-
PDF
- Split View
-
Views
-
Cite
Cite
Javier Eduardo Fernandez, Vincent Perreten, Sybille Schwendener, The novel macrolide resistance genes mef(F) and msr(G) are located on a plasmid in Macrococcus canis and a transposon in Macrococcus caseolyticus, Journal of Antimicrobial Chemotherapy, Volume 76, Issue 1, January 2021, Pages 48–54, https://doi.org/10.1093/jac/dkaa405
Close - Share Icon Share
Abstract
To analyse macrolide resistance in a Macrococcus canis strain isolated from a dog with an ear infection, and determine whether the resistance mechanism is also present in other bacteria, and associated with mobile genetic elements.
The whole genome of M. canis Epi0082 was sequenced using PacBio and Illumina technologies. Novel macrolide resistance determinants were identified through bioinformatic analysis, and functionality was demonstrated by expression in Staphylococcus aureus. Mobile genetic elements containing the novel genes were analysed in silico for strain Epi0082 as well as in other bacterial strains deposited in GenBank.
M. canis Epi0082 contained a 3212 bp operon with the novel macrolide resistance genes mef(F) and msr(G) encoding a efflux protein and an ABC-F ribosomal protection protein, respectively. Cloning in S. aureus confirmed that both genes individually confer resistance to the 14- and 15-membered ring macrolides erythromycin and azithromycin, but not the 16-membered ring macrolide tylosin. A reduced susceptibility to the streptogramin B pristinamycin IA was additionally observed when msr(G) was expressed in S. aureus under erythromycin induction. Epi0082 carried the mef(F)–msr(G) operon together with the chloramphenicol resistance gene fexB in a novel 39 302 bp plasmid pMiCAN82a. The mef(F)–msr(G) operon was also found in macrolide-resistant Macrococcus caseolyticus strains in the GenBank database, but was situated in the chromosome as part of a novel 13 820 bp or 13 894 bp transposon Tn6776.
The identification of mef(F) and msr(G) on different mobile genetic elements in Macrococcus species indicates that these genes hold potential for further dissemination of resistance to the clinically important macrolides in the bacterial population.
Introduction
Macrococcus is a genus of Gram-positive cocci closely related to Staphylococcus.1,2 Previously they were thought to include non-pathogenic species from the skin of mammals;1,3 however, some of them have recently been associated with diseases in animals4–6 and humans.7,Macrococcus has also been considered as a possible reservoir for antimicrobial resistance genes of pathogenic Staphylococcus species.2,8–10
Co-localization of mef(A) variants and msr(D) genes within an operon is well known in Streptococcus species that exhibit resistance against 14- and 15-membered ring macrolides but remain susceptible to 16-membered ring macrolides.11–15 Recently, a distantly related operon carrying the macrolide resistance genes mef(D) and msr(F) was identified in both Macrococcus canis and Staphylococcus aureus.16 Mef proteins belong to the major facilitator superfamily (MFS) proteins involved in macrolide efflux, and Msr proteins are ATP-binding cassette subfamily F (ABC-F) proteins that mediate ribosomal protection against macrolides and in some cases also against streptogramin B.17
Neither the new mef(D) and msr(F) genes nor any of the macrolide resistance genes commonly found in Gram-positive bacteria could be identified in the macrolide- and chloramphenicol-resistant M. canis strain Epi0082 isolated from a dog with otitis externa in Switzerland.4 We therefore conducted a next-generation sequencing (NGS)-based analysis to identify the antibiotic resistance genes in M. canis Epi0082 and tested possible new macrolide resistance genes for functionality in S. aureus. Furthermore, the mobile genetic elements carrying these genes in Epi0082 as well as in other bacterial strains deposited in GenBank were investigated.
Materials and methods
Bacterial strains, vectors and growth conditions
M. canis strain Epi0082 was isolated from a Newfoundland dog with otitis externa in Switzerland and assigned to ST5 in a previous study.4,Escherichia coli DH5α was used for cloning and amplification of pTSSCm-based shuttle plasmids.18,S. aureus strain RN4220 was used as the recipient of the pTSSCm-based constructs and for phenotypic gene expression.19M. canis was routinely cultivated on trypticase soy agar plates containing 5% sheep blood (TSA-SB) (Becton Dickinson Company). E. coli and S. aureus strains were grown either in LB broth with shaking or on LB agar plates. E. coli and S. aureus strains containing pTSSCm-based plasmids [tet(L)] were selected and grown on LB agar containing tetracycline 10 mg/L. All strains were grown under aerobic conditions at 37°C.
NGS, assembly, annotation and bioinformatic analysis
The genome of M. canis Epi0082 was sequenced by both long (Pacific Biosciences) and short reads (HiSeq Illumina, 2 × 150 bp paired-end) technologies. The initial de novo genome assembly was performed with Canu v1.920 using only long reads. This initial draft genome assembly was subsequently used as a scaffold in Unicycler v0.4.421 together with the Illumina short reads in order to obtain high-quality circular contigs.
ORFs were predicted and annotated using Prokka22 and the NCBI Prokaryotic Genome Annotation Pipeline.23 Antibiotic resistance genes were identified using ResFinder v3.224 and by manual BLAST analysis. The hypothetical functions of ORFs of plasmid pMiCAN82a were predicted with BLASTP,25 UniProt26 and PROSITE27,28 databases. For the mef(F)–msr(G) operon, promoter and transcription start sites (TSSs) were identified using BPROM,29 and the the mRNA secondary structure of the operon’s leader region was analysed using RNAfold30 and ARNold31 tools.
A search for mef(F)–msr(G)-like genes was performed against the nucleotide collection (nr/nt) and the whole-genome shotgun contigs (wgs) databases of the NCBI. Evolutionary analysis of the amino acid sequences of Mef and Msr protein was performed using the UPGMA method (Phylogeny test by Bootstrap method, 2000 replications) in MEGA v10.1.532 based on a multisequence alignment executed using MUSCLE33 (Gap open = −2.90, Gap extend = 0.0). The percentage amino acid identity was determined by multiple sequence alignment using ClustalO.34
Gene structures were visualized using DNAPlotter Release 18.1.035 for plasmid pMiCAN82a and Easyfig v2.136 for transposon Tn6776.
Plasmid construction
Plasmids used for gene expression were generated using the promoterless E. coli–S. aureus shuttle vector pTSSCm18 and DNA inserts amplified from M. canis Epi0082. Template DNA was extracted from strain Epi0082 with the DNeasy Blood & Tissue Kits (Qiagen GmBH) using the protocol for Gram-positive bacteria and enzymatic lysis buffer supplemented with 50 mg/L lysostaphin. Plasmids pJEFBG, pJEFMEF and pJEFMSR were constructed by restriction enzyme-based cloning (Figure S1, available as Supplementary data at JAC Online). DNA inserts were amplified with the Phusion Hot Start II High-Fidelity DNA polymerase (Thermo Scientific) using the primers and conditions listed in Table S1. PCR amplicons and vector pTSSCm were digested with restriction endonucleases KpnI and PstI to facilitate T4 DNA ligation (Table S1). Plasmid pJEFMSR2 was generated through PCR-based mutagenesis of pJEFBG that resulted in deletion of mef(F) and an amplicon with homologous ends for in vivo recombination (Table S1). The pJEFBG template DNA was digested with DpnI. Constructs were obtained in E. coli DH5α by heat shock transformation and selection on LB agar containing 10 mg/L tetracycline. Plasmid DNA from E. coli was isolated using the peqGOLD Plasmid Miniprep Kit I (Peqlab Biotechnologie GmbH). Generated plasmids were confirmed by restriction digestion and Sanger sequencing prior to electroporation into S. aureus RN4220.37
Antimicrobial resistance profiles
The MICs of one representative of each of the 14-, 15- and 16-membered ring macrolides, namely erythromycin, azithromycin and tylosin, as well as the streptogramin B pristinamycin IA, were determined for the S. aureus RN4220 and M. canis strains in Müller–Hinton broth by the microdilution method according to the CLSI recommendations (Table 1).38 Serial fold dilutions ranging from 0.125 to 64 mg/L were prepared in 96-well plates for erythromycin, azithromycin and pristinamycin IA. A Sensititre™ NLVET9 plate (Thermo Fisher Scientific) was used to determine the MIC for tylosin (range 0.06 to 128 mg/L). Inducible resistance to pristinamycin IA and tylosin was measured in the presence of 1 and 2 mg/L erythromycin.
MICs of 14-, 15- and 16-membered ring macrolides erythromycin, azithromycin, tylosin and the streptogramin B pristinamycin IA for Staphylococcus aureus and Macrococcus canis as determined by the broth microdilution method
| Strain/plasmid . | Origin and characteristicsa . | Reference . | Antibiotic resistance genesb . | MIC (mg/L) . | |||||
|---|---|---|---|---|---|---|---|---|---|
| ERY . | AZM . | TYL . | iTYL . | PIA . | iPIA . | ||||
| S. aureus | |||||||||
| RN4220 | Recipient strain for electrotransformation, plasmid free. | 19 | ≤0.125 | 0.5 | 1 | NA | 8 | NA | |
| RN4220/pTSSCm | RN4220 with S. aureus–E. coli shuttle vector pTSSCm. | 18 | tet(L) | ≤0.125 | 0.25 | 1 | NA | 8 | NA |
| RN4220/pJEFMEF | RN4220 with mef(F) and its regulatory region cloned into pTSSCm. | This study | mef(F), tet(L) | 4 | 8 | 0.5 | 1c | 8 | 8c |
| RN4220/pJEFMSR | RN4220 with msr(G) and upstream 113 bp intergenic region cloned into pTSSCm. | This study | msr(G), tet(L) | ≤0.125 | 0.5 | 0.5 | NA | 8 | NA |
| RN4220/ pJEFMSR2 | RN4220 with msr(G) and the regulatory region upstream of mef(F) cloned into pTSSCm. | This study | msr(G), tet(L) | 32 | 64 | 0.5 | 1 | 8 | 32 |
| RN4220/pJEFBG | RN4220 with mef(F), its regulatory region and msr(G) cloned into pTSSCm. | This study | mef(F), msr(G), tet(L) | 16 | 64 | 0.5 | 1 | 8 | 16 |
| M. canis | |||||||||
| KM45013 | M. canis type strain. | 5 | mecB | ≤0.125 | 0.5 | 0.5 | NA | 2 | NA |
| Epi0082 | M. canis strain with plasmid pMiCAN82a. | 4 | mef(F), msr(G), fexB | 8 | 16 | 0.5 | 0.25 | 2 | 2 |
| Strain/plasmid . | Origin and characteristicsa . | Reference . | Antibiotic resistance genesb . | MIC (mg/L) . | |||||
|---|---|---|---|---|---|---|---|---|---|
| ERY . | AZM . | TYL . | iTYL . | PIA . | iPIA . | ||||
| S. aureus | |||||||||
| RN4220 | Recipient strain for electrotransformation, plasmid free. | 19 | ≤0.125 | 0.5 | 1 | NA | 8 | NA | |
| RN4220/pTSSCm | RN4220 with S. aureus–E. coli shuttle vector pTSSCm. | 18 | tet(L) | ≤0.125 | 0.25 | 1 | NA | 8 | NA |
| RN4220/pJEFMEF | RN4220 with mef(F) and its regulatory region cloned into pTSSCm. | This study | mef(F), tet(L) | 4 | 8 | 0.5 | 1c | 8 | 8c |
| RN4220/pJEFMSR | RN4220 with msr(G) and upstream 113 bp intergenic region cloned into pTSSCm. | This study | msr(G), tet(L) | ≤0.125 | 0.5 | 0.5 | NA | 8 | NA |
| RN4220/ pJEFMSR2 | RN4220 with msr(G) and the regulatory region upstream of mef(F) cloned into pTSSCm. | This study | msr(G), tet(L) | 32 | 64 | 0.5 | 1 | 8 | 32 |
| RN4220/pJEFBG | RN4220 with mef(F), its regulatory region and msr(G) cloned into pTSSCm. | This study | mef(F), msr(G), tet(L) | 16 | 64 | 0.5 | 1 | 8 | 16 |
| M. canis | |||||||||
| KM45013 | M. canis type strain. | 5 | mecB | ≤0.125 | 0.5 | 0.5 | NA | 2 | NA |
| Epi0082 | M. canis strain with plasmid pMiCAN82a. | 4 | mef(F), msr(G), fexB | 8 | 16 | 0.5 | 0.25 | 2 | 2 |
ERY, erythromycin; AZM, azithromycin; TYL, tylosin; PIA, pristinamycin IA; iPIA and iTYL, tylosin and pristinamycin IA induced with erythromycin 2 mg/L; NA, not applicable.
pTSSCm is a promoterless cloning vector.
Antibiotic resistance genes and function: tet(L), tetracycline efflux; mef(F), macrolide efflux; msr(G), ABC-F ribosomal protection for macrolide and inducible streptogramin B resistance; fexB, phenicol exporter; mecB, alternative penicillin-binding protein PBP 2a for methicillin resistance.
Induced with 1 mg/L erythromycin.
MICs of 14-, 15- and 16-membered ring macrolides erythromycin, azithromycin, tylosin and the streptogramin B pristinamycin IA for Staphylococcus aureus and Macrococcus canis as determined by the broth microdilution method
| Strain/plasmid . | Origin and characteristicsa . | Reference . | Antibiotic resistance genesb . | MIC (mg/L) . | |||||
|---|---|---|---|---|---|---|---|---|---|
| ERY . | AZM . | TYL . | iTYL . | PIA . | iPIA . | ||||
| S. aureus | |||||||||
| RN4220 | Recipient strain for electrotransformation, plasmid free. | 19 | ≤0.125 | 0.5 | 1 | NA | 8 | NA | |
| RN4220/pTSSCm | RN4220 with S. aureus–E. coli shuttle vector pTSSCm. | 18 | tet(L) | ≤0.125 | 0.25 | 1 | NA | 8 | NA |
| RN4220/pJEFMEF | RN4220 with mef(F) and its regulatory region cloned into pTSSCm. | This study | mef(F), tet(L) | 4 | 8 | 0.5 | 1c | 8 | 8c |
| RN4220/pJEFMSR | RN4220 with msr(G) and upstream 113 bp intergenic region cloned into pTSSCm. | This study | msr(G), tet(L) | ≤0.125 | 0.5 | 0.5 | NA | 8 | NA |
| RN4220/ pJEFMSR2 | RN4220 with msr(G) and the regulatory region upstream of mef(F) cloned into pTSSCm. | This study | msr(G), tet(L) | 32 | 64 | 0.5 | 1 | 8 | 32 |
| RN4220/pJEFBG | RN4220 with mef(F), its regulatory region and msr(G) cloned into pTSSCm. | This study | mef(F), msr(G), tet(L) | 16 | 64 | 0.5 | 1 | 8 | 16 |
| M. canis | |||||||||
| KM45013 | M. canis type strain. | 5 | mecB | ≤0.125 | 0.5 | 0.5 | NA | 2 | NA |
| Epi0082 | M. canis strain with plasmid pMiCAN82a. | 4 | mef(F), msr(G), fexB | 8 | 16 | 0.5 | 0.25 | 2 | 2 |
| Strain/plasmid . | Origin and characteristicsa . | Reference . | Antibiotic resistance genesb . | MIC (mg/L) . | |||||
|---|---|---|---|---|---|---|---|---|---|
| ERY . | AZM . | TYL . | iTYL . | PIA . | iPIA . | ||||
| S. aureus | |||||||||
| RN4220 | Recipient strain for electrotransformation, plasmid free. | 19 | ≤0.125 | 0.5 | 1 | NA | 8 | NA | |
| RN4220/pTSSCm | RN4220 with S. aureus–E. coli shuttle vector pTSSCm. | 18 | tet(L) | ≤0.125 | 0.25 | 1 | NA | 8 | NA |
| RN4220/pJEFMEF | RN4220 with mef(F) and its regulatory region cloned into pTSSCm. | This study | mef(F), tet(L) | 4 | 8 | 0.5 | 1c | 8 | 8c |
| RN4220/pJEFMSR | RN4220 with msr(G) and upstream 113 bp intergenic region cloned into pTSSCm. | This study | msr(G), tet(L) | ≤0.125 | 0.5 | 0.5 | NA | 8 | NA |
| RN4220/ pJEFMSR2 | RN4220 with msr(G) and the regulatory region upstream of mef(F) cloned into pTSSCm. | This study | msr(G), tet(L) | 32 | 64 | 0.5 | 1 | 8 | 32 |
| RN4220/pJEFBG | RN4220 with mef(F), its regulatory region and msr(G) cloned into pTSSCm. | This study | mef(F), msr(G), tet(L) | 16 | 64 | 0.5 | 1 | 8 | 16 |
| M. canis | |||||||||
| KM45013 | M. canis type strain. | 5 | mecB | ≤0.125 | 0.5 | 0.5 | NA | 2 | NA |
| Epi0082 | M. canis strain with plasmid pMiCAN82a. | 4 | mef(F), msr(G), fexB | 8 | 16 | 0.5 | 0.25 | 2 | 2 |
ERY, erythromycin; AZM, azithromycin; TYL, tylosin; PIA, pristinamycin IA; iPIA and iTYL, tylosin and pristinamycin IA induced with erythromycin 2 mg/L; NA, not applicable.
pTSSCm is a promoterless cloning vector.
Antibiotic resistance genes and function: tet(L), tetracycline efflux; mef(F), macrolide efflux; msr(G), ABC-F ribosomal protection for macrolide and inducible streptogramin B resistance; fexB, phenicol exporter; mecB, alternative penicillin-binding protein PBP 2a for methicillin resistance.
Induced with 1 mg/L erythromycin.
Reverse transcription–PCR (RT–PCR)
To analyse the transcription of mef(F) and msr(G) genes, total RNA was extracted from strains M. canis Epi0082 and S. aureus RN4220 containing pJEFBG using the Quick-RNA Fungal/Bacterial Miniprep Kit (Zymo Research) and following the standard manufacturer’s protocol. RNA was extracted from a culture grown in the presence or absence of 2 mg/L erythromycin, followed by a DNA digestion treatment using DNase I (provided in the kit). cDNA was generated by reverse transcription using the murine leukaemia virus reverse transcriptase (Roche Diagnostics) and the leading region-targeting LR-F primer (Table S1). DNA fragments targeting a 170 bp leading region of the operon and a 404 bp region spanning the end of the mef(F) gene, the intergenic region and the beginning of the msr(G) gene were amplified by PCR using the Taq polymerase (FIREPol® DNA Polymerase, Solis BioDyne) with specific primers and conditions listed in Table S1. Samples not incubated with the reverse transcriptase during cDNA generation were included as negative controls to ensure the absence of genomic DNA.
Nucleotide sequence accession numbers
The complete genome of M. canis Epi0082 is available under GenBank accession numbers CP046363–CP046367 (NCBI Bioproject PRJNA590936). Plasmid pMiCAN82a was assigned accession number CP046364, and Tn6776 of M. caseolyticus 5459_5_49 was assigned accession number BK012114.
Results and discussion
Identification and structural organization of the novel macrolide resistance genes mef(F) and msr(G)
The genome of M. canis strain Epi0082 consisted of a 2 324 457 bp circular chromosome and four plasmids of 39 302 bp (pMiCAN82a), 19 741 bp (pMiCAN82b), 10 540 bp (pMiCAN82c) and 2565 bp (pMiCAN82d). The initial software-based search for antibiotic resistance genes only identified the chloramphenicol resistance gene fexB on plasmid pMiCAN82a that shared 99% nucleotide identity with fexB of Enterococcus faecium strain EFM-1 (GenBank accession number JN201336). Further individual analysis of the remaining ORFs of pMiCAN82a revealed the presence of an operon containing two adjacent genes related to macrolide resistance operons of the mef-msr type.11,13,16
The putative new mef gene found in pMiCAN82a encodes a 397 amino acid MFS protein that contains the PROSITE profile PS50850 and exhibits 71% amino acid identity with the Mef(D) protein of M. canis (GenBank accession number MN728681) and less than 40% amino acid identity with all other known macrolide efflux proteins (Figure 1a). The second gene located in the operon is the putative new msr gene, which encodes a 485 amino acid ABC-F protein that contains two nucleotide-binding domains (PS50893) separated by a 99 amino acid linker. This protein shares <67% amino acid identity with Msr(F) and Msr(H) of M. canis and <42% amino acid identity with all other characterized Msr proteins (Figure 1b). The new genes were assigned the names mef(F) and msr(G) according to the nomenclature for MLS genes, which defines a new gene if the deduced amino acid sequence shares <80% identity to any of the previously characterized genes (https://faculty.washington.edu/marilynr).39
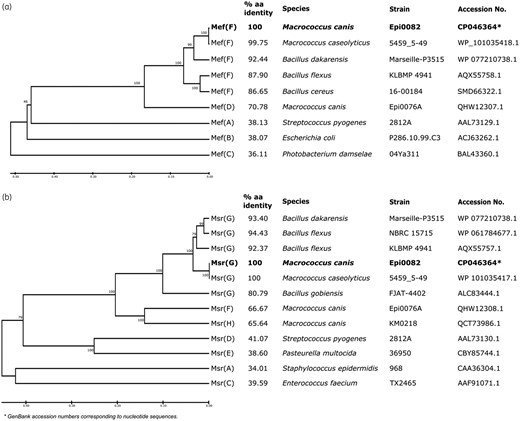
Evolutionary analysis of the amino acid (aa) sequences of Mef and Msr proteins from different families. (a) Clustering of Mef protein. (b) Clustering of Msr protein. The clustering of aa sequences was performed using MEGA v10.1.5.32 The percentage aa identity between the novel determinants Mef(D) and Msr(G) and related determinants is indicated.
The mef(F) and msr(G) genes were organized in an operon [mef(F)–msr(G)] with msr(G) situated 117 bp downstream of mef(F). The presence of a PCR product that spans the mef(F) and msr(G) genes in the RT–PCR analysis confirmed co-transcription of both genes (Figure S2). A promoter search predicted a putative TSS 443 bp upstream of the mef(F) start codon with −10 (5′-ATATATAAT) and −35 (5′-TTGACT) promoter sequences located 14 and 33 bp upstream of the TSS, respectively. Analysis of the mRNA secondary structure of the 443 bp leader region identified several stem–loops, two putative transcription terminators, two ribosomal binding sites (RBSs) and a 14 codon leader peptide (MTHSMRLRSLSLNK) (Figure S3). The similarity of these features to those found in the leader region of inducible expressed erm and (mef-)msr genes suggests that mef(F)–msr(G) expression depends on the presence of erythromycin.40 Our RT–PCR results further showed that the mef(F)–msr(G) transcript signal was stronger in the presence of erythromycin while the signal of the leader region was less affected by the presence or absence of the inducer (Figure S2). This observation suggests that expression of mef(F) and msr(G) is controlled by transcriptional attenuation similar to the mechanism described for erm(K) and mef(A)–msr(D) genes.41,42
Functional characterization of mef(F) and msr(G)
M. canis strain Epi0082 showed an at least 64-fold higher MIC of erythromycin and a 32-fold higher MIC of azithromycin compared with the type strain KM45013T (Table 1). To demonstrate the association of the mef(F)–msr(G) operon with macrolide resistance, the genes were inserted individually or together into the shuttle vector pTSSCm18 and expressed in S. aureus RN4220 (Table 1). Four different pTSSCm constructs were generated, each containing either mef(F)–msr(G) (pJEFBG), mef(F) (pJEFMEF) or msr(G) under the control of the regulatory sequence of the operon (pJEFMSR2) [including 761 bp of DNA upstream of mef(F)] or msr(G) with only 113 bp of upstream DNA (pJEFMSR) corresponding to the intergenic region between mef(F) and msr(G) (Figure S1). The MIC of the 14-membered ring erythromycin for RN4220 harbouring the complete mef(F)–msr(G) operon on pJEFBG was 16 mg/L, corresponding to an at least 128-fold increased resistance compared with the control strains RN4220 and RN4220/pTSSCm (Table 1). A lower MIC of 4 mg/L was measured for RN4220 expressing only mef(F) from pJEFMEF, whereas a higher MIC of 32 mg/L was observed when msr(G) alone was expressed from the regulatory sequence upstream of mef(F) on plasmid pJEFMSR2. However, when msr(G) was cloned with the intergenic sequence upstream of msr(G) in pJEFMSR (Table 1), the MIC remained unchanged, indicating that the promoter sequence upstream of mef(F) is also required for expression of msr(G). Determination of the MICs of 15- and 16-membered ring macrolides for the same constructs indicated that both genes can confer resistance to the 15-membered ring azithromycin but not to the 16-membered tylosin (Table 1). For S. aureus, MIC values increased 32-fold for RN4220 cells expressing mef(F) from pJEFMEF (MIC 8 mg/L) and 256-fold for cells expressing msr(G) from pJEFMSR2 (MIC 64 mg/L) compared with cells harbouring the empty vector pTSSCm (MIC 0.25 mg/L). The results also indicate that azithromycin functions as an inducer. Resistance to tylosin was not observed and could also not be induced with erythromycin (Table 1). Resistance against pristinamycin IA (with and without 2 mg/L erythromycin induction) was not observed for M. canis Epi0082. However, for RN4220 containing pJEFMSR2 and pJEFBG, an up to 2-fold higher MIC was observed under erythromycin induction, indicating that msr(G) also has an ability to mediate low-level streptogramin B resistance (Table 1).
Susceptibility testing performed with S. aureus strains clearly confirmed that the mef(F)–msr(G) operon from M. canis Epi0082 mediates resistance to macrolides. It also showed that both genes can function independently, although they are expressed together from the same operon promoter. The msr(G) gene appears to contribute more to the resistance phenotype than mef(F), similar to observations made before for mef–msr operons in M. canis and S. pyogenes.12,16
Characterization of genetic elements containing mef(F) and msr(G)
In M. canis Epi0082, the mef(F)–msr(G) operon was located on pMiCAN82a (Figure 2). This plasmid contained 42 ORFs and its G + C content was 28.3 mol% which was lower than that of the chromosome (36.5 mol%), except for a region containing the mef(F)–msr(G) operon and fexB that exhibited a higher GC content (32.3 mol%). This aforementioned 9317 bp region was bordered by 25 bp terminal inverted repeats (IRs) and flanking 6 bp direct repeats (DRs) (GAATCG), and contained ORFs similar to the resolvase binL (99% nt identity) and part of the replicative transposase of Tn552 (89% nt identity) (GenBank accession number X52734) (Figure 2). The remaining part of pMiCAN82a (29 985 bp) was additionally found (with 97% coverage and >99% identity) in the genome of M. caseolyticus strain 5798_EF375 deposited in the GenBank database (accession numbers PIXG01000020.1 and PIXG01000024.1).43 Interestingly, M. caseolyticus 5798_EF375 also carries the mef(F)–msr(G) operon (>99% nt identity), but in the chromosome (accession number PIXG01000018.1) (see below).
![Genomic circular representation of plasmid pMiCAN82a including the fexB gene and the macrolide resistance operon containing the novel genes mef(F) and msr(G). The inner circle represents GC% content, with the colour code corresponding to above/below average. Genes are represented by arrows and coloured by putative function: resistance genes are shown in green; putative conjugation-associated gene arrays are in blue; and recombinases are in yellow. Inverted repeats (IRs) [TTCTCATATATC(G/A)AGTAAAGTGACA] and direct repeats (DRs) (GAATCG) flanking the resistance region are indicated. Abbreviations: mtase, modification methylase; nuc, endonuclease; for other abbreviations, see text. The map was drawn using DNAPlotter (Release 18.1.0)35 and the sequence of M. canis Epi0082 (CP046364). This figure appears in colour in the online version of JAC and in black and white in the printed version of JAC.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jac/76/1/10.1093_jac_dkaa405/1/m_dkaa405f2.jpeg?Expires=1750211347&Signature=12I6pmbdx4xONKtEjWg19TlOlObF9ifj9YXOPqBMegYksaCR1Gf0Q0Z-z7b8yrZVhtExDpil~wkk6XQjq9L2QRE5rrNiTzw56rrACCYJjbj4Uv9m~~eChFvLUrWOAbMVkTHz04Mm3XdtWjqd8vTRmxk35eg35g075K-aZWAQyzXvH8xohw5w~Go4E7K9gx7Kf44OoCTL7hUItNxJsT9bOayDBwxa4Z1OHKi0xPLxOfGwZwknaOMHoOGnOqg9s-mkpe3IBPeMXyZ0W~Fa7Ibd8jXtNthqmNmfNasDN0GK1N6WFDwbcSpB6dVDX2YSncihgtbVzoHigHDTxGUQPpe8eA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Genomic circular representation of plasmid pMiCAN82a including the fexB gene and the macrolide resistance operon containing the novel genes mef(F) and msr(G). The inner circle represents GC% content, with the colour code corresponding to above/below average. Genes are represented by arrows and coloured by putative function: resistance genes are shown in green; putative conjugation-associated gene arrays are in blue; and recombinases are in yellow. Inverted repeats (IRs) [TTCTCATATATC(G/A)AGTAAAGTGACA] and direct repeats (DRs) (GAATCG) flanking the resistance region are indicated. Abbreviations: mtase, modification methylase; nuc, endonuclease; for other abbreviations, see text. The map was drawn using DNAPlotter (Release 18.1.0)35 and the sequence of M. canis Epi0082 (CP046364). This figure appears in colour in the online version of JAC and in black and white in the printed version of JAC.
In pMiCAN82a, it was not possible to identify any proteins containing the classical domain of replication initiation proteins for theta-mode replication [RepA_N (pfam06970), Rep_3 (pfam01051) and PriCT_1 (pfam08708)].44 Other features typically found in plasmids were present, including plasmid resolvase (Res) and partitioning proteins (ParA) as well as key proteins possibly involved in conjugative transfer, such as DNA relaxase (Rlx) (pfam03389: MobA_MobL), coupling protein (Cpl) (COG3505: VirD4), conjugative transfer ATPase (VirB4) (TIGR00929: VirB4_CagE) and lytic transglycosylase (Slt) (COG2951: MltB) (Figure 2).
In the M. caseolyticus strain 5798_EF375 and in another three M. caseolyticus strains, 5459_5_49, 5781_EF64 and 5782_EF_83, all macrolide resistant from bovine sources in the UK,43 the mef(F)–msr(G) operon was located in the chromosome downstream of the glutamine-fructose-6-phosphate transaminase gene (glmS) as part of a 13 820 bp or 13 894 bp element flanked by IRs (Figure 3). Recombinase genes unrelated to those found on plasmid pMiCAN82a were present, encoding proteins structurally related to transposition proteins TnsABCDE of Tn7 from E. coli (GenBank accession number KX117211).45 The chromosomal location and the presence of TnsABCDE-related products suggest that mef(F)–msr(G) are carried in M. caseolyticus by a novel transposon distantly related to Tn7 that was assigned the name Tn6776 (https://transposon.lstmed.ac.uk/).46 Furthermore, operons similar to mef(F)–msr(G) encoding proteins that share around 90% amino acid identities to Mef(F) and Msr(G) were identified in different Bacillus species (GenBank accession numbers CP016790.1, NZ_LT707408.1 and FWYT01000069.1) (Figure 1). These operons in Bacillus were located in the chromosome, and no further similarities to pMiCAN82a and Tn6776 were observed in their vicinity.
![Transposon Tn6776 containing the mef(F)–msr(G) operon in M. caseolyticus. The transposon was inserted into the chromosome downstream of the glutamine-fructose-6-phosphate transaminase gene (glmS). Genes distantly related to those of Tn7 of E. coli (tnsABCDE) as well as the flanking inverted repeat [IR: 5′-TGTTGGTTTA(T/A)AATAAAG(A/T)TTGAT] are indicated. The figure was generated using Easyfig (v2.1)36 and M. caseolyticus sequences of strains without the transposon IMD0819 (GenBank accession number CP021058.1) and 5785_EF123 (PIWZ00000000.1) and with the transposon 5459_5_49 (PIWU00000000.1) and 5798_EF375 (PIXG00000000.1). This figure appears in colour in the online version of JAC and in black and white in the printed version of JAC.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jac/76/1/10.1093_jac_dkaa405/1/m_dkaa405f3.jpeg?Expires=1750211347&Signature=g8AFFh5IP1qODEz8icgP1tUgneO18qfuWMu-ju9hniSOLvKJ3CTXI7Xe3MwRfdRqzK1ZwPBhkybsJkFT5JkmUbA~ipDws6zKM4S5pAg-W7cVKXDQHKt8XePKzGVJRGTs3CAsxl66l1x0iOcCe-aTAUniDP33ym~1pHh36exM6lrMZZv5PuNTy8JrnWDbVFCENFb0JHAYadVvhU5Hkr6Hdhr0wLKUagv25nKgp6bg03JLCLn0ZPadzZ7Pq2~HAnH9j-JmkDY2mj1SxoFjQWPbtWd4PctG8lgRIYlG-qThLwK74HDFDAaPkYT3cZvW~jtMRbqQnGlKAjMkvq~~WLNFLA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Transposon Tn6776 containing the mef(F)–msr(G) operon in M. caseolyticus. The transposon was inserted into the chromosome downstream of the glutamine-fructose-6-phosphate transaminase gene (glmS). Genes distantly related to those of Tn7 of E. coli (tnsABCDE) as well as the flanking inverted repeat [IR: 5′-TGTTGGTTTA(T/A)AATAAAG(A/T)TTGAT] are indicated. The figure was generated using Easyfig (v2.1)36 and M. caseolyticus sequences of strains without the transposon IMD0819 (GenBank accession number CP021058.1) and 5785_EF123 (PIWZ00000000.1) and with the transposon 5459_5_49 (PIWU00000000.1) and 5798_EF375 (PIXG00000000.1). This figure appears in colour in the online version of JAC and in black and white in the printed version of JAC.
The identification of an operon containing the new macrolide resistance genes mef(F) and msr(G) in different species of the Staphylococcaceae and Bacillaceae families, indicates that these genes might be widespread in the environment. Both elements carrying mef(F)–msr(G) in Macrococcus, the plasmid pMiCAN82a of M. canis and the transposon Tn6776 of M. caseolyticus, pose a risk of further dissemination of macrolide resistance across bacterial populations.
Acknowledgements
This study was partially conducted as part of the Master of Science thesis of J.E.F. during 2018–19 at the Institute of Veterinary Bacteriology, University of Bern. We thank Marilyn C. Roberts (University of Washington) for assigning names to the novel mef(F) and msr(G) genes, Adam Roberts (Liverpool School of Tropical Medicine) for the designation of the novel transposon Tn6776, and Alexandro Rossano (Institute of Veterinary Bacteriology) for technical assistance. We also thank the Institute of Genetics at the University of Bern for kindly providing PacBio sequencing.
Funding
This study was supported by internal funds from the Institute of Veterinary Bacteriology, University of Bern and by grant no. 1.18.10 of the Swiss Federal Food Safety and Veterinary Office (FSVO).
Transparency declarations
None to declare.
Supplementary data
Tables S1 and Figures S1 to S3 are available as Supplementary data at JAC Online.
References
NCBI Resource Coordinators.
UniProt Consortium.
CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically—Eleventh Edition: M07.



