-
PDF
- Split View
-
Views
-
Cite
Cite
Kai Peng, Ruichao Li, Tao He, Yuan Liu, Zhiqiang Wang, Characterization of a porcine Proteus cibarius strain co-harbouring tet(X6) and cfr, Journal of Antimicrobial Chemotherapy, Volume 75, Issue 6, June 2020, Pages 1652–1654, https://doi.org/10.1093/jac/dkaa047
Close - Share Icon Share
Sir,
Plasmid-mediated tigecycline-resistance genes tet(X3) and tet(X4) have been reported in Enterobacteriaceae isolated from food-producing animals.1,2 Recently, chromosomally mediated tet(X4) has been found in Escherichia coli 2ZN37-2 isolated from little egrets,3 which implied the great mobility of tet(X) variant genes. Besides, since the report of the multiresistance gene cfr in Proteus vulgaris,4 subsequent studies have shown that cfr can spread through plasmids and transposons in Gram-negative bacteria.5,6 Although the tet(X) variant genes and cfr were highly mobile, no convergence of the two genes in the same strain was reported. In this study, we characterized a novel variant of tet(X) located on the chromosome and a cfr gene located on a novel plasmid from a Proteus cibarius strain.
Strain ZF2 was isolated from a faeces sample of swine origin in May 2018 in Jiangsu, China, using an LB agar plate supplemented with tigecycline (4 mg/L), and further identified as P. cibarius by 16S rRNA gene sequencing and WGS analysis. Antimicrobial susceptibility testing was performed by broth microdilution with E. coli ATCC 25922 as the control and interpreted as per the EUCAST standard (http://www.eucast.org/clinical_breakpoints/). ZF2 was resistant to doxycycline, tigecycline, colistin, florfenicol and amoxicillin (Table S1, available as Supplementary data at JAC Online). A novel tet(X) gene was identified by PCR and sequencing using primers as previously described.1 The novel tet(X) variant, designated as tet(X6), encoded 378 amino acids, which showed 84.4%, 66.3%, 84.7%, 79.6%, 87.3% and 95% amino acid sequence identity to those of Tet(X) (M37699), Tet(X1) (AJ311171), Tet(X2) (AJ311171), Tet(X3) (MK134375), Tet(X4) (MK134376) and Tet(X5) (CP040912) respectively (Figure S1a). To confirm its resistance function, the intact ORF of tet(X6) was amplified by PCR with primers ZF2-tet(X)-F (cgagctcCCAGCGAACAAGAAT) and ZF2-tet(X)-R (cccaagcttCCTCGCCTTTCTGTT), cloned into the pET28a vector, transformed chemically into E. coli BL21 and the MICs of different tetracycline antibiotics were determined. Sanger sequencing was used to confirm the insertion of the tet(X6) gene in pET28a. Under IPTG induction, E. coli BL21 harbouring pET28a-tetX6 conferred resistance to tetracycline (MIC 128 mg/L), oxytetracycline (MIC 64 mg/L), doxycycline (MIC 16 mg/L), minocycline (MIC 16 mg/L), tigecycline (MIC 8 mg/L), eravacycline (MIC 8 mg/L) and omadacycline (MIC 32 mg/L) (Table S1), indicating that the tet(X6) gene can confer tigecycline resistance.
Conjugation assay was performed to verify the transferability of the tet(X6) gene with E. coli J53 AziR as the recipient strain. However, the experiment failed after three repeats. To investigate the genetic structure of the tet(X6) gene, genomic sequences of ZF2 were de novo assembled by short-read Illumina and Nanopore long-read MinION platforms7 and the complete genome sequences of ZF2 were annotated using RAST (http://rast.nmpdr.org/) automatically and modified manually. Strain ZF2 harboured one chromosome (4 237 246 bp, CP045008) and three plasmids: pZF2-cfr (59 167 bp, CP045009); pZF2-7kb (7081 bp, CP045010); and pZF2-qnrD (2683 bp, CP045011). The tet(X6) gene was detected in a chromosomal integrative and conjugative element (ICE), 130 143 bp in size, showing 73% coverage and 96.14% nucleotide identity to ICEPmiChn2 (KY437726.1) from Proteus mirabilis JN7.8 This region integrated into the 5′ end of the prfC gene (Figure S2), confirmed by PCR and Sanger sequencing, and possessed a conserved integrase gene int, which were specific features of the SXT/R391 ICE family. Also, this novel tet(X6)-bearing SXT/R391 ICE was designated as ICEPciChn1 according to the nomenclature system.9 Conjugation of ICEPmiChn2 was successful in strain JN7, but failed for the ICEPciChn1 carried by ZF2. The structural analysis of two ICEs showed that the lack of xis may lead to disruption of the conjugation ability of the ICE in ZF2, implying xis may play a critical role in facilitating ICE excision.10 BLASTn analysis showed that ICEPciChn1 also shared a similar backbone structure with ICEPmiCHN3300 (KX243415.1) from a clinical P. mirabilis strain (Figure 1a). Like the genetic environment of tet(X3) and tet(X4) described previously,1,2 the tet(X6) gene was located within a 7679 bp region with the gene arrangement ISCR2-ORF1-tet(X6)-ORF2-ORF3-ORF4-ISCR2 (Figure 1b), which suggested that ISCR2 was associated with the mobilization of the tet(X6) gene. Inverse PCR with primers P3-1 (ACTGTAATGTCCACGCCGTT) and P4 (TGCTCATTTGATGCCTCCTT) generated a circular intermediate, implying a possible role of ISCR2 in the transferability of the tet(X6) gene. Other resistance genes, including double floR genes, double strB genes, sul2, strA and aph(3′)-VIa, were found around the tet(X6) gene in ICEPciChn1. Interestingly, these genes were carried by a transposon-like structure flanked by two tnpA genes and integrated into variable region III of ICEPciChn1, which was similar to ICEAplChn1 (KX196444.1) (Figure 1b). This transposon-like structure may contribute to the transfer of antibiotic resistance genes. The emergence of a novel tet(X) variant in an ICE suggested that ICEs could serve as important carriers for tigecycline resistance genes and pose a potential risk of dissemination.
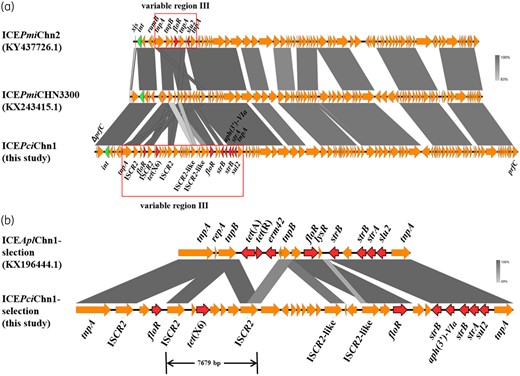
Comparative analysis of the tet(X6)-harbouring ICE and other similar ICEs. (a) Structure comparisons of ICEPmiChn2 (KY437726.1), ICEPmiCHN3300 (KX243415.1) and ICEPciChn1 (this study). (b) Comparative genetic environments of the tet(X6)-harbouring ICE and ICEAplChn1 (KX196444.1). Visualization of nucleotide sequence comparisons was generated with Easyfig (http://mjsull.github.io/Easyfig/files.html). This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
A transferable multiresistance gene, cfr, was found located on a plasmid designated as pZF2-cfr. BLASTn analysis of this plasmid showed no significant homology to any plasmids in the GenBank nr database. The cfr gene was flanked by IS26 elements and inserted into the backbone of pZF2-cfr (Figure S3a). A similar structure harbouring cfr was found in the NCBI database (JF969273.1) (Figure S3b), suggesting that IS26 could play an important role in cfr gene transfer. Subsequently, pZF2-cfr was transformed into E. coli EC600 by electrotransformation and the recipient E. coli EC600 showed a 32-fold increase in florfenicol MIC. To the best of our knowledge, this is the first report of a cfr-bearing plasmid in P. cibarius.
In conclusion, we identified a novel tet(X) variant gene within MDR ICEs on the chromosome of strain ZF2. The cfr gene was identified on a novel plasmid, pZF2-cfr, of the same strain, suggesting that P. cibarius played an important role in the spread of antibiotic resistance genes. Continuous surveillance of Proteus species, including P. cibarius, should be conducted for understanding and tackling the dissemination of tigecycline resistance.
Funding
This work was supported by the Natural Science Foundation of Jiangsu Province (no. BK20180900), the National Natural Science Foundation of China (no. 31872523 and no. 31872526) and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Transparency declarations
None to declare.
Supplementary data
Table S1 and Figures S1 to S3 are available as Supplementary data at JAC Online.
References
Author notes
Kai Peng and Ruichao Li contributed equally to the work.



