-
PDF
- Split View
-
Views
-
Cite
Cite
D Torumkuney, A Tunger, B Sancak, A Bıçakçıgil, B Altun, Z Aktas, C Kayacan, I Morrissey, Results from the Survey of Antibiotic Resistance (SOAR) 2015–17 in Turkey: data based on CLSI, EUCAST (dose-specific) and pharmacokinetic/pharmacodynamic (PK/PD) breakpoints, Journal of Antimicrobial Chemotherapy, Volume 75, Issue Supplement_1, April 2020, Pages i88–i99, https://doi.org/10.1093/jac/dkaa086
Close - Share Icon Share
Abstract
To determine antibiotic susceptibility of Streptococcus pneumoniae and Haemophilus influenzae isolates from community-acquired respiratory tract infections (CA-RTIs) collected in 2015–17 from Turkey.
MICs were determined by CLSI broth microdilution and susceptibility was assessed using CLSI, EUCAST (dose-specific) and pharmacokinetic/pharmacodynamic (PK/PD) breakpoints.
A total of 179 S. pneumoniae and 239 H. influenzae isolates were collected. Few (27.9%) pneumococci were penicillin susceptible by CLSI oral or EUCAST low-dose breakpoints, but by EUCAST high-dose or CLSI IV breakpoints 84.4% were susceptible. The most active antibiotics (excluding penicillin IV) by CLSI breakpoints were fluoroquinolones (98.9% of isolates susceptible), ceftriaxone (83.2%), amoxicillin (78.8%) and amoxicillin/clavulanic acid (78.8%). Pneumococcal susceptibility to amoxicillin and amoxicillin/clavulanic acid was lower using EUCAST low-dose breakpoints (49.7%), although susceptibility increased when using EUCAST high-dose (57.0%–58.1%) and PK/PD (78.8%–87.7%) breakpoints. Twenty-three H. influenzae isolates were β-lactamase positive, with 11 characterized as β-lactamase negative and ampicillin resistant following EUCAST criteria and 5 by CLSI criteria. Generally antibiotic susceptibility was high using CLSI breakpoints: ≥92.9% for all antibiotics except ampicillin (87% by CLSI and EUCAST breakpoints) and trimethoprim/sulfamethoxazole (67.4% and 72% by CLSI and EUCAST breakpoints, respectively). Susceptibility using EUCAST breakpoints (where these are published) was similar, except for cefuroxime (oral) with 3.8% of isolates susceptible. PK/PD breakpoints indicated low susceptibility to macrolides (5.9%–10%) and cefaclor (13%). The application of different EUCAST breakpoints for low and higher doses for some of the antibiotics (amoxicillin, amoxicillin/clavulanic acid, ampicillin, penicillin, ceftriaxone, clarithromycin, erythromycin, levofloxacin and trimethoprim/sulfamethoxazole) allowed, for the first time in a SOAR study, the effect of raising the dosage on susceptibility to be quantified.
Antibiotic susceptibility of S. pneumoniae was generally low, which is in keeping with evidence of inappropriate and high antibiotic use in Turkey. H. influenzae susceptibility was high. These data are important for empirical therapy of CA-RTIs.
Introduction
Community-acquired bacterial pneumonia is an important world health problem often requiring hospitalization and is clearly a major healthcare burden in terms of infrastructure and cost.1,2 Lower respiratory tract infections (LRTIs), despite being largely preventable, are a major cause of morbidity and mortality world-wide.3 Successful management of LRTIs relies on appropriate empirical antibiotic therapy through the use of both local and international guidelines,4 especially in this era of increasing global antimicrobial resistance.2 In a recent publication on antibiotic consumption in Eastern Europe during 2015, Turkey ranked highest in consumption of antibiotics for systemic use (amoxicillin with or without a β-lactamase inhibitor, ampicillin, cefepime, meropenem, ciprofloxacin and colistin) at 41.5 DDDs/1000 inhabitants.5 Turkey also had the highest DDDs/1000 inhabitants when the same analysis was performed in 2011, although for this time period it was slightly higher at 42.3.5 In a separate study, by splitting antibiotic consumption data by province or region in Turkey, there was approximately twice as much antibiotic use in western Turkey compared with eastern Turkey. This has partially been attributed to differing climate and seasonal weather variability in the different regions of Turkey.6 However, the main reason for variability was associated with poor access to healthcare.6 Resistance development is frequently associated with inappropriate antibiotic use,7 which is high in Turkey, with 29.4% of patients reported to use antibiotics without a prescription and 55.5% of patients discontinuing antibiotic medication before completing the course.8
Streptococcus pneumoniae and Haemophilus influenzae are the major bacteria associated with community-acquired respiratory tract infections (CA-RTIs).9,10 Both pathogens have shown increasing resistance to first-line antibiotics such as penicillin and ampicillin.2,11 As rates of resistance are variable from country to country, surveillance data can provide useful information to guide local antibiotic policies.
The Survey of Antibiotic Resistance (SOAR) is an international antibiotic resistance surveillance study that focuses on key respiratory pathogens from community-acquired infections and has been running since 2002 in the Middle East, Africa, Latin America, Asia-Pacific countries and Commonwealth of Independent States countries. For this study, recent SOAR data from hospitals in Turkey have been analysed to provide a picture of the current state of antibiotic susceptibility of S. pneumoniae and H. influenzae associated with CA-RTIs.
Materials and methods
Collaborating centres
The following three centres in Turkey took part in the study: Istanbul University Hospital, Ege University Hospital and Hacettepe University Hospital.
Clinical isolates
Isolates of H. influenzae and S. pneumoniae from CA-RTIs (patients with respiratory tract infections in the community, not hospitalized for more than 48 h) were sent to two central laboratories (LGC, Fordham, UK and IHMA Europe, Monthey, Switzerland) in transport swabs, where they were subcultured and re-identified. H. influenzae were re-identified by MALDI-TOF MS methodology and S. pneumoniae identity was confirmed by optochin susceptibility and bile solubility. β-Lactamase production was determined for each H. influenzae isolate by a chromogenic cephalosporin (nitrocefin) disc method. Duplicate isolates from the same patient were not accepted.
Susceptibility testing
Isolates were evaluated for antibiotic susceptibility using broth microdilution methodology recommended by CLSI.12 Both pathogens were assessed for susceptibility to amoxicillin, amoxicillin/clavulanic acid (2:1), ampicillin, azithromycin, cefaclor, cefdinir, cefditoren, cefixime, cefpodoxime, ceftriaxone, cefuroxime, clarithromycin, levofloxacin, moxifloxacin and trimethoprim/sulfamethoxazole (1:19). S. pneumoniae was also tested for susceptibility to penicillin and erythromycin.
Susceptibility to the study drugs was calculated based on CLSI breakpoints, EUCAST (dose-specific) breakpoints and pharmacokinetic/pharmacodynamic (PK/PD) breakpoints.13–15 These breakpoints are shown in Tables 1–3. To fully assess antibiotics where high-dose therapies are available, susceptibility using EUCAST criteria was also calculated by combining percentage susceptible and percentage intermediate (susceptible, increased exposure) into the susceptible category.14 The antibiotics with high-dose availability assessed in this way were as follows: amoxicillin (0.75–1 g oral, 3 × daily), amoxicillin/clavulanic acid (0.875 g amoxicillin/0.125 g clavulanic acid oral, 3 × daily), ampicillin (2 g IV, 4 × daily), penicillin (2.4 g IV, 2 MU 4–6 × daily), ceftriaxone (2 g IV, 2 × daily), clarithromycin (0.5 g oral, 2 × daily), erythromycin (1 g oral or IV, 4 × daily), levofloxacin (0.75 g oral, 2 × daily; or 0.4 g IV, 3 × daily) and trimethoprim/sulfamethoxazole (0.24 g trimethoprim/1.2 g sulfamethoxazole oral or IV, 2 × daily).14
CLSI MIC breakpoints (mg/L) used for S. pneumoniae and H. influenzae isolates
| Antibiotic . | S. pneumoniae . | H. influenzae . | ||||
|---|---|---|---|---|---|---|
| S . | I . | R . | S . | I . | R . | |
| Amoxicillin | ≤2 | 4 | ≥8 | – | – | – |
| Amoxicillin/ clavulanic acida | ≤2 | 4 | ≥8 | ≤4 | – | ≥8 |
| Ampicillin | – | – | – | ≤1 | 2 | ≥4 |
| Penicillin (2.4 g 2 MU × 4–6 IV) | ≤2 | 4 | ≥8 | – | – | – |
| Penicillin (oral) | ≤0.06 | 0.12–1 | ≥2 | – | – | – |
| Cefaclor | ≤1 | 2 | ≥4 | ≤8 | 16 | ≥32 |
| Cefdinir | ≤0.5 | 1 | ≥2 | ≤1 | – | – |
| Cefditoren | – | – | – | – | – | – |
| Cefixime | – | – | – | ≤1 | – | – |
| Cefpodoxime | ≤0.5 | 1 | ≥2 | ≤2 | – | – |
| Ceftriaxone | ≤1 | 2 | ≥4 | ≤2 | – | – |
| Cefuroximeb | ≤1 | 2 | ≥4 | ≤4 | 8 | ≥16 |
| Azithromycin | ≤0.5 | 1 | ≥2 | ≤4 | – | – |
| Clarithromycin | ≤0.25 | 0.5 | ≥1 | ≤8 | 16 | ≥32 |
| Erythromycin | ≤0.25 | 0.5 | ≥1 | – | – | – |
| Levofloxacin | ≤2 | 4 | ≥8 | ≤2 | – | – |
| Moxifloxacin | ≤1 | 2 | ≥4 | ≤1 | – | – |
| Trimethoprim/ sulfamethoxazolec | ≤0.5 | 1–2 | ≥4 | ≤0.5 | 1–2 | ≥4 |
| Antibiotic . | S. pneumoniae . | H. influenzae . | ||||
|---|---|---|---|---|---|---|
| S . | I . | R . | S . | I . | R . | |
| Amoxicillin | ≤2 | 4 | ≥8 | – | – | – |
| Amoxicillin/ clavulanic acida | ≤2 | 4 | ≥8 | ≤4 | – | ≥8 |
| Ampicillin | – | – | – | ≤1 | 2 | ≥4 |
| Penicillin (2.4 g 2 MU × 4–6 IV) | ≤2 | 4 | ≥8 | – | – | – |
| Penicillin (oral) | ≤0.06 | 0.12–1 | ≥2 | – | – | – |
| Cefaclor | ≤1 | 2 | ≥4 | ≤8 | 16 | ≥32 |
| Cefdinir | ≤0.5 | 1 | ≥2 | ≤1 | – | – |
| Cefditoren | – | – | – | – | – | – |
| Cefixime | – | – | – | ≤1 | – | – |
| Cefpodoxime | ≤0.5 | 1 | ≥2 | ≤2 | – | – |
| Ceftriaxone | ≤1 | 2 | ≥4 | ≤2 | – | – |
| Cefuroximeb | ≤1 | 2 | ≥4 | ≤4 | 8 | ≥16 |
| Azithromycin | ≤0.5 | 1 | ≥2 | ≤4 | – | – |
| Clarithromycin | ≤0.25 | 0.5 | ≥1 | ≤8 | 16 | ≥32 |
| Erythromycin | ≤0.25 | 0.5 | ≥1 | – | – | – |
| Levofloxacin | ≤2 | 4 | ≥8 | ≤2 | – | – |
| Moxifloxacin | ≤1 | 2 | ≥4 | ≤1 | – | – |
| Trimethoprim/ sulfamethoxazolec | ≤0.5 | 1–2 | ≥4 | ≤0.5 | 1–2 | ≥4 |
S, susceptible; I, intermediate; R, resistant; –, not applicable.
Amoxicillin/clavulanic acid was tested at a 2:1 amoxicillin to clavulanic acid ratio; breakpoints are expressed as the amoxicillin component.
Breakpoints used are for cefuroxime axetil (oral).
Trimethoprim/sulfamethoxazole was tested at a 1:19 trimethoprim to sulfamethoxazole ratio; breakpoints are expressed as the trimethoprim component.
CLSI MIC breakpoints (mg/L) used for S. pneumoniae and H. influenzae isolates
| Antibiotic . | S. pneumoniae . | H. influenzae . | ||||
|---|---|---|---|---|---|---|
| S . | I . | R . | S . | I . | R . | |
| Amoxicillin | ≤2 | 4 | ≥8 | – | – | – |
| Amoxicillin/ clavulanic acida | ≤2 | 4 | ≥8 | ≤4 | – | ≥8 |
| Ampicillin | – | – | – | ≤1 | 2 | ≥4 |
| Penicillin (2.4 g 2 MU × 4–6 IV) | ≤2 | 4 | ≥8 | – | – | – |
| Penicillin (oral) | ≤0.06 | 0.12–1 | ≥2 | – | – | – |
| Cefaclor | ≤1 | 2 | ≥4 | ≤8 | 16 | ≥32 |
| Cefdinir | ≤0.5 | 1 | ≥2 | ≤1 | – | – |
| Cefditoren | – | – | – | – | – | – |
| Cefixime | – | – | – | ≤1 | – | – |
| Cefpodoxime | ≤0.5 | 1 | ≥2 | ≤2 | – | – |
| Ceftriaxone | ≤1 | 2 | ≥4 | ≤2 | – | – |
| Cefuroximeb | ≤1 | 2 | ≥4 | ≤4 | 8 | ≥16 |
| Azithromycin | ≤0.5 | 1 | ≥2 | ≤4 | – | – |
| Clarithromycin | ≤0.25 | 0.5 | ≥1 | ≤8 | 16 | ≥32 |
| Erythromycin | ≤0.25 | 0.5 | ≥1 | – | – | – |
| Levofloxacin | ≤2 | 4 | ≥8 | ≤2 | – | – |
| Moxifloxacin | ≤1 | 2 | ≥4 | ≤1 | – | – |
| Trimethoprim/ sulfamethoxazolec | ≤0.5 | 1–2 | ≥4 | ≤0.5 | 1–2 | ≥4 |
| Antibiotic . | S. pneumoniae . | H. influenzae . | ||||
|---|---|---|---|---|---|---|
| S . | I . | R . | S . | I . | R . | |
| Amoxicillin | ≤2 | 4 | ≥8 | – | – | – |
| Amoxicillin/ clavulanic acida | ≤2 | 4 | ≥8 | ≤4 | – | ≥8 |
| Ampicillin | – | – | – | ≤1 | 2 | ≥4 |
| Penicillin (2.4 g 2 MU × 4–6 IV) | ≤2 | 4 | ≥8 | – | – | – |
| Penicillin (oral) | ≤0.06 | 0.12–1 | ≥2 | – | – | – |
| Cefaclor | ≤1 | 2 | ≥4 | ≤8 | 16 | ≥32 |
| Cefdinir | ≤0.5 | 1 | ≥2 | ≤1 | – | – |
| Cefditoren | – | – | – | – | – | – |
| Cefixime | – | – | – | ≤1 | – | – |
| Cefpodoxime | ≤0.5 | 1 | ≥2 | ≤2 | – | – |
| Ceftriaxone | ≤1 | 2 | ≥4 | ≤2 | – | – |
| Cefuroximeb | ≤1 | 2 | ≥4 | ≤4 | 8 | ≥16 |
| Azithromycin | ≤0.5 | 1 | ≥2 | ≤4 | – | – |
| Clarithromycin | ≤0.25 | 0.5 | ≥1 | ≤8 | 16 | ≥32 |
| Erythromycin | ≤0.25 | 0.5 | ≥1 | – | – | – |
| Levofloxacin | ≤2 | 4 | ≥8 | ≤2 | – | – |
| Moxifloxacin | ≤1 | 2 | ≥4 | ≤1 | – | – |
| Trimethoprim/ sulfamethoxazolec | ≤0.5 | 1–2 | ≥4 | ≤0.5 | 1–2 | ≥4 |
S, susceptible; I, intermediate; R, resistant; –, not applicable.
Amoxicillin/clavulanic acid was tested at a 2:1 amoxicillin to clavulanic acid ratio; breakpoints are expressed as the amoxicillin component.
Breakpoints used are for cefuroxime axetil (oral).
Trimethoprim/sulfamethoxazole was tested at a 1:19 trimethoprim to sulfamethoxazole ratio; breakpoints are expressed as the trimethoprim component.
EUCAST (dose-specific) MIC breakpoints (mg/L) used for S. pneumoniae and H. influenzae isolates
| Antibiotic . | S. pneumoniae . | H. influenzae . | ||
|---|---|---|---|---|
| S . | R . | S . | R . | |
| Amoxicillin (0.5 g × 3 oral) | ≤0.5 | >1 | – | – |
| Amoxicillin (0.75–1 g × 3 oral) | ≤1 | >1 | ≤2 | >2 |
| Amoxicillin/clavulanic acida (0.5 g/0.125 g × 3 oral) | ≤0.5 | >1 | – | – |
| Amoxicillin/clavulanic acida (0.875 g/0.125 g × 3 oral) | ≤1 | >1 | ≤2 | >2 |
| Ampicillin (2 g × 3 IV) | ≤0.5 | >2 | ≤1 | >1 |
| Ampicillin (2 g × 4 IV) | ≤2 | >2 | ≤1 | >1 |
| Penicillin (0.6 g 1 MU × 4 IV) | ≤0.06 | >2 | – | – |
| Penicillin (2.4 g 2 MU × 4–6 IV) | ≤2 | >2 | – | – |
| Cefaclor | ≤0.03 | >0.5 | – | – |
| Cefdinir | – | – | – | – |
| Cefditoren | – | – | – | – |
| Cefixime | – | – | ≤0.12 | >0.12 |
| Cefpodoxime | ≤0.25 | >0.5 | ≤0.25 | >0.25 |
| Ceftriaxone (1 g × 1 IV) | ≤0.5 | >2 | ≤0.12 | >0.12 |
| Ceftriaxone (2 g × 2 IV) | ≤2 | >2 | ≤0.12 | >0.12 |
| Cefuroximeb | ≤0.25 | >0.5 | ≤0.12 | >1 |
| Azithromycin | ≤0.25 | >0.5 | – | – |
| Clarithromycin (0.25 g × 2 oral) | ≤0.25 | >0.5 | – | – |
| Clarithromycin (0.5 g × 2 oral) | ≤0.5 | >0.5 | – | – |
| Erythromycin (0.5 g × 2–4 oral or 0.5 g × 2–4 IV) | ≤0.25 | >0.5 | – | – |
| Erythromycin (1 g × 4 oral or 1 g × 4 IV) | ≤0.5 | >0.5 | – | – |
| Levofloxacin (0.5 g × 2 oral or 0.4 g × 2 IV) | ≤2 | >2 | ≤0.06 | >0.06 |
| Levofloxacin (0.75 g × 2 oral or 0.4 g × 3 IV) | ≤2 | >2 | ≤0.06 | >0.06 |
| Moxifloxacin | ≤0.5 | >0.5 | ≤0.12 | >0.12 |
| Trimethoprim/sulfamethoxazolec (0.16 g/0.8 g × 2 oral or IV) | ≤1 | >2 | ≤0.5 | >1 |
| Trimethoprim/sulfamethoxazolec (0.24 g/1.2 g × 2 oral or IV) | ≤2 | >2 | ≤1 | >1 |
| Antibiotic . | S. pneumoniae . | H. influenzae . | ||
|---|---|---|---|---|
| S . | R . | S . | R . | |
| Amoxicillin (0.5 g × 3 oral) | ≤0.5 | >1 | – | – |
| Amoxicillin (0.75–1 g × 3 oral) | ≤1 | >1 | ≤2 | >2 |
| Amoxicillin/clavulanic acida (0.5 g/0.125 g × 3 oral) | ≤0.5 | >1 | – | – |
| Amoxicillin/clavulanic acida (0.875 g/0.125 g × 3 oral) | ≤1 | >1 | ≤2 | >2 |
| Ampicillin (2 g × 3 IV) | ≤0.5 | >2 | ≤1 | >1 |
| Ampicillin (2 g × 4 IV) | ≤2 | >2 | ≤1 | >1 |
| Penicillin (0.6 g 1 MU × 4 IV) | ≤0.06 | >2 | – | – |
| Penicillin (2.4 g 2 MU × 4–6 IV) | ≤2 | >2 | – | – |
| Cefaclor | ≤0.03 | >0.5 | – | – |
| Cefdinir | – | – | – | – |
| Cefditoren | – | – | – | – |
| Cefixime | – | – | ≤0.12 | >0.12 |
| Cefpodoxime | ≤0.25 | >0.5 | ≤0.25 | >0.25 |
| Ceftriaxone (1 g × 1 IV) | ≤0.5 | >2 | ≤0.12 | >0.12 |
| Ceftriaxone (2 g × 2 IV) | ≤2 | >2 | ≤0.12 | >0.12 |
| Cefuroximeb | ≤0.25 | >0.5 | ≤0.12 | >1 |
| Azithromycin | ≤0.25 | >0.5 | – | – |
| Clarithromycin (0.25 g × 2 oral) | ≤0.25 | >0.5 | – | – |
| Clarithromycin (0.5 g × 2 oral) | ≤0.5 | >0.5 | – | – |
| Erythromycin (0.5 g × 2–4 oral or 0.5 g × 2–4 IV) | ≤0.25 | >0.5 | – | – |
| Erythromycin (1 g × 4 oral or 1 g × 4 IV) | ≤0.5 | >0.5 | – | – |
| Levofloxacin (0.5 g × 2 oral or 0.4 g × 2 IV) | ≤2 | >2 | ≤0.06 | >0.06 |
| Levofloxacin (0.75 g × 2 oral or 0.4 g × 3 IV) | ≤2 | >2 | ≤0.06 | >0.06 |
| Moxifloxacin | ≤0.5 | >0.5 | ≤0.12 | >0.12 |
| Trimethoprim/sulfamethoxazolec (0.16 g/0.8 g × 2 oral or IV) | ≤1 | >2 | ≤0.5 | >1 |
| Trimethoprim/sulfamethoxazolec (0.24 g/1.2 g × 2 oral or IV) | ≤2 | >2 | ≤1 | >1 |
S, susceptible; R, resistant; –, not applicable.
The I category is not listed, but is interpreted as the values between the S and the R breakpoints. If the S and R breakpoints are the same value there is no I category.14
Amoxicillin/clavulanic acid was tested at a 2:1 amoxicillin to clavulanic acid ratio; breakpoints are expressed as the amoxicillin component.
Breakpoints used are for cefuroxime axetil (oral).
Trimethoprim/sulfamethoxazole was tested at a 1:19 trimethoprim to sulfamethoxazole ratio; breakpoints are expressed as the trimethoprim component.
EUCAST (dose-specific) MIC breakpoints (mg/L) used for S. pneumoniae and H. influenzae isolates
| Antibiotic . | S. pneumoniae . | H. influenzae . | ||
|---|---|---|---|---|
| S . | R . | S . | R . | |
| Amoxicillin (0.5 g × 3 oral) | ≤0.5 | >1 | – | – |
| Amoxicillin (0.75–1 g × 3 oral) | ≤1 | >1 | ≤2 | >2 |
| Amoxicillin/clavulanic acida (0.5 g/0.125 g × 3 oral) | ≤0.5 | >1 | – | – |
| Amoxicillin/clavulanic acida (0.875 g/0.125 g × 3 oral) | ≤1 | >1 | ≤2 | >2 |
| Ampicillin (2 g × 3 IV) | ≤0.5 | >2 | ≤1 | >1 |
| Ampicillin (2 g × 4 IV) | ≤2 | >2 | ≤1 | >1 |
| Penicillin (0.6 g 1 MU × 4 IV) | ≤0.06 | >2 | – | – |
| Penicillin (2.4 g 2 MU × 4–6 IV) | ≤2 | >2 | – | – |
| Cefaclor | ≤0.03 | >0.5 | – | – |
| Cefdinir | – | – | – | – |
| Cefditoren | – | – | – | – |
| Cefixime | – | – | ≤0.12 | >0.12 |
| Cefpodoxime | ≤0.25 | >0.5 | ≤0.25 | >0.25 |
| Ceftriaxone (1 g × 1 IV) | ≤0.5 | >2 | ≤0.12 | >0.12 |
| Ceftriaxone (2 g × 2 IV) | ≤2 | >2 | ≤0.12 | >0.12 |
| Cefuroximeb | ≤0.25 | >0.5 | ≤0.12 | >1 |
| Azithromycin | ≤0.25 | >0.5 | – | – |
| Clarithromycin (0.25 g × 2 oral) | ≤0.25 | >0.5 | – | – |
| Clarithromycin (0.5 g × 2 oral) | ≤0.5 | >0.5 | – | – |
| Erythromycin (0.5 g × 2–4 oral or 0.5 g × 2–4 IV) | ≤0.25 | >0.5 | – | – |
| Erythromycin (1 g × 4 oral or 1 g × 4 IV) | ≤0.5 | >0.5 | – | – |
| Levofloxacin (0.5 g × 2 oral or 0.4 g × 2 IV) | ≤2 | >2 | ≤0.06 | >0.06 |
| Levofloxacin (0.75 g × 2 oral or 0.4 g × 3 IV) | ≤2 | >2 | ≤0.06 | >0.06 |
| Moxifloxacin | ≤0.5 | >0.5 | ≤0.12 | >0.12 |
| Trimethoprim/sulfamethoxazolec (0.16 g/0.8 g × 2 oral or IV) | ≤1 | >2 | ≤0.5 | >1 |
| Trimethoprim/sulfamethoxazolec (0.24 g/1.2 g × 2 oral or IV) | ≤2 | >2 | ≤1 | >1 |
| Antibiotic . | S. pneumoniae . | H. influenzae . | ||
|---|---|---|---|---|
| S . | R . | S . | R . | |
| Amoxicillin (0.5 g × 3 oral) | ≤0.5 | >1 | – | – |
| Amoxicillin (0.75–1 g × 3 oral) | ≤1 | >1 | ≤2 | >2 |
| Amoxicillin/clavulanic acida (0.5 g/0.125 g × 3 oral) | ≤0.5 | >1 | – | – |
| Amoxicillin/clavulanic acida (0.875 g/0.125 g × 3 oral) | ≤1 | >1 | ≤2 | >2 |
| Ampicillin (2 g × 3 IV) | ≤0.5 | >2 | ≤1 | >1 |
| Ampicillin (2 g × 4 IV) | ≤2 | >2 | ≤1 | >1 |
| Penicillin (0.6 g 1 MU × 4 IV) | ≤0.06 | >2 | – | – |
| Penicillin (2.4 g 2 MU × 4–6 IV) | ≤2 | >2 | – | – |
| Cefaclor | ≤0.03 | >0.5 | – | – |
| Cefdinir | – | – | – | – |
| Cefditoren | – | – | – | – |
| Cefixime | – | – | ≤0.12 | >0.12 |
| Cefpodoxime | ≤0.25 | >0.5 | ≤0.25 | >0.25 |
| Ceftriaxone (1 g × 1 IV) | ≤0.5 | >2 | ≤0.12 | >0.12 |
| Ceftriaxone (2 g × 2 IV) | ≤2 | >2 | ≤0.12 | >0.12 |
| Cefuroximeb | ≤0.25 | >0.5 | ≤0.12 | >1 |
| Azithromycin | ≤0.25 | >0.5 | – | – |
| Clarithromycin (0.25 g × 2 oral) | ≤0.25 | >0.5 | – | – |
| Clarithromycin (0.5 g × 2 oral) | ≤0.5 | >0.5 | – | – |
| Erythromycin (0.5 g × 2–4 oral or 0.5 g × 2–4 IV) | ≤0.25 | >0.5 | – | – |
| Erythromycin (1 g × 4 oral or 1 g × 4 IV) | ≤0.5 | >0.5 | – | – |
| Levofloxacin (0.5 g × 2 oral or 0.4 g × 2 IV) | ≤2 | >2 | ≤0.06 | >0.06 |
| Levofloxacin (0.75 g × 2 oral or 0.4 g × 3 IV) | ≤2 | >2 | ≤0.06 | >0.06 |
| Moxifloxacin | ≤0.5 | >0.5 | ≤0.12 | >0.12 |
| Trimethoprim/sulfamethoxazolec (0.16 g/0.8 g × 2 oral or IV) | ≤1 | >2 | ≤0.5 | >1 |
| Trimethoprim/sulfamethoxazolec (0.24 g/1.2 g × 2 oral or IV) | ≤2 | >2 | ≤1 | >1 |
S, susceptible; R, resistant; –, not applicable.
The I category is not listed, but is interpreted as the values between the S and the R breakpoints. If the S and R breakpoints are the same value there is no I category.14
Amoxicillin/clavulanic acid was tested at a 2:1 amoxicillin to clavulanic acid ratio; breakpoints are expressed as the amoxicillin component.
Breakpoints used are for cefuroxime axetil (oral).
Trimethoprim/sulfamethoxazole was tested at a 1:19 trimethoprim to sulfamethoxazole ratio; breakpoints are expressed as the trimethoprim component.
PK/PD MIC breakpoints (mg/L) used for S. pneumoniae and H. influenzae isolates
| Antibiotic . | S. pneumoniae and H. influenzae . |
|---|---|
| S only . | |
| Amoxicillin (1.5 g/day) | ≤2 |
| Amoxicillin (4 g/day) | ≤4 |
| Amoxicillin/clavulanic acida (1.75 g/0.25 g/day adults; 45 mg/6.4 mg/kg/day children) | ≤2 |
| Amoxicillin/clavulanic acidb (4 g/0.25 g/day adults; 90 mg/6.4 mg/kg/day children) | ≤4 |
| Ampicillin | – |
| Penicillin | – |
| Cefaclor | ≤0.5 |
| Cefdinir | ≤0.25 |
| Cefditoren | – |
| Cefixime | ≤1 |
| Cefpodoxime | ≤0.5 |
| Ceftriaxone | ≤1 |
| Cefuroximec | ≤1 |
| Azithromycin | ≤0.12 |
| Clarithromycin | ≤0.25 |
| Erythromycin | ≤0.25 |
| Levofloxacin | ≤2 |
| Moxifloxacin | ≤1 |
| Trimethoprim/sulfamethoxazoled | ≤0.5 |
| Antibiotic . | S. pneumoniae and H. influenzae . |
|---|---|
| S only . | |
| Amoxicillin (1.5 g/day) | ≤2 |
| Amoxicillin (4 g/day) | ≤4 |
| Amoxicillin/clavulanic acida (1.75 g/0.25 g/day adults; 45 mg/6.4 mg/kg/day children) | ≤2 |
| Amoxicillin/clavulanic acidb (4 g/0.25 g/day adults; 90 mg/6.4 mg/kg/day children) | ≤4 |
| Ampicillin | – |
| Penicillin | – |
| Cefaclor | ≤0.5 |
| Cefdinir | ≤0.25 |
| Cefditoren | – |
| Cefixime | ≤1 |
| Cefpodoxime | ≤0.5 |
| Ceftriaxone | ≤1 |
| Cefuroximec | ≤1 |
| Azithromycin | ≤0.12 |
| Clarithromycin | ≤0.25 |
| Erythromycin | ≤0.25 |
| Levofloxacin | ≤2 |
| Moxifloxacin | ≤1 |
| Trimethoprim/sulfamethoxazoled | ≤0.5 |
S, susceptible; –, not applicable.
Amoxicillin/clavulanic acid for low dose in adults/children.
Amoxicillin/clavulanic acid for high dose in adults/children.
Breakpoints used are for cefuroxime axetil (oral).
Trimethoprim/sulfamethoxazole was tested at a 1:19 trimethoprim to sulfamethoxazole ratio; breakpoints are expressed as the trimethoprim component.
PK/PD MIC breakpoints (mg/L) used for S. pneumoniae and H. influenzae isolates
| Antibiotic . | S. pneumoniae and H. influenzae . |
|---|---|
| S only . | |
| Amoxicillin (1.5 g/day) | ≤2 |
| Amoxicillin (4 g/day) | ≤4 |
| Amoxicillin/clavulanic acida (1.75 g/0.25 g/day adults; 45 mg/6.4 mg/kg/day children) | ≤2 |
| Amoxicillin/clavulanic acidb (4 g/0.25 g/day adults; 90 mg/6.4 mg/kg/day children) | ≤4 |
| Ampicillin | – |
| Penicillin | – |
| Cefaclor | ≤0.5 |
| Cefdinir | ≤0.25 |
| Cefditoren | – |
| Cefixime | ≤1 |
| Cefpodoxime | ≤0.5 |
| Ceftriaxone | ≤1 |
| Cefuroximec | ≤1 |
| Azithromycin | ≤0.12 |
| Clarithromycin | ≤0.25 |
| Erythromycin | ≤0.25 |
| Levofloxacin | ≤2 |
| Moxifloxacin | ≤1 |
| Trimethoprim/sulfamethoxazoled | ≤0.5 |
| Antibiotic . | S. pneumoniae and H. influenzae . |
|---|---|
| S only . | |
| Amoxicillin (1.5 g/day) | ≤2 |
| Amoxicillin (4 g/day) | ≤4 |
| Amoxicillin/clavulanic acida (1.75 g/0.25 g/day adults; 45 mg/6.4 mg/kg/day children) | ≤2 |
| Amoxicillin/clavulanic acidb (4 g/0.25 g/day adults; 90 mg/6.4 mg/kg/day children) | ≤4 |
| Ampicillin | – |
| Penicillin | – |
| Cefaclor | ≤0.5 |
| Cefdinir | ≤0.25 |
| Cefditoren | – |
| Cefixime | ≤1 |
| Cefpodoxime | ≤0.5 |
| Ceftriaxone | ≤1 |
| Cefuroximec | ≤1 |
| Azithromycin | ≤0.12 |
| Clarithromycin | ≤0.25 |
| Erythromycin | ≤0.25 |
| Levofloxacin | ≤2 |
| Moxifloxacin | ≤1 |
| Trimethoprim/sulfamethoxazoled | ≤0.5 |
S, susceptible; –, not applicable.
Amoxicillin/clavulanic acid for low dose in adults/children.
Amoxicillin/clavulanic acid for high dose in adults/children.
Breakpoints used are for cefuroxime axetil (oral).
Trimethoprim/sulfamethoxazole was tested at a 1:19 trimethoprim to sulfamethoxazole ratio; breakpoints are expressed as the trimethoprim component.
Quality control and data analysis
Quality control strains S. pneumoniae ATCC 49619, Escherichia coli ATCC 25922, H. influenzae ATCC 49247, H. influenzae ATCC 49766 and E. coli ATCC 35218 were included on each day of testing. Results of susceptibility testing were accepted if the results for the control strains were within published limits. Differences in susceptibility (using CLSI criteria) across penicillin susceptibility (S. pneumoniae only) were assessed for statistical significance with Fisher’s exact test using XLSTAT version 2019.1.3.57796. A P value <0.05 was considered statistically significant.
Ethics
SOAR studies are not human subject studies. During the study, only microorganisms were examined.
Results
S. pneumoniae isolates
A total of 179 S. pneumoniae isolates were collected from three centres in Turkey from 2015 to 2017. Most isolates came from sputum (n = 108; 60.3%), with the remainder from blood (n = 24; 13.4%), transtracheal aspirate (n = 23; 12.8%), bronchoalveolar lavage (n = 20; 11.2%) and middle ear effusion (n = 4; 2.2%). Most isolates (n = 94; 52.5%) came from adults (aged 13–64 years). Of the remaining isolates, 56 (31.3%) were from elderly patients (aged ≥65 years) and 29 (16.2%) from paediatric patients (aged ≤12 years).
Summary MIC and susceptibility data for all 179 S. pneumoniae isolates are shown in Tables 4–6 and Figures 1–3. MIC distribution data are shown in Table S1 (available as Supplementary data at JAC Online).
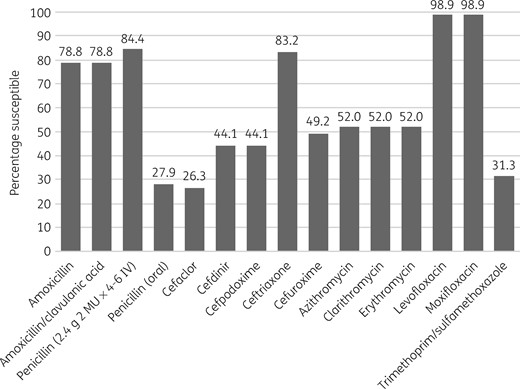
Percentage antibiotic susceptibility rates of S. pneumoniae isolates (n = 179) from Turkey based on CLSI breakpoints.
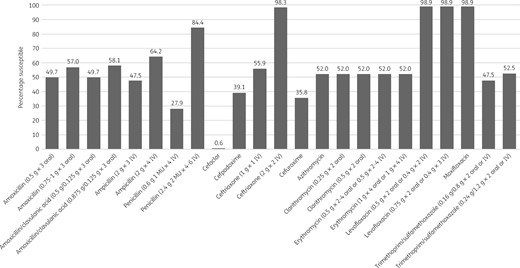
Percentage antibiotic susceptibility rates of S. pneumoniae isolates (n = 179) from Turkey based on EUCAST (dose-specific) breakpoints.
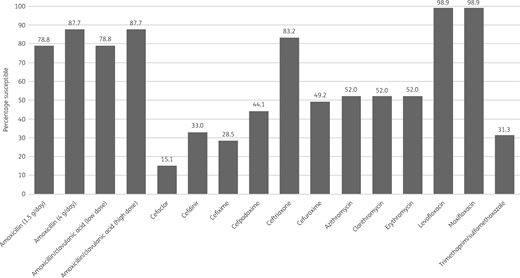
Percentage antibiotic susceptibility rates of S. pneumoniae isolates (n = 179) from Turkey based on PK/PD breakpoints. Low-dose amoxicillin/clavulanic acid = 1.75 g/0.25 g/day adults; 45 mg/6.4 mg/kg/day children. High-dose amoxicillin/clavulanic acid = 4 g/0.25 g/day adults; 90 mg/6.4 mg/kg/day children.
MIC and susceptibility data for S. pneumoniae isolates (n = 179) from Turkey using CLSI breakpoints
| Antibiotic . | MIC (mg/L) . | CLSI susceptibility . | |||||
|---|---|---|---|---|---|---|---|
| 50% . | 90% . | min . | max . | %S . | %I . | %R . | |
| Amoxicillin | 1 | 8 | ≤0.015 | 8 | 78.8 | 8.9 | 12.3 |
| Amoxicillin/clavulanic acid | 1 | 8 | ≤0.015 | 8 | 78.8 | 8.9 | 12.3 |
| Penicillin (2.4 g 2 MU × 4–6 IV) | 0.5 | 4 | ≤0.03 | 4 | 84.4 | 15.6 | 0.0 |
| Penicillin (oral) | 0.5 | 4 | ≤0.03 | 4 | 27.9 | 29.1 | 43.0 |
| Cefaclor | 16 | >32 | ≤0.03 | >32 | 26.3 | 10.1 | 63.7 |
| Cefdinir | 2 | 16 | ≤0.015 | 16 | 44.1 | 5.0 | 50.8 |
| Cefditoren | 0.25 | 1 | ≤0.015 | 4 | – | – | – |
| Cefixime | 8 | 64 | ≤0.06 | >64 | – | – | – |
| Cefpodoxime | 2 | 16 | ≤0.015 | >16 | 44.1 | 5.6 | 50.3 |
| Ceftriaxone | 0.5 | 2 | ≤0.015 | 8 | 83.2 | 15.1 | 1.7 |
| Cefuroxime | 2 | 16 | ≤0.015 | >16 | 49.2 | 2.8 | 48.0 |
| Azithromycin | 0.12 | >32 | ≤0.03 | >32 | 52.0 | 1.1 | 46.9 |
| Clarithromycin | 0.03 | >16 | ≤0.015 | >16 | 52.0 | 0.0 | 48.0 |
| Erythromycin | 0.06 | >16 | ≤0.015 | >16 | 52.0 | 0.0 | 48.0 |
| Levofloxacin | 1 | 1 | ≤0.06 | 32 | 98.9 | 0.0 | 1.1 |
| Moxifloxacin | 0.12 | 0.25 | ≤0.03 | 4 | 98.9 | 0.6 | 0.6 |
| Trimethoprim/sulfamethoxazole | 2 | 16 | 0.12 | 32 | 31.3 | 21.2 | 47.5 |
| Antibiotic . | MIC (mg/L) . | CLSI susceptibility . | |||||
|---|---|---|---|---|---|---|---|
| 50% . | 90% . | min . | max . | %S . | %I . | %R . | |
| Amoxicillin | 1 | 8 | ≤0.015 | 8 | 78.8 | 8.9 | 12.3 |
| Amoxicillin/clavulanic acid | 1 | 8 | ≤0.015 | 8 | 78.8 | 8.9 | 12.3 |
| Penicillin (2.4 g 2 MU × 4–6 IV) | 0.5 | 4 | ≤0.03 | 4 | 84.4 | 15.6 | 0.0 |
| Penicillin (oral) | 0.5 | 4 | ≤0.03 | 4 | 27.9 | 29.1 | 43.0 |
| Cefaclor | 16 | >32 | ≤0.03 | >32 | 26.3 | 10.1 | 63.7 |
| Cefdinir | 2 | 16 | ≤0.015 | 16 | 44.1 | 5.0 | 50.8 |
| Cefditoren | 0.25 | 1 | ≤0.015 | 4 | – | – | – |
| Cefixime | 8 | 64 | ≤0.06 | >64 | – | – | – |
| Cefpodoxime | 2 | 16 | ≤0.015 | >16 | 44.1 | 5.6 | 50.3 |
| Ceftriaxone | 0.5 | 2 | ≤0.015 | 8 | 83.2 | 15.1 | 1.7 |
| Cefuroxime | 2 | 16 | ≤0.015 | >16 | 49.2 | 2.8 | 48.0 |
| Azithromycin | 0.12 | >32 | ≤0.03 | >32 | 52.0 | 1.1 | 46.9 |
| Clarithromycin | 0.03 | >16 | ≤0.015 | >16 | 52.0 | 0.0 | 48.0 |
| Erythromycin | 0.06 | >16 | ≤0.015 | >16 | 52.0 | 0.0 | 48.0 |
| Levofloxacin | 1 | 1 | ≤0.06 | 32 | 98.9 | 0.0 | 1.1 |
| Moxifloxacin | 0.12 | 0.25 | ≤0.03 | 4 | 98.9 | 0.6 | 0.6 |
| Trimethoprim/sulfamethoxazole | 2 | 16 | 0.12 | 32 | 31.3 | 21.2 | 47.5 |
min, minimum; max, maximum; S, susceptible; I, intermediate; R, resistant; –, not applicable.
MIC and susceptibility data for S. pneumoniae isolates (n = 179) from Turkey using CLSI breakpoints
| Antibiotic . | MIC (mg/L) . | CLSI susceptibility . | |||||
|---|---|---|---|---|---|---|---|
| 50% . | 90% . | min . | max . | %S . | %I . | %R . | |
| Amoxicillin | 1 | 8 | ≤0.015 | 8 | 78.8 | 8.9 | 12.3 |
| Amoxicillin/clavulanic acid | 1 | 8 | ≤0.015 | 8 | 78.8 | 8.9 | 12.3 |
| Penicillin (2.4 g 2 MU × 4–6 IV) | 0.5 | 4 | ≤0.03 | 4 | 84.4 | 15.6 | 0.0 |
| Penicillin (oral) | 0.5 | 4 | ≤0.03 | 4 | 27.9 | 29.1 | 43.0 |
| Cefaclor | 16 | >32 | ≤0.03 | >32 | 26.3 | 10.1 | 63.7 |
| Cefdinir | 2 | 16 | ≤0.015 | 16 | 44.1 | 5.0 | 50.8 |
| Cefditoren | 0.25 | 1 | ≤0.015 | 4 | – | – | – |
| Cefixime | 8 | 64 | ≤0.06 | >64 | – | – | – |
| Cefpodoxime | 2 | 16 | ≤0.015 | >16 | 44.1 | 5.6 | 50.3 |
| Ceftriaxone | 0.5 | 2 | ≤0.015 | 8 | 83.2 | 15.1 | 1.7 |
| Cefuroxime | 2 | 16 | ≤0.015 | >16 | 49.2 | 2.8 | 48.0 |
| Azithromycin | 0.12 | >32 | ≤0.03 | >32 | 52.0 | 1.1 | 46.9 |
| Clarithromycin | 0.03 | >16 | ≤0.015 | >16 | 52.0 | 0.0 | 48.0 |
| Erythromycin | 0.06 | >16 | ≤0.015 | >16 | 52.0 | 0.0 | 48.0 |
| Levofloxacin | 1 | 1 | ≤0.06 | 32 | 98.9 | 0.0 | 1.1 |
| Moxifloxacin | 0.12 | 0.25 | ≤0.03 | 4 | 98.9 | 0.6 | 0.6 |
| Trimethoprim/sulfamethoxazole | 2 | 16 | 0.12 | 32 | 31.3 | 21.2 | 47.5 |
| Antibiotic . | MIC (mg/L) . | CLSI susceptibility . | |||||
|---|---|---|---|---|---|---|---|
| 50% . | 90% . | min . | max . | %S . | %I . | %R . | |
| Amoxicillin | 1 | 8 | ≤0.015 | 8 | 78.8 | 8.9 | 12.3 |
| Amoxicillin/clavulanic acid | 1 | 8 | ≤0.015 | 8 | 78.8 | 8.9 | 12.3 |
| Penicillin (2.4 g 2 MU × 4–6 IV) | 0.5 | 4 | ≤0.03 | 4 | 84.4 | 15.6 | 0.0 |
| Penicillin (oral) | 0.5 | 4 | ≤0.03 | 4 | 27.9 | 29.1 | 43.0 |
| Cefaclor | 16 | >32 | ≤0.03 | >32 | 26.3 | 10.1 | 63.7 |
| Cefdinir | 2 | 16 | ≤0.015 | 16 | 44.1 | 5.0 | 50.8 |
| Cefditoren | 0.25 | 1 | ≤0.015 | 4 | – | – | – |
| Cefixime | 8 | 64 | ≤0.06 | >64 | – | – | – |
| Cefpodoxime | 2 | 16 | ≤0.015 | >16 | 44.1 | 5.6 | 50.3 |
| Ceftriaxone | 0.5 | 2 | ≤0.015 | 8 | 83.2 | 15.1 | 1.7 |
| Cefuroxime | 2 | 16 | ≤0.015 | >16 | 49.2 | 2.8 | 48.0 |
| Azithromycin | 0.12 | >32 | ≤0.03 | >32 | 52.0 | 1.1 | 46.9 |
| Clarithromycin | 0.03 | >16 | ≤0.015 | >16 | 52.0 | 0.0 | 48.0 |
| Erythromycin | 0.06 | >16 | ≤0.015 | >16 | 52.0 | 0.0 | 48.0 |
| Levofloxacin | 1 | 1 | ≤0.06 | 32 | 98.9 | 0.0 | 1.1 |
| Moxifloxacin | 0.12 | 0.25 | ≤0.03 | 4 | 98.9 | 0.6 | 0.6 |
| Trimethoprim/sulfamethoxazole | 2 | 16 | 0.12 | 32 | 31.3 | 21.2 | 47.5 |
min, minimum; max, maximum; S, susceptible; I, intermediate; R, resistant; –, not applicable.
MIC and susceptibility data for S. pneumoniae isolates (n = 179) from Turkey using EUCAST (dose-specific) breakpoints
| Antibiotic . | MIC (mg/L) . | EUCAST susceptibility . | |||||
|---|---|---|---|---|---|---|---|
| 50% . | 90% . | min . | max . | %S . | %I . | %R . | |
| Amoxicillin (0.5 g × 3 oral) | 1 | 8 | ≤0.015 | 8 | 49.7 | 7.3 | 43.0 |
| Amoxicillin (0.75–1 g × 3 oral) | 1 | 8 | ≤0.015 | 8 | 57.0 | – | 43.0 |
| Amoxicillin/clavulanic acid (0. 5 g/0.125 g × 3 oral) | 1 | 8 | ≤0.015 | 8 | 49.7 | 8.4 | 41.9 |
| Amoxicillin/clavulanic acid (0.875 g/0.125 g × 3 oral) | 1 | 8 | ≤0.015 | 8 | 58.1 | – | 41.9 |
| Ampicillin (2 g × 3 IV) | 1 | 8 | ≤0.03 | 16 | 47.5 | 16.8 | 35.8 |
| Ampicillin (2 g × 4 IV) | 1 | 8 | ≤0.03 | 16 | 64.2 | – | 35.8 |
| Penicillin (0.6 g 1 MU × 4 IV) | 0.5 | 4 | ≤0.03 | 4 | 27.9 | 56.4 | 15.6 |
| Penicillin (2.4 g 2 MU × 4–6 IV) | 0.5 | 4 | ≤0.03 | 4 | 84.4 | – | 15.6 |
| Cefaclor | 16 | >32 | ≤0.03 | >32 | 0.6 | 14.5 | 84.9 |
| Cefdinir | 2 | 16 | ≤0.015 | 16 | – | – | – |
| Cefditoren | 0.25 | 1 | ≤0.015 | 4 | – | – | – |
| Cefixime | 8 | 64 | ≤0.06 | >64 | – | – | – |
| Cefpodoxime | 2 | 16 | ≤0.015 | >16 | 39.1 | 5.0 | 55.9 |
| Ceftriaxone (1 g × 1 IV) | 0.5 | 2 | ≤0.015 | 8 | 55.9 | 42.5 | 1.7 |
| Ceftriaxone (2 g × 2 IV) | 0.5 | 2 | ≤0.015 | 8 | 98.3 | – | 1.7 |
| Cefuroxime | 2 | 16 | ≤0.015 | >16 | 35.8 | 11.2 | 53.1 |
| Azithromycin | 0.12 | >32 | ≤0.03 | >32 | 52.0 | 0.0 | 48.0 |
| Clarithromycin (0.25 g × 2 oral) | 0.03 | >16 | ≤0.015 | >16 | 52.0 | 0.0 | 48.0 |
| Clarithromycin (0.5 g × 2 oral) | 0.03 | >16 | ≤0.015 | >16 | 52.0 | – | 48.0 |
| Erythromycin (0.5 g × 2–4 oral or 0.5 g × 2–4 IV) | 0.06 | >16 | ≤0.015 | >16 | 52.0 | 0.0 | 48.0 |
| Erythromycin (1 g × 4 oral or 1 g × 4 IV) | 0.06 | >16 | ≤0.015 | >16 | 52.0 | – | 48.0 |
| Levofloxacin (0.5 g × 2 oral or 0.4 g × 2 IV) | 1 | 1 | ≤0.06 | 32 | 98.9 | – | 1.1 |
| Levofloxacin (0.75 g × 2 oral or 0.4 g × 3 IV) | 1 | 1 | ≤0.06 | 32 | 98.9 | – | 1.1 |
| Moxifloxacin | 0.12 | 0.25 | ≤0.03 | 4 | 98.9 | – | 1.1 |
| Trimethoprim/sulfamethoxazole (0.16 g/0.8 g × 2 oral or IV) | 2 | 16 | 0.12 | 32 | 47.5 | 5.0 | 47.5 |
| Trimethoprim/sulfamethoxazole (0.24 g/1.2 g × 2 oral or IV) | 2 | 16 | 0.12 | 32 | 52.5 | – | 47.5 |
| Antibiotic . | MIC (mg/L) . | EUCAST susceptibility . | |||||
|---|---|---|---|---|---|---|---|
| 50% . | 90% . | min . | max . | %S . | %I . | %R . | |
| Amoxicillin (0.5 g × 3 oral) | 1 | 8 | ≤0.015 | 8 | 49.7 | 7.3 | 43.0 |
| Amoxicillin (0.75–1 g × 3 oral) | 1 | 8 | ≤0.015 | 8 | 57.0 | – | 43.0 |
| Amoxicillin/clavulanic acid (0. 5 g/0.125 g × 3 oral) | 1 | 8 | ≤0.015 | 8 | 49.7 | 8.4 | 41.9 |
| Amoxicillin/clavulanic acid (0.875 g/0.125 g × 3 oral) | 1 | 8 | ≤0.015 | 8 | 58.1 | – | 41.9 |
| Ampicillin (2 g × 3 IV) | 1 | 8 | ≤0.03 | 16 | 47.5 | 16.8 | 35.8 |
| Ampicillin (2 g × 4 IV) | 1 | 8 | ≤0.03 | 16 | 64.2 | – | 35.8 |
| Penicillin (0.6 g 1 MU × 4 IV) | 0.5 | 4 | ≤0.03 | 4 | 27.9 | 56.4 | 15.6 |
| Penicillin (2.4 g 2 MU × 4–6 IV) | 0.5 | 4 | ≤0.03 | 4 | 84.4 | – | 15.6 |
| Cefaclor | 16 | >32 | ≤0.03 | >32 | 0.6 | 14.5 | 84.9 |
| Cefdinir | 2 | 16 | ≤0.015 | 16 | – | – | – |
| Cefditoren | 0.25 | 1 | ≤0.015 | 4 | – | – | – |
| Cefixime | 8 | 64 | ≤0.06 | >64 | – | – | – |
| Cefpodoxime | 2 | 16 | ≤0.015 | >16 | 39.1 | 5.0 | 55.9 |
| Ceftriaxone (1 g × 1 IV) | 0.5 | 2 | ≤0.015 | 8 | 55.9 | 42.5 | 1.7 |
| Ceftriaxone (2 g × 2 IV) | 0.5 | 2 | ≤0.015 | 8 | 98.3 | – | 1.7 |
| Cefuroxime | 2 | 16 | ≤0.015 | >16 | 35.8 | 11.2 | 53.1 |
| Azithromycin | 0.12 | >32 | ≤0.03 | >32 | 52.0 | 0.0 | 48.0 |
| Clarithromycin (0.25 g × 2 oral) | 0.03 | >16 | ≤0.015 | >16 | 52.0 | 0.0 | 48.0 |
| Clarithromycin (0.5 g × 2 oral) | 0.03 | >16 | ≤0.015 | >16 | 52.0 | – | 48.0 |
| Erythromycin (0.5 g × 2–4 oral or 0.5 g × 2–4 IV) | 0.06 | >16 | ≤0.015 | >16 | 52.0 | 0.0 | 48.0 |
| Erythromycin (1 g × 4 oral or 1 g × 4 IV) | 0.06 | >16 | ≤0.015 | >16 | 52.0 | – | 48.0 |
| Levofloxacin (0.5 g × 2 oral or 0.4 g × 2 IV) | 1 | 1 | ≤0.06 | 32 | 98.9 | – | 1.1 |
| Levofloxacin (0.75 g × 2 oral or 0.4 g × 3 IV) | 1 | 1 | ≤0.06 | 32 | 98.9 | – | 1.1 |
| Moxifloxacin | 0.12 | 0.25 | ≤0.03 | 4 | 98.9 | – | 1.1 |
| Trimethoprim/sulfamethoxazole (0.16 g/0.8 g × 2 oral or IV) | 2 | 16 | 0.12 | 32 | 47.5 | 5.0 | 47.5 |
| Trimethoprim/sulfamethoxazole (0.24 g/1.2 g × 2 oral or IV) | 2 | 16 | 0.12 | 32 | 52.5 | – | 47.5 |
min, minimum; max, maximum; S, susceptible; I, intermediate; R, resistant; –, not applicable.
MIC and susceptibility data for S. pneumoniae isolates (n = 179) from Turkey using EUCAST (dose-specific) breakpoints
| Antibiotic . | MIC (mg/L) . | EUCAST susceptibility . | |||||
|---|---|---|---|---|---|---|---|
| 50% . | 90% . | min . | max . | %S . | %I . | %R . | |
| Amoxicillin (0.5 g × 3 oral) | 1 | 8 | ≤0.015 | 8 | 49.7 | 7.3 | 43.0 |
| Amoxicillin (0.75–1 g × 3 oral) | 1 | 8 | ≤0.015 | 8 | 57.0 | – | 43.0 |
| Amoxicillin/clavulanic acid (0. 5 g/0.125 g × 3 oral) | 1 | 8 | ≤0.015 | 8 | 49.7 | 8.4 | 41.9 |
| Amoxicillin/clavulanic acid (0.875 g/0.125 g × 3 oral) | 1 | 8 | ≤0.015 | 8 | 58.1 | – | 41.9 |
| Ampicillin (2 g × 3 IV) | 1 | 8 | ≤0.03 | 16 | 47.5 | 16.8 | 35.8 |
| Ampicillin (2 g × 4 IV) | 1 | 8 | ≤0.03 | 16 | 64.2 | – | 35.8 |
| Penicillin (0.6 g 1 MU × 4 IV) | 0.5 | 4 | ≤0.03 | 4 | 27.9 | 56.4 | 15.6 |
| Penicillin (2.4 g 2 MU × 4–6 IV) | 0.5 | 4 | ≤0.03 | 4 | 84.4 | – | 15.6 |
| Cefaclor | 16 | >32 | ≤0.03 | >32 | 0.6 | 14.5 | 84.9 |
| Cefdinir | 2 | 16 | ≤0.015 | 16 | – | – | – |
| Cefditoren | 0.25 | 1 | ≤0.015 | 4 | – | – | – |
| Cefixime | 8 | 64 | ≤0.06 | >64 | – | – | – |
| Cefpodoxime | 2 | 16 | ≤0.015 | >16 | 39.1 | 5.0 | 55.9 |
| Ceftriaxone (1 g × 1 IV) | 0.5 | 2 | ≤0.015 | 8 | 55.9 | 42.5 | 1.7 |
| Ceftriaxone (2 g × 2 IV) | 0.5 | 2 | ≤0.015 | 8 | 98.3 | – | 1.7 |
| Cefuroxime | 2 | 16 | ≤0.015 | >16 | 35.8 | 11.2 | 53.1 |
| Azithromycin | 0.12 | >32 | ≤0.03 | >32 | 52.0 | 0.0 | 48.0 |
| Clarithromycin (0.25 g × 2 oral) | 0.03 | >16 | ≤0.015 | >16 | 52.0 | 0.0 | 48.0 |
| Clarithromycin (0.5 g × 2 oral) | 0.03 | >16 | ≤0.015 | >16 | 52.0 | – | 48.0 |
| Erythromycin (0.5 g × 2–4 oral or 0.5 g × 2–4 IV) | 0.06 | >16 | ≤0.015 | >16 | 52.0 | 0.0 | 48.0 |
| Erythromycin (1 g × 4 oral or 1 g × 4 IV) | 0.06 | >16 | ≤0.015 | >16 | 52.0 | – | 48.0 |
| Levofloxacin (0.5 g × 2 oral or 0.4 g × 2 IV) | 1 | 1 | ≤0.06 | 32 | 98.9 | – | 1.1 |
| Levofloxacin (0.75 g × 2 oral or 0.4 g × 3 IV) | 1 | 1 | ≤0.06 | 32 | 98.9 | – | 1.1 |
| Moxifloxacin | 0.12 | 0.25 | ≤0.03 | 4 | 98.9 | – | 1.1 |
| Trimethoprim/sulfamethoxazole (0.16 g/0.8 g × 2 oral or IV) | 2 | 16 | 0.12 | 32 | 47.5 | 5.0 | 47.5 |
| Trimethoprim/sulfamethoxazole (0.24 g/1.2 g × 2 oral or IV) | 2 | 16 | 0.12 | 32 | 52.5 | – | 47.5 |
| Antibiotic . | MIC (mg/L) . | EUCAST susceptibility . | |||||
|---|---|---|---|---|---|---|---|
| 50% . | 90% . | min . | max . | %S . | %I . | %R . | |
| Amoxicillin (0.5 g × 3 oral) | 1 | 8 | ≤0.015 | 8 | 49.7 | 7.3 | 43.0 |
| Amoxicillin (0.75–1 g × 3 oral) | 1 | 8 | ≤0.015 | 8 | 57.0 | – | 43.0 |
| Amoxicillin/clavulanic acid (0. 5 g/0.125 g × 3 oral) | 1 | 8 | ≤0.015 | 8 | 49.7 | 8.4 | 41.9 |
| Amoxicillin/clavulanic acid (0.875 g/0.125 g × 3 oral) | 1 | 8 | ≤0.015 | 8 | 58.1 | – | 41.9 |
| Ampicillin (2 g × 3 IV) | 1 | 8 | ≤0.03 | 16 | 47.5 | 16.8 | 35.8 |
| Ampicillin (2 g × 4 IV) | 1 | 8 | ≤0.03 | 16 | 64.2 | – | 35.8 |
| Penicillin (0.6 g 1 MU × 4 IV) | 0.5 | 4 | ≤0.03 | 4 | 27.9 | 56.4 | 15.6 |
| Penicillin (2.4 g 2 MU × 4–6 IV) | 0.5 | 4 | ≤0.03 | 4 | 84.4 | – | 15.6 |
| Cefaclor | 16 | >32 | ≤0.03 | >32 | 0.6 | 14.5 | 84.9 |
| Cefdinir | 2 | 16 | ≤0.015 | 16 | – | – | – |
| Cefditoren | 0.25 | 1 | ≤0.015 | 4 | – | – | – |
| Cefixime | 8 | 64 | ≤0.06 | >64 | – | – | – |
| Cefpodoxime | 2 | 16 | ≤0.015 | >16 | 39.1 | 5.0 | 55.9 |
| Ceftriaxone (1 g × 1 IV) | 0.5 | 2 | ≤0.015 | 8 | 55.9 | 42.5 | 1.7 |
| Ceftriaxone (2 g × 2 IV) | 0.5 | 2 | ≤0.015 | 8 | 98.3 | – | 1.7 |
| Cefuroxime | 2 | 16 | ≤0.015 | >16 | 35.8 | 11.2 | 53.1 |
| Azithromycin | 0.12 | >32 | ≤0.03 | >32 | 52.0 | 0.0 | 48.0 |
| Clarithromycin (0.25 g × 2 oral) | 0.03 | >16 | ≤0.015 | >16 | 52.0 | 0.0 | 48.0 |
| Clarithromycin (0.5 g × 2 oral) | 0.03 | >16 | ≤0.015 | >16 | 52.0 | – | 48.0 |
| Erythromycin (0.5 g × 2–4 oral or 0.5 g × 2–4 IV) | 0.06 | >16 | ≤0.015 | >16 | 52.0 | 0.0 | 48.0 |
| Erythromycin (1 g × 4 oral or 1 g × 4 IV) | 0.06 | >16 | ≤0.015 | >16 | 52.0 | – | 48.0 |
| Levofloxacin (0.5 g × 2 oral or 0.4 g × 2 IV) | 1 | 1 | ≤0.06 | 32 | 98.9 | – | 1.1 |
| Levofloxacin (0.75 g × 2 oral or 0.4 g × 3 IV) | 1 | 1 | ≤0.06 | 32 | 98.9 | – | 1.1 |
| Moxifloxacin | 0.12 | 0.25 | ≤0.03 | 4 | 98.9 | – | 1.1 |
| Trimethoprim/sulfamethoxazole (0.16 g/0.8 g × 2 oral or IV) | 2 | 16 | 0.12 | 32 | 47.5 | 5.0 | 47.5 |
| Trimethoprim/sulfamethoxazole (0.24 g/1.2 g × 2 oral or IV) | 2 | 16 | 0.12 | 32 | 52.5 | – | 47.5 |
min, minimum; max, maximum; S, susceptible; I, intermediate; R, resistant; –, not applicable.
MIC and susceptibility data for S. pneumoniae isolates (n = 179) from Turkey using PK/PD breakpoints
| Antibiotic . | MIC (mg/L) . | PK/PD susceptibility . | |||
|---|---|---|---|---|---|
| 50% . | 90% . | min . | max . | %S only . | |
| Amoxicillin (1.5 g/day) | 1 | 8 | ≤0.015 | 8 | 78.8 |
| Amoxicillin (4 g/day) | 1 | 8 | ≤0.015 | 8 | 87.7 |
| Amoxicillin/clavulanic acid (1.75 g/0.25 g/day adults; 45 mg/6.4 mg/kg/day children) | 1 | 8 | ≤0.015 | 8 | 78.8 |
| Amoxicillin/clavulanic acid (4 g/0.25 g/day adults; 90 mg/6.4 mg/kg/day children) | 1 | 8 | ≤0.015 | 8 | 87.7 |
| Ampicillin | 1 | 8 | ≤0.03 | 16 | – |
| Penicillin | 0.5 | 4 | ≤0.03 | 4 | – |
| Cefaclor | 16 | >32 | ≤0.03 | >32 | 15.1 |
| Cefdinir | 2 | 16 | ≤0.015 | 16 | 33.0 |
| Cefditoren | 0.25 | 1 | ≤0.015 | 4 | – |
| Cefixime | 8 | 64 | ≤0.06 | >64 | 28.5 |
| Cefpodoxime | 2 | 16 | ≤0.015 | >16 | 44.1 |
| Ceftriaxone | 0.5 | 2 | ≤0.015 | 8 | 83.2 |
| Cefuroxime | 2 | 16 | ≤0.015 | >16 | 49.2 |
| Azithromycin | 0.12 | >32 | ≤0.03 | >32 | 52.0 |
| Clarithromycin | 0.03 | >16 | ≤0.015 | >16 | 52.0 |
| Erythromycin | 0.06 | >16 | ≤0.015 | >16 | 52.0 |
| Levofloxacin | 1 | 1 | ≤0.06 | 32 | 98.9 |
| Moxifloxacin | 0.12 | 0.25 | ≤0.03 | 4 | 98.9 |
| Trimethoprim/sulfamethoxazole | 2 | 16 | 0.12 | 32 | 31.3 |
| Antibiotic . | MIC (mg/L) . | PK/PD susceptibility . | |||
|---|---|---|---|---|---|
| 50% . | 90% . | min . | max . | %S only . | |
| Amoxicillin (1.5 g/day) | 1 | 8 | ≤0.015 | 8 | 78.8 |
| Amoxicillin (4 g/day) | 1 | 8 | ≤0.015 | 8 | 87.7 |
| Amoxicillin/clavulanic acid (1.75 g/0.25 g/day adults; 45 mg/6.4 mg/kg/day children) | 1 | 8 | ≤0.015 | 8 | 78.8 |
| Amoxicillin/clavulanic acid (4 g/0.25 g/day adults; 90 mg/6.4 mg/kg/day children) | 1 | 8 | ≤0.015 | 8 | 87.7 |
| Ampicillin | 1 | 8 | ≤0.03 | 16 | – |
| Penicillin | 0.5 | 4 | ≤0.03 | 4 | – |
| Cefaclor | 16 | >32 | ≤0.03 | >32 | 15.1 |
| Cefdinir | 2 | 16 | ≤0.015 | 16 | 33.0 |
| Cefditoren | 0.25 | 1 | ≤0.015 | 4 | – |
| Cefixime | 8 | 64 | ≤0.06 | >64 | 28.5 |
| Cefpodoxime | 2 | 16 | ≤0.015 | >16 | 44.1 |
| Ceftriaxone | 0.5 | 2 | ≤0.015 | 8 | 83.2 |
| Cefuroxime | 2 | 16 | ≤0.015 | >16 | 49.2 |
| Azithromycin | 0.12 | >32 | ≤0.03 | >32 | 52.0 |
| Clarithromycin | 0.03 | >16 | ≤0.015 | >16 | 52.0 |
| Erythromycin | 0.06 | >16 | ≤0.015 | >16 | 52.0 |
| Levofloxacin | 1 | 1 | ≤0.06 | 32 | 98.9 |
| Moxifloxacin | 0.12 | 0.25 | ≤0.03 | 4 | 98.9 |
| Trimethoprim/sulfamethoxazole | 2 | 16 | 0.12 | 32 | 31.3 |
min, minimum; max, maximum; S, susceptible; –, not applicable.
MIC and susceptibility data for S. pneumoniae isolates (n = 179) from Turkey using PK/PD breakpoints
| Antibiotic . | MIC (mg/L) . | PK/PD susceptibility . | |||
|---|---|---|---|---|---|
| 50% . | 90% . | min . | max . | %S only . | |
| Amoxicillin (1.5 g/day) | 1 | 8 | ≤0.015 | 8 | 78.8 |
| Amoxicillin (4 g/day) | 1 | 8 | ≤0.015 | 8 | 87.7 |
| Amoxicillin/clavulanic acid (1.75 g/0.25 g/day adults; 45 mg/6.4 mg/kg/day children) | 1 | 8 | ≤0.015 | 8 | 78.8 |
| Amoxicillin/clavulanic acid (4 g/0.25 g/day adults; 90 mg/6.4 mg/kg/day children) | 1 | 8 | ≤0.015 | 8 | 87.7 |
| Ampicillin | 1 | 8 | ≤0.03 | 16 | – |
| Penicillin | 0.5 | 4 | ≤0.03 | 4 | – |
| Cefaclor | 16 | >32 | ≤0.03 | >32 | 15.1 |
| Cefdinir | 2 | 16 | ≤0.015 | 16 | 33.0 |
| Cefditoren | 0.25 | 1 | ≤0.015 | 4 | – |
| Cefixime | 8 | 64 | ≤0.06 | >64 | 28.5 |
| Cefpodoxime | 2 | 16 | ≤0.015 | >16 | 44.1 |
| Ceftriaxone | 0.5 | 2 | ≤0.015 | 8 | 83.2 |
| Cefuroxime | 2 | 16 | ≤0.015 | >16 | 49.2 |
| Azithromycin | 0.12 | >32 | ≤0.03 | >32 | 52.0 |
| Clarithromycin | 0.03 | >16 | ≤0.015 | >16 | 52.0 |
| Erythromycin | 0.06 | >16 | ≤0.015 | >16 | 52.0 |
| Levofloxacin | 1 | 1 | ≤0.06 | 32 | 98.9 |
| Moxifloxacin | 0.12 | 0.25 | ≤0.03 | 4 | 98.9 |
| Trimethoprim/sulfamethoxazole | 2 | 16 | 0.12 | 32 | 31.3 |
| Antibiotic . | MIC (mg/L) . | PK/PD susceptibility . | |||
|---|---|---|---|---|---|
| 50% . | 90% . | min . | max . | %S only . | |
| Amoxicillin (1.5 g/day) | 1 | 8 | ≤0.015 | 8 | 78.8 |
| Amoxicillin (4 g/day) | 1 | 8 | ≤0.015 | 8 | 87.7 |
| Amoxicillin/clavulanic acid (1.75 g/0.25 g/day adults; 45 mg/6.4 mg/kg/day children) | 1 | 8 | ≤0.015 | 8 | 78.8 |
| Amoxicillin/clavulanic acid (4 g/0.25 g/day adults; 90 mg/6.4 mg/kg/day children) | 1 | 8 | ≤0.015 | 8 | 87.7 |
| Ampicillin | 1 | 8 | ≤0.03 | 16 | – |
| Penicillin | 0.5 | 4 | ≤0.03 | 4 | – |
| Cefaclor | 16 | >32 | ≤0.03 | >32 | 15.1 |
| Cefdinir | 2 | 16 | ≤0.015 | 16 | 33.0 |
| Cefditoren | 0.25 | 1 | ≤0.015 | 4 | – |
| Cefixime | 8 | 64 | ≤0.06 | >64 | 28.5 |
| Cefpodoxime | 2 | 16 | ≤0.015 | >16 | 44.1 |
| Ceftriaxone | 0.5 | 2 | ≤0.015 | 8 | 83.2 |
| Cefuroxime | 2 | 16 | ≤0.015 | >16 | 49.2 |
| Azithromycin | 0.12 | >32 | ≤0.03 | >32 | 52.0 |
| Clarithromycin | 0.03 | >16 | ≤0.015 | >16 | 52.0 |
| Erythromycin | 0.06 | >16 | ≤0.015 | >16 | 52.0 |
| Levofloxacin | 1 | 1 | ≤0.06 | 32 | 98.9 |
| Moxifloxacin | 0.12 | 0.25 | ≤0.03 | 4 | 98.9 |
| Trimethoprim/sulfamethoxazole | 2 | 16 | 0.12 | 32 | 31.3 |
min, minimum; max, maximum; S, susceptible; –, not applicable.
S. pneumoniae susceptibility
Only 27.9% (n = 50) of the 179 pneumococci collected in Turkey were susceptible to penicillin according to CLSI oral or EUCAST low-dose IV breakpoints. However, based on EUCAST high-dose and CLSI IV breakpoints, 84.4% of isolates were considered penicillin susceptible. The most active antibiotics were the fluoroquinolones levofloxacin and moxifloxacin, with 98.9% of isolates susceptible by all breakpoints. Susceptibility of isolates to ceftriaxone by CLSI or PK/PD breakpoints was 83.2%; 98.3% were susceptible by EUCAST high-dose breakpoints but only 55.9% were susceptible by EUCAST low-dose breakpoints. Susceptibility to amoxicillin and amoxicillin/clavulanic acid showed a similar pattern, with 78.8% of pneumococci susceptible by CLSI and PK/PD low-dose breakpoints, but by EUCAST low-dose breakpoints 49.7% were susceptible. Using EUCAST high-dose breakpoints, susceptibility to amoxicillin and amoxicillin/clavulanic acid was 57% and 58.1%, respectively, and susceptibility of isolates to both antibiotics using PK/PD high-dose breakpoints was 87.7%. The activity of the remaining antibiotics was low: 52% of pneumococci were susceptible to macrolides (all breakpoints), 49.2% were susceptible to cefuroxime (oral) by CLSI and PK/PD breakpoints (35.8% by EUCAST breakpoints), 47.5% and 64.2% were susceptible to ampicillin by EUCAST low-dose and high-dose breakpoints, respectively, 44.1% and 33% were susceptible to cefdinir by CLSI and PK/PD breakpoints, respectively, 44.1% were susceptible to cefpodoxime by CLSI and PK/PD breakpoints (39.1% by EUCAST breakpoints) and 31.3% were susceptible to trimethoprim/sulfamethoxazole by CLSI and PK/PD breakpoints (47.5% and 52.5% by EUCAST low-dose and high-dose breakpoints, respectively). The lowest susceptibility was observed for cefaclor, with only 26.3% of isolates susceptible by CLSI breakpoints, 15.1% by PK/PD breakpoints and 0.6% by EUCAST breakpoints. See Tables 4–6 and Figures 1–3.
Comparative susceptibility of S. pneumoniae by penicillin susceptibility
Among the 179 S. pneumoniae isolates, 50 (27.9%) were penicillin susceptible S. pneumoniae (PSSP), 52 (29.1%) were penicillin intermediate S. pneumoniae (PISP) and 77 (43%) were penicillin resistant S. pneumoniae (PRSP) according to CLSI oral breakpoints. Using CLSI breakpoints, all PSSP isolates were susceptible to amoxicillin, amoxicillin/clavulanic acid, cefdinir, ceftriaxone and oral cefuroxime (Figure 4). Susceptibility to cefpodoxime, the macrolides and the fluoroquinolones was 98%, to cefaclor 90% and to trimethoprim/sulfamethoxazole 72%. PSSP isolates showed significantly higher (P < 0.0001) susceptibility rates than PISP isolates for all antibiotics except amoxicillin, amoxicillin/clavulanic acid, ceftriaxone, levofloxacin and moxifloxacin, all of which were active against all PISP isolates. Susceptibility of PISP isolates to the remaining tested antibiotics ranged from 3.9% for cefaclor to 73.1% for cefuroxime (oral). PSSP isolates were also significantly more susceptible (P < 0.0001) than PRSP isolates to all antibiotics except the fluoroquinolones, which retained activity against 98.7% of PRSP isolates. Susceptibility rates of PRSP isolates to ceftriaxone, amoxicillin and amoxicillin/clavulanic acid were reduced to 61%, 50.7% and 50.7%, respectively, and further reduced to <21% to all other antibiotics tested.

Percentage susceptibility rates (with 95% CI) based on CLSI breakpoints for antibiotics against PSSP, PISP and PRSP from Turkey. Penicillin susceptibility categories are based on oral penicillin CLSI breakpoints. aSusceptibility was significantly higher among PSSP and PISP isolates than PRSP isolates (P < 0.0001). bSusceptibility was significantly higher among PSSP isolates than PISP isolates and among PISP isolates than PRSP isolates (P < 0.001). cSusceptibility was significantly higher among PSSP isolates than PISP and PRSP isolates (P < 0.0001).
H. influenzae isolates
A total of 239 H. influenzae isolates were collected in Turkey from 2015 to 2017. Most isolates originated from sputum (n = 200; 83.7%). The remaining isolates were from bronchoalveolar lavage (n = 21; 8.8%), transtracheal aspirate (n = 15; 6.3%), middle ear effusion (n = 2; 0.8%) and blood (n = 1; 0.4%). Just over half of the isolates (n = 128; 53.6%) came from adults (aged 13–64 years), while isolates from paediatric patients (≤12 years old) represented 27.2% (n = 65) and the remaining 19.2% (n = 46) were from elderly patients (aged ≥65 years).
Summary MIC and susceptibility data for all 239 H. influenzae isolates are shown in Tables 7–9 and Figures 5–7. MIC distribution data are shown in Table S2. Susceptibility to ampicillin and amoxicillin was determined solely on the basis of the MIC rather than on production of β-lactamase. Consequently, a small number of β-lactamase producers were classified as susceptible to these agents. The likely treatment outcomes for such strains remain unclear.
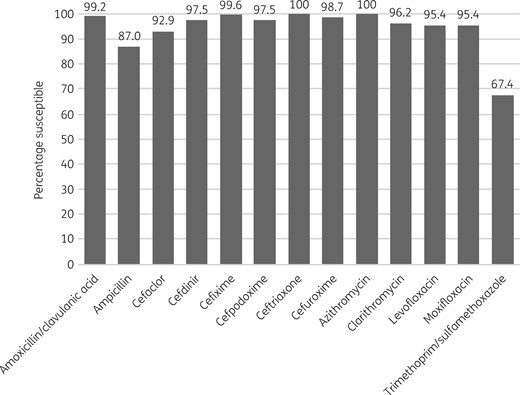
Percentage antibiotic susceptibility rates of H. influenzae isolates (n = 239) from Turkey based on CLSI breakpoints.
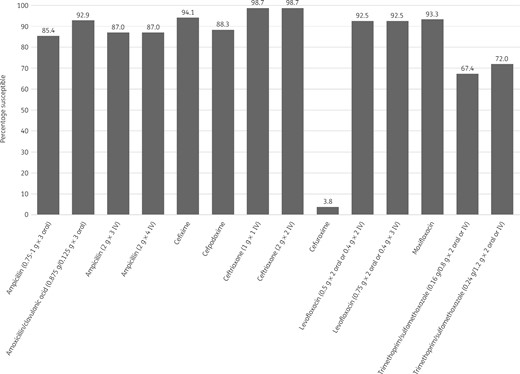
Percentage antibiotic susceptibility rates of H. influenzae isolates (n = 239) from Turkey based on EUCAST (dose-specific) breakpoints.
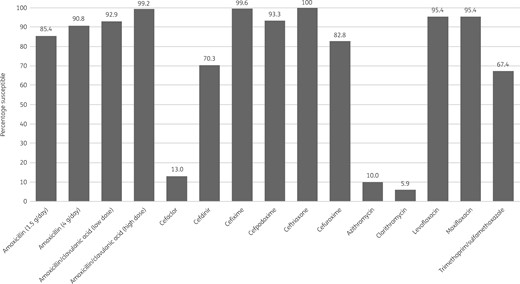
Percentage antibiotic susceptibility rates of H. influenzae isolates (n = 239) from Turkey based on PK/PD breakpoints. Low-dose amoxicillin/clavulanic acid = 1.75 g/0.25 g/day adults; 45 mg/6.4 mg/kg/day children. High-dose amoxicillin/clavulanic acid = 4 g/0.25 g/day adults; 90 mg/6.4 mg/kg/day children.
MIC and susceptibility data for H. influenzae isolates (n = 239) from Turkey using CLSI breakpoints
| Antibiotic . | MIC (mg/L) . | CLSI susceptibility . | |||||
|---|---|---|---|---|---|---|---|
| 50% . | 90% . | min . | max . | %S . | %I . | %R . | |
| Amoxicillin | 0.5 | 4 | ≤0.12 | >128 | – | – | – |
| Amoxicillin/clavulanic acid | 0.5 | 2 | ≤0.06 | 8 | 99.2 | – | 0.8 |
| Ampicillin | 0.25 | 4 | 0.12 | >128 | 87.0 | 2.5 | 10.5 |
| Cefaclor | 2 | 8 | 0.12 | 32 | 92.9 | 6.7 | 0.4 |
| Cefdinir | 0.25 | 0.5 | 0.06 | 2 | 97.5 | – | – |
| Cefditoren | ≤0.03 | 0.06 | ≤0.03 | 2 | – | – | – |
| Cefixime | 0.03 | 0.12 | ≤0.015 | 2 | 99.6 | – | – |
| Cefpodoxime | 0.12 | 0.5 | ≤0.015 | >16 | 97.5 | – | – |
| Ceftriaxone | ≤0.015 | ≤0.015 | ≤0.015 | 0.5 | 100 | – | – |
| Cefuroxime | 0.5 | 2 | 0.06 | 8 | 98.7 | 1.3 | 0.0 |
| Azithromycin | 0.5 | 1 | 0.06 | 4 | 100 | – | – |
| Clarithromycin | 4 | 8 | ≤0.03 | >32 | 96.2 | 2.9 | 0.8 |
| Levofloxacin | 0.015 | 0.06 | ≤0.004 | >8 | 95.4 | – | – |
| Moxifloxacin | 0.015 | 0.06 | ≤0.004 | >8 | 95.4 | – | – |
| Trimethoprim/sulfamethoxazole | 0.25 | 16 | ≤0.015 | >16 | 67.4 | 4.6 | 28.0 |
| Antibiotic . | MIC (mg/L) . | CLSI susceptibility . | |||||
|---|---|---|---|---|---|---|---|
| 50% . | 90% . | min . | max . | %S . | %I . | %R . | |
| Amoxicillin | 0.5 | 4 | ≤0.12 | >128 | – | – | – |
| Amoxicillin/clavulanic acid | 0.5 | 2 | ≤0.06 | 8 | 99.2 | – | 0.8 |
| Ampicillin | 0.25 | 4 | 0.12 | >128 | 87.0 | 2.5 | 10.5 |
| Cefaclor | 2 | 8 | 0.12 | 32 | 92.9 | 6.7 | 0.4 |
| Cefdinir | 0.25 | 0.5 | 0.06 | 2 | 97.5 | – | – |
| Cefditoren | ≤0.03 | 0.06 | ≤0.03 | 2 | – | – | – |
| Cefixime | 0.03 | 0.12 | ≤0.015 | 2 | 99.6 | – | – |
| Cefpodoxime | 0.12 | 0.5 | ≤0.015 | >16 | 97.5 | – | – |
| Ceftriaxone | ≤0.015 | ≤0.015 | ≤0.015 | 0.5 | 100 | – | – |
| Cefuroxime | 0.5 | 2 | 0.06 | 8 | 98.7 | 1.3 | 0.0 |
| Azithromycin | 0.5 | 1 | 0.06 | 4 | 100 | – | – |
| Clarithromycin | 4 | 8 | ≤0.03 | >32 | 96.2 | 2.9 | 0.8 |
| Levofloxacin | 0.015 | 0.06 | ≤0.004 | >8 | 95.4 | – | – |
| Moxifloxacin | 0.015 | 0.06 | ≤0.004 | >8 | 95.4 | – | – |
| Trimethoprim/sulfamethoxazole | 0.25 | 16 | ≤0.015 | >16 | 67.4 | 4.6 | 28.0 |
min, minimum; max, maximum; S, susceptible; I, intermediate; R, resistant; –, not applicable.
MIC and susceptibility data for H. influenzae isolates (n = 239) from Turkey using CLSI breakpoints
| Antibiotic . | MIC (mg/L) . | CLSI susceptibility . | |||||
|---|---|---|---|---|---|---|---|
| 50% . | 90% . | min . | max . | %S . | %I . | %R . | |
| Amoxicillin | 0.5 | 4 | ≤0.12 | >128 | – | – | – |
| Amoxicillin/clavulanic acid | 0.5 | 2 | ≤0.06 | 8 | 99.2 | – | 0.8 |
| Ampicillin | 0.25 | 4 | 0.12 | >128 | 87.0 | 2.5 | 10.5 |
| Cefaclor | 2 | 8 | 0.12 | 32 | 92.9 | 6.7 | 0.4 |
| Cefdinir | 0.25 | 0.5 | 0.06 | 2 | 97.5 | – | – |
| Cefditoren | ≤0.03 | 0.06 | ≤0.03 | 2 | – | – | – |
| Cefixime | 0.03 | 0.12 | ≤0.015 | 2 | 99.6 | – | – |
| Cefpodoxime | 0.12 | 0.5 | ≤0.015 | >16 | 97.5 | – | – |
| Ceftriaxone | ≤0.015 | ≤0.015 | ≤0.015 | 0.5 | 100 | – | – |
| Cefuroxime | 0.5 | 2 | 0.06 | 8 | 98.7 | 1.3 | 0.0 |
| Azithromycin | 0.5 | 1 | 0.06 | 4 | 100 | – | – |
| Clarithromycin | 4 | 8 | ≤0.03 | >32 | 96.2 | 2.9 | 0.8 |
| Levofloxacin | 0.015 | 0.06 | ≤0.004 | >8 | 95.4 | – | – |
| Moxifloxacin | 0.015 | 0.06 | ≤0.004 | >8 | 95.4 | – | – |
| Trimethoprim/sulfamethoxazole | 0.25 | 16 | ≤0.015 | >16 | 67.4 | 4.6 | 28.0 |
| Antibiotic . | MIC (mg/L) . | CLSI susceptibility . | |||||
|---|---|---|---|---|---|---|---|
| 50% . | 90% . | min . | max . | %S . | %I . | %R . | |
| Amoxicillin | 0.5 | 4 | ≤0.12 | >128 | – | – | – |
| Amoxicillin/clavulanic acid | 0.5 | 2 | ≤0.06 | 8 | 99.2 | – | 0.8 |
| Ampicillin | 0.25 | 4 | 0.12 | >128 | 87.0 | 2.5 | 10.5 |
| Cefaclor | 2 | 8 | 0.12 | 32 | 92.9 | 6.7 | 0.4 |
| Cefdinir | 0.25 | 0.5 | 0.06 | 2 | 97.5 | – | – |
| Cefditoren | ≤0.03 | 0.06 | ≤0.03 | 2 | – | – | – |
| Cefixime | 0.03 | 0.12 | ≤0.015 | 2 | 99.6 | – | – |
| Cefpodoxime | 0.12 | 0.5 | ≤0.015 | >16 | 97.5 | – | – |
| Ceftriaxone | ≤0.015 | ≤0.015 | ≤0.015 | 0.5 | 100 | – | – |
| Cefuroxime | 0.5 | 2 | 0.06 | 8 | 98.7 | 1.3 | 0.0 |
| Azithromycin | 0.5 | 1 | 0.06 | 4 | 100 | – | – |
| Clarithromycin | 4 | 8 | ≤0.03 | >32 | 96.2 | 2.9 | 0.8 |
| Levofloxacin | 0.015 | 0.06 | ≤0.004 | >8 | 95.4 | – | – |
| Moxifloxacin | 0.015 | 0.06 | ≤0.004 | >8 | 95.4 | – | – |
| Trimethoprim/sulfamethoxazole | 0.25 | 16 | ≤0.015 | >16 | 67.4 | 4.6 | 28.0 |
min, minimum; max, maximum; S, susceptible; I, intermediate; R, resistant; –, not applicable.
MIC and susceptibility data for H. influenzae isolates (n = 239) from Turkey using EUCAST (dose-specific) breakpoints
| Antibiotic . | MIC (mg/L) . | EUCAST susceptibility . | |||||
|---|---|---|---|---|---|---|---|
| 50% . | 90% . | min . | max . | %S . | %I . | %R . | |
| Amoxicillin (0.75–1 g × 3 oral) | 0.5 | 4 | ≤0.12 | >128 | 85.4 | – | 14.6 |
| Amoxicillin/clavulanic acid (0.875 g/0.125 g × 3 oral) | 0.5 | 2 | ≤0.06 | 8 | 92.9 | – | 7.1 |
| Ampicillin (2 g × 3 IV) | 0.25 | 4 | 0.12 | >128 | 87.0 | – | 13.0 |
| Ampicillin (2 g × 4 IV) | 0.25 | 4 | 0.12 | >128 | 87.0 | – | 13.0 |
| Cefaclor | 2 | 8 | 0.12 | 32 | – | – | – |
| Cefdinir | 0.25 | 0.5 | 0.06 | 2 | – | – | – |
| Cefditoren | ≤0.03 | 0.06 | ≤0.03 | 2 | – | – | – |
| Cefixime | 0.03 | 0.12 | ≤0.015 | 2 | 94.1 | – | 5.9 |
| Cefpodoxime | 0.12 | 0.5 | ≤0.015 | >16 | 88.3 | – | 11.7 |
| Ceftriaxone (1 g × 1 IV) | ≤0.015 | ≤0.015 | ≤0.015 | 0.5 | 98.7 | – | 1.3 |
| Ceftriaxone (2 g × 2 IV) | ≤0.015 | ≤0.015 | ≤0.015 | 0.5 | 98.7 | – | 1.3 |
| Cefuroxime | 0.5 | 2 | 0.06 | 8 | 3.8 | – | 96.2 |
| Azithromycin | 0.5 | 1 | 0.06 | 4 | – | – | – |
| Clarithromycin | 4 | 8 | ≤0.03 | >32 | – | – | – |
| Levofloxacin (0.5 g × 2 oral or 0.4 g × 2 IV) | 0.015 | 0.06 | ≤0.004 | >8 | 92.5 | – | 7.5 |
| Levofloxacin (0.75 g × 2 oral or 0.4 g × 3 IV) | 0.015 | 0.06 | ≤0.004 | >8 | 92.5 | – | 7.5 |
| Moxifloxacin | 0.015 | 0.06 | ≤0.004 | >8 | 93.3 | – | 6.7 |
| Trimethoprim/sulfamethoxazole (0.16 g/0.8 g × 2 oral or IV) | 0.25 | 16 | ≤0.015 | >16 | 67.4 | 4.6 | 28.0 |
| Trimethoprim/sulfamethoxazole (0.24 g/1.2 g × 2 oral or IV) | 0.25 | 16 | ≤0.015 | >16 | 72.0 | – | 28.0 |
| Antibiotic . | MIC (mg/L) . | EUCAST susceptibility . | |||||
|---|---|---|---|---|---|---|---|
| 50% . | 90% . | min . | max . | %S . | %I . | %R . | |
| Amoxicillin (0.75–1 g × 3 oral) | 0.5 | 4 | ≤0.12 | >128 | 85.4 | – | 14.6 |
| Amoxicillin/clavulanic acid (0.875 g/0.125 g × 3 oral) | 0.5 | 2 | ≤0.06 | 8 | 92.9 | – | 7.1 |
| Ampicillin (2 g × 3 IV) | 0.25 | 4 | 0.12 | >128 | 87.0 | – | 13.0 |
| Ampicillin (2 g × 4 IV) | 0.25 | 4 | 0.12 | >128 | 87.0 | – | 13.0 |
| Cefaclor | 2 | 8 | 0.12 | 32 | – | – | – |
| Cefdinir | 0.25 | 0.5 | 0.06 | 2 | – | – | – |
| Cefditoren | ≤0.03 | 0.06 | ≤0.03 | 2 | – | – | – |
| Cefixime | 0.03 | 0.12 | ≤0.015 | 2 | 94.1 | – | 5.9 |
| Cefpodoxime | 0.12 | 0.5 | ≤0.015 | >16 | 88.3 | – | 11.7 |
| Ceftriaxone (1 g × 1 IV) | ≤0.015 | ≤0.015 | ≤0.015 | 0.5 | 98.7 | – | 1.3 |
| Ceftriaxone (2 g × 2 IV) | ≤0.015 | ≤0.015 | ≤0.015 | 0.5 | 98.7 | – | 1.3 |
| Cefuroxime | 0.5 | 2 | 0.06 | 8 | 3.8 | – | 96.2 |
| Azithromycin | 0.5 | 1 | 0.06 | 4 | – | – | – |
| Clarithromycin | 4 | 8 | ≤0.03 | >32 | – | – | – |
| Levofloxacin (0.5 g × 2 oral or 0.4 g × 2 IV) | 0.015 | 0.06 | ≤0.004 | >8 | 92.5 | – | 7.5 |
| Levofloxacin (0.75 g × 2 oral or 0.4 g × 3 IV) | 0.015 | 0.06 | ≤0.004 | >8 | 92.5 | – | 7.5 |
| Moxifloxacin | 0.015 | 0.06 | ≤0.004 | >8 | 93.3 | – | 6.7 |
| Trimethoprim/sulfamethoxazole (0.16 g/0.8 g × 2 oral or IV) | 0.25 | 16 | ≤0.015 | >16 | 67.4 | 4.6 | 28.0 |
| Trimethoprim/sulfamethoxazole (0.24 g/1.2 g × 2 oral or IV) | 0.25 | 16 | ≤0.015 | >16 | 72.0 | – | 28.0 |
min, minimum; max, maximum; S, susceptible; I, intermediate; R, resistant; –, not applicable.
MIC and susceptibility data for H. influenzae isolates (n = 239) from Turkey using EUCAST (dose-specific) breakpoints
| Antibiotic . | MIC (mg/L) . | EUCAST susceptibility . | |||||
|---|---|---|---|---|---|---|---|
| 50% . | 90% . | min . | max . | %S . | %I . | %R . | |
| Amoxicillin (0.75–1 g × 3 oral) | 0.5 | 4 | ≤0.12 | >128 | 85.4 | – | 14.6 |
| Amoxicillin/clavulanic acid (0.875 g/0.125 g × 3 oral) | 0.5 | 2 | ≤0.06 | 8 | 92.9 | – | 7.1 |
| Ampicillin (2 g × 3 IV) | 0.25 | 4 | 0.12 | >128 | 87.0 | – | 13.0 |
| Ampicillin (2 g × 4 IV) | 0.25 | 4 | 0.12 | >128 | 87.0 | – | 13.0 |
| Cefaclor | 2 | 8 | 0.12 | 32 | – | – | – |
| Cefdinir | 0.25 | 0.5 | 0.06 | 2 | – | – | – |
| Cefditoren | ≤0.03 | 0.06 | ≤0.03 | 2 | – | – | – |
| Cefixime | 0.03 | 0.12 | ≤0.015 | 2 | 94.1 | – | 5.9 |
| Cefpodoxime | 0.12 | 0.5 | ≤0.015 | >16 | 88.3 | – | 11.7 |
| Ceftriaxone (1 g × 1 IV) | ≤0.015 | ≤0.015 | ≤0.015 | 0.5 | 98.7 | – | 1.3 |
| Ceftriaxone (2 g × 2 IV) | ≤0.015 | ≤0.015 | ≤0.015 | 0.5 | 98.7 | – | 1.3 |
| Cefuroxime | 0.5 | 2 | 0.06 | 8 | 3.8 | – | 96.2 |
| Azithromycin | 0.5 | 1 | 0.06 | 4 | – | – | – |
| Clarithromycin | 4 | 8 | ≤0.03 | >32 | – | – | – |
| Levofloxacin (0.5 g × 2 oral or 0.4 g × 2 IV) | 0.015 | 0.06 | ≤0.004 | >8 | 92.5 | – | 7.5 |
| Levofloxacin (0.75 g × 2 oral or 0.4 g × 3 IV) | 0.015 | 0.06 | ≤0.004 | >8 | 92.5 | – | 7.5 |
| Moxifloxacin | 0.015 | 0.06 | ≤0.004 | >8 | 93.3 | – | 6.7 |
| Trimethoprim/sulfamethoxazole (0.16 g/0.8 g × 2 oral or IV) | 0.25 | 16 | ≤0.015 | >16 | 67.4 | 4.6 | 28.0 |
| Trimethoprim/sulfamethoxazole (0.24 g/1.2 g × 2 oral or IV) | 0.25 | 16 | ≤0.015 | >16 | 72.0 | – | 28.0 |
| Antibiotic . | MIC (mg/L) . | EUCAST susceptibility . | |||||
|---|---|---|---|---|---|---|---|
| 50% . | 90% . | min . | max . | %S . | %I . | %R . | |
| Amoxicillin (0.75–1 g × 3 oral) | 0.5 | 4 | ≤0.12 | >128 | 85.4 | – | 14.6 |
| Amoxicillin/clavulanic acid (0.875 g/0.125 g × 3 oral) | 0.5 | 2 | ≤0.06 | 8 | 92.9 | – | 7.1 |
| Ampicillin (2 g × 3 IV) | 0.25 | 4 | 0.12 | >128 | 87.0 | – | 13.0 |
| Ampicillin (2 g × 4 IV) | 0.25 | 4 | 0.12 | >128 | 87.0 | – | 13.0 |
| Cefaclor | 2 | 8 | 0.12 | 32 | – | – | – |
| Cefdinir | 0.25 | 0.5 | 0.06 | 2 | – | – | – |
| Cefditoren | ≤0.03 | 0.06 | ≤0.03 | 2 | – | – | – |
| Cefixime | 0.03 | 0.12 | ≤0.015 | 2 | 94.1 | – | 5.9 |
| Cefpodoxime | 0.12 | 0.5 | ≤0.015 | >16 | 88.3 | – | 11.7 |
| Ceftriaxone (1 g × 1 IV) | ≤0.015 | ≤0.015 | ≤0.015 | 0.5 | 98.7 | – | 1.3 |
| Ceftriaxone (2 g × 2 IV) | ≤0.015 | ≤0.015 | ≤0.015 | 0.5 | 98.7 | – | 1.3 |
| Cefuroxime | 0.5 | 2 | 0.06 | 8 | 3.8 | – | 96.2 |
| Azithromycin | 0.5 | 1 | 0.06 | 4 | – | – | – |
| Clarithromycin | 4 | 8 | ≤0.03 | >32 | – | – | – |
| Levofloxacin (0.5 g × 2 oral or 0.4 g × 2 IV) | 0.015 | 0.06 | ≤0.004 | >8 | 92.5 | – | 7.5 |
| Levofloxacin (0.75 g × 2 oral or 0.4 g × 3 IV) | 0.015 | 0.06 | ≤0.004 | >8 | 92.5 | – | 7.5 |
| Moxifloxacin | 0.015 | 0.06 | ≤0.004 | >8 | 93.3 | – | 6.7 |
| Trimethoprim/sulfamethoxazole (0.16 g/0.8 g × 2 oral or IV) | 0.25 | 16 | ≤0.015 | >16 | 67.4 | 4.6 | 28.0 |
| Trimethoprim/sulfamethoxazole (0.24 g/1.2 g × 2 oral or IV) | 0.25 | 16 | ≤0.015 | >16 | 72.0 | – | 28.0 |
min, minimum; max, maximum; S, susceptible; I, intermediate; R, resistant; –, not applicable.
MIC and susceptibility data for H. influenzae isolates (n = 239) from Turkey using PK/PD breakpoints
| Antibiotic . | MIC (mg/L) . | PK/PD susceptibility . | |||
|---|---|---|---|---|---|
| 50% . | 90% . | min . | max . | %S only . | |
| Amoxicillin (1.5 g/day) | 0.5 | 4 | ≤0.12 | >128 | 85.4 |
| Amoxicillin (4 g/day) | 0.5 | 4 | ≤0.12 | >128 | 90.8 |
| Amoxicillin/clavulanic acid (1.75 g/0.25 g/day adults; 45 mg/6.4 mg/kg/day children) | 0.5 | 2 | ≤0.06 | 8 | 92.9 |
| Amoxicillin/clavulanic acid (4 g/0.25 g/day adults; 90 mg/6.4 mg/kg/day children) | 0.5 | 2 | ≤0.06 | 8 | 99.2 |
| Ampicillin | 0.25 | 4 | 0.12 | >128 | – |
| Cefaclor | 2 | 8 | 0.12 | 32 | 13.0 |
| Cefdinir | 0.25 | 0.5 | 0.06 | 2 | 70.3 |
| Cefditoren | ≤0.03 | 0.06 | ≤0.03 | 2 | – |
| Cefixime | 0.03 | 0.12 | ≤0.015 | 2 | 99.6 |
| Cefpodoxime | 0.12 | 0.5 | ≤0.015 | >16 | 93.3 |
| Ceftriaxone | ≤0.015 | ≤0.015 | ≤0.015 | 0.5 | 100 |
| Cefuroxime | 0.5 | 2 | 0.06 | 8 | 82.8 |
| Azithromycin | 0.5 | 1 | 0.06 | 4 | 10.0 |
| Clarithromycin | 4 | 8 | ≤0.03 | >32 | 5.9 |
| Levofloxacin | 0.015 | 0.06 | ≤0.004 | >8 | 95.4 |
| Moxifloxacin | 0.015 | 0.06 | ≤0.004 | >8 | 95.4 |
| Trimethoprim/sulfamethoxazole | 0.25 | 16 | ≤0.015 | >16 | 67.4 |
| Antibiotic . | MIC (mg/L) . | PK/PD susceptibility . | |||
|---|---|---|---|---|---|
| 50% . | 90% . | min . | max . | %S only . | |
| Amoxicillin (1.5 g/day) | 0.5 | 4 | ≤0.12 | >128 | 85.4 |
| Amoxicillin (4 g/day) | 0.5 | 4 | ≤0.12 | >128 | 90.8 |
| Amoxicillin/clavulanic acid (1.75 g/0.25 g/day adults; 45 mg/6.4 mg/kg/day children) | 0.5 | 2 | ≤0.06 | 8 | 92.9 |
| Amoxicillin/clavulanic acid (4 g/0.25 g/day adults; 90 mg/6.4 mg/kg/day children) | 0.5 | 2 | ≤0.06 | 8 | 99.2 |
| Ampicillin | 0.25 | 4 | 0.12 | >128 | – |
| Cefaclor | 2 | 8 | 0.12 | 32 | 13.0 |
| Cefdinir | 0.25 | 0.5 | 0.06 | 2 | 70.3 |
| Cefditoren | ≤0.03 | 0.06 | ≤0.03 | 2 | – |
| Cefixime | 0.03 | 0.12 | ≤0.015 | 2 | 99.6 |
| Cefpodoxime | 0.12 | 0.5 | ≤0.015 | >16 | 93.3 |
| Ceftriaxone | ≤0.015 | ≤0.015 | ≤0.015 | 0.5 | 100 |
| Cefuroxime | 0.5 | 2 | 0.06 | 8 | 82.8 |
| Azithromycin | 0.5 | 1 | 0.06 | 4 | 10.0 |
| Clarithromycin | 4 | 8 | ≤0.03 | >32 | 5.9 |
| Levofloxacin | 0.015 | 0.06 | ≤0.004 | >8 | 95.4 |
| Moxifloxacin | 0.015 | 0.06 | ≤0.004 | >8 | 95.4 |
| Trimethoprim/sulfamethoxazole | 0.25 | 16 | ≤0.015 | >16 | 67.4 |
min, minimum; max, maximum; S, susceptible; –, not applicable.
MIC and susceptibility data for H. influenzae isolates (n = 239) from Turkey using PK/PD breakpoints
| Antibiotic . | MIC (mg/L) . | PK/PD susceptibility . | |||
|---|---|---|---|---|---|
| 50% . | 90% . | min . | max . | %S only . | |
| Amoxicillin (1.5 g/day) | 0.5 | 4 | ≤0.12 | >128 | 85.4 |
| Amoxicillin (4 g/day) | 0.5 | 4 | ≤0.12 | >128 | 90.8 |
| Amoxicillin/clavulanic acid (1.75 g/0.25 g/day adults; 45 mg/6.4 mg/kg/day children) | 0.5 | 2 | ≤0.06 | 8 | 92.9 |
| Amoxicillin/clavulanic acid (4 g/0.25 g/day adults; 90 mg/6.4 mg/kg/day children) | 0.5 | 2 | ≤0.06 | 8 | 99.2 |
| Ampicillin | 0.25 | 4 | 0.12 | >128 | – |
| Cefaclor | 2 | 8 | 0.12 | 32 | 13.0 |
| Cefdinir | 0.25 | 0.5 | 0.06 | 2 | 70.3 |
| Cefditoren | ≤0.03 | 0.06 | ≤0.03 | 2 | – |
| Cefixime | 0.03 | 0.12 | ≤0.015 | 2 | 99.6 |
| Cefpodoxime | 0.12 | 0.5 | ≤0.015 | >16 | 93.3 |
| Ceftriaxone | ≤0.015 | ≤0.015 | ≤0.015 | 0.5 | 100 |
| Cefuroxime | 0.5 | 2 | 0.06 | 8 | 82.8 |
| Azithromycin | 0.5 | 1 | 0.06 | 4 | 10.0 |
| Clarithromycin | 4 | 8 | ≤0.03 | >32 | 5.9 |
| Levofloxacin | 0.015 | 0.06 | ≤0.004 | >8 | 95.4 |
| Moxifloxacin | 0.015 | 0.06 | ≤0.004 | >8 | 95.4 |
| Trimethoprim/sulfamethoxazole | 0.25 | 16 | ≤0.015 | >16 | 67.4 |
| Antibiotic . | MIC (mg/L) . | PK/PD susceptibility . | |||
|---|---|---|---|---|---|
| 50% . | 90% . | min . | max . | %S only . | |
| Amoxicillin (1.5 g/day) | 0.5 | 4 | ≤0.12 | >128 | 85.4 |
| Amoxicillin (4 g/day) | 0.5 | 4 | ≤0.12 | >128 | 90.8 |
| Amoxicillin/clavulanic acid (1.75 g/0.25 g/day adults; 45 mg/6.4 mg/kg/day children) | 0.5 | 2 | ≤0.06 | 8 | 92.9 |
| Amoxicillin/clavulanic acid (4 g/0.25 g/day adults; 90 mg/6.4 mg/kg/day children) | 0.5 | 2 | ≤0.06 | 8 | 99.2 |
| Ampicillin | 0.25 | 4 | 0.12 | >128 | – |
| Cefaclor | 2 | 8 | 0.12 | 32 | 13.0 |
| Cefdinir | 0.25 | 0.5 | 0.06 | 2 | 70.3 |
| Cefditoren | ≤0.03 | 0.06 | ≤0.03 | 2 | – |
| Cefixime | 0.03 | 0.12 | ≤0.015 | 2 | 99.6 |
| Cefpodoxime | 0.12 | 0.5 | ≤0.015 | >16 | 93.3 |
| Ceftriaxone | ≤0.015 | ≤0.015 | ≤0.015 | 0.5 | 100 |
| Cefuroxime | 0.5 | 2 | 0.06 | 8 | 82.8 |
| Azithromycin | 0.5 | 1 | 0.06 | 4 | 10.0 |
| Clarithromycin | 4 | 8 | ≤0.03 | >32 | 5.9 |
| Levofloxacin | 0.015 | 0.06 | ≤0.004 | >8 | 95.4 |
| Moxifloxacin | 0.015 | 0.06 | ≤0.004 | >8 | 95.4 |
| Trimethoprim/sulfamethoxazole | 0.25 | 16 | ≤0.015 | >16 | 67.4 |
min, minimum; max, maximum; S, susceptible; –, not applicable.
H. influenzae susceptibility
Most isolates of H. influenzae from Turkey were β-lactamase negative (216/239, 90.4%). Within this population 11 isolates were β-lactamase negative and ampicillin resistant (BLNAR) by EUCAST breakpoints (ampicillin MIC >1 mg/L) and 5 by CLSI breakpoints (ampicillin MIC ≥4 mg/L). In keeping with this, 85.4%–90.8% of isolates were susceptible to ampicillin or amoxicillin, depending on the breakpoint used. All isolates were susceptible to ceftriaxone by CLSI and PK/PD breakpoints and only slightly less susceptible (98.7%) by EUCAST breakpoints. By CLSI and PK/PD breakpoints 99.6% of isolates were susceptible to cefixime and 94.1% were susceptible by EUCAST breakpoints. Susceptibility to amoxicillin/clavulanic acid was 99.2% by CLSI and PK/PD high-dose breakpoints and 92.9% by EUCAST high-dose and PK/PD low-dose breakpoints. Similar high susceptibility (98.7%) was observed to cefuroxime (oral) by CLSI breakpoints, but susceptibility was lower at 82.8% by PK/PD breakpoints and much lower (3.8%) according to EUCAST breakpoints. Isolate susceptibility to cefpodoxime was also lower for PK/PD and EUCAST breakpoints compared with CLSI breakpoints, but the effect was not as great as that seen with cefuroxime (oral): 93.3%, 88.3% and 97.5% susceptible, respectively. EUCAST breakpoints are not published for cefaclor or macrolides, but, while isolate susceptibility using CLSI breakpoints was high (92.9%–100%), susceptibility was much lower using PK/PD breakpoints (5.9%–13%). Susceptibility to cefdinir was high at 97.5% by CLSI breakpoints and lower by PK/PD breakpoints at 70.3% (no EUCAST breakpoints are published). Susceptibility to fluoroquinolones was 95.4% by CLSI and PK/PD breakpoints, but by EUCAST breakpoints 93.3% of isolates were susceptible to moxifloxacin and 92.5% were susceptible to levofloxacin (high or low dose). By PK/PD breakpoints susceptibility to clarithromycin and azithromycin was 5.9% and 10%, respectively. Susceptibility to trimethoprim/sulfamethoxazole was 67.4% by CLSI, PK/PD and EUCAST low-dose breakpoints, but 72% by EUCAST high-dose breakpoints. See Tables 7–9 and Figures 5–7.
Discussion
SOAR is an ongoing global surveillance study that originated in 2002, focusing on the two main CA-RTI pathogens, S. pneumoniae and H. influenzae. The data presented here are an analysis of the antibiotic susceptibility of S. pneumoniae and H. influenzae isolates collected in Turkey from three centres between 2015 and 2017. As with all surveillance studies using sentinel centres it is possible that susceptibility rates elsewhere in Turkey may differ, as demonstrated in an analysis of antibiotic susceptibility in Turkey during the SOAR study 2011–13.16
Penicillin susceptibility in S. pneumoniae was very low in Turkey using EUCAST low-dose IV or CLSI oral breakpoints (27.9%) and this is a continuation of a trend of reduced penicillin susceptibility observed since 2002 in SOAR surveillance in Turkey.16,17 In 2002–03 oral penicillin susceptibility was 74.7% in Turkey.17 Cefaclor was also tested between 2002 and 2015 in SOAR and showed reduced susceptibility over time (85.3% of isolates susceptible in 2002–03 versus 26.3% in 2015–17).16,17 Apart from fluoroquinolones, susceptibility of pneumococci to all other antibiotics was reduced between 2011–13 and 2015–17.16 By penicillin IV breakpoints using CLSI criteria, isolates from Turkey were mainly penicillin susceptible (84.4%) in 2015–17, which is less than that observed in 2011–13 through the SOAR programme in Turkey (97.3% susceptible).16 Similarly, ceftriaxone, amoxicillin and amoxicillin/clavulanic acid susceptibility by CLSI breakpoints was relatively high compared with most other antibiotics in 2015–17, but the susceptibility rate was much lower than in 2011–13: 83.2% versus 97.3% for ceftriaxone and 78.8% versus 91.3% for amoxicillin and amoxicillin/clavulanic acid. Other study data published for 2011–13 from Turkey very closely match those found in SOAR during the same time period.18 Apart from fluoroquinolones, there was a clear association between penicillin susceptibility and susceptibility to other antibiotics. These susceptibility data fit with the continuous high consumption rates and inappropriate antibiotic use in Turkey.5,6,8
In this study we compared the effect of differing breakpoints on antibiotic susceptibility. When using EUCAST breakpoints cefaclor was almost inactive against pneumococci (0.6% of isolates susceptible). Although higher susceptibility was observed by PK/PD (15.1%) and CLSI (26.3%) breakpoints, no criteria would support the use of this oral cephalosporin. A similar discrepancy was seen with cefdinir; 33% of isolates were susceptible by PK/PD breakpoints and 44.1% by CLSI breakpoints (EUCAST does not provide breakpoints for this antibiotic). By PK/PD breakpoints, susceptibility to cefixime was 28.5%. The high susceptibility of isolates to ceftriaxone (83.2% by CLSI or PK/PD breakpoints) was replicated using EUCAST breakpoints, where 98.3% susceptibility was observed, but only at the high dose recommended by EUCAST. Susceptibility to amoxicillin and amoxicillin/clavulanic acid was lower in pneumococci by EUCAST breakpoints: 49.7% using EUCAST low-dose breakpoints and 57.0%/58.1% using EUCAST high-dose breakpoints compared with 78.8% by CLSI and PK/PD low-dose breakpoints and 87.7% by PK/PD high-dose breakpoints.
H. influenzae from Turkey were mainly β-lactamase negative (90.4%), with 11 isolates characterized as BLNAR using EUCAST breakpoints and 5 by CLSI breakpoints. With the exception of trimethoprim/sulfamethoxazole (67.4% susceptible), susceptibility to antibiotics was high by CLSI breakpoints (≥87%). Previous SOAR surveillance from 2002 to 2013 also indicated generally high antibiotic susceptibility.16,17 Surveillance data published elsewhere have also shown high levels of antimicrobial agent susceptibility in Turkey during 2011–12.19 These data contrast considerably with the low susceptibility observed with pneumococci in this study. However, there were large differences in susceptibility depending on the breakpoints used. Susceptibility of H. influenzae to the macrolides was 96.2%–100% by CLSI breakpoints, but only 5.9%–10% by PK/PD breakpoints. Furthermore, susceptibility to cefaclor was 92.9% by CLSI breakpoints, but 13% by PK/PD breakpoints. EUCAST does not publish macrolide or cefaclor breakpoints for H. influenzae. Susceptibility to cefuroxime (oral) was 98.7% by CLSI breakpoints and 82.8% by PK/PD breakpoints, but only 3.8% by EUCAST breakpoints.
Following discussion and collaboration with EUCAST, this study also employed, for the first time in the SOAR investigations, different EUCAST breakpoints for low and high doses of several antibiotics tested so that the effect of different dosages on the susceptibility of the CA-RTI pathogens could be observed. Correct antibiotic dosing remains a challenge for the clinician, particularly since PK/PD parameters may alter during serious illness.20 Personalized antibiotic treatment may be an option; this is currently only considered for patients in intensive care.21 The ability to now assess pathogen susceptibility at different antibiotic doses, along with advances in diagnostics and monitoring, could allow possible progress in this area and the subsequent benefit for a wider range of patient groups.
To conclude, low susceptibility was observed for many antibiotics in S. pneumoniae from Turkey, but not H. influenzae. Continued surveillance of antibiotic susceptibility in Turkey is required to regularly assess any future changes.
Acknowledgements
We would like to thank: the participating laboratories for supplying the isolates; Professor Rafael Canton (Ramón y Cajal University Hospital, Spain) for reviewing the manuscript and EUCAST breakpoint tables; Dr Federica Monti (IHMA) and Livewire Editorial Communications for editorial assistance; Dr Sibylle Lob (IHMA) for the statistical analysis; Dr Devrim Ulusal (GSK-Turkey) and Dr Yalcin Seyhun (GSK-Turkey) for helping to facilitate the SOAR study in Turkey; Karen Langfeld (GSK) for reviewing the manuscript; and the SOAR Team (Subhashri Kundu, Cristiana Beltrame, Rendani Manenzhe and Nergis Keles) for their continuous support of SOAR.
Funding
This study was funded by GlaxoSmithKline.
Transparency declarations
This article forms part of a Supplement sponsored by GlaxoSmithKline. D. Torumkuney is an employee of GlaxoSmithKline and holds shares in GlaxoSmithKline. I. Morrissey is an employee of IHMA, a medical communication and consultancy company, who participated in the exploration, interpretation of the results and preparation of this manuscript on behalf of GlaxoSmithKline. All other authors: none to declare. Editorial assistance was provided by Livewire Editorial Communications.
References
CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically—Eleventh Edition: M07.
CLSI. Performance Standards for Antimicrobial Susceptibility Testing—Twenty-Ninth Edition: M100.
EUCAST. Breakpoint Tables for Interpretation of MICs and Zone Diameters, Version 9.0.
- amoxicillin
- ampicillin
- antibiotics
- ceftriaxone
- erythromycin
- antibiotic resistance, bacterial
- penicillin
- cefuroxime
- clarithromycin
- haemophilus influenzae
- amoxicillin-potassium clavulanate combination
- cefaclor
- cefixime
- fluoroquinolones
- pneumococcal infections
- respiratory tract infections
- streptococcus pneumoniae
- trimethoprim-sulfamethoxazole combination
- macrolides
- levofloxacin
- pharmacodynamics
- cefdinir
- moxifloxacin
- antimicrobial susceptibility
- cefpodoxime
- multi-antibiotic resistance
- community
- malnutrition-inflammation-cachexia syndrome



