-
PDF
- Split View
-
Views
-
Cite
Cite
Maria Diletta Pezzani, Fulvia Mazzaferri, Monica Compri, Liliana Galia, Nico T Mutters, Gunnar Kahlmeter, Theoklis E Zaoutis, Mitchell J Schwaber, Jesús Rodríguez-Baño, Stephan Harbarth, Evelina Tacconelli, the COACH working group , Linking antimicrobial resistance surveillance to antibiotic policy in healthcare settings: the COMBACTE-Magnet EPI-Net COACH project, Journal of Antimicrobial Chemotherapy, Volume 75, Issue Supplement_2, December 2020, Pages ii2–ii19, https://doi.org/10.1093/jac/dkaa425
Close - Share Icon Share
Abstract
To systematically summarize the evidence on how to collect, analyse and report antimicrobial resistance (AMR) surveillance data to inform antimicrobial stewardship (AMS) teams providing guidance on empirical antibiotic treatment in healthcare settings.
The research group identified 10 key questions about the link between AMR surveillance and AMS using a checklist of 9 elements for good practice in health research priority settings and a modified 3D combined approach matrix, and conducted a systematic review of published original studies and guidelines on the link between AMR surveillance and AMS.
The questions identified focused on AMS team composition; minimum infrastructure requirements for AMR surveillance; organisms, samples and susceptibility patterns to report; data stratification strategies; reporting frequency; resistance thresholds to drive empirical therapy; surveillance in high-risk hospital units, long-term care, outpatient and veterinary settings; and surveillance data from other countries. Twenty guidelines and seven original studies on the implementation of AMR surveillance as part of an AMS programme were included in the literature review.
The evidence summarized in this review provides a useful basis for a more integrated process of developing procedures to report AMR surveillance data to drive AMS interventions. These procedures should be extended to settings outside the acute-care institutions, such as long-term care, outpatient and veterinary. Without proper AMR surveillance, implementation of AMS policies cannot contribute effectively to the fight against MDR pathogens and may even worsen the burden of adverse events from such interventions.
Introduction
High-quality and timely antimicrobial resistance (AMR) surveillance plays a pivotal role in administering appropriate empirical antimicrobial therapy and implementing antimicrobial stewardship (AMS) programmes. Although IDSA1,2 and European Commission (EC)3 guidelines emphasize the importance of AMR surveillance in assisting AMS teams to develop empirical therapy protocols, no clear guidance exists on AMR surveillance or reporting for this purpose.2,3 Major limitations include lack of adequate and comprehensive AMR surveillance systems as well as poor integration between laboratory and clinical data due to limited information technology platforms.4
This work is part of the Epidemiology Network (EPI-Net) project and the basis for the development of the collaboration between EPI-Net and the JPIAMR ARCH network (http://archnet-surveillance.eu). EPI-Net was launched in 2015 to improve surveillance of AMR and healthcare-associated infections in Europe, under the COMBACTE-MAGNET consortium of the New Drugs for Bad Bugs (ND4BB) programme (https://www.combacte.com/about/epi-net). ND4BB is funded by the Innovative Medicines Initiative with the EC and the European Federation of Pharmaceutical Industries and Associations to address the European AMR crisis and accelerate development of and access to new medications (http://www.nd4bb.eu).
Our objective was to systematically summarize the evidence on collection, analysis and reporting of AMR surveillance data to optimize antibiotic recommendations and empirical prescribing policies by AMS teams.
Methods
The research group set priorities for our recommendations using a checklist of nine elements for good practice in health research priority settings5 and a modified 3D combined approach matrix.6 From this analysis, we identified 10 key questions about the link between AMR surveillance and AMS and conducted a systematic literature review. Relevant English-language articles published from July 2008 to August 2019 were retrieved through searches of PubMed, Embase, the Cochrane Central Register of Controlled Trials, the Database of Abstracts of Reviews of Effects and the Cochrane Database of Systematic Reviews. A combination of Medical Subject Headings and equivalent terms was used in the search strategy (Figure 1). The review protocol is available on the EPI-Net website (https://EPI-net.eu).
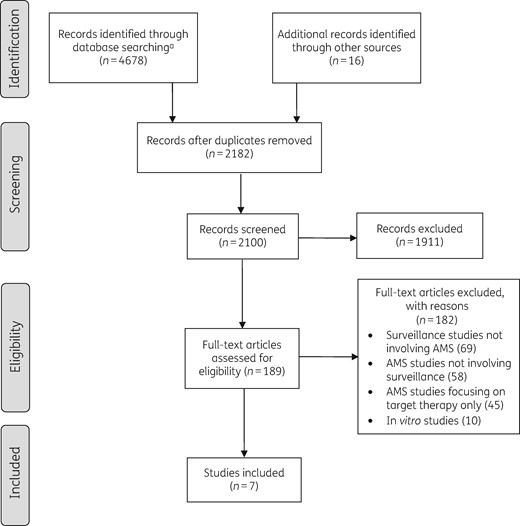
Study selection process. aIndex search terms: (surveillance) AND (epidemiol* OR prevalence OR incidence OR rate) AND (susceptib* OR resist* OR isolat* OR pathogen OR pathogens OR bacteri*) AND (antimicrobial stewardship OR anti-microbial stewardship OR antibiotic stewardship OR antimicrobial policy OR antimicrobial policies OR anti-microbial policy OR antimicrobial policies OR antibiotic policy OR antibiotic policies OR antimicrobial prescript* OR anti-microbial prescript* OR antibiotic prescript*).
Reviewers used a two-stage selection process. First, abstracts were screened against eligibility criteria and duplicate and irrelevant documents were excluded. Next, full-text articles were assessed, study data (design, setting, population, intervention, comparison, outcomes) were extracted from eligible articles, and references were screened on titles and abstracts for further inclusion (Figure 1). No restriction on study design, population or setting was applied. We included both original articles assessing implementation of AMR surveillance reports as part of an AMS programme and guidelines providing recommendations on reporting AMR surveillance data to the AMS team. The PICO framework is shown in Table 1. Quality of original articles was assessed using the Effective Practice and Organisation of Care quality criteria for interrupted time series7 and the Newcastle-Ottawa Scale for cohort and before–after studies.8
| Patients | Any patient in any community or healthcare setting undergoing antibiotic prophylaxis or treatment |
| Interventions | Articles pertaining to surveillance interventions that aimed to improve antibiotic prescribing in healthcare settings |
| Comparison | Standard of care |
| Outcome | Any assessed AMS outcome:
|
| Patients | Any patient in any community or healthcare setting undergoing antibiotic prophylaxis or treatment |
| Interventions | Articles pertaining to surveillance interventions that aimed to improve antibiotic prescribing in healthcare settings |
| Comparison | Standard of care |
| Outcome | Any assessed AMS outcome:
|
DOT, days of therapy; CDI, Clostridioides difficile infection; LOS, length of stay.
| Patients | Any patient in any community or healthcare setting undergoing antibiotic prophylaxis or treatment |
| Interventions | Articles pertaining to surveillance interventions that aimed to improve antibiotic prescribing in healthcare settings |
| Comparison | Standard of care |
| Outcome | Any assessed AMS outcome:
|
| Patients | Any patient in any community or healthcare setting undergoing antibiotic prophylaxis or treatment |
| Interventions | Articles pertaining to surveillance interventions that aimed to improve antibiotic prescribing in healthcare settings |
| Comparison | Standard of care |
| Outcome | Any assessed AMS outcome:
|
DOT, days of therapy; CDI, Clostridioides difficile infection; LOS, length of stay.
Results and discussion
We identified 20 guidelines with recommendations on the implementation of AMR surveillance as part of an AMS programme. All recommendations were supported by only low-quality evidence (expert opinion or small observational studies)1–3,9–25 (Table 2).
Recommendations and/or statements from guidelines (2007–18) on how to link antimicrobial resistance surveillance data to antimicrobial stewardship, classified into 10 key questions
| First author, year . | Title . | Recommendation . |
|---|---|---|
| Question 1 - What is the most appropriate AMS team composition to facilitate implementation of surveillance systems and to inform AMS interventions? | ||
| Dellit, 20071 | Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional programme to enhance antimicrobial stewardship |
|
| National Institute for Health and Care Excellence, 201513 | Antimicrobial stewardship: systems and processes for effective antimicrobial medicine use |
|
| de With, 20169 | Strategies to enhance rational use of antibiotics in hospital: a guideline by the German Society for Infectious Diseases |
|
| Department of Health, Republic of South Africa, 201714 | Guidelines on implementation of the antimicrobial strategy in South Africa: one health approach & governance |
|
| Australian Commission on Safety and Quality in Health Care, 201811 | Antimicrobial stewardship in Australian healthcare | TERTIARY CARE
|
| British Society for Antimicrobial Chemotherapy, 201812 | Antimicrobial stewardship: from principles to practice |
|
| Castro-Sánchez, 20183 | European Commission guidelines for the prudent use of antimicrobials in human health |
|
| Question 2 - What are the minimum infrastructural requirements of AMR surveillance to inform AMS interventions? | ||
| ||
| Question 3 - Which bacteria and samples should be included in the AMR surveillance report and how should susceptibility patterns be reported to inform AMS interventions? | ||
| SARI Hospital Antimicrobial Stewardship Working Group, 200916 | Guidelines for antimicrobial stewardship in hospitals in Ireland |
|
| de With, 20169 | Strategies to enhance rational use of antibiotics in hospital: a guideline by the German Society for Infectious Diseases |
|
| Department of Health, Republic of South Africa, 201714 | Guidelines on implementation of the antimicrobial strategy in South Africa: one health approach & governance |
|
| Australian Commission on Safety and Quality in Health Care, 201811 | Antimicrobial stewardship in Australian health care |
|
| Castro-Sánchez, 20183 | European Commission guidelines for the prudent use of antimicrobials in human health |
|
| Centers for Disease Control and Prevention, 201815 | Antimicrobial stewardship core elements at small and critical access hospitals |
|
| Question 4 - How should AMR surveillance data be stratified to inform AMS interventions? | ||
| SARI Hospital Antimicrobial Stewardship Working Group16 | Guidelines for antimicrobial stewardship in hospitals in Ireland |
|
| Barlam, 20162 | Implementing an antimicrobial stewardship programme: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America |
|
| de With, 20169 | Strategies to enhance rational use of antibiotics in hospital: a guideline by the German Society for Infectious Diseases |
|
| Australian Commission on Safety and Quality in Health Care, 201811 | Antimicrobial stewardship in Australian health care |
|
| Question 5 - What is the frequency of reporting AMR surveillance data to inform AMS interventions? | ||
| Dellit, 20071 | Infectious Diseases Society of America and the Society of Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship |
|
| SARI Hospital Antimicrobial Stewardship Working Group, 200916 | Guidelines for antimicrobial stewardship in hospitals in Ireland |
|
| de With, 20169 | Strategies to enhance rational use of antibiotics in hospital: a guideline by the German Society for Infectious Diseases |
|
| Department of Health Republic of South Africa, 201714 | Guidelines on implementation of the antimicrobial strategy in South Africa: one health approach & governance |
|
| Australian Commission on Safety and Quality in Health Care, 201811 | Antimicrobial stewardship in Australian health care |
|
| Question 6 - What are the threshold levels of resistance for changing the empirical antimicrobial treatment recommendation? | ||
| Gupta, 201117 | International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases |
|
| Kalil, 201618 | Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society |
|
| Torres, 201719 | International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia: guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociación Latinoamericana del Tórax (ALAT) |
|
| Hawkey, 201810 | Treatment of infections caused by multidrug-resistant Gram-negative bacteria: report of the British Society for Antimicrobial Chemotherapy |
|
| Question 7 - How should AMR surveillance be tailored to AMS in settings with patients at high risk of AMR colonization and infection? | ||
| ||
| Question 8 - Should AMR surveillance reports include data from long-term care facility and outpatient settings to inform AMS interventions? | ||
| Johnson, 201625 | Improving feedback of surveillance data on antimicrobial consumption, resistance and stewardship in England: putting the data at your fingertips |
|
| Centers for Disease Control and Prevention, 201715 | The core elements for antimicrobial stewardship in nursing homes |
|
| Jump, 201720 | Template for an antimicrobial stewardship policy for post-acute and long-term care settings |
|
| Klepser, 201723 | A call to action for outpatient antimicrobial stewardship |
|
| McElligott, 201722 | Antimicrobial stewardship in nursing facilities |
|
| Australian Commission on Safety and Quality in Health Care, 201811 | Antimicrobial stewardship in Australian health care |
|
| Quality Innovation Network National Coordinating Center (USA), 201824 | A field guide to antimicrobial stewardship in outpatient settings |
|
| Question 9 - Should AMR surveillance include data from other countries to inform AMS interventions? | ||
| No guideline reports specifically on this topic | ||
| Question 10 - Should AMR surveillance reports include regional and/or national surveillance data from companion and food-producing animals to inform AMS interventions in human healthcare? | ||
| No guideline reports specifically on this topic | ||
| First author, year . | Title . | Recommendation . |
|---|---|---|
| Question 1 - What is the most appropriate AMS team composition to facilitate implementation of surveillance systems and to inform AMS interventions? | ||
| Dellit, 20071 | Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional programme to enhance antimicrobial stewardship |
|
| National Institute for Health and Care Excellence, 201513 | Antimicrobial stewardship: systems and processes for effective antimicrobial medicine use |
|
| de With, 20169 | Strategies to enhance rational use of antibiotics in hospital: a guideline by the German Society for Infectious Diseases |
|
| Department of Health, Republic of South Africa, 201714 | Guidelines on implementation of the antimicrobial strategy in South Africa: one health approach & governance |
|
| Australian Commission on Safety and Quality in Health Care, 201811 | Antimicrobial stewardship in Australian healthcare | TERTIARY CARE
|
| British Society for Antimicrobial Chemotherapy, 201812 | Antimicrobial stewardship: from principles to practice |
|
| Castro-Sánchez, 20183 | European Commission guidelines for the prudent use of antimicrobials in human health |
|
| Question 2 - What are the minimum infrastructural requirements of AMR surveillance to inform AMS interventions? | ||
| ||
| Question 3 - Which bacteria and samples should be included in the AMR surveillance report and how should susceptibility patterns be reported to inform AMS interventions? | ||
| SARI Hospital Antimicrobial Stewardship Working Group, 200916 | Guidelines for antimicrobial stewardship in hospitals in Ireland |
|
| de With, 20169 | Strategies to enhance rational use of antibiotics in hospital: a guideline by the German Society for Infectious Diseases |
|
| Department of Health, Republic of South Africa, 201714 | Guidelines on implementation of the antimicrobial strategy in South Africa: one health approach & governance |
|
| Australian Commission on Safety and Quality in Health Care, 201811 | Antimicrobial stewardship in Australian health care |
|
| Castro-Sánchez, 20183 | European Commission guidelines for the prudent use of antimicrobials in human health |
|
| Centers for Disease Control and Prevention, 201815 | Antimicrobial stewardship core elements at small and critical access hospitals |
|
| Question 4 - How should AMR surveillance data be stratified to inform AMS interventions? | ||
| SARI Hospital Antimicrobial Stewardship Working Group16 | Guidelines for antimicrobial stewardship in hospitals in Ireland |
|
| Barlam, 20162 | Implementing an antimicrobial stewardship programme: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America |
|
| de With, 20169 | Strategies to enhance rational use of antibiotics in hospital: a guideline by the German Society for Infectious Diseases |
|
| Australian Commission on Safety and Quality in Health Care, 201811 | Antimicrobial stewardship in Australian health care |
|
| Question 5 - What is the frequency of reporting AMR surveillance data to inform AMS interventions? | ||
| Dellit, 20071 | Infectious Diseases Society of America and the Society of Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship |
|
| SARI Hospital Antimicrobial Stewardship Working Group, 200916 | Guidelines for antimicrobial stewardship in hospitals in Ireland |
|
| de With, 20169 | Strategies to enhance rational use of antibiotics in hospital: a guideline by the German Society for Infectious Diseases |
|
| Department of Health Republic of South Africa, 201714 | Guidelines on implementation of the antimicrobial strategy in South Africa: one health approach & governance |
|
| Australian Commission on Safety and Quality in Health Care, 201811 | Antimicrobial stewardship in Australian health care |
|
| Question 6 - What are the threshold levels of resistance for changing the empirical antimicrobial treatment recommendation? | ||
| Gupta, 201117 | International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases |
|
| Kalil, 201618 | Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society |
|
| Torres, 201719 | International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia: guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociación Latinoamericana del Tórax (ALAT) |
|
| Hawkey, 201810 | Treatment of infections caused by multidrug-resistant Gram-negative bacteria: report of the British Society for Antimicrobial Chemotherapy |
|
| Question 7 - How should AMR surveillance be tailored to AMS in settings with patients at high risk of AMR colonization and infection? | ||
| ||
| Question 8 - Should AMR surveillance reports include data from long-term care facility and outpatient settings to inform AMS interventions? | ||
| Johnson, 201625 | Improving feedback of surveillance data on antimicrobial consumption, resistance and stewardship in England: putting the data at your fingertips |
|
| Centers for Disease Control and Prevention, 201715 | The core elements for antimicrobial stewardship in nursing homes |
|
| Jump, 201720 | Template for an antimicrobial stewardship policy for post-acute and long-term care settings |
|
| Klepser, 201723 | A call to action for outpatient antimicrobial stewardship |
|
| McElligott, 201722 | Antimicrobial stewardship in nursing facilities |
|
| Australian Commission on Safety and Quality in Health Care, 201811 | Antimicrobial stewardship in Australian health care |
|
| Quality Innovation Network National Coordinating Center (USA), 201824 | A field guide to antimicrobial stewardship in outpatient settings |
|
| Question 9 - Should AMR surveillance include data from other countries to inform AMS interventions? | ||
| No guideline reports specifically on this topic | ||
| Question 10 - Should AMR surveillance reports include regional and/or national surveillance data from companion and food-producing animals to inform AMS interventions in human healthcare? | ||
| No guideline reports specifically on this topic | ||
CRE, carbapenem-resistant Enterobacteriaceae; ESKAPE, Enterococcus species, Staphylococcus aureus, K. pneumoniae, A. baumannii, P. aeruginosa, E. coli; IPC; infection prevention and control; PD, pharmacodynamics; PK, pharmacokinetics.
Recommendations and/or statements from guidelines (2007–18) on how to link antimicrobial resistance surveillance data to antimicrobial stewardship, classified into 10 key questions
| First author, year . | Title . | Recommendation . |
|---|---|---|
| Question 1 - What is the most appropriate AMS team composition to facilitate implementation of surveillance systems and to inform AMS interventions? | ||
| Dellit, 20071 | Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional programme to enhance antimicrobial stewardship |
|
| National Institute for Health and Care Excellence, 201513 | Antimicrobial stewardship: systems and processes for effective antimicrobial medicine use |
|
| de With, 20169 | Strategies to enhance rational use of antibiotics in hospital: a guideline by the German Society for Infectious Diseases |
|
| Department of Health, Republic of South Africa, 201714 | Guidelines on implementation of the antimicrobial strategy in South Africa: one health approach & governance |
|
| Australian Commission on Safety and Quality in Health Care, 201811 | Antimicrobial stewardship in Australian healthcare | TERTIARY CARE
|
| British Society for Antimicrobial Chemotherapy, 201812 | Antimicrobial stewardship: from principles to practice |
|
| Castro-Sánchez, 20183 | European Commission guidelines for the prudent use of antimicrobials in human health |
|
| Question 2 - What are the minimum infrastructural requirements of AMR surveillance to inform AMS interventions? | ||
| ||
| Question 3 - Which bacteria and samples should be included in the AMR surveillance report and how should susceptibility patterns be reported to inform AMS interventions? | ||
| SARI Hospital Antimicrobial Stewardship Working Group, 200916 | Guidelines for antimicrobial stewardship in hospitals in Ireland |
|
| de With, 20169 | Strategies to enhance rational use of antibiotics in hospital: a guideline by the German Society for Infectious Diseases |
|
| Department of Health, Republic of South Africa, 201714 | Guidelines on implementation of the antimicrobial strategy in South Africa: one health approach & governance |
|
| Australian Commission on Safety and Quality in Health Care, 201811 | Antimicrobial stewardship in Australian health care |
|
| Castro-Sánchez, 20183 | European Commission guidelines for the prudent use of antimicrobials in human health |
|
| Centers for Disease Control and Prevention, 201815 | Antimicrobial stewardship core elements at small and critical access hospitals |
|
| Question 4 - How should AMR surveillance data be stratified to inform AMS interventions? | ||
| SARI Hospital Antimicrobial Stewardship Working Group16 | Guidelines for antimicrobial stewardship in hospitals in Ireland |
|
| Barlam, 20162 | Implementing an antimicrobial stewardship programme: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America |
|
| de With, 20169 | Strategies to enhance rational use of antibiotics in hospital: a guideline by the German Society for Infectious Diseases |
|
| Australian Commission on Safety and Quality in Health Care, 201811 | Antimicrobial stewardship in Australian health care |
|
| Question 5 - What is the frequency of reporting AMR surveillance data to inform AMS interventions? | ||
| Dellit, 20071 | Infectious Diseases Society of America and the Society of Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship |
|
| SARI Hospital Antimicrobial Stewardship Working Group, 200916 | Guidelines for antimicrobial stewardship in hospitals in Ireland |
|
| de With, 20169 | Strategies to enhance rational use of antibiotics in hospital: a guideline by the German Society for Infectious Diseases |
|
| Department of Health Republic of South Africa, 201714 | Guidelines on implementation of the antimicrobial strategy in South Africa: one health approach & governance |
|
| Australian Commission on Safety and Quality in Health Care, 201811 | Antimicrobial stewardship in Australian health care |
|
| Question 6 - What are the threshold levels of resistance for changing the empirical antimicrobial treatment recommendation? | ||
| Gupta, 201117 | International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases |
|
| Kalil, 201618 | Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society |
|
| Torres, 201719 | International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia: guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociación Latinoamericana del Tórax (ALAT) |
|
| Hawkey, 201810 | Treatment of infections caused by multidrug-resistant Gram-negative bacteria: report of the British Society for Antimicrobial Chemotherapy |
|
| Question 7 - How should AMR surveillance be tailored to AMS in settings with patients at high risk of AMR colonization and infection? | ||
| ||
| Question 8 - Should AMR surveillance reports include data from long-term care facility and outpatient settings to inform AMS interventions? | ||
| Johnson, 201625 | Improving feedback of surveillance data on antimicrobial consumption, resistance and stewardship in England: putting the data at your fingertips |
|
| Centers for Disease Control and Prevention, 201715 | The core elements for antimicrobial stewardship in nursing homes |
|
| Jump, 201720 | Template for an antimicrobial stewardship policy for post-acute and long-term care settings |
|
| Klepser, 201723 | A call to action for outpatient antimicrobial stewardship |
|
| McElligott, 201722 | Antimicrobial stewardship in nursing facilities |
|
| Australian Commission on Safety and Quality in Health Care, 201811 | Antimicrobial stewardship in Australian health care |
|
| Quality Innovation Network National Coordinating Center (USA), 201824 | A field guide to antimicrobial stewardship in outpatient settings |
|
| Question 9 - Should AMR surveillance include data from other countries to inform AMS interventions? | ||
| No guideline reports specifically on this topic | ||
| Question 10 - Should AMR surveillance reports include regional and/or national surveillance data from companion and food-producing animals to inform AMS interventions in human healthcare? | ||
| No guideline reports specifically on this topic | ||
| First author, year . | Title . | Recommendation . |
|---|---|---|
| Question 1 - What is the most appropriate AMS team composition to facilitate implementation of surveillance systems and to inform AMS interventions? | ||
| Dellit, 20071 | Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional programme to enhance antimicrobial stewardship |
|
| National Institute for Health and Care Excellence, 201513 | Antimicrobial stewardship: systems and processes for effective antimicrobial medicine use |
|
| de With, 20169 | Strategies to enhance rational use of antibiotics in hospital: a guideline by the German Society for Infectious Diseases |
|
| Department of Health, Republic of South Africa, 201714 | Guidelines on implementation of the antimicrobial strategy in South Africa: one health approach & governance |
|
| Australian Commission on Safety and Quality in Health Care, 201811 | Antimicrobial stewardship in Australian healthcare | TERTIARY CARE
|
| British Society for Antimicrobial Chemotherapy, 201812 | Antimicrobial stewardship: from principles to practice |
|
| Castro-Sánchez, 20183 | European Commission guidelines for the prudent use of antimicrobials in human health |
|
| Question 2 - What are the minimum infrastructural requirements of AMR surveillance to inform AMS interventions? | ||
| ||
| Question 3 - Which bacteria and samples should be included in the AMR surveillance report and how should susceptibility patterns be reported to inform AMS interventions? | ||
| SARI Hospital Antimicrobial Stewardship Working Group, 200916 | Guidelines for antimicrobial stewardship in hospitals in Ireland |
|
| de With, 20169 | Strategies to enhance rational use of antibiotics in hospital: a guideline by the German Society for Infectious Diseases |
|
| Department of Health, Republic of South Africa, 201714 | Guidelines on implementation of the antimicrobial strategy in South Africa: one health approach & governance |
|
| Australian Commission on Safety and Quality in Health Care, 201811 | Antimicrobial stewardship in Australian health care |
|
| Castro-Sánchez, 20183 | European Commission guidelines for the prudent use of antimicrobials in human health |
|
| Centers for Disease Control and Prevention, 201815 | Antimicrobial stewardship core elements at small and critical access hospitals |
|
| Question 4 - How should AMR surveillance data be stratified to inform AMS interventions? | ||
| SARI Hospital Antimicrobial Stewardship Working Group16 | Guidelines for antimicrobial stewardship in hospitals in Ireland |
|
| Barlam, 20162 | Implementing an antimicrobial stewardship programme: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America |
|
| de With, 20169 | Strategies to enhance rational use of antibiotics in hospital: a guideline by the German Society for Infectious Diseases |
|
| Australian Commission on Safety and Quality in Health Care, 201811 | Antimicrobial stewardship in Australian health care |
|
| Question 5 - What is the frequency of reporting AMR surveillance data to inform AMS interventions? | ||
| Dellit, 20071 | Infectious Diseases Society of America and the Society of Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship |
|
| SARI Hospital Antimicrobial Stewardship Working Group, 200916 | Guidelines for antimicrobial stewardship in hospitals in Ireland |
|
| de With, 20169 | Strategies to enhance rational use of antibiotics in hospital: a guideline by the German Society for Infectious Diseases |
|
| Department of Health Republic of South Africa, 201714 | Guidelines on implementation of the antimicrobial strategy in South Africa: one health approach & governance |
|
| Australian Commission on Safety and Quality in Health Care, 201811 | Antimicrobial stewardship in Australian health care |
|
| Question 6 - What are the threshold levels of resistance for changing the empirical antimicrobial treatment recommendation? | ||
| Gupta, 201117 | International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases |
|
| Kalil, 201618 | Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society |
|
| Torres, 201719 | International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia: guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociación Latinoamericana del Tórax (ALAT) |
|
| Hawkey, 201810 | Treatment of infections caused by multidrug-resistant Gram-negative bacteria: report of the British Society for Antimicrobial Chemotherapy |
|
| Question 7 - How should AMR surveillance be tailored to AMS in settings with patients at high risk of AMR colonization and infection? | ||
| ||
| Question 8 - Should AMR surveillance reports include data from long-term care facility and outpatient settings to inform AMS interventions? | ||
| Johnson, 201625 | Improving feedback of surveillance data on antimicrobial consumption, resistance and stewardship in England: putting the data at your fingertips |
|
| Centers for Disease Control and Prevention, 201715 | The core elements for antimicrobial stewardship in nursing homes |
|
| Jump, 201720 | Template for an antimicrobial stewardship policy for post-acute and long-term care settings |
|
| Klepser, 201723 | A call to action for outpatient antimicrobial stewardship |
|
| McElligott, 201722 | Antimicrobial stewardship in nursing facilities |
|
| Australian Commission on Safety and Quality in Health Care, 201811 | Antimicrobial stewardship in Australian health care |
|
| Quality Innovation Network National Coordinating Center (USA), 201824 | A field guide to antimicrobial stewardship in outpatient settings |
|
| Question 9 - Should AMR surveillance include data from other countries to inform AMS interventions? | ||
| No guideline reports specifically on this topic | ||
| Question 10 - Should AMR surveillance reports include regional and/or national surveillance data from companion and food-producing animals to inform AMS interventions in human healthcare? | ||
| No guideline reports specifically on this topic | ||
CRE, carbapenem-resistant Enterobacteriaceae; ESKAPE, Enterococcus species, Staphylococcus aureus, K. pneumoniae, A. baumannii, P. aeruginosa, E. coli; IPC; infection prevention and control; PD, pharmacodynamics; PK, pharmacokinetics.
Database searching retrieved 2182 unique study records. Initial screening identified 182 full-text articles, of which 7 studies were eligible: 2 interrupted time series analyses,26,27 1 prospective cohort study,28 1 retrospective cohort study,29,30 1 controlled before–after study31 and 2 uncontrolled before–after studies.32,33 Six studies found that AMS interventions linked to surveillance were effective in reducing AMR rates,26,27,29–33 and two studies showed a significant reduction in 30 day mortality.28,33 Study design, sample size, type of intervention, outcome and quality assessment are shown in Table 3.
| Author, year . | Study design (time period) . | Sample size . | Intervention . | Comparison . | Clinical outcome . | Results . | P value . | Quality assessment (tool) . |
|---|---|---|---|---|---|---|---|---|
| Meyer, 200926 | Interrupted time series (2004) | 16 bed ICU | Changes in antibiotic prescription guidelines based on microbiological data | Previous antibiotic prescription guidelines | Prevalence of third-generation cephalosporin-resistant K. pneumoniae (1) and E. coli (2) | (1) 21.2% vs 33.3% (2) 6.2% vs 5.7% | (1) 0.047 (2) 0.856 | Medium (EPOC) |
| Tuon, 201727 | Interrupted time series (2014 vs 2015) | 186 bed hospital | Mobile guidance manual for the choice of the empirical therapy, based on a real-time update of laboratory culture results and susceptibility profiles (stratified by site of infection) | NA | Consumption of aminoglycosides Consumption of cefepime Consumption of piperacillin/tazobactam Consumption of meropenem Consumption of ciprofloxacin Consumption of polymyxin Susceptibility to meropenema Susceptibility to polymyxina Susceptibility to cefepimea Susceptibility to amikacina Susceptibility to ciprofloxacina Susceptibility to gentamicina | Increase Increase Reduction Reduction Reduction Reduction 73% vs 83% 69% vs 83% 62% vs 57% 79% vs 83% 52% vs 49% 68% vs 69% | 0.02 0.01 0.02 0.44 0.08 0.34 <0.05 <0.05 <0.05 NS NS NS | Low (EPOC) |
| Rodriguez- Maresca, 201428 | Prospective cohort study (2008–10) | Intervention: 44 ICU patients; Control: 129 ICU patients | Empirical treatment of lower respiratory tract infection, urinary tract infection and bacteraemia according to a real-time updated local resistance map (an antibiotic was recommended when active against >75% of all bacteria isolated in the same infection) | Empirical treatment according to clinical criteria | Mortality Length of stay Appropriateness | 20% vs 27% 13.8 vs 19.5 days 80% vs 26% | 0.75 0.16 0.005 | Low (NOS) |
| Palmer, 201129 | Retrospective cohort study (2002–6) | Intervention: 27; Control: 7 | Antibiotic prescription based on different MICs for P. aeruginosa | Antibiotic prescription based on different MICs for P. aeruginosa | 30 day all-cause mortality | 22% vs 85% | 0.004 | Medium (NOS) |
| Knudsen, 201431 | Controlled before–after study (2008–12) | Intervention: university hospital; Control: four other hospitals | Antimicrobial stewardship programme with antibiotic guidelines | No antimicrobial stewardship programme or antibiotic guidelines | Incidence of ESBL K. pneumoniae ESBL carrier rate All-cause 30 day mortality | Reduction Reduction Similar | <0.02 <0.023 NS | High (NOS) |
| Wong-Beringer, 200932 | Uncontrolled before (1997–2004)–after (2005–7) study | 565-bed hospital | Yearly reporting of links between the institutional antibiogram and the antibiotic prescribing patterns to the medical staff | NA | Empirical prescribing of quinolones Susceptibility to anti- P. aeruginosa Mortality associated with P. aeruginosa infections | 30% reduction 10% increase 2-fold reduction | NA NA NA | Low (NOS) |
| Nachtigall, 201433 | Uncontrolled before–after study (2006–10) | Pre: 328 ICU patients Post (third period): 293 ICU patients | Computerized decision support systems with updated microbiological findings | Paper-based guidelines for antibiotic therapy | Antibiotic-free days All-cause mortality | 32% vs 42% 10.5% vs 8.9% | <0.01 0.624 | Medium (NOS) |
| Author, year . | Study design (time period) . | Sample size . | Intervention . | Comparison . | Clinical outcome . | Results . | P value . | Quality assessment (tool) . |
|---|---|---|---|---|---|---|---|---|
| Meyer, 200926 | Interrupted time series (2004) | 16 bed ICU | Changes in antibiotic prescription guidelines based on microbiological data | Previous antibiotic prescription guidelines | Prevalence of third-generation cephalosporin-resistant K. pneumoniae (1) and E. coli (2) | (1) 21.2% vs 33.3% (2) 6.2% vs 5.7% | (1) 0.047 (2) 0.856 | Medium (EPOC) |
| Tuon, 201727 | Interrupted time series (2014 vs 2015) | 186 bed hospital | Mobile guidance manual for the choice of the empirical therapy, based on a real-time update of laboratory culture results and susceptibility profiles (stratified by site of infection) | NA | Consumption of aminoglycosides Consumption of cefepime Consumption of piperacillin/tazobactam Consumption of meropenem Consumption of ciprofloxacin Consumption of polymyxin Susceptibility to meropenema Susceptibility to polymyxina Susceptibility to cefepimea Susceptibility to amikacina Susceptibility to ciprofloxacina Susceptibility to gentamicina | Increase Increase Reduction Reduction Reduction Reduction 73% vs 83% 69% vs 83% 62% vs 57% 79% vs 83% 52% vs 49% 68% vs 69% | 0.02 0.01 0.02 0.44 0.08 0.34 <0.05 <0.05 <0.05 NS NS NS | Low (EPOC) |
| Rodriguez- Maresca, 201428 | Prospective cohort study (2008–10) | Intervention: 44 ICU patients; Control: 129 ICU patients | Empirical treatment of lower respiratory tract infection, urinary tract infection and bacteraemia according to a real-time updated local resistance map (an antibiotic was recommended when active against >75% of all bacteria isolated in the same infection) | Empirical treatment according to clinical criteria | Mortality Length of stay Appropriateness | 20% vs 27% 13.8 vs 19.5 days 80% vs 26% | 0.75 0.16 0.005 | Low (NOS) |
| Palmer, 201129 | Retrospective cohort study (2002–6) | Intervention: 27; Control: 7 | Antibiotic prescription based on different MICs for P. aeruginosa | Antibiotic prescription based on different MICs for P. aeruginosa | 30 day all-cause mortality | 22% vs 85% | 0.004 | Medium (NOS) |
| Knudsen, 201431 | Controlled before–after study (2008–12) | Intervention: university hospital; Control: four other hospitals | Antimicrobial stewardship programme with antibiotic guidelines | No antimicrobial stewardship programme or antibiotic guidelines | Incidence of ESBL K. pneumoniae ESBL carrier rate All-cause 30 day mortality | Reduction Reduction Similar | <0.02 <0.023 NS | High (NOS) |
| Wong-Beringer, 200932 | Uncontrolled before (1997–2004)–after (2005–7) study | 565-bed hospital | Yearly reporting of links between the institutional antibiogram and the antibiotic prescribing patterns to the medical staff | NA | Empirical prescribing of quinolones Susceptibility to anti- P. aeruginosa Mortality associated with P. aeruginosa infections | 30% reduction 10% increase 2-fold reduction | NA NA NA | Low (NOS) |
| Nachtigall, 201433 | Uncontrolled before–after study (2006–10) | Pre: 328 ICU patients Post (third period): 293 ICU patients | Computerized decision support systems with updated microbiological findings | Paper-based guidelines for antibiotic therapy | Antibiotic-free days All-cause mortality | 32% vs 42% 10.5% vs 8.9% | <0.01 0.624 | Medium (NOS) |
EPOC, Effective Practice and Organisation of Care quality criteria; NOS, Newcastle-Ottawa Scale; NA, not applicable; NS, not significant.
922 cultures positive for Gram-negative bacilli.
| Author, year . | Study design (time period) . | Sample size . | Intervention . | Comparison . | Clinical outcome . | Results . | P value . | Quality assessment (tool) . |
|---|---|---|---|---|---|---|---|---|
| Meyer, 200926 | Interrupted time series (2004) | 16 bed ICU | Changes in antibiotic prescription guidelines based on microbiological data | Previous antibiotic prescription guidelines | Prevalence of third-generation cephalosporin-resistant K. pneumoniae (1) and E. coli (2) | (1) 21.2% vs 33.3% (2) 6.2% vs 5.7% | (1) 0.047 (2) 0.856 | Medium (EPOC) |
| Tuon, 201727 | Interrupted time series (2014 vs 2015) | 186 bed hospital | Mobile guidance manual for the choice of the empirical therapy, based on a real-time update of laboratory culture results and susceptibility profiles (stratified by site of infection) | NA | Consumption of aminoglycosides Consumption of cefepime Consumption of piperacillin/tazobactam Consumption of meropenem Consumption of ciprofloxacin Consumption of polymyxin Susceptibility to meropenema Susceptibility to polymyxina Susceptibility to cefepimea Susceptibility to amikacina Susceptibility to ciprofloxacina Susceptibility to gentamicina | Increase Increase Reduction Reduction Reduction Reduction 73% vs 83% 69% vs 83% 62% vs 57% 79% vs 83% 52% vs 49% 68% vs 69% | 0.02 0.01 0.02 0.44 0.08 0.34 <0.05 <0.05 <0.05 NS NS NS | Low (EPOC) |
| Rodriguez- Maresca, 201428 | Prospective cohort study (2008–10) | Intervention: 44 ICU patients; Control: 129 ICU patients | Empirical treatment of lower respiratory tract infection, urinary tract infection and bacteraemia according to a real-time updated local resistance map (an antibiotic was recommended when active against >75% of all bacteria isolated in the same infection) | Empirical treatment according to clinical criteria | Mortality Length of stay Appropriateness | 20% vs 27% 13.8 vs 19.5 days 80% vs 26% | 0.75 0.16 0.005 | Low (NOS) |
| Palmer, 201129 | Retrospective cohort study (2002–6) | Intervention: 27; Control: 7 | Antibiotic prescription based on different MICs for P. aeruginosa | Antibiotic prescription based on different MICs for P. aeruginosa | 30 day all-cause mortality | 22% vs 85% | 0.004 | Medium (NOS) |
| Knudsen, 201431 | Controlled before–after study (2008–12) | Intervention: university hospital; Control: four other hospitals | Antimicrobial stewardship programme with antibiotic guidelines | No antimicrobial stewardship programme or antibiotic guidelines | Incidence of ESBL K. pneumoniae ESBL carrier rate All-cause 30 day mortality | Reduction Reduction Similar | <0.02 <0.023 NS | High (NOS) |
| Wong-Beringer, 200932 | Uncontrolled before (1997–2004)–after (2005–7) study | 565-bed hospital | Yearly reporting of links between the institutional antibiogram and the antibiotic prescribing patterns to the medical staff | NA | Empirical prescribing of quinolones Susceptibility to anti- P. aeruginosa Mortality associated with P. aeruginosa infections | 30% reduction 10% increase 2-fold reduction | NA NA NA | Low (NOS) |
| Nachtigall, 201433 | Uncontrolled before–after study (2006–10) | Pre: 328 ICU patients Post (third period): 293 ICU patients | Computerized decision support systems with updated microbiological findings | Paper-based guidelines for antibiotic therapy | Antibiotic-free days All-cause mortality | 32% vs 42% 10.5% vs 8.9% | <0.01 0.624 | Medium (NOS) |
| Author, year . | Study design (time period) . | Sample size . | Intervention . | Comparison . | Clinical outcome . | Results . | P value . | Quality assessment (tool) . |
|---|---|---|---|---|---|---|---|---|
| Meyer, 200926 | Interrupted time series (2004) | 16 bed ICU | Changes in antibiotic prescription guidelines based on microbiological data | Previous antibiotic prescription guidelines | Prevalence of third-generation cephalosporin-resistant K. pneumoniae (1) and E. coli (2) | (1) 21.2% vs 33.3% (2) 6.2% vs 5.7% | (1) 0.047 (2) 0.856 | Medium (EPOC) |
| Tuon, 201727 | Interrupted time series (2014 vs 2015) | 186 bed hospital | Mobile guidance manual for the choice of the empirical therapy, based on a real-time update of laboratory culture results and susceptibility profiles (stratified by site of infection) | NA | Consumption of aminoglycosides Consumption of cefepime Consumption of piperacillin/tazobactam Consumption of meropenem Consumption of ciprofloxacin Consumption of polymyxin Susceptibility to meropenema Susceptibility to polymyxina Susceptibility to cefepimea Susceptibility to amikacina Susceptibility to ciprofloxacina Susceptibility to gentamicina | Increase Increase Reduction Reduction Reduction Reduction 73% vs 83% 69% vs 83% 62% vs 57% 79% vs 83% 52% vs 49% 68% vs 69% | 0.02 0.01 0.02 0.44 0.08 0.34 <0.05 <0.05 <0.05 NS NS NS | Low (EPOC) |
| Rodriguez- Maresca, 201428 | Prospective cohort study (2008–10) | Intervention: 44 ICU patients; Control: 129 ICU patients | Empirical treatment of lower respiratory tract infection, urinary tract infection and bacteraemia according to a real-time updated local resistance map (an antibiotic was recommended when active against >75% of all bacteria isolated in the same infection) | Empirical treatment according to clinical criteria | Mortality Length of stay Appropriateness | 20% vs 27% 13.8 vs 19.5 days 80% vs 26% | 0.75 0.16 0.005 | Low (NOS) |
| Palmer, 201129 | Retrospective cohort study (2002–6) | Intervention: 27; Control: 7 | Antibiotic prescription based on different MICs for P. aeruginosa | Antibiotic prescription based on different MICs for P. aeruginosa | 30 day all-cause mortality | 22% vs 85% | 0.004 | Medium (NOS) |
| Knudsen, 201431 | Controlled before–after study (2008–12) | Intervention: university hospital; Control: four other hospitals | Antimicrobial stewardship programme with antibiotic guidelines | No antimicrobial stewardship programme or antibiotic guidelines | Incidence of ESBL K. pneumoniae ESBL carrier rate All-cause 30 day mortality | Reduction Reduction Similar | <0.02 <0.023 NS | High (NOS) |
| Wong-Beringer, 200932 | Uncontrolled before (1997–2004)–after (2005–7) study | 565-bed hospital | Yearly reporting of links between the institutional antibiogram and the antibiotic prescribing patterns to the medical staff | NA | Empirical prescribing of quinolones Susceptibility to anti- P. aeruginosa Mortality associated with P. aeruginosa infections | 30% reduction 10% increase 2-fold reduction | NA NA NA | Low (NOS) |
| Nachtigall, 201433 | Uncontrolled before–after study (2006–10) | Pre: 328 ICU patients Post (third period): 293 ICU patients | Computerized decision support systems with updated microbiological findings | Paper-based guidelines for antibiotic therapy | Antibiotic-free days All-cause mortality | 32% vs 42% 10.5% vs 8.9% | <0.01 0.624 | Medium (NOS) |
EPOC, Effective Practice and Organisation of Care quality criteria; NOS, Newcastle-Ottawa Scale; NA, not applicable; NS, not significant.
922 cultures positive for Gram-negative bacilli.
Basic and additional requirements for providing AMR data are summarized in Table 4.
| Question . | Basic requirements for providing AMR data . | Additional requirements for providing AMR data . |
|---|---|---|
| 1 - What is the most appropriate AMS team composition to facilitate implementation of surveillance systems and to inform AMS interventions? | Include infectious diseases clinicians, clinical microbiologists and pharmacists in a multidisciplinary AMS team | Include infectious diseases clinicians, clinical microbiologists, pharmacists, nurses, psychologists, epidemiologists and infection control specialists in a multidisciplinary AMS team |
| 2 - What are the minimum infrastructural requirements of AMR surveillance to inform AMS interventions? |
| Link the laboratory and information technology platforms to integrate laboratory and clinical/demographical data |
| 3 - Which bacteria and samples should be included in the AMR surveillance report and how should susceptibility patterns be reported to inform AMS interventions? |
|
|
| 4 - How should AMR surveillance data be stratified to inform AMS interventions? |
|
|
| 5 - What is the frequency of reporting AMR surveillance data to inform AMS interventions? | Provision of comprehensive routine data at least on yearly basis |
|
| 6 - What are the threshold levels of resistance for changing the empirical antimicrobial treatment recommendation? |
| On the basis of local AMR rates, set resistance thresholds at a local level according to:
|
| 7 - How should AMR surveillance be tailored to AMS in settings with patients at high risk of AMR colonization and infection? | No evidence on this topic | No evidence on this topic |
| 8 - Should AMR surveillance reports include data from long-term care facility and outpatient settings to inform AMS interventions? |
| Provide a facility-specific/outpatient antibiogram, at least quarterly |
| 9 - Should AMR surveillance include data from other countries to inform AMS interventions? | No evidence on this topic | No evidence on this topic |
| 10 - Should AMR surveillance reports include regional and/or national surveillance data from companion and food-producing animals to inform AMS interventions in human healthcare? | No evidence on this topic | No evidence on this topic |
| Question . | Basic requirements for providing AMR data . | Additional requirements for providing AMR data . |
|---|---|---|
| 1 - What is the most appropriate AMS team composition to facilitate implementation of surveillance systems and to inform AMS interventions? | Include infectious diseases clinicians, clinical microbiologists and pharmacists in a multidisciplinary AMS team | Include infectious diseases clinicians, clinical microbiologists, pharmacists, nurses, psychologists, epidemiologists and infection control specialists in a multidisciplinary AMS team |
| 2 - What are the minimum infrastructural requirements of AMR surveillance to inform AMS interventions? |
| Link the laboratory and information technology platforms to integrate laboratory and clinical/demographical data |
| 3 - Which bacteria and samples should be included in the AMR surveillance report and how should susceptibility patterns be reported to inform AMS interventions? |
|
|
| 4 - How should AMR surveillance data be stratified to inform AMS interventions? |
|
|
| 5 - What is the frequency of reporting AMR surveillance data to inform AMS interventions? | Provision of comprehensive routine data at least on yearly basis |
|
| 6 - What are the threshold levels of resistance for changing the empirical antimicrobial treatment recommendation? |
| On the basis of local AMR rates, set resistance thresholds at a local level according to:
|
| 7 - How should AMR surveillance be tailored to AMS in settings with patients at high risk of AMR colonization and infection? | No evidence on this topic | No evidence on this topic |
| 8 - Should AMR surveillance reports include data from long-term care facility and outpatient settings to inform AMS interventions? |
| Provide a facility-specific/outpatient antibiogram, at least quarterly |
| 9 - Should AMR surveillance include data from other countries to inform AMS interventions? | No evidence on this topic | No evidence on this topic |
| 10 - Should AMR surveillance reports include regional and/or national surveillance data from companion and food-producing animals to inform AMS interventions in human healthcare? | No evidence on this topic | No evidence on this topic |
CRE, carbapenem-resistant Enterobacteriaceae.
| Question . | Basic requirements for providing AMR data . | Additional requirements for providing AMR data . |
|---|---|---|
| 1 - What is the most appropriate AMS team composition to facilitate implementation of surveillance systems and to inform AMS interventions? | Include infectious diseases clinicians, clinical microbiologists and pharmacists in a multidisciplinary AMS team | Include infectious diseases clinicians, clinical microbiologists, pharmacists, nurses, psychologists, epidemiologists and infection control specialists in a multidisciplinary AMS team |
| 2 - What are the minimum infrastructural requirements of AMR surveillance to inform AMS interventions? |
| Link the laboratory and information technology platforms to integrate laboratory and clinical/demographical data |
| 3 - Which bacteria and samples should be included in the AMR surveillance report and how should susceptibility patterns be reported to inform AMS interventions? |
|
|
| 4 - How should AMR surveillance data be stratified to inform AMS interventions? |
|
|
| 5 - What is the frequency of reporting AMR surveillance data to inform AMS interventions? | Provision of comprehensive routine data at least on yearly basis |
|
| 6 - What are the threshold levels of resistance for changing the empirical antimicrobial treatment recommendation? |
| On the basis of local AMR rates, set resistance thresholds at a local level according to:
|
| 7 - How should AMR surveillance be tailored to AMS in settings with patients at high risk of AMR colonization and infection? | No evidence on this topic | No evidence on this topic |
| 8 - Should AMR surveillance reports include data from long-term care facility and outpatient settings to inform AMS interventions? |
| Provide a facility-specific/outpatient antibiogram, at least quarterly |
| 9 - Should AMR surveillance include data from other countries to inform AMS interventions? | No evidence on this topic | No evidence on this topic |
| 10 - Should AMR surveillance reports include regional and/or national surveillance data from companion and food-producing animals to inform AMS interventions in human healthcare? | No evidence on this topic | No evidence on this topic |
| Question . | Basic requirements for providing AMR data . | Additional requirements for providing AMR data . |
|---|---|---|
| 1 - What is the most appropriate AMS team composition to facilitate implementation of surveillance systems and to inform AMS interventions? | Include infectious diseases clinicians, clinical microbiologists and pharmacists in a multidisciplinary AMS team | Include infectious diseases clinicians, clinical microbiologists, pharmacists, nurses, psychologists, epidemiologists and infection control specialists in a multidisciplinary AMS team |
| 2 - What are the minimum infrastructural requirements of AMR surveillance to inform AMS interventions? |
| Link the laboratory and information technology platforms to integrate laboratory and clinical/demographical data |
| 3 - Which bacteria and samples should be included in the AMR surveillance report and how should susceptibility patterns be reported to inform AMS interventions? |
|
|
| 4 - How should AMR surveillance data be stratified to inform AMS interventions? |
|
|
| 5 - What is the frequency of reporting AMR surveillance data to inform AMS interventions? | Provision of comprehensive routine data at least on yearly basis |
|
| 6 - What are the threshold levels of resistance for changing the empirical antimicrobial treatment recommendation? |
| On the basis of local AMR rates, set resistance thresholds at a local level according to:
|
| 7 - How should AMR surveillance be tailored to AMS in settings with patients at high risk of AMR colonization and infection? | No evidence on this topic | No evidence on this topic |
| 8 - Should AMR surveillance reports include data from long-term care facility and outpatient settings to inform AMS interventions? |
| Provide a facility-specific/outpatient antibiogram, at least quarterly |
| 9 - Should AMR surveillance include data from other countries to inform AMS interventions? | No evidence on this topic | No evidence on this topic |
| 10 - Should AMR surveillance reports include regional and/or national surveillance data from companion and food-producing animals to inform AMS interventions in human healthcare? | No evidence on this topic | No evidence on this topic |
CRE, carbapenem-resistant Enterobacteriaceae.
1. What is the most appropriate AMS team composition to facilitate implementation of surveillance systems and inform AMS interventions?
Seven guidelines underlined the benefits of a multidisciplinary AMS team, including infectious diseases specialist, clinical microbiologist, pharmacist, nurse, psychologist, epidemiologist and infection control specialist1,3,9,11–14 Six studies assessed an AMS intervention with a clinical microbiologist included in the team.26,28–33
To link surveillance data with clinical recommendations, involvement of a clinical microbiologist, pharmacist and infectious diseases specialist is fundamental. In settings where these specialists are not available, educational activities supporting establishment of qualified personnel trained in AMR and antimicrobial use should be a priority. The hub-and-spoke network model, in which a primary centre (hub) supports secondary centres with limited services (spokes), is often used to optimize the utilization of healthcare services in resource-constrained settings.34 For AMS, experts in infectious diseases, clinical microbiologists and pharmacists in a hub hospital assist trained personnel in spoke hospitals to overcome resource limitations and implement effective, efficient collaboration and quality control of AMS activities.
2. What are the minimum infrastructure requirements of AMR surveillance to inform AMS interventions?
No guidelines or studies addressed structural requirements for appropriate hospital AMR surveillance to inform AMS intervention.
Fulfilment of good laboratory practices (i.e. processes that assure the integrity, safety and efficacy of laboratory activities) is the cornerstone. A quality management system should supervise the coordination and realization of quality objectives.34–36 According to the research group, the medical director should be responsible for ensuring that adequate staffing and resources are allocated to support the functions and efforts of the quality management system. The international core set of quality-system essentials includes the following components: organization; facilities and safety; personnel and customer focus; purchasing, inventory and equipment; process management; documents and records and information management; occurrence management and assessment; and continual improvement (Table 5).
| Task . | Activity . |
|---|---|
| Organization | Management and organizational structure of the laboratory. |
| Facilities and safety | Analysis of potential harm from pathogens/chemicals and assessment of requirements for laboratory design and safety to prevent and control exposure to physical, chemical and biological hazards. |
| Personnel and customer focus | Choice and provision of qualified and skilled staff also in the context of interaction with potential customers (i.e. physicians, patients, public health services and community). |
| Purchasing, inventory and equipment | Proper equipment management to ensure reliable and timely testing to reduce variations in test results, thus maintaining laboratory performance and avoiding waste. |
| Process management | Control of different actions/activities (e.g. sample management and examination processes) to ensure accurate testing and valid results. It includes implementation of an internal quality control programme and participation in national and/or international external quality assurance. |
| Documents and records and information management | Control of safety and availability of documents and records, storage, ensuring accessibility whenever needed. The information management system is responsible for the processes needed to effectively manage data by guaranteeing unique identifiers for patients and samples, standard request forms and the patient’s privacy. |
| Occurrence management and assessment | Identification of errors, involving either testing or other processes, and application of appropriate corrections to prevent their further occurrence. Assessment is defined as the systematic examination of the quality management system to demonstrate that the laboratory is meeting regulatory and customer requirements through internal and external audits. |
| Continual improvement | Ensuring continual improvement in laboratory quality over time. |
| Task . | Activity . |
|---|---|
| Organization | Management and organizational structure of the laboratory. |
| Facilities and safety | Analysis of potential harm from pathogens/chemicals and assessment of requirements for laboratory design and safety to prevent and control exposure to physical, chemical and biological hazards. |
| Personnel and customer focus | Choice and provision of qualified and skilled staff also in the context of interaction with potential customers (i.e. physicians, patients, public health services and community). |
| Purchasing, inventory and equipment | Proper equipment management to ensure reliable and timely testing to reduce variations in test results, thus maintaining laboratory performance and avoiding waste. |
| Process management | Control of different actions/activities (e.g. sample management and examination processes) to ensure accurate testing and valid results. It includes implementation of an internal quality control programme and participation in national and/or international external quality assurance. |
| Documents and records and information management | Control of safety and availability of documents and records, storage, ensuring accessibility whenever needed. The information management system is responsible for the processes needed to effectively manage data by guaranteeing unique identifiers for patients and samples, standard request forms and the patient’s privacy. |
| Occurrence management and assessment | Identification of errors, involving either testing or other processes, and application of appropriate corrections to prevent their further occurrence. Assessment is defined as the systematic examination of the quality management system to demonstrate that the laboratory is meeting regulatory and customer requirements through internal and external audits. |
| Continual improvement | Ensuring continual improvement in laboratory quality over time. |
| Task . | Activity . |
|---|---|
| Organization | Management and organizational structure of the laboratory. |
| Facilities and safety | Analysis of potential harm from pathogens/chemicals and assessment of requirements for laboratory design and safety to prevent and control exposure to physical, chemical and biological hazards. |
| Personnel and customer focus | Choice and provision of qualified and skilled staff also in the context of interaction with potential customers (i.e. physicians, patients, public health services and community). |
| Purchasing, inventory and equipment | Proper equipment management to ensure reliable and timely testing to reduce variations in test results, thus maintaining laboratory performance and avoiding waste. |
| Process management | Control of different actions/activities (e.g. sample management and examination processes) to ensure accurate testing and valid results. It includes implementation of an internal quality control programme and participation in national and/or international external quality assurance. |
| Documents and records and information management | Control of safety and availability of documents and records, storage, ensuring accessibility whenever needed. The information management system is responsible for the processes needed to effectively manage data by guaranteeing unique identifiers for patients and samples, standard request forms and the patient’s privacy. |
| Occurrence management and assessment | Identification of errors, involving either testing or other processes, and application of appropriate corrections to prevent their further occurrence. Assessment is defined as the systematic examination of the quality management system to demonstrate that the laboratory is meeting regulatory and customer requirements through internal and external audits. |
| Continual improvement | Ensuring continual improvement in laboratory quality over time. |
| Task . | Activity . |
|---|---|
| Organization | Management and organizational structure of the laboratory. |
| Facilities and safety | Analysis of potential harm from pathogens/chemicals and assessment of requirements for laboratory design and safety to prevent and control exposure to physical, chemical and biological hazards. |
| Personnel and customer focus | Choice and provision of qualified and skilled staff also in the context of interaction with potential customers (i.e. physicians, patients, public health services and community). |
| Purchasing, inventory and equipment | Proper equipment management to ensure reliable and timely testing to reduce variations in test results, thus maintaining laboratory performance and avoiding waste. |
| Process management | Control of different actions/activities (e.g. sample management and examination processes) to ensure accurate testing and valid results. It includes implementation of an internal quality control programme and participation in national and/or international external quality assurance. |
| Documents and records and information management | Control of safety and availability of documents and records, storage, ensuring accessibility whenever needed. The information management system is responsible for the processes needed to effectively manage data by guaranteeing unique identifiers for patients and samples, standard request forms and the patient’s privacy. |
| Occurrence management and assessment | Identification of errors, involving either testing or other processes, and application of appropriate corrections to prevent their further occurrence. Assessment is defined as the systematic examination of the quality management system to demonstrate that the laboratory is meeting regulatory and customer requirements through internal and external audits. |
| Continual improvement | Ensuring continual improvement in laboratory quality over time. |
For AMR surveillance, it is useful to establish a memorandum of understanding for data sharing with other national/regional institutions and a linkage with a national/central reference laboratory for technical support. The connection between hospital patient data from different healthcare settings allows comparison of AMR rates and helps AMS teams develop recommendations for patients with a history of hospitalization elsewhere. External sources of AMR rates in European countries include EARS-Net for invasive isolates and the EPI-Net website (https://EPI-net.eu), on which all publicly available AMR surveillance data (including monitoring of AMR to new antibiotics) are continually updated.
Still, these international standards are not always applicable. Logistic barriers (e.g. geographical spread of hospitals) can affect communication and reporting by limiting access to laboratory services.37 Low/middle income countries (LMICs) are often characterized by small-scale laboratories, lack of appropriate training and the absence of laboratory information systems. Development of national quality regulations based on international standards but also informed by country-specific characteristics and available resources is encouraged.38,39
3. Which bacteria and samples should be included in the AMR surveillance report and how should susceptibility patterns be reported to inform AMS interventions?
Six guidelines indicated that the criteria for the selection of pathogens to target in AMR surveillance should be based on local epidemiology and the major clinical impact attributable to a specific AMR profile,3,9,11,14,16,21 one specified priority specimens for microbiological analysis,16 and one underlined the relevance of separate reporting of screening samples.9 One guideline specifically stated a minimum number of isolates for the construction of cumulative antibiograms,16 and two recommended molecular diagnostics as a tool to focus appropriate AMS interventions.9,11 Five studies assessed an AMS intervention providing an MIC based on cumulative antibiograms.26–30,32
The most common Gram-negative (e.g. Escherichia coli) and Gram-positive (e.g. Staphylococcus aureus) pathogens have been suggested as proxies for hospitals unable to compute their AMR rates on a Gram-stain basis, although this practice is less precise and accurate.40 Pathogens can be selected on the basis of hospital case mix composition and service type. Knowledge of the highest priorities at international and national levels can be taken as a first step of selection.41 Data on Clostridioides difficile infections should be included in the surveillance programme because they have been shown to be an important quality indicator for assessment of AMS intervention impact at the patient level.42,43
Whether antibiograms are an appropriate tool to measure AMS intervention effectiveness on AMR rates is debatable.44 Antibiograms are usually reported as cumulative results of all susceptibility tests,45,46 based on different stratification criteria and over predefined time intervals. Reporting of a cumulative antibiogram with ≥30 isolates tested during the analysis period is recommended to produce an appropriate statistical estimate of cumulative susceptibility rates.46 Smaller numbers are generally not suitable because random fluctuations of uncertain significance may occur and AMR rates are thus easily biased. To achieve this minimum, it may be appropriate to either include isolates collected over a longer period or limit the combination of stratification criteria.
Invasive isolates should always be included, and screening isolates from surveillance cultures should be reported separately.9,47 Colonization status data should be interpreted carefully and may be taken into account only in selected cases (e.g. for post-transplantation infection prophylaxis or neutropenic fever treatment).48,49
The choice among strategies depends strongly on what is most feasible and least time-consuming for the laboratory.46,50 The first-isolate strategy, which includes the first isolate of a given species per patient per analysis period (e.g. 1 year), is simple and is generally recommended.46,50 However, eliminating subsequent isolates from the same patient does not account for subsequent occurrence of resistant mutants or strains, which may be particularly important for some pathogens, such as Enterobacter species, Serratia species, Pseudomonas aeruginosa and Acinetobacter baumannii.
Antimicrobial susceptibility test data can be displayed using qualitative categories (susceptible/intermediate/resistant) or MIC.46,51 Qualitative results are simpler for clinicians but are poorly comparable among different laboratories because of the variety of testing methods and adoption of different interpretative criteria. Importantly, the latest EUCAST interpretative categories classify non-resistant isolates in relation to antimicrobial exposure level on the basis of administration route, dose, dosing interval, infusion time and pharmacokinetics profile, emphasizing the relationship between the drug exposure of the microorganism at the infection site and the interpretative breakpoint.51
Despite the known clonal distribution of antibiotic resistance in many bacteria, empirical antibiotic selection still relies heavily on cumulative antibiograms, resulting in overuse of broad-spectrum agents. Antibiotic selection based on a genotype-specific antibiogram merges epidemiological surveillance and antimicrobial stewardship, possibly reducing the relative likelihood of antibiotic/pathogen mismatch.1,9,11 Genotyping is relevant for both infection control and AMS intervention, so it can be useful to guide therapy for severe infections (i.e. sepsis),10 but it is not strictly essential for AMS interventions. Some guidelines suggest rapid diagnostic typing methods for investigation of clonality among resistant strains to drive AMS interventions.1,9,11
4. How should AMR surveillance data be stratified to inform AMS interventions?
Four guidelines suggested AMR rates stratified by hospital unit or department,1,2,9,11 specimen type9 or age group.2 Five studies evaluated AMS interventions with AMR surveillance data stratified by hospital unit or department,26,28,31,33 specimen type,27,28 risk of MDR pathogen colonization/infection28,31 or infection type.32
Observational studies assessed AMR rates against different stratification criteria, revealing substantial differences across hospital units, specimen type, infection type and population characteristics, specifically inpatient versus outpatient and adult versus paediatric.40,47,52–55
Stratification is recommended to enhance data consistency, assuming adequate numbers of tested organisms (Table 6). Stratification based on timing of specimen collection during the course of hospitalization has revealed significant differences in AMR data. It is essential to provide better guidance for empirical therapy decisions, representing a valuable proxy for infection acquisition (community acquired versus hospital acquired).52,54–56 Hospital-acquired infections are defined as infections that occur ≥48 h after admission.40,57 Nevertheless, patients with early-onset hospital-acquired infections, variably defined as occurring within 4–7 days after admission, have lower AMR rates than patients with late onset.
Stratification strategies of antimicrobial resistance surveillance data: benefits and drawbacks
| Stratification strategy . | Benefits . | Drawbacks . |
|---|---|---|
| Timing of specimen collection |
|
|
| Unit or department |
|
|
| Sample type |
|
|
| Infection type |
|
|
| Patients’ risk (i.e. solid or haematological malignancies, cystic fibrosis, recent antibiotic administration or recent hospitalizations) |
|
|
| Age categories |
|
|
| Stratification strategy . | Benefits . | Drawbacks . |
|---|---|---|
| Timing of specimen collection |
|
|
| Unit or department |
|
|
| Sample type |
|
|
| Infection type |
|
|
| Patients’ risk (i.e. solid or haematological malignancies, cystic fibrosis, recent antibiotic administration or recent hospitalizations) |
|
|
| Age categories |
|
|
Stratification strategies of antimicrobial resistance surveillance data: benefits and drawbacks
| Stratification strategy . | Benefits . | Drawbacks . |
|---|---|---|
| Timing of specimen collection |
|
|
| Unit or department |
|
|
| Sample type |
|
|
| Infection type |
|
|
| Patients’ risk (i.e. solid or haematological malignancies, cystic fibrosis, recent antibiotic administration or recent hospitalizations) |
|
|
| Age categories |
|
|
| Stratification strategy . | Benefits . | Drawbacks . |
|---|---|---|
| Timing of specimen collection |
|
|
| Unit or department |
|
|
| Sample type |
|
|
| Infection type |
|
|
| Patients’ risk (i.e. solid or haematological malignancies, cystic fibrosis, recent antibiotic administration or recent hospitalizations) |
|
|
| Age categories |
|
|
Unit- or department-based stratification addresses case-mix differences more appropriately than hospital-wide data, representing a valuable proxy for stratifying by both age and immune status with no need for integrated demographic or background data.40,52,55,58–60 However, in units or departments where samples are collected only for severe infections or those not responding to first-line treatment, AMR rates might be inflated and lead to inappropriate therapy choice and increased AMR and cost.
Sample type-based stratification is another option.40,47,52,55 Using data from sterile sites obviates the need for integrated clinical data on case definition, which requires specific software not available in most laboratories. Reporting data from non-sterile sites (e.g. wounds) should be avoided because it can inflate AMR rates.
Adoption of AMR surveillance based either on infection type [e.g. pneumonia, intra-abdominal infections or urinary tract infections (UTIs)] or on groups of patients at high/low risk of MDR pathogen colonization/infection (e.g. solid or haematological malignancies, cystic fibrosis, recent antibiotic administrations or recent hospitalizations) has been shown to provide informative reports by combining laboratory data with clinical or background data.18,47,55,61–63 Nevertheless, the lack of information system support together with the need for a more intensive workload in the case of manual data entry often represents a serious barrier to implementation, making this option unrealistic on a routine basis in the absence of sophisticated computerized decision support systems.
Age categories (i.e. paediatrics, adults, elderly) are a feasible stratification strategy and may help to avoid AMR overestimation in paediatrics and AMR underestimation in the elderly, in whom high rates of MDR E. coli and S. aureus are well documented.53,54,64,65 Nevertheless, unit/department-based AMR surveillance represents a valuable proxy for stratification by age because it considers paediatrics as well as units that focus on elderly patients (e.g. general medicine).
5. What is the frequency of reporting AMR surveillance data to inform AMS interventions?
Five guidelines recommended reporting AMR surveillance data at least annually to inform AMS interventions.1,9,11,14,16 Three studies assessed a bundled AMS intervention providing AMR surveillance reports monthly to yearly26,31 or yearly.32
AMR surveillance reports should provide regularly updated overviews of local epidemiology. Nevertheless, a recent review of European surveillance systems highlighted that most AMR surveillance systems provide outdated reports, thus reducing their value in driving clinical decisions.4 Delayed reporting leads to suboptimal empirical prescribing that may jeopardize patient outcomes and increase MDR bacteria transmission risk. Annual reporting provides sufficient data to drive AMS,1,9,11,14,16 but in the presence of a new intervention or outbreak a higher frequency might be considered.14 The suitable time interval for reporting AMR data in high-risk patients (i.e. immunocompromised hosts) is still a matter of debate. Of note, development of automated information systems providing real-time updates on AMR data allows AMS interventions tailored to real-time antibiotic consumption data.66
6. What are the threshold levels of resistance for changing the empirical antimicrobial treatment recommendation?
Four guidelines defined a resistance level above which further empirical use of an antimicrobial drug is no longer appropriate for uncomplicated UTIs,17 hospital-acquired pneumonia (HAP), ventilator-associated pneumonia (VAP)18,19 and sepsis.10 IDSA guidelines on uncomplicated UTIs recommend against empirical use of cotrimoxazole when the resistance rate exceeds 20%,17 on the basis of trials showing that in women with acute cystitis caused by cotrimoxazole-resistant pathogens the drug has a failure rate of approximately 50%.63,67–69 Fluoroquinolones are also not recommended as empirical therapy for pyelonephritis in areas where more than 10% of UTI pathogens demonstrate resistance, primarily based on expert opinion.17 IDSA guidelines on HAP/VAP management suggest including an agent active against MRSA, either vancomycin or linezolid, for empirical treatment of suspected HAP/VAP when >10%–20% of S. aureus are MRSA.18 Prescription of two antibiotics active against P. aeruginosa is recommended for empirical treatment of suspected VAP when >10% of P. aeruginosa are resistant to the monotherapy agent. The ERS/ESICM/ESCMID/ALAT guidelines for HAP/VAP management recommend a higher cut-off rate of 25% for both Gram-negative pathogens and MRSA19 on the basis of a study identifying a resistance rate >25% as an independent variable associated with treatment failure of monotherapies for HAP caused by resistant pathogens.70 In patients with sepsis, experts suggest applying a lower threshold, not exceeding 10%–20%, which should be further reduced to 5% for immunocompromised patients.10
One study assessed resistance thresholds in the framework of an AMS intervention, changing the recommendation when the resistance rate to an antibiotic was over 25% of all isolates for the same infection during the previous year.28
Threshold definition needs to balance the risk of excessive antibiotic use against the need for effective initial antibiotic therapy, especially for invasive infections.57,67,68,71,72 Furthermore, thresholds should be adjusted for high-risk groups and vary according to infection type and severity. On the basis of the limited evidence available, 25% may represent a reasonable threshold level of resistance for using alternative agents, whereas 5%–10% may be considered for higher-risk situations, such as septic shock and neutropenia with severe infections. These or similar thresholds can be applied to updated, appropriately stratified and carefully deduplicated AMR surveillance data at the local level.
Although there is evidence to recommend a change in surgical prophylaxis in settings with a high risk of MRSA surgical site infections when nasal and skin decolonization is not performed, a clear threshold definition is lacking.73,74 There are also uncertainties regarding whether AMR surveillance should drive antibiotic surgical prophylaxis against MDR Gram-negative bacteria, although more evidence is available on the increased risk of surgical site infections in MDR Gram-negative carriers.75,76 AMR surveillance data on screening isolates can be useful for the AMS team to individualize surgical prophylaxis practice in selected cases.
7. How should AMR surveillance be tailored to AMS in settings with patients at high risk of colonization and infection by antimicrobial-resistant bacteria?
Neither guidelines nor studies addressed AMR surveillance for AMS specifically in immunocompromised patients, intensive care or paediatric units.
Development of AMS programmes in such high-risk settings is associated with unique challenges because of the complexity of management in these populations. Benefits of aggregate versus individual data must be carefully weighed. Individual-level data are preferable because individual risk factors play an important role in these populations and cannot be accounted for by aggregate data reporting (e.g. ecological bias).77 However, because of small numbers, stratification may be problematic, and a yearly report may miss critical trends. Some authors suggested limiting AMR surveillance to locally relevant resistant pathogens twice yearly.48,49 Identification of priority resistant bacteria to target at the local level is fundamental and should be based on consideration of the trends of high-priority bacteria at the national and international levels (e.g. carbapenem-resistant Enterobacteriaceae and carbapenem-resistant P. aeruginosa). Resistance rate thresholds among carriers to guide changes in empirical treatment are still difficult to establish since no evidence is available. Surveillance data from screening procedures at the unit level should be provided, as these can be helpful in making decisions for prophylaxis regimens and/or empirical treatment of invasive infections.49 Computerized tools providing time-series analyses of AMR surveillance and antimicrobial consumption can help AMS teams build clinical decision pathways by analysing temporal relationships and the effect of antimicrobial usage on AMR and forecasting variations in AMR accordingly.66,78–80
8. Should AMR surveillance reports include data from long-term care facility and outpatient settings to inform AMS interventions?
Six guidelines addressed AMS in long-term care facility (LTCF) and outpatient settings with regard to AMR surveillance: two focus on LTCFs20,22 and four on outpatient settings.11,23–25 No studies assessing an AMS intervention were performed in these settings.
Despite several studies reporting high rates of MDR bacteria in LTCF and outpatient settings, local AMR surveillance data are rarely recorded (<20% of cases in Europe).80,81 Some centres send so few cultures that numbers of bacterial isolates are insufficient to generate an AMR surveillance report yearly, while others may have sufficient data to develop only urine antibiograms.20 Moreover, they often lack on-site sampling equipment, which affects surveillance quality.82 Nevertheless, updated AMR surveillance reports from LTCF and outpatient settings can inform AMS programmes to drive appropriate empirical antimicrobial therapy not only in these settings but also in affiliated acute-care hospitals.
9. Should AMR surveillance include data from other countries to inform AMS interventions?
No guideline addressed AMS with specific reference to AMR surveillance data availability in other countries, although assessment of patient travel history has been suggested and active surveillance for patients transferred from hospitals abroad has been recommended.83
Travel (including medical tourism) is an important risk factor for AMR spread.84 The risk of acquiring new colonization with MDR Gram-negative bacteria depends on several factors (e.g. travel destination, digestive disorders, antibiotic intake), and it has been reported to vary from 21% to 85%.85,86 Thus, the latest ECDC guidance suggests surveillance by rectal screening of patients transferred across borders into a healthcare facility in another country.83 AMR rates at the global level should be made available and shared among countries, particularly in LMICs where knowledge of AMR burden is still fragmentary.87,88
10. Should AMR surveillance reports include regional and/or national surveillance data from companion and food-producing animals to inform AMS interventions in human healthcare?
No guideline or study addressed integration of AMR data on bacteria circulating in humans and animals to inform AMS interventions in human healthcare.
Global increase in MRSA has clearly shown the role of livestock MRSA in human infections.89,90 In many countries, particularly in northern Europe, critical areas and workers have been periodically screened, and WGS has been used to compare and connect human and animal strains.91 For MDR Gram-negative pathogens, several studies on ESBL-producing E. coli in poultry and pigs have reported similarities and transfer from animals to humans.92–94 AMR surveillance in both companion and terrestrial food-producing animals is an important public health objective,95 and several national authorities have introduced regulations to prevent antimicrobial overuse in the veterinary field.96
Conclusions
The link with AMR surveillance is essential for any AMS programme and should be clearly defined before starting an AMS intervention. The evidence summarized in this review provides a useful basis for a more integrated process of developing procedures to report AMR surveillance data to drive AMS interventions. These procedures should be extended to settings outside acute-care institutions, such as to outpatient and veterinary settings and LTCFs. Without proper AMR surveillance in any setting, implementation of AMS policies cannot contribute effectively to the fight against MDR pathogens and may even worsen the burden of adverse events from such interventions.
Acknowledgements
The paper is based on the discussion during the annual meeting of the EPI-Net project in Verona in September 2018. We are grateful to Francesca Chiara, Frank Leus and Ghada Zoubiane for their participation in the meeting and their important collaboration.
Members of the COACH working group
Blanca Anaya, Unit of Infectious Diseases and Clinical Microbiology, Hospital Universitario Virgen Macarena, Institute of Biomedicine of Seville (IBIS), Seville, Spain; Fabiana Arieti, Infectious Diseases Section, Department of Diagnostic and Public Health, University of Verona, Verona, Italy; Nithya Babu Rajendran, Infectious Diseases, Department of Internal Medicine I, Tübingen University Hospital, Tubingen, Germany and German Centre for Infection Research (DZIF), Clinical Research Unit for healthcare associated infections, Tübingen, Germany; Zaira R. Palacios Baena, Unit of Infectious Diseases and Clinical Microbiology, Hospital Universitario Virgen Macarena, Institute of Biomedicine of Seville (IBIS), Seville, Spain; Jesús Rodríguez-Baño, Division of Infectious Diseases, Microbiology and Preventive Medicine, Hospital Universitario Virgen Macarena, Department of Medicine, University of Seville, Biomedicine Institute of Seville (IBIS), Seville, Spain; Silvio Brusaferro, Istituto Superiore di Sanità, Rome, Italy and Department of Medicine, University of Udine, Udine, Italy; Elena Carrara, Infectious Diseases Section, Department of Diagnostic and Public Health, University of Verona, Verona, Italy; Dario Cattaneo, Unit of Clinical Pharmacology, ASST Fatebenefratelli Sacco University Hospital, Milan, Italy; Esmita Charani, NIHR Health Protection Research Unit in Healthcare Associated Infections and Antimicrobial Resistance, Faculty of Medicine, Imperial College London, London, UK; Monica Compri, Infectious Diseases Section, Department of Diagnostic and Public Health, University of Verona, Verona, Italy; Sergey Eremin, Surveillance, Prevention and Control Department, AMR Division, World Health Organization, Geneva, Switzerland; Liliana Galia, Infectious Diseases Section, Department of Diagnostic and Public Health, University of Verona, Verona, Italy; Daniele Roberto Giacobbe, Infectious Diseases Unit, Ospedale Policlinico San Martino - IRCCS, Genoa, Italy; Aina Gomila-Grange, Department of Infectious Diseases, Hospital Universitari Parc Taulí Sabadell, Barcelona, Spain; Stephan Harbarth, Infection Control Program, World Health Organization Collaborating Centre on Patient Safety, Geneva University Hospitals and Faculty of Medicine, Geneva, Switzerland; Gunnar Kahlmeter, Department of Clinical Microbiology, Växjö Central Hospital, Växjö, Sweden; Ramanan Laxminarayan, Centre for Disease Dynamics, Economics & Policy, New Delhi, India and Princeton Environmental Institute, Princeton University, Princeton, NJ, USA; Giuliana Lo Cascio, Microbiology and Virology Unit, Department of Pathology, Azienda Ospedaliera Universitaria Integrata di Verona, Verona, Italy; Fulvia Mazzaferri, Infectious Diseases Section, Department of Diagnostic and Public Health, University of Verona, Verona, Italy; Elena Mazzolini, Department of Epidemiology, Istituto Zooprofilattico Sperimentale delle Venezie, Legnaro, Padua, Italy; Michael McCarthy, AstraZeneca, Early Cardiovascular, Renal and Metabolism, Research and Development, USA; Rafael Canton, Hospital Universitario Ramón y Cajal and Instituto Ramón y Cajal de Investigación Sanitaria (IRYCIS), Madrid, Spain; Nico T. Mutters, Bonn University Hospital, Institute for Hygiene and Public Health, Bonn, Germany; Olaf Neth, Pediatric Infectious Diseases, Rheumatology and Immunology Unit, Hospital Infantil Virgen del Rocío, Instituto de Biomedicina de Sevilla (IBIS), Sevilla, Spain; Abdelhak Oualim, Sanofi-Pasteur, Swiftwater, PA, USA; Maria Diletta Pezzani, Infectious Diseases Section, Department of Diagnostic and Public Health, University of Verona, Verona, Italy; Adelina Prioteasa, Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, The Netherlands; Katia Saris, Department of Medical Microbiology and Infectious Diseases, Canisius-Wilhelmina Hospital (CWZ), REshape Center for Innovation, Radboudumc, Nijmegen, The Netherlands; Mitchell J. Schwaber, National Centre for Infection Control, Israel Ministry of Health and Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel; Remco Schrijver, VetEffecT, Bilthoven, The Netherlands; Frangiscos Sifakis, Boehringer Ingelheim Pharmaceuticals, Inc., Ridgefield, CT, USA; AstraZeneca LP, Gaithersburg, Maryland, USA; Evelina Tacconelli, Infectious Diseases Section, Department of Diagnostic and Public Health, University of Verona, Verona, Italy and Infectious Diseases, Department of Internal Medicine I, Tübingen University Hospital, Tubingen, Germany and German Centre for Infection Research (DZIF), Clinical Research Unit for healthcare associated infections, Tübingen, Germany; Cuong Vuong, AiCuris Anti-infective Cures GmbH, Wuppertal, Germany; Martin Wolkewitz, Institute of Medical Biometry and Statistics, Faculty of Medicine and Medical Center, University of Freiburg, Freiburg, Germany; Theoklis E. Zaoutis, Perelman School of Medicine at the University of Pennsylvania, Infectious Diseases Division, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA.
Funding
This work was supported by the Innovative Medicines Initiative Joint Undertaking under grant agreement number 115737, resources of which are composed of financial contribution from the European Union Seventh Framework Program (FP7/2007-2013) and European Federation of Pharmaceutical Industries and Associations (EFPIA) companies in-kind contribution. The funders had no role in the writing of this work.
Transparency declarations
All authors: none to declare. Anne McDonough, a professional medical writer, provided medical writing services for editing and proofing of this service on behalf of EPI-Net. This article forms part of a Supplement.
References
Cochrane Effective Practice and Organisation of Care (EPOC). What Study Designs Can be Considered for Inclusion in an EPOC Review and What Should They be Called? https://epoc.cochrane.org/sites/epoc.cochrane.org/files/public/uploads/Resources-for-authors2017/what_study_designs_should_be_included_in_an_epoc_review.pdf.
Australian Commission on Safety and Quality in Health Care. Antimicrobial Stewardship in Australian Health Care 2018. https://www.safetyandquality.gov.au/our-work/antimicrobial-stewardship/antimicrobial-stewardship-australian-health-care-2018.
British Society for Antimicrobial Chemotherapy. Antimicrobial Stewardship: From Principles to Practice. http://www.bsac.org.uk/antimicrobialstewardshipebook/BSAC-AntimicrobialStewardship-FromPrinciplestoPractice-eBook.pdf.
National Institute for Health and Care Excellence. Antimicrobial Stewardship: Systems and Processes for Effective Antimicrobial Medicine Use: NICE guideline [NG15]. https://www.nice.org.uk/guidance/ng15.
Department of Health Republic of South Africa. Guidelines on Implementation of the Antimicrobial Strategy in South Africa: One Health Approach & Governance. http://www.health.gov.za/index.php/antimicrobial-resistance?download=2194: antimicrobial-stewardship-guidelines-governance-june2017.
Centers for Disease Control and Prevention. Implementation of Antibiotic Stewardship Core Elements at Small and Critical Access Hospitals. https://www.cdc.gov/antibiotic-use/core-elements/small-critical.html.
SARI Hospital Antimicrobial Stewardship Working Group. Strategy for Guidelines for Antimicrobial Stewardship in Hospitals in Ireland. https://www.hpsc.ie/a-z/microbiologyantimicrobialresistance/infectioncontrolandhai/guidelines/File,4116,en.pdf.
Centers for Disease Control and Prevention. The Core Elements for Antibiotic Stewardship in Nursing Homes. https://www.cdc.gov/longtermcare/pdfs/core-elements-antibiotic-stewardship.pdf.
Quality Innovation Network National Coordinating Center. A Field Guide to Antibiotic Stewardship in Outpatient Settings. https://qioprogram.org/sites/default/files/editors/141/C310_Field_Guide_20180730_FNL.pdf.
World Health Organization. Laboratory Quality Management System: Handbook.
World Health Organization Regional Office for Africa. Guide for Establishing Laboratory-Based Surveillance for Antimicrobial Resistance. https://www.afro.who.int/sites/default/files/2017-06/guide-for-establishing-lab-based-surveillance-for-amr.pdf.
CLSI. Analysis and Presentation of Cumulative Antimicrobial Susceptibility Test Data—Fourth Edition: M39-A4.
European Committee on Antimicrobial Susceptibility Testing. Redefining Susceptibility Testing Categories S, I and R. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/EUCAST_Presentations/2018/EUCAST_-_Intermediate_category_-_information_for_all.pdf.
Center for Diseases Control, Atlanta. Multidrug-Resistant Organism & Clostridioides difficile Infection (MDRO/CDI) Module. 2020. https://www.cdc.gov/nhsn/PDFs/pscManual/12pscMDRO_CDADcurrent.pdf.
World Health Organization.
European Centre for Disease Prevention and Control. Risk Assessment on the Spread of Carbapenemase-Producing Enterobacteriaceae (CPE) through Patient Transfer between Healthcare Facilities, with Special Emphasis on Cross-border Transfer. Stockholm: ECDC,
Author notes
Maria Diletta Pezzani and Fulvia Mazzaferri Equally contributing authors.
Stephan Harbarth and Evelina Tacconelli Equally contributing last authors.
Members are listed in the Acknowledgements section.



