both | Culture | 5.5 | CTX 500 mg, NA CRO 250 mg N: 1.7% (1/58) V: NA D: 3.4% (2/58) |
| Judson73 | 1983 | CRO 250 mg PPEN 4.8 MU (×2 doses)+PROB 1 g | 100.0 (10/10) 100.0 (11/11) | Setting and country not stated | F | Culture | 5.5 | |
| Judson41 | 1985 | CRO 125 mg SPT 2 g | 100.0 (52/52) 100.0 (9/9) | STI Clinic, USA | M | Culture | 6 | |
| Kaplowitz42 | 1987 | AMP 3.5 g+PROB 1 g | 87.5 (7/8) | STI Clinic, USA | Both | Culture | 5 | AMP 3.5 g+PROB 1 g N: 2.7% (2/75) V: 1.3% (1/75) D: NA |
| Kirkcaldy43 | 2014 | GEN 240 mg+AZM 2 g GEM 320 mg+AZM 2 g | 100.0 (1/1) 100.0 (5/5) | STI Clinic, USA | Both | Culture | 13.5 | GEN 240 mg+AZM 2 g N: 27.7% (56/202) V: 7.4% (15/202) D: 19.3% (39/202) GEM 320 mg+AZM 2 g N: 37.2% (74/199) V: 5.0% (10/199) D: 23.1% (46/199) |
| Lossick44 | 1982 | CFX 1.5 g+PROB 1 g PPEN 4.8 MU+PROB 1 g | 97.1 (34/35) 85.2 (23/27) | STI Clinic, USA | F | Culture | 5 | |
| McCormack45 | 1993 | CTX 500 mg CRO 250 mg | 100.0 (16/16) 100.0 (19/19) | Setting not stated, USA | Both | Culture | 5.5 | |
| Mogabgab46 | 1994 | CTX 500 mg CRO 250 mg | 100.0 (3/3) 100.0 (3/3) | STI Clinic, USA | Both | Culture | 5.5 | |
| Mohanty60 | 1988 | ATM 1 g | 100.0 (8/8) | Hospital, UK | Both | Culture | 14 | |
| Mroczkowski47 | 1997 | CFM 400 mg | 97.4 (37/38) | STI Clinic, USA | F | Culture | 7.5 | CFM 400 mg N: 2.1% (4/189) V: 1.1% (2/189) D: 0.5% (1/189) |
| Obaid48 | 1983 | PPEN 4.8 MU (×2 doses)+PROB 1 g | 100.0 (4/4) | STI Clinic, USA | M | Culture | 5 | |
| Pabst49 | 1989 | CRO 250 mg | 100.0 (5/5) | STI Clinic, USA | Both | Culture | 8.5 | |
| Raad58 | 1988 | PPEN 4.8 MU+PROB 1 g | 100.0 (1/1) | STI Clinic, USA and Peru | Both | Culture | 5 | |
| Ramus50 | 2001 | CRO 125 mg CFM 400 mg | 100.0 (23/23) 100.0 (16/16) | Hospital, USA | F (pregnant females only) | Culture | 7 | |
| Rob66 | 2019 | GEN 240 mg+AZM 2 g CRO 500 mg+AZM 2 g | 100.0 (40/40) 100.0 (38/38) | Hospital, Czech Republic | Both | NAAT | 10 | GEN 240 mg+AZM 2 g N: 19.4% (14/72) V: 0.0% (0/72) D: 23.6% (17/72) CRO 500 mg+AZM 2 g N: 26.8% (19/71) V: 1.4% (1/71) D: 26.8% (19/71) |
| Romanowski65 | 1984 | AMP 3.5 g+PROB 1 g | 100.0 (23/23) | Setting not stated, Canada | Both | Culture | 5.5 | |
| Ross61 | 2019 | GEN 240 mg+AZM 1 g CRO 500 mg+AZM 1 g | 89.9 (107/119) 97.8 (134/137) | STI Clinic, UK | Both | Culture+NAAT | 14 | GEN 240 mg+AZM 1 g N: 13.8% (41/298) V: 4.0% (12/298) D: NA CRO 500 mg+AZM 1 g N: 11.9% (38/320) V: 0.9% (3/320) D: NA |
| Scott62 | 1987 | CIP 250 mg AMP 500 mg q6h for 5/7+PROB 1 g | 100.0 (3/3) 100.0 (3/3) | Hospital, UK | M | Culture | 14 | |
| Simpson51 | 1981 | PPEN 4.8 MU+PROB 1 g CTX 1 g | 100.0 (9/9) 100.0 (9/9) | STI Clinic, USA | Both | Culture | 5 | |
| Simpson52 | 1982 | PIP 2 g+PROB 1 g PPEN 4.8 MU+PROB 1 g | 100.0 (1/1) 100.0 (4/4) | STI Clinic, USA | M | Culture | 5 | |
| Sinanian53 | 1973 | SPT 4 g TET 9 g | 98.2 (54/55) 93.0 (53/57) | STI Clinic, USA | F | Culture | 7 | SPT 4 g, NA TET 9 g N: 3.0% (6/198) V: NA D: NA |
| Slutkin54 | 1982 | PPEN 4.8 MU+PROB 1 g | 87.5 (14/16) | STI Clinic, USA | Both | Culture | 6 | |
| Smith55 | 1993 | CRO 250 mg | 100.0 (24/24) | STI Clinic, USA | F | Culture | 7 | CRO 250 mg N: 0.3% (1/380) V: NA D: 0.5% (2/380) |
| Stolz67 | 1984 | CTX 1 g CFX 1.5 g | 100.0 (103/103) 92.2 (71/77) | Setting not stated, The Netherlands | Both | Culture | 10.5 | CTX 1 g N: 0.3% (2/590) V: NA D: NA CFX 1.5 g N: 0.3% (2/647) V: NA D: NA |
| Stoner56 | 2001 | GAT 400 mg GAT 600 mg OFX 400 mg | 100.0 (20/20) 100.0 (16/16) 100.0 (7/7) | Setting not stated, USA | F | Culture | 7 | GAT 400 mg N: 8.1% (24/295) V: 0.7% (2/295) D: 2.0% (6/295) GAT 600 mg N: 10.7% (31/291) V: 1.7% (5/291) D: 3.4% (10/291) OFX 400 mg N: 4.2% (6/142) V: 1.4% (2/142) D: 2.8% (4/142) |
| Taylor18 | 2018 | GEP 1500 mg GEP 3000 mg | 100.0 (1/1) 100.0 (2/2) | Setting not stated, USA and UK | M | Culture+NAAT | 6 | GEP 1500 mg N: 5.8% (3/52) V: NA D: 17.3% (9/52) GEP 3000 mg N: 20.8% (11/53) V: NA D: 35.8% (19/53) |
| Taylor20 | 2018 | ZOL 2 g ZOL 3 g CRO 500 mg | 100.0 (4/4) 100.0 (6/6) 100.0 (3/3) | STI Clinic, USA | Both | Culture+NAAT | 6 | |
| Thorpe57 | 1996 | CFX 1000 mg CIP 500 mg | 96.8 (30/31) 100.0 (26/26) | STI Clinic, USA and Puerto Rico | Both | Culture | 6 |
AMX, amoxicillin; AMP, ampicillin; AZM, azithromycin; ATM, aztreonam; BPEN, benzylpenicillin; CRO, ceftriaxone; CFX, cefuroxime axetil/sodium; CFM, cefixime; FOX, cefoxitin; CIP, ciprofloxacin; CTX, cefotaxime; DEL, delafloxacin; GAT, gatifloxacin; GEM, Gemifloxacin; GEN, gentamicin; GEP, gepotidacin; OFX, ofloxacin; PIP, piperacillin; PPEN, procaine penicillin; PROB, probenecid; SOL, solithromycin; SPT, spectinomycin; SUL, sulbactam; TET, tetracycline; ZOL, zoliflodacin; NA, not available.
Gender of participants in the trial who contributed to the rectal endpoint.
Side effects are nausea (N), vomiting (V) or diarrhoea (D) among all participants and not specifically among those with rectal infection.
Study characteristics
Among the 54 included studies, 35 (64.8%) studies were conducted in the USA,17,20,24–56 one (1.9%) in both USA and Australia,19 one (1.9%) in both USA and UK,18 one (1.9%) in both USA and Puerto Rico,57 one (1.9%) in both USA and Peru,58 four (7.4%) in the UK only,59–62 2 (3.7%) in Finland,63,64 one (1.9%) in Canada,65 one (1.9%) in the Czech Republic,66 one (1.9%) in the Netherlands,67 and for six (11.1%) studies, the country was not specified.68–73 A total of 37 (68.5%) studies were based in STI clinics,19,20,24–27,29–44,46–49,51–55,57–59,61,70,71 eight (14.8%) were hospital-based,28,50,60,62–64,66,72 and nine (16.7%) did not specify their setting.17,18,45,56,65,67–69,73 Ten (18.5%) studies included rectal data from men only,18,34,39,41,48,52,62,70–72 16 (29.6%) had data from women only,25–27,29,30,37,38,44,47,53,55,56,63,64,69,73 26 (48.1%) had data from both men and women,17,19,20,24,31–33,35,36,40,42,43,45,46,49,51,54,57–61,65–68 and two (3.7%) studies included pregnant women only.28,50 Overall, a total of 1813 participants and 44 treatment regimens were assessed for microbial cure for rectal NG with 19 (35.2%) studies comparing monotherapy with dual therapy,19,24–28,34,42,48,51,54,58–60,62,65,68,69,73 32 (59.3%) assessing the efficacy of monotherapies only,17,18,20,29–33,35–41,44–47,49,50,52,53,55–57,63,64,67,70–72 and three (5.6%) assessed dual therapies.43,61,66 Previously trialled treatments were assessed in 32 (59.3%) studies,24–28,30,32,34,35,40,42–46,48,51–54,56–59,62–65,67–69,73 current treatments in 32 (59.3%) studies,17,19,20,24,28–31,33,36–41,43,45–47,49,50,53,55,57,59,61,62,66,70–73 and emerging treatments in six (11.1%) studies.17–20,33,60 The duration of follow up post treatment ranged from 5 to 16 days (median of 6 days). The diagnostic methods for the test of cure were culture only in 48 studies,24–60,62–65,67–73 NAAT only in one study,66 and both culture and NAAT in five studies.17–20,61 Overall, 19 studies reported nausea, vomiting or diarrhoea data alongside efficacy data.17–19,24,25,28,36,37,40,42,43,47,53,55,56,61,66,67,69
Most of the included studies were from 1981 onwards with the exception of a single paper on spectinomycin from 1973.53
Overall efficacy
The overall summary treatment efficacy for rectal NG was 100.00% (95% CI: 99.89%–100.00%) with low heterogeneity (I2 = 0.00%; P = 0.86) (Figure 2). The treatment efficacy estimates for all studies stratified by treatment regimen are shown in Table S1.
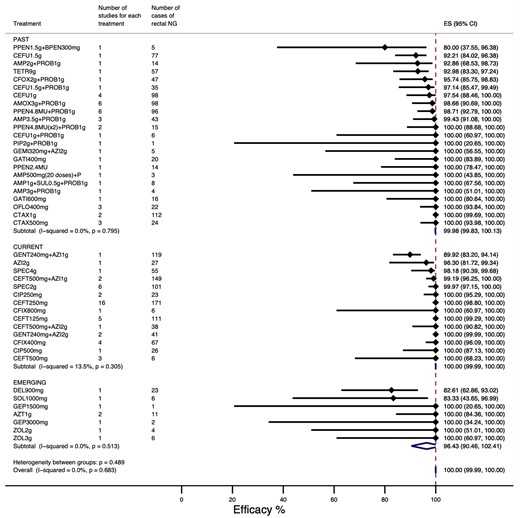
Overall treatment efficacy (lowest to highest efficacy). ES, effect size measured as treatment efficacy, defined as the percentage with microbial cure at follow-up. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Subgroup analyses
Treatment efficacy was higher in those studies using culture to assess microbiological cure with negligible heterogeneity (100.00%; 95% CI: 99.91%–100.00%; I2 = 0.00%; P = 0.95) (Figure S1A) than in those studies using NAAT only (100.00%; 95% CI: 84.82%–100.00%) or culture and NAAT (97.77%; 95% CI: 92.29%–100.00%; I2 = 77.61%; P = 0.00) (Figure S1B).
Summary efficacy estimates for mono or dual therapies were similar and both had low heterogeneity. Summary estimate for monotherapies was 100.00% (95% CI: 99.88%–100.00%; I2 = 0.00%; P = 0.97) (Figure S2A) and for dual therapies it was 100.00% (95% CI: 97.65%–100.00%; I2 = 56.24%; P = 0.03) (Figure S2B).
The summary efficacies for previously trialled treatments and current treatments were 99.91% (95% CI: 99.13%–100.00%, I2 = 0.50%; P = 0.46) (Figure S3A) and 100.00% (95% CI: 99.96%–100.00%; I2 = 0.00%; P = 0.98) (Figure S3B) respectively. The summary efficacies for currently recommended ceftriaxone as monotherapy were 100.00% (95% CI: 99.29%–100.00%, I2 = 0.00%; P = 0.96) for 125 mg dose, 100% (95% CI: 98.80%–100.00%, I2 = 0.00%; P = 0.99) for 250 mg dose and 100.00% (95% CI: 68.23%–100.00%, I2 = 0.00%; P = 0.96) for 500 mg dose. Similarly high efficacies were observed when ceftriaxone was administered as dual therapy with azithromycin (Table S1). The summary efficacy for emerging treatments was 97.16% (95% CI: 86.79%–100.00%; I2 = 0.00%; P = 0.84) (Figure S3C).
Side effects of treatments
Overall, 7.15% (95% CI: 4.14%–10.86%) reported nausea, with high heterogeneity between studies (I2 = 95.98%, P < 0.01). Nausea was higher for emerging treatments and dual therapies with azithromycin 2 g (Figure S4). Vomiting was reported with a summary estimate of 1.99% (95% CI: 1.17%–3.00%) with high heterogeneity between studies (I2 = 72.01%, P < 0.01) (Figure S5). Overall, 9.42% (95% CI: 5.59%–14.08%) reported diarrhoea, with high heterogeneity between studies (I2 = 95.22%, P < 0.01). Diarrhoea was more commonly seen with emerging treatments and dual therapies with azithromycin 2 g (Figure S6).
Risk of bias in studies
Overall, the studies had low to medium bias with deviation from the allocated treatment being the greatest issue. Sample size varied considerably across studies ranging from 1 to 137, with 29 studies having <20 cases of rectal NG included in the analysis.18–20,24,26,30–34,40,42,43,46,48,49,51,52,54,58–60,62–64,68,69,71,72 Rectal NG was a secondary outcome in all studies and no studies stratified randomization by infection site, meaning confounding cannot be excluded from the results and contributing moderate bias (Table 2 and Table S2).
| Author, year . | Bias from randomization . | Deviation from allocated treatment . | Missing outcome data . | Measure ment bias . | Selection of the reported result . | Sample size bias . | Overall risk . |
|---|---|---|---|---|---|---|---|
| Baddour, 198925 | Low to medium | High | Unclear | Low | Unclear | Low | Low to medium |
| Baddour, 199224 | Low to medium | Unclear | Unclear | Low | Unclear | High | Low to medium |
| Batteiger, 198526 | Low to medium | Low | Unclear | Low | Unclear | Medium | Low |
| Black, 198927 | Low | High | Unclear | Low | Unclear | Medium | Low to medium |
| Cavenee, 199328 | Low | High | Unclear | Low | Unclear | Low to medium | Low to medium |
| Chen, 201919 | Low | High | Medium | Low | Low | Medium to high | Medium |
| Collier, 198429 | Low | Low to medium | Unclear | Low | Unclear | Low to medium | Low to medium |
| Covino, 199331 | Low to medium | High | Unclear | Low | Unclear | High | Medium |
| Covino, 199030 | Low to medium | High | Unclear | Low | Unclear | High | Medium |
| Das, 198968 | Low | Unclear | Unclear | Low | Unclear | High | Low to medium |
| Dixon, 198659 | Medium | Unclear | Unclear | Low | Unclear | High | Medium |
| Dubois, 199069 | Low | Low | Unclear | Low | Unclear | High | Medium |
| Edwards, 198432 | Low | Low to medium | Low | Low | Unclear | High | Low to medium |
| Forstrom, 197264 | Low to medium | High | Low | Low | Unclear | Medium | Medium |
| Forstrom, 197463 | Low to medium | Unclear | High | Low | Unclear | Medium | Medium |
| Gottlieb, 198533 | Low to medium | Medium | Unclear | Low | Unclear | High | Medium |
| Gottlieb, 198634 | Low to medium | Medium | Unclear | Low | Unclear | High | Medium |
| Greaves, 198335 | Low to medium | Unclear | High | Low | Unclear | Low | Low to medium |
| Handsfield, 198171 | Low | Medium | Unclear | Low | Unclear | High | Medium |
| Handsfield, 198370 | Low | Medium | Unclear | Low | Unclear | Medium | Low to medium |
| Handsfield, 199137 | Medium | High | Unclear | Low | Unclear | High | Medium |
| Handsfield, 199436 | Low | High | Unclear | Low | Unclear | Low to medium | Low to medium |
| Hook, 198639 | Low to medium | Unclear | Unclear | Low | Unclear | Low | Low |
| Hook, 199338 | Low to medium | Low | Unclear | Low | Unclear | Low | Low to medium |
| Hook, 199772 | Low to medium | High | Unclear | Low | Unclear | High | Low to medium |
| Hook, 201917 | Low | High | Unclear | Low | Low | Low to medium | Low to medium |
| Jones, 199140 | Low | Unclear | Unclear | Low | Unclear | High | Low to medium |
| Judson, 198373 | Low | Unclear | Unclear | Low | Unclear | High | Low to medium |
| Judson, 198541 | Low | Unclear | Unclear | Low | Unclear | Medium | Low to medium |
| Kaplowitz, 198742 | Low | Unclear | Unclear | Low | Unclear | High | Low to medium |
| Kirkcaldy, 201443 | Low | High | Unclear | Low | Unclear | High | Low to medium |
| Lossick, 198244 | Low | Low | Unclear | Low | Unclear | Low | Low |
| McCormack, 199345 | Low to medium | Unclear | Unclear | Low | Unclear | Medium | Low to medium |
| Mogabgab, 199446 | Low to medium | Unclear | Unclear | Low | Unclear | High | Low to medium |
| Mohanty, 198860 | Low | Unclear | Unclear | Low | Unclear | High | Low to medium |
| Mroczkowski, 199747 | Low | High | Unclear | Low | Unclear | Low | Low to medium |
| Obaid, 198348 | Low | High | High | Low | Unclear | High | Medium |
| Pabst, 198949 | Low | High | Unclear | Low | Unclear | High | Low to medium |
| Raad, 198858 | Low | Low | Unclear | Low | Unclear | High | Low to medium |
| Ramus, 200150 | High | High | Unclear | Low | Unclear | Low to medium | Medium to high |
| Rob, 201966 | Low | High | Unclear | Low | Unclear | Low | Low to medium |
| Romanowski, 198465 | Low | High | Unclear | Low | Unclear | Low | Low to medium |
| Ross, 201961 | Low | Low to medium | Low | Low | Low | Low | Low to medium |
| Scott, 198762 | Low | Unclear | Unclear | Low | Unclear | High | Low to medium |
| Simpson, 198151 | Low | High | Unclear | Low | Unclear | High | Medium |
| Simpson, 198252 | Low | Unclear | Unclear | Low | Unclear | High | Low to medium |
| Sinanian, 197353 | Low to medium | High | Unclear | Low | Unclear | Low | Low to medium |
| Slutkin, 198254 | Low to medium | Medium | Unclear | Low | Unclear | Medium | Low to medium |
| Smith, 199355 | Low to medium | High | Unclear | Low | Unclear | Low | Low to medium |
| Stolz, 198467 | Low to medium | Unclear | Unclear | Low | Unclear | Low | Low to medium |
| Stoner, 200156 | Low | Low | Unclear | Low | Unclear | Medium to high | Low to medium |
| Taylor, 201818 | Low to medium | High | Unclear | Low | Unclear | High | Low to medium |
| Taylor, 201820 | Low | High | Medium to high | Low | Low | High | Medium |
| Thorpe, 199657 | Low | Medium | Unclear | Low | Unclear | Low | Low to medium |
| Author, year . | Bias from randomization . | Deviation from allocated treatment . | Missing outcome data . | Measure ment bias . | Selection of the reported result . | Sample size bias . | Overall risk . |
|---|---|---|---|---|---|---|---|
| Baddour, 198925 | Low to medium | High | Unclear | Low | Unclear | Low | Low to medium |
| Baddour, 199224 | Low to medium | Unclear | Unclear | Low | Unclear | High | Low to medium |
| Batteiger, 198526 | Low to medium | Low | Unclear | Low | Unclear | Medium | Low |
| Black, 198927 | Low | High | Unclear | Low | Unclear | Medium | Low to medium |
| Cavenee, 199328 | Low | High | Unclear | Low | Unclear | Low to medium | Low to medium |
| Chen, 201919 | Low | High | Medium | Low | Low | Medium to high | Medium |
| Collier, 198429 | Low | Low to medium | Unclear | Low | Unclear | Low to medium | Low to medium |
| Covino, 199331 | Low to medium | High | Unclear | Low | Unclear | High | Medium |
| Covino, 199030 | Low to medium | High | Unclear | Low | Unclear | High | Medium |
| Das, 198968 | Low | Unclear | Unclear | Low | Unclear | High | Low to medium |
| Dixon, 198659 | Medium | Unclear | Unclear | Low | Unclear | High | Medium |
| Dubois, 199069 | Low | Low | Unclear | Low | Unclear | High | Medium |
| Edwards, 198432 | Low | Low to medium | Low | Low | Unclear | High | Low to medium |
| Forstrom, 197264 | Low to medium | High | Low | Low | Unclear | Medium | Medium |
| Forstrom, 197463 | Low to medium | Unclear | High | Low | Unclear | Medium | Medium |
| Gottlieb, 198533 | Low to medium | Medium | Unclear | Low | Unclear | High | Medium |
| Gottlieb, 198634 | Low to medium | Medium | Unclear | Low | Unclear | High | Medium |
| Greaves, 198335 | Low to medium | Unclear | High | Low | Unclear | Low | Low to medium |
| Handsfield, 198171 | Low | Medium | Unclear | Low | Unclear | High | Medium |
| Handsfield, 198370 | Low | Medium | Unclear | Low | Unclear | Medium | Low to medium |
| Handsfield, 199137 | Medium | High | Unclear | Low | Unclear | High | Medium |
| Handsfield, 199436 | Low | High | Unclear | Low | Unclear | Low to medium | Low to medium |
| Hook, 198639 | Low to medium | Unclear | Unclear | Low | Unclear | Low | Low |
| Hook, 199338 | Low to medium | Low | Unclear | Low | Unclear | Low | Low to medium |
| Hook, 199772 | Low to medium | High | Unclear | Low | Unclear | High | Low to medium |
| Hook, 201917 | Low | High | Unclear | Low | Low | Low to medium | Low to medium |
| Jones, 199140 | Low | Unclear | Unclear | Low | Unclear | High | Low to medium |
| Judson, 198373 | Low | Unclear | Unclear | Low | Unclear | High | Low to medium |
| Judson, 198541 | Low | Unclear | Unclear | Low | Unclear | Medium | Low to medium |
| Kaplowitz, 198742 | Low | Unclear | Unclear | Low | Unclear | High | Low to medium |
| Kirkcaldy, 201443 | Low | High | Unclear | Low | Unclear | High | Low to medium |
| Lossick, 198244 | Low | Low | Unclear | Low | Unclear | Low | Low |
| McCormack, 199345 | Low to medium | Unclear | Unclear | Low | Unclear | Medium | Low to medium |
| Mogabgab, 199446 | Low to medium | Unclear | Unclear | Low | Unclear | High | Low to medium |
| Mohanty, 198860 | Low | Unclear | Unclear | Low | Unclear | High | Low to medium |
| Mroczkowski, 199747 | Low | High | Unclear | Low | Unclear | Low | Low to medium |
| Obaid, 198348 | Low | High | High | Low | Unclear | High | Medium |
| Pabst, 198949 | Low | High | Unclear | Low | Unclear | High | Low to medium |
| Raad, 198858 | Low | Low | Unclear | Low | Unclear | High | Low to medium |
| Ramus, 200150 | High | High | Unclear | Low | Unclear | Low to medium | Medium to high |
| Rob, 201966 | Low | High | Unclear | Low | Unclear | Low | Low to medium |
| Romanowski, 198465 | Low | High | Unclear | Low | Unclear | Low | Low to medium |
| Ross, 201961 | Low | Low to medium | Low | Low | Low | Low | Low to medium |
| Scott, 198762 | Low | Unclear | Unclear | Low | Unclear | High | Low to medium |
| Simpson, 198151 | Low | High | Unclear | Low | Unclear | High | Medium |
| Simpson, 198252 | Low | Unclear | Unclear | Low | Unclear | High | Low to medium |
| Sinanian, 197353 | Low to medium | High | Unclear | Low | Unclear | Low | Low to medium |
| Slutkin, 198254 | Low to medium | Medium | Unclear | Low | Unclear | Medium | Low to medium |
| Smith, 199355 | Low to medium | High | Unclear | Low | Unclear | Low | Low to medium |
| Stolz, 198467 | Low to medium | Unclear | Unclear | Low | Unclear | Low | Low to medium |
| Stoner, 200156 | Low | Low | Unclear | Low | Unclear | Medium to high | Low to medium |
| Taylor, 201818 | Low to medium | High | Unclear | Low | Unclear | High | Low to medium |
| Taylor, 201820 | Low | High | Medium to high | Low | Low | High | Medium |
| Thorpe, 199657 | Low | Medium | Unclear | Low | Unclear | Low | Low to medium |
| Author, year . | Bias from randomization . | Deviation from allocated treatment . | Missing outcome data . | Measure ment bias . | Selection of the reported result . | Sample size bias . | Overall risk . |
|---|---|---|---|---|---|---|---|
| Baddour, 198925 | Low to medium | High | Unclear | Low | Unclear | Low | Low to medium |
| Baddour, 199224 | Low to medium | Unclear | Unclear | Low | Unclear | High | Low to medium |
| Batteiger, 198526 | Low to medium | Low | Unclear | Low | Unclear | Medium | Low |
| Black, 198927 | Low | High | Unclear | Low | Unclear | Medium | Low to medium |
| Cavenee, 199328 | Low | High | Unclear | Low | Unclear | Low to medium | Low to medium |
| Chen, 201919 | Low | High | Medium | Low | Low | Medium to high | Medium |
| Collier, 198429 | Low | Low to medium | Unclear | Low | Unclear | Low to medium | Low to medium |
| Covino, 199331 | Low to medium | High | Unclear | Low | Unclear | High | Medium |
| Covino, 199030 | Low to medium | High | Unclear | Low | Unclear | High | Medium |
| Das, 198968 | Low | Unclear | Unclear | Low | Unclear | High | Low to medium |
| Dixon, 198659 | Medium | Unclear | Unclear | Low | Unclear | High | Medium |
| Dubois, 199069 | Low | Low | Unclear | Low | Unclear | High | Medium |
| Edwards, 198432 | Low | Low to medium | Low | Low | Unclear | High | Low to medium |
| Forstrom, 197264 | Low to medium | High | Low | Low | Unclear | Medium | Medium |
| Forstrom, 197463 | Low to medium | Unclear | High | Low | Unclear | Medium | Medium |
| Gottlieb, 198533 | Low to medium | Medium | Unclear | Low | Unclear | High | Medium |
| Gottlieb, 198634 | Low to medium | Medium | Unclear | Low | Unclear | High | Medium |
| Greaves, 198335 | Low to medium | Unclear | High | Low | Unclear | Low | Low to medium |
| Handsfield, 198171 | Low | Medium | Unclear | Low | Unclear | High | Medium |
| Handsfield, 198370 | Low | Medium | Unclear | Low | Unclear | Medium | Low to medium |
| Handsfield, 199137 | Medium | High | Unclear | Low | Unclear | High | Medium |
| Handsfield, 199436 | Low | High | Unclear | Low | Unclear | Low to medium | Low to medium |
| Hook, 198639 | Low to medium | Unclear | Unclear | Low | Unclear | Low | Low |
| Hook, 199338 | Low to medium | Low | Unclear | Low | Unclear | Low | Low to medium |
| Hook, 199772 | Low to medium | High | Unclear | Low | Unclear | High | Low to medium |
| Hook, 201917 | Low | High | Unclear | Low | Low | Low to medium | Low to medium |
| Jones, 199140 | Low | Unclear | Unclear | Low | Unclear | High | Low to medium |
| Judson, 198373 | Low | Unclear | Unclear | Low | Unclear | High | Low to medium |
| Judson, 198541 | Low | Unclear | Unclear | Low | Unclear | Medium | Low to medium |
| Kaplowitz, 198742 | Low | Unclear | Unclear | Low | Unclear | High | Low to medium |
| Kirkcaldy, 201443 | Low | High | Unclear | Low | Unclear | High | Low to medium |
| Lossick, 198244 | Low | Low | Unclear | Low | Unclear | Low | Low |
| McCormack, 199345 | Low to medium | Unclear | Unclear | Low | Unclear | Medium | Low to medium |
| Mogabgab, 199446 | Low to medium | Unclear | Unclear | Low | Unclear | High | Low to medium |
| Mohanty, 198860 | Low | Unclear | Unclear | Low | Unclear | High | Low to medium |
| Mroczkowski, 199747 | Low | High | Unclear | Low | Unclear | Low | Low to medium |
| Obaid, 198348 | Low | High | High | Low | Unclear | High | Medium |
| Pabst, 198949 | Low | High | Unclear | Low | Unclear | High | Low to medium |
| Raad, 198858 | Low | Low | Unclear | Low | Unclear | High | Low to medium |
| Ramus, 200150 | High | High | Unclear | Low | Unclear | Low to medium | Medium to high |
| Rob, 201966 | Low | High | Unclear | Low | Unclear | Low | Low to medium |
| Romanowski, 198465 | Low | High | Unclear | Low | Unclear | Low | Low to medium |
| Ross, 201961 | Low | Low to medium | Low | Low | Low | Low | Low to medium |
| Scott, 198762 | Low | Unclear | Unclear | Low | Unclear | High | Low to medium |
| Simpson, 198151 | Low | High | Unclear | Low | Unclear | High | Medium |
| Simpson, 198252 | Low | Unclear | Unclear | Low | Unclear | High | Low to medium |
| Sinanian, 197353 | Low to medium | High | Unclear | Low | Unclear | Low | Low to medium |
| Slutkin, 198254 | Low to medium | Medium | Unclear | Low | Unclear | Medium | Low to medium |
| Smith, 199355 | Low to medium | High | Unclear | Low | Unclear | Low | Low to medium |
| Stolz, 198467 | Low to medium | Unclear | Unclear | Low | Unclear | Low | Low to medium |
| Stoner, 200156 | Low | Low | Unclear | Low | Unclear | Medium to high | Low to medium |
| Taylor, 201818 | Low to medium | High | Unclear | Low | Unclear | High | Low to medium |
| Taylor, 201820 | Low | High | Medium to high | Low | Low | High | Medium |
| Thorpe, 199657 | Low | Medium | Unclear | Low | Unclear | Low | Low to medium |
| Author, year . | Bias from randomization . | Deviation from allocated treatment . | Missing outcome data . | Measure ment bias . | Selection of the reported result . | Sample size bias . | Overall risk . |
|---|---|---|---|---|---|---|---|
| Baddour, 198925 | Low to medium | High | Unclear | Low | Unclear | Low | Low to medium |
| Baddour, 199224 | Low to medium | Unclear | Unclear | Low | Unclear | High | Low to medium |
| Batteiger, 198526 | Low to medium | Low | Unclear | Low | Unclear | Medium | Low |
| Black, 198927 | Low | High | Unclear | Low | Unclear | Medium | Low to medium |
| Cavenee, 199328 | Low | High | Unclear | Low | Unclear | Low to medium | Low to medium |
| Chen, 201919 | Low | High | Medium | Low | Low | Medium to high | Medium |
| Collier, 198429 | Low | Low to medium | Unclear | Low | Unclear | Low to medium | Low to medium |
| Covino, 199331 | Low to medium | High | Unclear | Low | Unclear | High | Medium |
| Covino, 199030 | Low to medium | High | Unclear | Low | Unclear | High | Medium |
| Das, 198968 | Low | Unclear | Unclear | Low | Unclear | High | Low to medium |
| Dixon, 198659 | Medium | Unclear | Unclear | Low | Unclear | High | Medium |
| Dubois, 199069 | Low | Low | Unclear | Low | Unclear | High | Medium |
| Edwards, 198432 | Low | Low to medium | Low | Low | Unclear | High | Low to medium |
| Forstrom, 197264 | Low to medium | High | Low | Low | Unclear | Medium | Medium |
| Forstrom, 197463 | Low to medium | Unclear | High | Low | Unclear | Medium | Medium |
| Gottlieb, 198533 | Low to medium | Medium | Unclear | Low | Unclear | High | Medium |
| Gottlieb, 198634 | Low to medium | Medium | Unclear | Low | Unclear | High | Medium |
| Greaves, 198335 | Low to medium | Unclear | High | Low | Unclear | Low | Low to medium |
| Handsfield, 198171 | Low | Medium | Unclear | Low | Unclear | High | Medium |
| Handsfield, 198370 | Low | Medium | Unclear | Low | Unclear | Medium | Low to medium |
| Handsfield, 199137 | Medium | High | Unclear | Low | Unclear | High | Medium |
| Handsfield, 199436 | Low | High | Unclear | Low | Unclear | Low to medium | Low to medium |
| Hook, 198639 | Low to medium | Unclear | Unclear | Low | Unclear | Low | Low |
| Hook, 199338 | Low to medium | Low | Unclear | Low | Unclear | Low | Low to medium |
| Hook, 199772 | Low to medium | High | Unclear | Low | Unclear | High | Low to medium |
| Hook, 201917 | Low | High | Unclear | Low | Low | Low to medium | Low to medium |
| Jones, 199140 | Low | Unclear | Unclear | Low | Unclear | High | Low to medium |
| Judson, 198373 | Low | Unclear | Unclear | Low | Unclear | High | Low to medium |
| Judson, 198541 | Low | Unclear | Unclear | Low | Unclear | Medium | Low to medium |
| Kaplowitz, 198742 | Low | Unclear | Unclear | Low | Unclear | High | Low to medium |
| Kirkcaldy, 201443 | Low | High | Unclear | Low | Unclear | High | Low to medium |
| Lossick, 198244 | Low | Low | Unclear | Low | Unclear | Low | Low |
| McCormack, 199345 | Low to medium | Unclear | Unclear | Low | Unclear | Medium | Low to medium |
| Mogabgab, 199446 | Low to medium | Unclear | Unclear | Low | Unclear | High | Low to medium |
| Mohanty, 198860 | Low | Unclear | Unclear | Low | Unclear | High | Low to medium |
| Mroczkowski, 199747 | Low | High | Unclear | Low | Unclear | Low | Low to medium |
| Obaid, 198348 | Low | High | High | Low | Unclear | High | Medium |
| Pabst, 198949 | Low | High | Unclear | Low | Unclear | High | Low to medium |
| Raad, 198858 | Low | Low | Unclear | Low | Unclear | High | Low to medium |
| Ramus, 200150 | High | High | Unclear | Low | Unclear | Low to medium | Medium to high |
| Rob, 201966 | Low | High | Unclear | Low | Unclear | Low | Low to medium |
| Romanowski, 198465 | Low | High | Unclear | Low | Unclear | Low | Low to medium |
| Ross, 201961 | Low | Low to medium | Low | Low | Low | Low | Low to medium |
| Scott, 198762 | Low | Unclear | Unclear | Low | Unclear | High | Low to medium |
| Simpson, 198151 | Low | High | Unclear | Low | Unclear | High | Medium |
| Simpson, 198252 | Low | Unclear | Unclear | Low | Unclear | High | Low to medium |
| Sinanian, 197353 | Low to medium | High | Unclear | Low | Unclear | Low | Low to medium |
| Slutkin, 198254 | Low to medium | Medium | Unclear | Low | Unclear | Medium | Low to medium |
| Smith, 199355 | Low to medium | High | Unclear | Low | Unclear | Low | Low to medium |
| Stolz, 198467 | Low to medium | Unclear | Unclear | Low | Unclear | Low | Low to medium |
| Stoner, 200156 | Low | Low | Unclear | Low | Unclear | Medium to high | Low to medium |
| Taylor, 201818 | Low to medium | High | Unclear | Low | Unclear | High | Low to medium |
| Taylor, 201820 | Low | High | Medium to high | Low | Low | High | Medium |
| Thorpe, 199657 | Low | Medium | Unclear | Low | Unclear | Low | Low to medium |
Discussion
This review evaluated treatment efficacy specifically for rectal NG infection and included 1813 cases assessing 44 treatment regimens and our meta-analysis found an overall treatment efficacy of 100.00% (95% CI: 99.89%–100.00%; I2 = 0.00%; P = 0.86) with low heterogeneity. The caveat to this statement is that these efficacies are accurate at the time of publication and antimicrobial resistance may have occurred since the trials had been conducted.
We found that regimens containing the currently recommended ceftriaxone, as mono or dual therapy, were highly efficacious in treating rectal NG. Ceftriaxone monotherapy and dual therapy meet the CDC’s criteria of acceptable efficacy criteria of ≥95% efficacy with a lower CI of ≥90%.11 This suggests dual therapy may not be required for rectal NG when pharyngeal infection has been excluded.
It is contentious whether dual therapy with ceftriaxone and azithromycin is warranted as first line treatment for rectal NG. Increasing the ceftriaxone dose has remained a successful strategy to address rising MICs.74 For instance, the UK has seen an upward trend in MICs (with the percentage of isolates with MICs ≥0.03 mg/L having increased from 16.60% in 2017 to 24.60% in 2018) and now recommends monotherapy ceftriaxone 1 g.7 Additionally, the use of dual therapy is predicated on NG being susceptible to azithromycin and surveillance data has shown that azithromycin resistance has increased.75 Maintaining susceptibility to azithromycin should be a priority as it could be used as a second line treatment in the event of widespread ceftriaxone failure. On balance, it appears that ceftriaxone as dual or monotherapy is effective but guidelines recommending monotherapy are preferable for antimicrobial stewardship.
We did not identify any RCTs for alternative regimens such as combination cefixime/azithromycin. This distinct literature gap disproportionally disadvantages low-resource settings as cefixime is an important oral alternative to ceftriaxone, which requires a skilled healthcare worker to administer. The UK guidelines recommend cefixime 400 mg/azithromycin 2 g as alternatives to first line therapy,5 however there remains concern regarding monotherapy with cefixime to select for both cefixime and ceftriaxone resistance.76 In addition, it remains uncertain what the role of cefixime is when we found that monotherapy with azithromycin 2 g can be highly effective against rectal NG.36,66 This degree of uncertainty surrounding dual therapy cefixime/azithromycin emphasizes the need for further studies investigating the role of cefixime.
Given rising concern regarding the future efficacy of current treatments, attention has turned towards the performance of novel drugs in Phase 2/3 trials, which this review has shown do not look promising at these regimens. Treatments with solithromycin, delafloxacin, gepotidacin, and zoliflodacin all failed to reach the CDC standard for acceptable efficacy. A trial comparing solithromycin 1000 mg against ceftriaxone 500 mg/azithromycin 1 g showed efficacy rates of 83.3% (5/6) and 100.0% (12/12) respectively for treating rectal infections.19 Similarly, a trial comparing delafloxacin 900 mg against ceftriaxone 250 mg reported efficacies of 82.6% (19/23) and 100.0% (13/13), respectively.17 Further studies are necessary to draw reliable conclusions regarding efficacy as both studies had small rectal sample sizes.17,19 A possible way to increase the performance of solithromycin would be to increase its dose, yet this may not be feasible as higher doses attract greater side effects of diarrhoea and vomiting as seen in a Phase 2 trial.77 Likewise, the delafloxacin trial also had consistently higher side effect rates for delafloxacin than for ceftriaxone.17 Trials with gepotidacin 1500 mg (1/1), gepotidacin 3000 mg (2/2), zoliflodacin 2 g (4/4), zoliflodacin 3 g (6/6) both demonstrated 100% efficacy rates against rectal infections, however, the interpretation of these studies is limited by their small sample sizes and will require further RCTs to robustly estimate the true efficacy of these new drugs.18,20 Similar to the other emerging drugs, gepotidacin will likely face issues of balancing increasing side effect profiles with increasing doses as diarrhoea and nausea rates were higher for the 3000 mg gepotidacin regimen (20.8% and 35.8% respectively) compared with the 1500 mg dose (5.8% and 17.3% respectively).18
Given ongoing concern about the efficacy of drugs currently in clinical trials, there is renewed interest in utilizing previously effective antibiotics for NG where there is known susceptibility data. Spectinomycin was extensively used during the 1970s and 1980s.11 However, high levels of resistance developed and use of the drug was discontinued.11 Resistance levels eventually decreased and spectinomycin 2 g is now recommended by the WHO if there is susceptibility to it in the local region.5 Spectinomycin 2 g demonstrated a summary estimate of 99.97% (95% CI: 97.15%–100.0%; I2 = 0.0%; P = 0.89) in our review. This estimate only reflects the drug’s historically high efficacy as all the spectinomycin 2 g trials were published before 1993.28,29,33,39,41,70 Given its prior effectiveness, it may be an appropriate treatment option if systems are in place for resistance-guided therapy. This is especially crucial for spectinomycin, as resistance can develop quickly with a single-step mutation.11
Another example of adopting historically effective antibiotics in the context of resistance-guided therapy is ciprofloxacin. Ciprofloxacin 250 mg has a summary estimate of 100% (95% CI: 95.29%–100%) based on trials conducted prior to 2000. Ciprofloxacin experienced a similar fate to spectinomycin where widespread resistance developed and it was then discontinued. It has now resurfaced as a first-line treatment in the UK if antimicrobial susceptibility is shown.7,11 Selective use of ciprofloxacin can mitigate NG resistance to first line dual therapy, and also has the additional advantage over spectinomycin of being oral in formulation which is more useful in resource-poor areas.
This review had several strengths. It captured all RCTs assessing treatment efficacy specifically for rectal NG. Additionally, the review not only considered the evidence for current recommended treatments, but it also compared more recent trials of emerging treatments and novel drug combinations with first line treatments. The review also had several limitations. Firstly, many trials had small sample sizes that limited reliable conclusions to be drawn regarding efficacy. Secondly, treatments were not randomly allocated by infection site. Thirdly, we were not able to draw conclusions about the current clinical efficacy of some drugs as many of the included trials were older and do not reflect changing resistance patterns. Ideally the monitoring of resistance would be assessed with clinical failure rates, however, MIC data are more easily obtainable. For the most up to date assessment of resistance, MICs need to be monitored and guideline recommendations need to reflect these changes accordingly.
Conclusions
This review has demonstrated that current first line WHO-recommended regimens containing ceftriaxone with or without azithromycin are likely to be effective in treating rectal NG. Ongoing surveillance of ceftriaxone resistance remains even more important today given multiple countries are now recommending ceftriaxone monotherapy. Monotherapy with spectinomycin and ciprofloxacin have been historically efficacious and ciprofloxacin could reasonably be adopted in settings where resistance guided therapies are possible. Emerging drugs have shown variable results but further trials with larger rectal NG sample sizes are needed. These drugs will need to be optimized in order to reach sufficient effectiveness whilst balancing side effects.
Funding
This study was completed as part of routine work for all authors. J.S.H is supported by a NHMRC Senior Research Fellowship (GNT1042907) and F.Y.S.K. by Ideas Grant (APP1181057).
Transparency declarations
F.Y.S.K. holds a consultancy contract with GSK. All other authors have none to declare.
Supplementary data
Figure S1 to S6 and Tables S1 and S2 are available as Supplementary data at JAC Online.
References
WHO. WHO guidelines for the treatment of Neisseria gonorrhoeae.
Australian Sexual Health Alliance. Australian STI management guidelines for use in primary care. http://www.sti.guidelines.org.au/sexually-transmissible-infections/chlamydia#management.
Public Health Agency of Canada. Section 5–6: Canadian Guidelines on Sexually Transmitted Infections – Management and treatment of specific infections – Gonococcal Infections.
US FDA. Drugs@FDA: FDA-Approved Drugs. https://www.accessdata.fda.gov/scripts/cder/daf/.
Author notes
Fabian Yuh Shiong Kong and Jane S. Hocking Joint senior authors.



