-
PDF
- Split View
-
Views
-
Cite
Cite
Daniela Sánchez, Solange Arazi Caillaud, Ines Zapiola, Silvina Fernandez Giuliano, Rosa Bologna, Andrea Mangano, Paula C Aulicino, Impact of genotypic diversity on selection of subtype-specific drug resistance profiles during raltegravir-based therapy in individuals infected with B and BF recombinant HIV-1 strains, Journal of Antimicrobial Chemotherapy, Volume 75, Issue 6, June 2020, Pages 1567–1574, https://doi.org/10.1093/jac/dkaa042
Close - Share Icon Share
Abstract
Current knowledge on HIV-1 resistance to integrase inhibitors (INIs) is based mostly on subtype B strains. This contrasts with the increasing use of INIs in low- and middle-income countries, where non-B subtypes predominate.
HIV-1 drug resistance genotyping was performed in 30 HIV-1-infected individuals undergoing virological failure to raltegravir. Drug resistance mutations (DRMs) and HIV-1 subtype were characterized using Stanford HIVdb and phylogenetic analyses.
Of the 30 integrase (IN) sequences, 14 were characterized as subtype F (47%), 8 as subtype B (27%), 7 as BF recombinants (23%) and 1 as a putative CRF05_DF (3%). In 25 cases (83%), protease and reverse transcriptase (PR-RT) sequences from the same individuals confirmed the presence of different BF recombinants. Stanford HIVdb genotyping was concordant with phylogenetic inference in 70% of IN and 60% of PR-RT sequences. INI DRMs differed between B and F IN subtypes, with Q148K/R/H, G140S and E138K/A being more prevalent in subtype B (63% versus 0%, P = 0.0021; 50% versus 0%, P = 0.0096; and 50% versus 0%, P = 0.0096, respectively). These differences were independent of the time on raltegravir therapy or viral load at the time of genotyping. INI DRMs in subtype F IN genomes predicted a lower level of resistance to raltegravir and no cross-resistance to second-generation INIs.
Alternative resistance pathways to raltegravir develop in subtypes B and F IN genomes, with implications for clinical practice. Evaluating the role of HIV-1 subtype in development and persistence of mutations that confer resistance to INIs will be important to improve algorithms for resistance testing and optimize the use of INIs.
Introduction
HIV integrase inhibitors (INIs) are among the most promising and potent antiretroviral (ARV) drugs currently available for treatment of HIV-1 infection. Their antiviral activity is exerted by blocking HIV genome transfer and integration into the host cell DNA.1 Currently, four INIs are available (raltegravir, dolutegravir, elvitegravir and bictegravir) and a fifth one—cabotegravir—is under clinical trial. While mutations in the integrase (IN) gene selected during exposure to raltegravir can also limit the activity of elvitegravir, they have little or no effect on second-generation INIs dolutegravir and bictegravir. However, there is still limited experience in clinical settings with these new ARVs and most of the current knowledge on HIV-1 resistance to INIs is based on subtype B strains.2,3
Approved in 2007, raltegravir was initially restricted to ARV-experienced patients requiring salvage ART regimens. A few years later, it was also approved for treatment of ART-naive populations. Currently, raltegravir is the preferred first-line regimen for neonates and an alternative first-line regimen for infants and children for whom approved dolutegravir dosing is not yet available (under 6 years old). In addition, raltegravir is the only INI recommended for treatment of HIV-1 infection in pregnant women and also for those who require treatment for coinfection with drugs that often cannot be used with many ARV medications, such as HIV patients coinfected with HCV, TB or HBV.4
Despite its high clinical efficacy, raltegravir shows a low genetic barrier to resistance, as a few mutations in the IN gene can render the drug ineffective against these viruses. In subtype B strains, resistance to raltegravir occurs through one of three major mutations in the IN HIV-1 gene: N155H, Q148K/H/R or Y143C/R.5–8 Each of these mutations can be accompanied by secondary mutations that compensate for fitness loss of the single mutants, with Y143C/R ± T97A, Q148H/K/R ± G140S/A and N155H ± E92Q being the most frequent combinations of mutations. While all three major mutations confer high-level resistance to raltegravir and elvitegravir, only those associated with the Q148H/K/R pathway substantially affect the activity of dolutegravir. Using a panel of 50 HIV-1 molecular clones with different IN substitutions, Kobayashi et al.9 showed that only three combinations of raltegravir-associated mutations (Q148H + G140S, Q148R + E138K and T66I + L74M) had an impact on the susceptibility to dolutegravir, decreasing it by 2- to 4-fold. In agreement with the in vitro experiments, results from the VIKING clinical study showed that viruses with Q148X + ≥1 raltegravir resistance-associated mutation were more prone to developing virological failure to dolutegravir, independently of the new mutations emerging in the IN gene.10–13 Therefore, individuals with raltegravir-resistant infection may still benefit from the use of the second-generation INIs dolutegravir and bictegravir, depending on the mutations present at baseline.
Until now, HIV-1 subtype has not been considered in the choice of ART regimen. However, increasing evidence shows that HIV-1 subtype differences can modulate the development and/or persistence of resistance mutations selected during exposure to INIs.14,15 Information on resistance to INIs in non-B subtypes is still scarce and based on a limited number of studies that compare a minority of different non-B strains against the predominant subtype B variants.16–18 This contrasts with the increasing use of INIs in clinical practice in low- and middle-income countries, where non-B subtypes predominate.19 Therefore, understanding the role of HIV-1 subtype on the development of resistance mutations can impact on ARV strategies.
In Argentina, the HIV-1 epidemic is characterized by co-circulation of subtype B strains together with different BF recombinant forms. Despite CRF12_BF being the prototypic BF recombinant from Argentina, we and others have shown that the majority of BF recombinants responsible for new infections in Argentina are in fact unique recombinant forms (URFs).20,21 While these studies were based on the characterization of the protease and reverse transcriptase (PR-RT) genomic region, the genetic diversity in the IN genomic region has not yet been determined.
Our aim was to investigate the prevalence and determinants of resistance mutations to INIs in a population of individuals failing raltegravir-based therapy in Argentina.
Patients and methods
Patients
HIV-1 genotypic resistance testing was performed in 30 HIV-1-infected individuals with virological failure to a raltegravir-based ART regimen. Of them, 14 (47%) were vertically HIV-1-infected children and adolescents from the Garrahan Hospital HIV cohort from Buenos Aires, Argentina. The remaining 16 (53%) were adult patients from Muñiz Hospital, from Buenos Aires, Argentina. Available clinical data included HIV-1 RNA load at the time of PR-RT and IN sequencing, as well as time of initiation of the raltegravir-based ART. The majority of the cases were receiving raltegravir as part of a salvage ART regimen and showed mutations to one or more classes of classical ARVs: to NRTIs in 24 cases (80%), to PIs in 12 cases (40%) and to NNRTIs in 13 cases (43%).
Ethics
The study was reviewed and approved by the Garrahan Hospital Ethics Committee (IRB00004240) before it began. Informed consent was obtained in all cases.
HIV-1 pol genotyping and identification of drug resistance mutations (DRMs)
HIV-1 genotyping was performed by PCR amplification and Sanger sequencing of plasma-derived HIV-1 genomic fragments. Samples were processed for RNA extraction, reverse transcription and subsequent PCR amplification of PR-RT and IN HIV-1 genomic segments using previously described primers and PCR conditions.22–25 Sanger sequencing was performed bidirectionally using the BigDye Terminator 3.1 Cycle Sequencing Kit from Applied Biosystems (Life Technology Corporation, Austin, TX, USA) on an AB Applied Biosystems 3130 or 3500xL Genetic Analyzer. PR-RT sequences were aligned and trimmed to PR codons 1–99 and RT codons 1–220 (nucleotide positions 2250 to 3205 relative to HXB2). IN sequences were aligned and trimmed to codons 1–288 (nucleotides 4230–5093 relative to HXB2). HIV-1 IN sequences were analysed for the presence of INI resistance-associated DRMs (INI DRMs) by submitting them to the Stanford University HIVdb program v8.8 (http://hivdb.stanford.edu). The following mutations were considered INI DRMs: T66K, L74F/I/M, E92Q, Q95K, T97A, G118R, E138A/K/T, G140A/S/C, P142T, Y143C/H/R, Q148H/K/R, V151I, N155H, E157Q, G163R/K, S230R, D232N and R263K.
Statistical analysis
Differences in proportions between groups were evaluated using Fisher’s exact test. Differences in medians between groups were assessed using the Mann–Whitney U-test.
Phylogenetic analyses and characterization of HIV-1 subtype
Characterization of HIV-1 genotype in PR-RT and IN sequences was performed as previously described.19 Briefly, HIV-1 sequences were aligned to HIV-1 subtype reference sequences obtained from Los Alamos National Laboratory HIV sequence database. A neighbour-joining phylogenetic tree was built using the Tamura–Nei model implemented in the MEGA 6.0 program.26 Similarity and bootscan analyses were performed with Simplot program v2.527 to evidence recombination breakpoints. PR-RT and IN sequences were also submitted to the Stanford HIVdb genotyping tool for comparison. In this tool, the subtype of the submitted sequence is determined by examining the closest matching reference sequence and applying a set of rules that make use of known properties of the different subtypes and circulating recombinant forms (CRFs).
Results
In a total of 30 individuals failing a raltegravir-based ART regimen, IN sequences were analysed for the presence of mutations associated with INI resistance and for characterization of HIV-1 subtype. IN genotyping was performed a median of 22 months (IQR: 11–35 months) after initiation of raltegravir-based ART. Median HIV-1 viral load measured at the time of genotyping was 4.72 log10 copies/mL (IQR: 4.08–4.97 log10 copies/mL). Twenty-four of the 30 cases (80%) presented one or more INI DRMs (Table 1). Major INI DRMs were identified in 20 cases (67%): Q148H/R (9 cases, 30%), G140S (8 cases, 27%), N155H (6 cases, 20%), Y143R (6 cases, 20%) and E138A/K (5 cases, 17%). According to the Stanford HIVdb program, INI DRMs predicted high-level resistance in 70% of the cases for raltegravir, in 50% of the cases for elvitegravir and in 13% of the cases for dolutegravir and bictegravir. Six cases (20%) maintained full susceptibility to raltegravir, 7 (23%) to elvitegravir and 20 (67%) to dolutegravir and bictegravir. The remaining cases showed low- to intermediate-level resistance.
Characteristics, mutational profiles and HIV-1 subtype identified in the study population
| ID . | HIV-1 pVL (log10 copies/mL) . | Time on RAL (months) . | INI DRMs . | Predicted drug resistance to: . | HIV-1 subtype in IN . | HIV-1 subtype in PR-RT . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| RAL . | EVG . | DTG . | BIC . | phylogenetic and recombination analysis . | Stanford HIVdb genotyping . | phylogenetic and recombination analysis . | Stanford HIVdb genotyping . | ||||
| AR_461008 | 4.92 | 30 | T97A, Y143R | H | L | S | S | B | B | B | B |
| AR_470449BIS | 5.54 | 57 | G140S, Q148H | H | H | I | I | B | B | URF_BF | B |
| AR_485196 | 5.08 | 14 | L74M, T97A, Y143R, E138K | H | I | L | L | B | B | B | B |
| AR_485630 | 5.15 | 27 | Y143R | H | S | S | S | B | B | B | B |
| AR_488090 | 4.76 | 8 | G140S, Q148H, E138K | H | H | H | H | B | B | URF_BF | B |
| AR_497339 | 4.79 | 11 | G140S, Q148H, E138K | H | H | H | H | B | B | B | B |
| AR_5856_AA323RED | 4.73 | 42 | G140S, Q148H | H | H | I | I | B | B | URF_BF | B |
| AR_481594 | 4.41 | 21 | T97A, Q148R, E138K, S147G, V151I/V | H | H | H | H | B | B | F | F |
| AR_475736 | 2.85 | 23 | L74M, N155H, V151I, G163R | H | H | S | S | F | B | CRF29_BF | CRF29_BF |
| AR_477551 | 3.69 | 30 | — | S | S | S | S | F | F | URF_BF | CRF12_BF |
| AR_481960 | 4.79 | — | — | S | S | S | S | F | F | CRF12_BF | CRF12_BF |
| AR_487095 | 5.31 | 7 | L74I/M, T97A, P142T, G163K | L | L | S | S | F | F | URF_BF | B + F |
| AR_490082 | 4.49 | 11 | T97A, Y143R | H | L | S | S | F | F | CRF12_BF | CRF12_BF |
| AR_495852 | 4.60 | 42 | L74M, T97A, Y143R | H | I | S | S | F | F | URF_BF | B + F |
| AR_598_X785RYL | 4.26 | 36 | G163K | L | L | S | S | F | F | URF_BF | B + F |
| AR_497397 | 6.01 | 76 | — | S | S | S | S | F | F | URF_BF | B + F |
| AR_5155_T723GM | 6.02 | 12 | L74M, T97A, Y143R | H | I | S | S | F | F | URF_BF | B+F |
| AR_4363_W359PMME | 3.68 | 10 | N155N/H | H | H | S | S | F | F | CRF12_BF | CRF12_BF |
| AR_3080_AA714DTJL | 3.28 | 28 | T97A, G163R | L | L | S | S | F | F | CRF17_BF | B |
| AR_5793_AA770PG | 2.95 | 22 | N155H, G163R | H | H | S | S | F | F | URF_BF | B + F |
| AR_2337_AA807FLA | 4.72 | 8 | — | S | S | S | S | F | F | URF_BF | B + F |
| AR_3174_AA185BIL | 4.08 | 8 | — | S | S | S | S | F | F | URF_BF | B |
| AR_490047 | — | 73 | G140S, Q148H | H | H | I | I | URF_BF | F | URF_BF | B |
| AR_3013_W812BDMS | 3.63 | 42 | V151I, N155H | H | H | S | S | URF_BF | B | URF_BF | B |
| AR_3302_Y937LLN | 4.52 | 19 | G140S, Q148H | H | H | I | I | URF_BF | B | URF_BF | B |
| AR_568_Z708GGA | 4.97 | 27 | G140S, Q148H | H | H | I | I | URF_BF | B | URF_BF | B |
| AR_6462_AA893CP | 4.62 | 17 | L74I, G140S, Q148H, E138A | H | H | H | H | URF_BF | F | CRF12_BF | CRF12_BF |
| AR_6286_AA416RBBJ | 4.76 | 12 | N155H | H | H | S | S | URF_BF | F | URF_BF | B + F |
| AR_3128_Z059UTA | 3.42 | 31 | — | S | S | S | S | URF_BF | F | URF_BF | B |
| AR_472584 | 6.62 | 35 | T97A, V151I, N155H | H | H | S | S | ? | CRF05_DF | CRF05_DF | CRF05_DF |
| ID . | HIV-1 pVL (log10 copies/mL) . | Time on RAL (months) . | INI DRMs . | Predicted drug resistance to: . | HIV-1 subtype in IN . | HIV-1 subtype in PR-RT . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| RAL . | EVG . | DTG . | BIC . | phylogenetic and recombination analysis . | Stanford HIVdb genotyping . | phylogenetic and recombination analysis . | Stanford HIVdb genotyping . | ||||
| AR_461008 | 4.92 | 30 | T97A, Y143R | H | L | S | S | B | B | B | B |
| AR_470449BIS | 5.54 | 57 | G140S, Q148H | H | H | I | I | B | B | URF_BF | B |
| AR_485196 | 5.08 | 14 | L74M, T97A, Y143R, E138K | H | I | L | L | B | B | B | B |
| AR_485630 | 5.15 | 27 | Y143R | H | S | S | S | B | B | B | B |
| AR_488090 | 4.76 | 8 | G140S, Q148H, E138K | H | H | H | H | B | B | URF_BF | B |
| AR_497339 | 4.79 | 11 | G140S, Q148H, E138K | H | H | H | H | B | B | B | B |
| AR_5856_AA323RED | 4.73 | 42 | G140S, Q148H | H | H | I | I | B | B | URF_BF | B |
| AR_481594 | 4.41 | 21 | T97A, Q148R, E138K, S147G, V151I/V | H | H | H | H | B | B | F | F |
| AR_475736 | 2.85 | 23 | L74M, N155H, V151I, G163R | H | H | S | S | F | B | CRF29_BF | CRF29_BF |
| AR_477551 | 3.69 | 30 | — | S | S | S | S | F | F | URF_BF | CRF12_BF |
| AR_481960 | 4.79 | — | — | S | S | S | S | F | F | CRF12_BF | CRF12_BF |
| AR_487095 | 5.31 | 7 | L74I/M, T97A, P142T, G163K | L | L | S | S | F | F | URF_BF | B + F |
| AR_490082 | 4.49 | 11 | T97A, Y143R | H | L | S | S | F | F | CRF12_BF | CRF12_BF |
| AR_495852 | 4.60 | 42 | L74M, T97A, Y143R | H | I | S | S | F | F | URF_BF | B + F |
| AR_598_X785RYL | 4.26 | 36 | G163K | L | L | S | S | F | F | URF_BF | B + F |
| AR_497397 | 6.01 | 76 | — | S | S | S | S | F | F | URF_BF | B + F |
| AR_5155_T723GM | 6.02 | 12 | L74M, T97A, Y143R | H | I | S | S | F | F | URF_BF | B+F |
| AR_4363_W359PMME | 3.68 | 10 | N155N/H | H | H | S | S | F | F | CRF12_BF | CRF12_BF |
| AR_3080_AA714DTJL | 3.28 | 28 | T97A, G163R | L | L | S | S | F | F | CRF17_BF | B |
| AR_5793_AA770PG | 2.95 | 22 | N155H, G163R | H | H | S | S | F | F | URF_BF | B + F |
| AR_2337_AA807FLA | 4.72 | 8 | — | S | S | S | S | F | F | URF_BF | B + F |
| AR_3174_AA185BIL | 4.08 | 8 | — | S | S | S | S | F | F | URF_BF | B |
| AR_490047 | — | 73 | G140S, Q148H | H | H | I | I | URF_BF | F | URF_BF | B |
| AR_3013_W812BDMS | 3.63 | 42 | V151I, N155H | H | H | S | S | URF_BF | B | URF_BF | B |
| AR_3302_Y937LLN | 4.52 | 19 | G140S, Q148H | H | H | I | I | URF_BF | B | URF_BF | B |
| AR_568_Z708GGA | 4.97 | 27 | G140S, Q148H | H | H | I | I | URF_BF | B | URF_BF | B |
| AR_6462_AA893CP | 4.62 | 17 | L74I, G140S, Q148H, E138A | H | H | H | H | URF_BF | F | CRF12_BF | CRF12_BF |
| AR_6286_AA416RBBJ | 4.76 | 12 | N155H | H | H | S | S | URF_BF | F | URF_BF | B + F |
| AR_3128_Z059UTA | 3.42 | 31 | — | S | S | S | S | URF_BF | F | URF_BF | B |
| AR_472584 | 6.62 | 35 | T97A, V151I, N155H | H | H | S | S | ? | CRF05_DF | CRF05_DF | CRF05_DF |
Mutations contributing to INI susceptibility according to the Stanford HIVdb program are shown in bold. Mutations known as major are underlined.
pVL, plasma viral load; RAL, raltegravir; EVG, elvitegravir; DTG, dolutegravir; BIC, bictegravir; S, susceptible; H, high-level resistant; I, intermediate-level resistant; L, low-level resistant;—, no mutation detected by Sanger sequencing; ?, could not be confirmed by phylogenetic analysis.
Characteristics, mutational profiles and HIV-1 subtype identified in the study population
| ID . | HIV-1 pVL (log10 copies/mL) . | Time on RAL (months) . | INI DRMs . | Predicted drug resistance to: . | HIV-1 subtype in IN . | HIV-1 subtype in PR-RT . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| RAL . | EVG . | DTG . | BIC . | phylogenetic and recombination analysis . | Stanford HIVdb genotyping . | phylogenetic and recombination analysis . | Stanford HIVdb genotyping . | ||||
| AR_461008 | 4.92 | 30 | T97A, Y143R | H | L | S | S | B | B | B | B |
| AR_470449BIS | 5.54 | 57 | G140S, Q148H | H | H | I | I | B | B | URF_BF | B |
| AR_485196 | 5.08 | 14 | L74M, T97A, Y143R, E138K | H | I | L | L | B | B | B | B |
| AR_485630 | 5.15 | 27 | Y143R | H | S | S | S | B | B | B | B |
| AR_488090 | 4.76 | 8 | G140S, Q148H, E138K | H | H | H | H | B | B | URF_BF | B |
| AR_497339 | 4.79 | 11 | G140S, Q148H, E138K | H | H | H | H | B | B | B | B |
| AR_5856_AA323RED | 4.73 | 42 | G140S, Q148H | H | H | I | I | B | B | URF_BF | B |
| AR_481594 | 4.41 | 21 | T97A, Q148R, E138K, S147G, V151I/V | H | H | H | H | B | B | F | F |
| AR_475736 | 2.85 | 23 | L74M, N155H, V151I, G163R | H | H | S | S | F | B | CRF29_BF | CRF29_BF |
| AR_477551 | 3.69 | 30 | — | S | S | S | S | F | F | URF_BF | CRF12_BF |
| AR_481960 | 4.79 | — | — | S | S | S | S | F | F | CRF12_BF | CRF12_BF |
| AR_487095 | 5.31 | 7 | L74I/M, T97A, P142T, G163K | L | L | S | S | F | F | URF_BF | B + F |
| AR_490082 | 4.49 | 11 | T97A, Y143R | H | L | S | S | F | F | CRF12_BF | CRF12_BF |
| AR_495852 | 4.60 | 42 | L74M, T97A, Y143R | H | I | S | S | F | F | URF_BF | B + F |
| AR_598_X785RYL | 4.26 | 36 | G163K | L | L | S | S | F | F | URF_BF | B + F |
| AR_497397 | 6.01 | 76 | — | S | S | S | S | F | F | URF_BF | B + F |
| AR_5155_T723GM | 6.02 | 12 | L74M, T97A, Y143R | H | I | S | S | F | F | URF_BF | B+F |
| AR_4363_W359PMME | 3.68 | 10 | N155N/H | H | H | S | S | F | F | CRF12_BF | CRF12_BF |
| AR_3080_AA714DTJL | 3.28 | 28 | T97A, G163R | L | L | S | S | F | F | CRF17_BF | B |
| AR_5793_AA770PG | 2.95 | 22 | N155H, G163R | H | H | S | S | F | F | URF_BF | B + F |
| AR_2337_AA807FLA | 4.72 | 8 | — | S | S | S | S | F | F | URF_BF | B + F |
| AR_3174_AA185BIL | 4.08 | 8 | — | S | S | S | S | F | F | URF_BF | B |
| AR_490047 | — | 73 | G140S, Q148H | H | H | I | I | URF_BF | F | URF_BF | B |
| AR_3013_W812BDMS | 3.63 | 42 | V151I, N155H | H | H | S | S | URF_BF | B | URF_BF | B |
| AR_3302_Y937LLN | 4.52 | 19 | G140S, Q148H | H | H | I | I | URF_BF | B | URF_BF | B |
| AR_568_Z708GGA | 4.97 | 27 | G140S, Q148H | H | H | I | I | URF_BF | B | URF_BF | B |
| AR_6462_AA893CP | 4.62 | 17 | L74I, G140S, Q148H, E138A | H | H | H | H | URF_BF | F | CRF12_BF | CRF12_BF |
| AR_6286_AA416RBBJ | 4.76 | 12 | N155H | H | H | S | S | URF_BF | F | URF_BF | B + F |
| AR_3128_Z059UTA | 3.42 | 31 | — | S | S | S | S | URF_BF | F | URF_BF | B |
| AR_472584 | 6.62 | 35 | T97A, V151I, N155H | H | H | S | S | ? | CRF05_DF | CRF05_DF | CRF05_DF |
| ID . | HIV-1 pVL (log10 copies/mL) . | Time on RAL (months) . | INI DRMs . | Predicted drug resistance to: . | HIV-1 subtype in IN . | HIV-1 subtype in PR-RT . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| RAL . | EVG . | DTG . | BIC . | phylogenetic and recombination analysis . | Stanford HIVdb genotyping . | phylogenetic and recombination analysis . | Stanford HIVdb genotyping . | ||||
| AR_461008 | 4.92 | 30 | T97A, Y143R | H | L | S | S | B | B | B | B |
| AR_470449BIS | 5.54 | 57 | G140S, Q148H | H | H | I | I | B | B | URF_BF | B |
| AR_485196 | 5.08 | 14 | L74M, T97A, Y143R, E138K | H | I | L | L | B | B | B | B |
| AR_485630 | 5.15 | 27 | Y143R | H | S | S | S | B | B | B | B |
| AR_488090 | 4.76 | 8 | G140S, Q148H, E138K | H | H | H | H | B | B | URF_BF | B |
| AR_497339 | 4.79 | 11 | G140S, Q148H, E138K | H | H | H | H | B | B | B | B |
| AR_5856_AA323RED | 4.73 | 42 | G140S, Q148H | H | H | I | I | B | B | URF_BF | B |
| AR_481594 | 4.41 | 21 | T97A, Q148R, E138K, S147G, V151I/V | H | H | H | H | B | B | F | F |
| AR_475736 | 2.85 | 23 | L74M, N155H, V151I, G163R | H | H | S | S | F | B | CRF29_BF | CRF29_BF |
| AR_477551 | 3.69 | 30 | — | S | S | S | S | F | F | URF_BF | CRF12_BF |
| AR_481960 | 4.79 | — | — | S | S | S | S | F | F | CRF12_BF | CRF12_BF |
| AR_487095 | 5.31 | 7 | L74I/M, T97A, P142T, G163K | L | L | S | S | F | F | URF_BF | B + F |
| AR_490082 | 4.49 | 11 | T97A, Y143R | H | L | S | S | F | F | CRF12_BF | CRF12_BF |
| AR_495852 | 4.60 | 42 | L74M, T97A, Y143R | H | I | S | S | F | F | URF_BF | B + F |
| AR_598_X785RYL | 4.26 | 36 | G163K | L | L | S | S | F | F | URF_BF | B + F |
| AR_497397 | 6.01 | 76 | — | S | S | S | S | F | F | URF_BF | B + F |
| AR_5155_T723GM | 6.02 | 12 | L74M, T97A, Y143R | H | I | S | S | F | F | URF_BF | B+F |
| AR_4363_W359PMME | 3.68 | 10 | N155N/H | H | H | S | S | F | F | CRF12_BF | CRF12_BF |
| AR_3080_AA714DTJL | 3.28 | 28 | T97A, G163R | L | L | S | S | F | F | CRF17_BF | B |
| AR_5793_AA770PG | 2.95 | 22 | N155H, G163R | H | H | S | S | F | F | URF_BF | B + F |
| AR_2337_AA807FLA | 4.72 | 8 | — | S | S | S | S | F | F | URF_BF | B + F |
| AR_3174_AA185BIL | 4.08 | 8 | — | S | S | S | S | F | F | URF_BF | B |
| AR_490047 | — | 73 | G140S, Q148H | H | H | I | I | URF_BF | F | URF_BF | B |
| AR_3013_W812BDMS | 3.63 | 42 | V151I, N155H | H | H | S | S | URF_BF | B | URF_BF | B |
| AR_3302_Y937LLN | 4.52 | 19 | G140S, Q148H | H | H | I | I | URF_BF | B | URF_BF | B |
| AR_568_Z708GGA | 4.97 | 27 | G140S, Q148H | H | H | I | I | URF_BF | B | URF_BF | B |
| AR_6462_AA893CP | 4.62 | 17 | L74I, G140S, Q148H, E138A | H | H | H | H | URF_BF | F | CRF12_BF | CRF12_BF |
| AR_6286_AA416RBBJ | 4.76 | 12 | N155H | H | H | S | S | URF_BF | F | URF_BF | B + F |
| AR_3128_Z059UTA | 3.42 | 31 | — | S | S | S | S | URF_BF | F | URF_BF | B |
| AR_472584 | 6.62 | 35 | T97A, V151I, N155H | H | H | S | S | ? | CRF05_DF | CRF05_DF | CRF05_DF |
Mutations contributing to INI susceptibility according to the Stanford HIVdb program are shown in bold. Mutations known as major are underlined.
pVL, plasma viral load; RAL, raltegravir; EVG, elvitegravir; DTG, dolutegravir; BIC, bictegravir; S, susceptible; H, high-level resistant; I, intermediate-level resistant; L, low-level resistant;—, no mutation detected by Sanger sequencing; ?, could not be confirmed by phylogenetic analysis.
According to the Stanford HIVdb genotyping tool, IN sequences were classified as subtype F (17 cases), B (12 cases) or CRF05_DF (1 case) (Table 1). However, important differences were identified in the subtype classification of IN sequences by phylogenetic analysis. Twenty-two of the 30 IN sequences formed well-supported phylogenetic clusters with subtype F (n = 14) or B (n = 8) references (Figure 1a). The remaining eight IN sequences failed to cluster with any of the HIV-1 subtype references. Further recombination analyses revealed different BF mosaic structures in seven cases (Figure 1b). Sequence AR_472584, characterized as CRF05_DF by the Stanford HIVdb genotyping tool, could not be confirmed by phylogenetic analysis, since both AR_472584 and the CRF05_DF reference disrupted the tree topology. Overall, results obtained by the Stanford HIVdb genotyping tool and phylogenetic analysis were consistent in 21 of the 30 IN sequences (70%). The Stanford HIVdb genotyping tool failed to distinguish BF recombinants and misclassified a subtype F strain as subtype B (AR_475736), while phylogenetic analysis was unable to confirm a putative CRF05_DF strain.
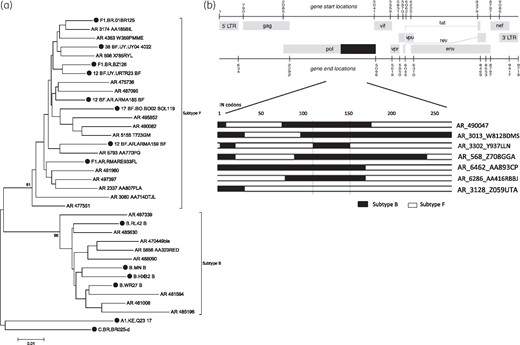
Characterization of HIV-1 subtype in the IN region. (a) Neighbour-joining phylogenetic tree, showing clustering of 863 bp IN sequences (codons 1–288) with subtype B or F reference strains. Subtype reference sequences are shown with filled symbols. (b) Schematic representation of BF intersubtype mosaic structures in the HIV-1 IN genomic region.
Based on the phylogenetic classification of HIV-1 subtypes, INI DRMs and susceptibility to raltegravir and dolutegravir were compared between those individuals with subtype B (n = 8) versus those with subtype F IN (n = 14) (Figure 2). Recombinants were excluded from the analyses. Statistically significant differences were observed for three major INI DRMs: E138A/K (50% B versus 0% F, P = 0.0096), G140S (50% B versus 0% F, P = 0.0096) and Q148H/R (63% B versus 0% F, P = 0.0021). Of note, mutations E138K/A + G140S and/or Q148H/R were also observed in four URF_BF structures with a subtype B segment including positions 138 to 148. Four of the 30 individuals studied (13%) presented high-level predicted resistance to all four INIs. Three of them presented mutations G140S, Q148H and E138K/A and the other one showed mutations Q148R, E138K and T97A. Mutations N155H and G163R/K were found at a high frequency exclusively in subtype F IN genomes (21% and 36%, respectively), although differences did not reach statistical significance. G163R was the most frequent accessory mutation in N155H mutants (two of six cases). Other mutations with a minor contribution to raltegravir resistance were: L74M/I (12% subtype B versus 29% subtype F) and V151I (12% subtype B versus 7% subtype F). According to the mutational profiles, the proportion of individuals with high-level resistance to raltegravir was higher for subtype B versus F (100% versus 43%, P = 0.0177). Also, subtype B was associated with a higher frequency of at least low-level resistance to dolutegravir (75% subtype B versus 0% subtype F, P = 0.0004). No differences were observed between the groups for median time of raltegravir exposure (17 months for subtype B versus 22 months for subtype F, P > 0.05) or median HIV-1 viral load at the time of genotyping (4.85 log10 copies/mL for subtype B versus 4.37 log10 copies/mL for subtype F, P > 0.05).
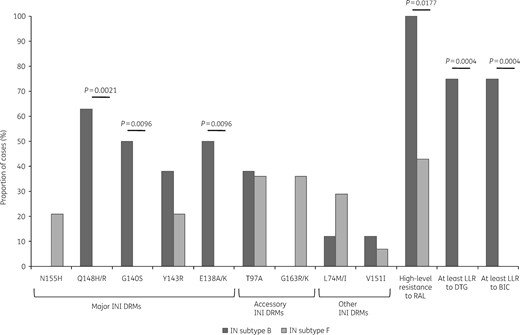
Frequency of resistance to INIs according to HIV-1 subtype of the IN genomic region. RAL, raltegravir; DTG, dolutegravir; BIC, bictegravir; LLR, low-level resistance.
In order to identify and distinguish different CRF_BFs, we performed additional subtype characterization of the pol PR-RT region. Through phylogenetic analysis, 4 of the 30 PR-RT sequences clustered with subtype B references, 1 with subtype F1 references and 7 were classified as different CRF_BFs: CRF12_BF (n = 4), CRF17_BF (n = 1), CRF05_DF (n = 1) or CRF29_BF (n = 1) (Figure S1, available as Supplementary data at JAC Online). The remaining 18 pol PR-RT sequences were identified as URFs_BF. For the PR-RT region, comparison between HIV-1 subtyping by phylogenetic inference and the Stanford HIVdb genotyping tool revealed concordance in 18 of 30 cases (60%). Overall, the following subtypes were found in our study population: 20 URF_BFs (67%), 4 subtype B (13%), 3 CRF12_BF (10%), 1 CRF17_BF (3%), 1 CRF29_BF (3%) and 1 putative CRF05_DF (3%).
Discussion
Data on DRMs during treatment with INI-based regimens in clinical practice are scarce, particularly for non-B HIV-1 subtypes and recombinant forms. In agreement with the known molecular epidemiology of HIV-1 in Argentina, different BF recombinants were found as the most prevalent HIV-1 subtype (84%). While most of them were entirely F (46%) or B (27%) in the IN genomic region, BF recombination was also observed in one in four IN sequences (23%). In our study, INI DRMs emerging in individuals failing a raltegravir-based ART regimen differed between B and F IN subtypes, with Q148K/R/H, G140S and E138K/A being more prevalent in subtype B.
We and others have shown that phylogenetic analysis of the PR-RT region allows the identification of the different CRF_BFs known to circulate in the Latin American region.21,28 Consistent with these studies, we observed a high frequency of multiple URF_BFs, as well as the presence of different CRF_BFs (including CRF12_BF, CRF17_BF and CRF29_BF). Of interest, we report for the first time, to our knowledge, circulation of CRF05_DF in Argentina. The CRF05 chimera was initially described in two Belgian individuals likely infected by partners from the Democratic Republic of the Congo.29 A third genome found in Spain was published in 2003 by Casado et al.30 According to the CRF compendium available at LANL (https://www.hiv.lanl.gov/content/sequence/HIV/CRFs/CRFs.html), the mosaic structure of CRF05_BF is still incomplete and subtype assignment is missing in the IN CDS. This could explain the inability of phylogenetic tools to classify this CRF structure through analysis of the HIV-1 IN genomic region, whereas it was identified by the Stanford HIVdb genotyping tool. This tool, incorporated into the HIVdb program, is freely available online. By submitting our sequences for characterization of HIV-1 subtype, we observed a partial concordance between Stanford tools and phylogenetic analyses (63% for PR-RT, 70% for IN). Importantly, the Stanford HIVdb genotyping tool was unable to identify BF recombinants in the IN region. However, BF recombination is more frequent than expected in IN. In conclusion, while easy-to-use genotyping tools can provide HIV-1 subtype in a rapid and simple way, the growing complexity of the HIV-1 epidemic will require more frequent updating of the HIV drug resistance genotyping tools.
High-level resistance to raltegravir was significantly less frequent in individuals carrying viral strains with subtype F IN (43% F versus 100% B). These results were mostly associated with the absence of major raltegravir INI DRMs in eight individuals. Of them, three showed only accessory mutations and five showed no INI DRMs at all. Although unidentified resistance mutations might exist, the absence of INI DRMs in these individuals may also result from poor drug compliance, as previously reported.31 Unfortunately, compliance was not recorded, resulting in a limitation of our study. Of note, subtype F IN also showed a higher frequency of mutations that, by themselves, have little or no effect on raltegravir susceptibility, namely G163R/K and L74M/I (36% and 29%, respectively). While G163R/K has previously been shown to occur as a natural polymorphism of subtype F—including BF recombinant viruses with subtype F IN genomes—L74M is minimally polymorphic in subtypes other than CRF02_AG.32 In our study, the role of these mutations as polymorphisms or raltegravir-selected mutations could not be evaluated due to the lack of IN genotyping prior to raltegravir use. Whether subtype F IN mutants generate different levels of resistance to INIs than the ones observed in subtype B is unknown. It is also possible that major raltegravir-resistant IN mutants develop less frequently in subtype F. In most non-B subtypes—including F—two nucleotide substitutions are required to change glycine to serine at position 140, whereas in subtype B only one change is required. Since 140 has been shown to be the position of a key mutation that restores the fitness of Q148 mutants, it has been suggested that differences in codon usage between B and non-B subtypes at position 140 could be responsible for the restriction of non-B subtypes to select for Q148 + G140 mutations.18 However, it is unclear whether codon usage alone can account for the development of HIV-1 resistance to raltegravir through mutational pathways other than Q148, or if other mechanisms yet to be identified are also involved.
The three major recognized pathways of genotypic resistance to raltegravir—Q148H/R/K, N155H and Y143R/C/H—were represented in our study. However, differences were observed according to HIV-1 subtype in the IN genomic region. In subtype F, development of resistance to raltegravir was associated with the N155H and Y143R mutational pathways, but not with Q148K/R. On the contrary, resistance to raltegravir in subtype B was associated with the mutations Y143R and Q148H/R. This is in agreement with previous reports showing that despite N155H appearing first, the pathway that eventually becomes predominant upon continued exposure to raltegravir is determined by substitutions at Q148 + G140 or Y143.33 Also, the association between Q148H and secondary mutations G140S and E138K found in subtype B from our study is consistent with previous reports from clinical observational cohorts18 and with in vitro selection experiments that show a role for these secondary mutations in the recovery of viral infectivity and/or INI resistance of Q148H/K/R mutants.34
The resilience to the emergence of Q148X in non-B subtypes had previously been observed in several non-B subtypes including subtypes A, C, D, F, G, CRF01_AE and CRF02_AG.18,31,35,36 However, it was unclear whether this was a relative or absolute phenomenon, since no information was provided on the time of raltegravir exposure. For the first time, our study shows that differences in resistance mutations developed by subtype B and F INs are independent of the time on raltegravir therapy. This observation is further supported by the absence of changes after 1 year of continued exposure to raltegravir in one individual carrying the N155H mutation in the context of a subtype F IN (data not shown) and the lack of reports of development of Q148H/R/K in subtype F IN genomes. Altogether, our results suggest that HIV-1 subtype can condition the mutational pathways, with clinical implications in relation to the likelihood of cross-resistance to dolutegravir in raltegravir-experienced individuals from our population. Whether HIV-1 subtype can impact clinical outcomes in individuals from Argentina undergoing therapy with INIs merits further research.
Our results have clinical implications in relation to the use of INIs in HIV-1 vertically infected children from Argentina. Due to the high levels of pretreatment drug resistance to NNRTIs in the paediatric population (22%),37 there is an urgent need to use raltegravir instead of nevirapine as the preferred choice in first-line ART in neonates. In addition, raltegravir is currently recommended as an alternative to lopinavir/ritonavir-based ART regimens, the first-line regimen currently used for infants and children for whom approved dolutegravir dosing is not yet available (under 6 years old).38 According to our results, HIV-1 subtyping of the IN CDS could be useful to guide the choice of first-line regimen in children and preserve future treatment options with second-generation INIs.
Characterization of HIV-1 subtype in the IN genomic segment could provide useful information to guide the use of INIs in individuals from Latin America. Despite the low number of cases included in our study, our results raise an interesting and important issue as the use of INIs will continue to increase in Argentina and Latin America. Appropriate use of resistance testing provides valuable information useful in constructing ARV regimens for maximal and continued suppression of HIV-1 replication. Evaluating the role of HIV-1 subtype in development and persistence of mutations that confer resistance to INIs will result in improved algorithms for resistance testing.
Acknowledgements
We gratefully thank Mrs Silvina Juarez and Miss Romina Guelho for technical assistance.
Funding
Data presented in this work have been generated as part of the routine work of the organizations listed. D.S. was supported by the National Ministry of Health from Argentina through the program Becas Abraam Sonis Salud Investiga 2018.
Transparency declarations
None to declare.
Supplementary data
Figure S1 is available as Supplementary data at JAC Online.
References
WHO. Updated Recommendations on First-Line and Second-Line Antiretroviral Regimens and Post-Exposure Prophylaxis and Recommendations on Early Infant Diagnosis of HIV.
WHO. Guidelines on the Public Health Response to Pretreatment HIV Drug Resistance.



