-
PDF
- Split View
-
Views
-
Cite
Cite
E J Growcott, L Gamboa, T Roth, S Lopez, C S Osborne, Efficacy of piperacillin in combination with novel β-lactamase inhibitor IID572 against β-lactamase-producing strains of Enterobacteriaceae and Staphylococcus aureus in murine neutropenic thigh infection models, Journal of Antimicrobial Chemotherapy, Volume 75, Issue 6, June 2020, Pages 1530–1536, https://doi.org/10.1093/jac/dkaa026
Close - Share Icon Share
Abstract
The neutropenic murine thigh infection model was used to assess the effectiveness of IID572, a novel β-lactamase inhibitor, in rescuing piperacillin activity against bacterial strains expressing various β-lactamase enzymes.
Mice (n = 4/group) were inoculated with Enterobacteriaceae or Staphylococcus aureus bacterial strains expressing a range of β-lactamases via intramuscular injection. Two hours after bacterial inoculation, subcutaneous treatment with piperacillin/IID572 or piperacillin/tazobactam every 3 h was initiated. Animals were euthanized via CO2 24 h after the start of therapy and bacterial cfu (log10 cfu) per thigh was determined, and the static dose was calculated.
In a dose-dependent manner, piperacillin/IID572 reduced the thigh bacterial burden in models established with Enterobacteriaceae producing class A, C and D β-lactamases (e.g. ESBLs, KPC, CMY-2 and OXA-48). Piperacillin/IID572 was also efficacious against MSSA strains, including one producing β-lactamase. Static doses of piperacillin/IID572 were calculable from animals infected with all strains tested and the calculated static doses ranged from 195 to 4612 mg/kg/day piperacillin, the active component in the combination. Of the 13 strains investigated, a 1 log10 bacterial reduction was achieved for 9 isolates and a 2 log10 reduction was achieved for 3 isolates; piperacillin/tazobactam was not efficacious against 6 of the 13 isolates tested.
In contrast to tazobactam, IID572 was able to rescue piperacillin efficacy in murine thigh infection models established with β-lactamase-producing strains of Enterobacteriaceae and S. aureus, including those expressing ESBLs or serine carbapenemases.
Introduction
The β-lactams are the most commonly used class of antibacterial agents in the USA, accounting for 65% of all injectable antibiotic prescriptions.1 In 2017, penicillins were the most frequently used antibiotics in Europe, ranging from 33% to 67% of community consumption.2 However, the ongoing emergence of MDR strains of bacteria highlights an essential unmet medical need to continue to develop new antimicrobial therapies; in particular carbapenem-resistant Enterobacteriaceae are a serious healthcare problem.3 One mechanism by which bacteria have developed resistance to antibiotics is via the production of β-lactamase enzymes, which hydrolyse the β-lactam ring present in the chemical structures of these antibiotics. Klebsiella pneumoniae carbapenemases (KPCs) have caused many carbapenem-resistant epidemics globally.4 In addition, resistance to colistin, one of the last lines of antibiotic defence, is emerging among β-lactamase-expressing strains in Europe5–7 and there is evidence of a correlation between β-lactamase production and colistin resistance.8
In an effort to overcome resistance induced by expression of β-lactamase enzymes there are two possible strategies. The first is to develop novel β-lactams with increased stability against these enzymes, such as LYS228,9 while the second is to partner a β-lactamase inhibitor (BLI) with a β-lactam antibiotic to rescue antibacterial activity. The combination of piperacillin/tazobactam is widely used as a broad-spectrum agent to treat infections, while more recently avibactam was combined with ceftazidime.10 However, resistance has emerged to this combination11 and thus new agents are still required. IID572 is a novel broad-spectrum BLI designed to overcome the current limitations of tazobactam and potentially replace tazobactam to restore the activity of piperacillin against drug-resistant Enterobacteriaceae.12 IID572 is a member of the diazabicyclooctane (DBO) class of BLIs and differs from other DBOs such as avibactam because of its broader inhibition of β-lactamase enzymes and the fact it does not have intrinsic antibacterial activity.12
Here the neutropenic mouse thigh infection model, which can be used to determine antibiotic efficacy,13–17 is utilized to assess the effectiveness of IID572 in rescuing piperacillin activity against bacterial strains expressing various β-lactamase enzymes.
Materials and methods
Bacterial strains
Strains used in this study were obtained from ATCC or were part of the Novartis strain collection, and are described in Table 1.
| Bacterial species . | Novartis strain . | Relevant characteristics . | Source . | MIC (mg/L) . | ||
|---|---|---|---|---|---|---|
| piperacillin/IID572 . | piperacillin/tazobactam . | piperacillin . | ||||
| E. coli | NB27001 | WT | ATCC 25922 | 4/4 | 4/4 | 4 |
| NB27235 | CTX-M-15 | JMI Labs | 2/4 | 4/4 | >64 | |
| NB27169 | KPC-3 | William Weiss | 4/4 | >64/4 | >64 | |
| K. pneumoniae | NB29002 | WT | ATCC 43816 | 2/4 | 4/4 | 8 |
| NB29257 | CMY-2, SHV-1, TEM-1 | Creighton University | 4/4 | 16/4 | >64 | |
| NB29001 | SHV-18 | ATCC 700603 | 8/4 | 8/4 | >64 | |
| NB29293 | SHV-12, CTX-M-15, DHA-1 | IHMA | 8/4 | >64/4 | >64 | |
| NB29355 | KPC-2 | IHMA | 8/4 | >64/4 | >64 | |
| NB29084 | CTX-M-14 | JMI Labs | 16/4 | 16/4 | >64 | |
| NB29316 | SHV-12, KPC-11, ST258 | IHMA | 16/4 | >64/4 | >64 | |
| NB29339 | OXA-48 | IHMA | 16/4 | >64/4 | >64 | |
| S. aureus | NB01001 | WT | ATCC 29213 | 0.5/2 | 1/4 | 2 |
| NB01421 | β-lactamase producing | Novartis | 1/2 | 1/4 | 32 | |
| Bacterial species . | Novartis strain . | Relevant characteristics . | Source . | MIC (mg/L) . | ||
|---|---|---|---|---|---|---|
| piperacillin/IID572 . | piperacillin/tazobactam . | piperacillin . | ||||
| E. coli | NB27001 | WT | ATCC 25922 | 4/4 | 4/4 | 4 |
| NB27235 | CTX-M-15 | JMI Labs | 2/4 | 4/4 | >64 | |
| NB27169 | KPC-3 | William Weiss | 4/4 | >64/4 | >64 | |
| K. pneumoniae | NB29002 | WT | ATCC 43816 | 2/4 | 4/4 | 8 |
| NB29257 | CMY-2, SHV-1, TEM-1 | Creighton University | 4/4 | 16/4 | >64 | |
| NB29001 | SHV-18 | ATCC 700603 | 8/4 | 8/4 | >64 | |
| NB29293 | SHV-12, CTX-M-15, DHA-1 | IHMA | 8/4 | >64/4 | >64 | |
| NB29355 | KPC-2 | IHMA | 8/4 | >64/4 | >64 | |
| NB29084 | CTX-M-14 | JMI Labs | 16/4 | 16/4 | >64 | |
| NB29316 | SHV-12, KPC-11, ST258 | IHMA | 16/4 | >64/4 | >64 | |
| NB29339 | OXA-48 | IHMA | 16/4 | >64/4 | >64 | |
| S. aureus | NB01001 | WT | ATCC 29213 | 0.5/2 | 1/4 | 2 |
| NB01421 | β-lactamase producing | Novartis | 1/2 | 1/4 | 32 | |
| Bacterial species . | Novartis strain . | Relevant characteristics . | Source . | MIC (mg/L) . | ||
|---|---|---|---|---|---|---|
| piperacillin/IID572 . | piperacillin/tazobactam . | piperacillin . | ||||
| E. coli | NB27001 | WT | ATCC 25922 | 4/4 | 4/4 | 4 |
| NB27235 | CTX-M-15 | JMI Labs | 2/4 | 4/4 | >64 | |
| NB27169 | KPC-3 | William Weiss | 4/4 | >64/4 | >64 | |
| K. pneumoniae | NB29002 | WT | ATCC 43816 | 2/4 | 4/4 | 8 |
| NB29257 | CMY-2, SHV-1, TEM-1 | Creighton University | 4/4 | 16/4 | >64 | |
| NB29001 | SHV-18 | ATCC 700603 | 8/4 | 8/4 | >64 | |
| NB29293 | SHV-12, CTX-M-15, DHA-1 | IHMA | 8/4 | >64/4 | >64 | |
| NB29355 | KPC-2 | IHMA | 8/4 | >64/4 | >64 | |
| NB29084 | CTX-M-14 | JMI Labs | 16/4 | 16/4 | >64 | |
| NB29316 | SHV-12, KPC-11, ST258 | IHMA | 16/4 | >64/4 | >64 | |
| NB29339 | OXA-48 | IHMA | 16/4 | >64/4 | >64 | |
| S. aureus | NB01001 | WT | ATCC 29213 | 0.5/2 | 1/4 | 2 |
| NB01421 | β-lactamase producing | Novartis | 1/2 | 1/4 | 32 | |
| Bacterial species . | Novartis strain . | Relevant characteristics . | Source . | MIC (mg/L) . | ||
|---|---|---|---|---|---|---|
| piperacillin/IID572 . | piperacillin/tazobactam . | piperacillin . | ||||
| E. coli | NB27001 | WT | ATCC 25922 | 4/4 | 4/4 | 4 |
| NB27235 | CTX-M-15 | JMI Labs | 2/4 | 4/4 | >64 | |
| NB27169 | KPC-3 | William Weiss | 4/4 | >64/4 | >64 | |
| K. pneumoniae | NB29002 | WT | ATCC 43816 | 2/4 | 4/4 | 8 |
| NB29257 | CMY-2, SHV-1, TEM-1 | Creighton University | 4/4 | 16/4 | >64 | |
| NB29001 | SHV-18 | ATCC 700603 | 8/4 | 8/4 | >64 | |
| NB29293 | SHV-12, CTX-M-15, DHA-1 | IHMA | 8/4 | >64/4 | >64 | |
| NB29355 | KPC-2 | IHMA | 8/4 | >64/4 | >64 | |
| NB29084 | CTX-M-14 | JMI Labs | 16/4 | 16/4 | >64 | |
| NB29316 | SHV-12, KPC-11, ST258 | IHMA | 16/4 | >64/4 | >64 | |
| NB29339 | OXA-48 | IHMA | 16/4 | >64/4 | >64 | |
| S. aureus | NB01001 | WT | ATCC 29213 | 0.5/2 | 1/4 | 2 |
| NB01421 | β-lactamase producing | Novartis | 1/2 | 1/4 | 32 | |
Test agent
IID572 was synthesized at Novartis, while piperacillin and piperacillin/tazobactam were obtained commercially. For in vitro studies, piperacillin and tazobactam were purchased from US Pharmacopeia (Rockville, MD, USA) and for in vivo studies piperacillin was purchased from Sigma–Aldrich (St Louis, MO, USA) and piperacillin/tazobactam from Sagent (Schaumburg, IL, USA).
Susceptibility testing
Antibacterial assays to determine MICs were performed according to CLSI procedures.18 When examining combinations against Gram-negative bacteria both IID57212 and tazobactam were used at a fixed concentration of 4 mg/L. Against Staphylococcus aureus, IID572 was tested at a fixed concentration of 2 mg/L, while a fixed concentration of 4 mg/L tazobactam was used.
Animals
All studies were approved by the Institutional Animal Care and Use Committee of Novartis Institutes for BioMedical Research, Inc., Emeryville, CA, USA. Female CD-1 mice (20–25 g; Envigo, Livermore, CA, USA) were kept under controlled SPF conditions with 12 h dark–12 h light cycles, 22°C constant temperature and 30%–70% relative humidity, with food and water ad libitum. Animals were housed n = 4/cage in IVC Innovive disposable caging (Innovive, San Diego, CA, USA).
Inoculum preparation
For each bacterial strain, an overnight culture was prepared by inoculating 25 mL of Mueller–Hinton broth with 20 μL of bacterial strain from frozen stock. The culture was allowed to grow for 16–18 h at 37°C with agitation at 150 rpm (I2400 Incubator Shaker, New Brunswick Scientific). Ten millilitres of the overnight culture was centrifuged at 2330 g for 10 min at 4°C and the bacterial pellet was re-suspended in 10 mL of sterile saline (0.9% w/v). Using OD:cfu correlations, the inoculum was prepared at 2 × 106–2 × 107 cfu/mL by diluting with sterile saline. To confirm titre, inoculum was diluted with sterile saline and plated on Trypticase Soy Agar (TSA) plates and incubated overnight at 37°C for bacterial enumeration.
Neutropenic murine thigh infection
Animals were rendered neutropenic by intraperitoneal injections of 150 and 100 mg/kg cyclophosphamide (Sigma–Aldrich) on days −4 and −1, respectively, prior to infection. Two hours prior to treatment (−2 h), 50 μL containing ∼105–106 cfu of bacterial inoculum was administered into the left gastrocnemius muscle via an intramuscular injection. At 0 h, animals were treated with piperacillin/IID572 or piperacillin/tazobactam via the subcutaneous route of administration (5 mL/kg) in alternating left and right inguinal areas. The dose of piperacillin ranged from 2.5 to 5120 mg/kg/day in combination with a fixed ratio of BLI (4:1, 8:1 or 16:1). The doses were divided equally and administered every 3 h for 24 h. At 0 h a cohort of animals were sacrificed via CO2 to determine the bacterial levels at the start of treatment. The remaining animals were euthanized 24 h after the start of therapy. The infected thighs were excised and homogenized in sterile saline (0.9% w/v) until the tissue was completely homogenized. The homogenates were serially diluted in sterile saline before dilutions were plated on TSA plates and incubated overnight at 37°C, and the colonies counted. The number of cfu/thigh was calculated and transformed to log10. All data are presented as mean ± SD. Data were analysed in GraphPad Prism 7.0 (GraphPad Software, San Diego, CA, USA).
Calculation of static dose
The static dose (mg/kg/day) is that which is required to keep the bacterial load at the same level as when therapy was initiated. Dose/day was plotted against log10 cfu and analysed using a four-parametric logistic curve (SigmaPlot 13.0, SyStat Software, San Jose, CA, USA). The static dose was calculated using the following equation: log10 static dose = {log10[E/(Emax − E)]/N} + log10 ED50, where E is the control growth (log10 change in cfu per thigh in untreated controls after the 24 h period of study), Emax is the maximum effect, ED50 is the dose required to achieve 50% of Emax and N is the slope of the dose–effect curve. In addition for some studies, the dose required to achieve 1 and 2 log10 reductions below bacterial stasis was calculated (E + 1 log10, E + 2 log10).
Results
In vitro activity
The MIC values of piperacillin/IID572, piperacillin/tazobactam and piperacillin are shown in Table 1. For Escherichia coli ATCC 25922 and S. aureus ATCC 29213 the MIC values were within the CLSI-approved quality control (QC) ranges for piperacillin.19 The MIC value of piperacillin/tazobactam was also within QC range for K. pneumoniae ATCC 700603 (8/4–32/4 mg/L).19 Of the Gram-negative strains tested, nine were resistant to piperacillin and five were resistant to piperacillin/tazobactam as defined by the EUCAST and CLSI breakpoints.19,20 Both S. aureus strains were susceptible to oxacillin, as defined by the CLSI19 (data not shown), and piperacillin had an elevated MIC for the isolate expressing β-lactamase (32 mg/L). IID572 was able to restore piperacillin activity against all the strains expressing β-lactamase enzymes as indicated by a minimum of 4- to 32-fold reduction in MIC values.
In vivo efficacy
To determine the in vivo efficacy of piperacillin/IID572, the combination was tested in neutropenic murine thigh infection models. First, to confirm that IID572 was able to restore the antibacterial activity of piperacillin against piperacillin/tazobactam-resistant clinical isolates, piperacillin/IID572 was tested against a strain of K. pneumoniae (NB29316) that expressed an ESBL (SHV-12) and a KPC (KPC-11) and was resistant to piperacillin/tazobactam as evidenced by an MIC >64 mg/L. Concurrently, a range of piperacillin to IID572 ratios was tested to determine the optimum combination. Treatment with piperacillin/IID572 was efficacious and reduced bacterial burden in the thigh in a dose-dependent manner (Figure 1). When using 4:1, 8:1 and 16:1 piperacillin/IID572, the doses of piperacillin required for bacterial stasis were calculated as 2468, 2297 and 3386 mg/kg/day, respectively. A dose of 4213 or 4463 mg/kg/day piperacillin was required to reduce bacterial burden 1 log10 below stasis when ratios of 4:1 or 8:1 were dosed. Treatment with 16:1 piperacillin/IID572 did not reduce bacterial load by 1 log10 cfu/thigh. Given that a 4:1 ratio did not improve efficacy over an 8:1 ratio, while a 16:1 ratio was unable to reduce bacterial load 1 log10 from stasis, an 8:1 ratio of piperacillin to IID572 was chosen for subsequent in vivo efficacy studies.
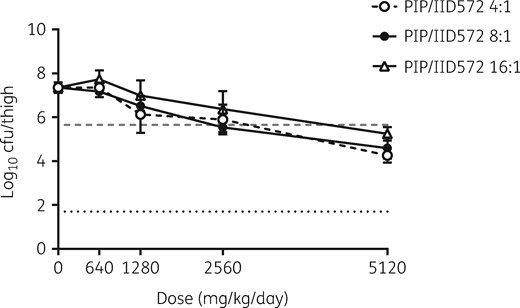
Effect of altering the piperacillin/IID572 ratio on efficacy in the neutropenic murine thigh model. Log10 cfu of K. pneumoniae NB29316 present in the thigh after 24 h of treatment with piperacillin/IID572. Mice were treated subcutaneously using different treatment ratios of piperacillin/IID572 every 3 h. The x-axis represents the dose of piperacillin, the dashed grey line represents bacterial levels at the start of therapy (0 h) and the dotted black line represents the limit of detection. Data are presented as mean ± SD; n = 4/group. PIP, piperacillin.
Continuing to use the neutropenic thigh model, the efficacy of piperacillin/IID572 was also tested against a range of bacterial strains, including Gram-negative (Enterobacteriaceae) and Gram-positive (S. aureus) bacteria, expressing various β-lactamase enzymes. A single high dose of 5120/640 mg/kg/day piperacillin/tazobactam was used for comparison.
First, piperacillin/IID572 was tested against Enterobacteriaceae. Bacterial burden in the thigh was reduced in a dose-dependent manner when reference strains E. coli ATCC 29522 (NB27001) and K. pneumoniae ATCC 700603 (NB29001) were treated (Figure 2a and b). The static dose and doses required for log10 reductions below stasis were calculated (Table 2); against E. coli ATCC 29522 the treatment enabled bacterial stasis as well as inducing 1 and 2 log10 reductions in bacterial load; this required piperacillin doses of 1870, 3187 and 3721 mg/kg/day, respectively. In a thigh model established with K. pneumoniae ATCC 700603, 1271 mg/kg/day was required to achieve bacterial stasis, while 4530 mg/kg/day was required for a 1 log10 bacterial reduction. This demonstrated the ability of IID572 to rescue piperacillin when treating an SHV-18-expressing strain. A single dose of 5120/640 mg/kg/day piperacillin/tazobactam demonstrated similar efficacy when compared with the highest dose tested of piperacillin/IID572 against these isolates. Piperacillin/IID572 combination treatment of thigh infections established with other strains of E. coli or K. pneumoniae expressing β-lactamase enzymes was found to be efficacious (Table 2), demonstrating IID572 was able to rescue piperacillin in the presence of these enzymes. Taken together with the reference strains the mean static dose was calculated; for E. coli and K. pneumoniae the mean static doses were 1721 ± 329 (n = 3) and 2645 ± 1405 mg/kg/day (n = 8), respectively.
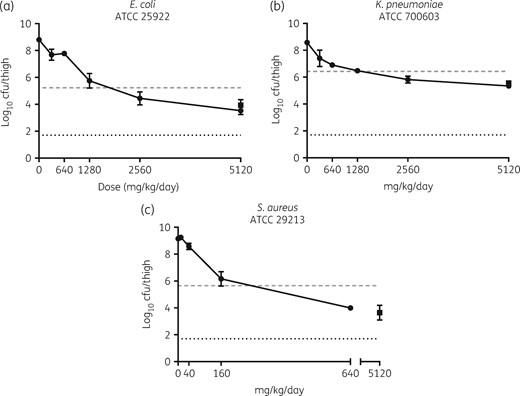
Efficacy of piperacillin in combination with IID572 in neutropenic murine thigh models of infection. Log10 cfu of E. coli NB27001 (a), K. pneumoniae NB29001 (b) and S. aureus NB01001 (c) present in the thigh after 24 h of treatment with piperacillin/IID572 (filled circles) or piperacillin/tazobactam (filled squares). Mice were treated subcutaneously every 3 h with piperacillin dosed in an 8:1 ratio with the BLI. The x-axis represents the dose of piperacillin, the dashed grey line represents bacterial levels at the start of therapy (0 h) and the dotted black line represents the limit of detection. Data are presented as mean ± SD; n = 4/group.
Doses calculated for stasis or 1 and 2 log10 kill for the piperacillin/IID572 combination against bacterial strains tested in vivo
| Species . | Novartis strain . | MIC (mg/L) . | Highest dose piperacillin:IID572 (mg/kg/day) . | Stasisa (mg/kg/day) . | 1 Log10 killa (mg/kg/day) . | 2 Log10 killa (mg/kg/day) . |
|---|---|---|---|---|---|---|
| E. coli | NB27001 | 4/4 | 5120:640 | 1870 | 3187 | 3721 |
| NB27235 | 2/4 | 5120:640 | 1344 | 2521 | 4393 | |
| NB27169 | 4/4 | 5120:640 | 1948 | 3511 | NR | |
| K. pneumoniae | NB29002 | 2/4 | 2560:320 | 2196 | NR | NR |
| NB29257 | 4/4 | 5120:640 | 495 | NR | NR | |
| NB29001 | 8/4 | 5120:640 | 1271 | 4530 | NR | |
| NB29293 | 8/4 | 5120:640 | 3379 | NR | NR | |
| NB29355 | 8/4 | 5120:640 | 4612 | NR | NR | |
| NB29084 | 16/4 | 5120:640 | 2663 | 4301 | NR | |
| NB29316 | 16/4 | 5120:640 | 2297 | 4664 | NR | |
| NB29339 | 16/4 | 5120:640 | 4249 | 5529 | NR | |
| S. aureus | NB01001 | 0.5/2 | 640:80 | 195 | 332 | NR |
| NB01421 | 1/2 | 5120:640 | 222 | 267 | 342 |
| Species . | Novartis strain . | MIC (mg/L) . | Highest dose piperacillin:IID572 (mg/kg/day) . | Stasisa (mg/kg/day) . | 1 Log10 killa (mg/kg/day) . | 2 Log10 killa (mg/kg/day) . |
|---|---|---|---|---|---|---|
| E. coli | NB27001 | 4/4 | 5120:640 | 1870 | 3187 | 3721 |
| NB27235 | 2/4 | 5120:640 | 1344 | 2521 | 4393 | |
| NB27169 | 4/4 | 5120:640 | 1948 | 3511 | NR | |
| K. pneumoniae | NB29002 | 2/4 | 2560:320 | 2196 | NR | NR |
| NB29257 | 4/4 | 5120:640 | 495 | NR | NR | |
| NB29001 | 8/4 | 5120:640 | 1271 | 4530 | NR | |
| NB29293 | 8/4 | 5120:640 | 3379 | NR | NR | |
| NB29355 | 8/4 | 5120:640 | 4612 | NR | NR | |
| NB29084 | 16/4 | 5120:640 | 2663 | 4301 | NR | |
| NB29316 | 16/4 | 5120:640 | 2297 | 4664 | NR | |
| NB29339 | 16/4 | 5120:640 | 4249 | 5529 | NR | |
| S. aureus | NB01001 | 0.5/2 | 640:80 | 195 | 332 | NR |
| NB01421 | 1/2 | 5120:640 | 222 | 267 | 342 |
NR, not reached.
As piperacillin is the active component of the combination, only the dose of piperacillin is recorded; piperacillin was dosed in a ratio of 8:1 with IID572.
Doses calculated for stasis or 1 and 2 log10 kill for the piperacillin/IID572 combination against bacterial strains tested in vivo
| Species . | Novartis strain . | MIC (mg/L) . | Highest dose piperacillin:IID572 (mg/kg/day) . | Stasisa (mg/kg/day) . | 1 Log10 killa (mg/kg/day) . | 2 Log10 killa (mg/kg/day) . |
|---|---|---|---|---|---|---|
| E. coli | NB27001 | 4/4 | 5120:640 | 1870 | 3187 | 3721 |
| NB27235 | 2/4 | 5120:640 | 1344 | 2521 | 4393 | |
| NB27169 | 4/4 | 5120:640 | 1948 | 3511 | NR | |
| K. pneumoniae | NB29002 | 2/4 | 2560:320 | 2196 | NR | NR |
| NB29257 | 4/4 | 5120:640 | 495 | NR | NR | |
| NB29001 | 8/4 | 5120:640 | 1271 | 4530 | NR | |
| NB29293 | 8/4 | 5120:640 | 3379 | NR | NR | |
| NB29355 | 8/4 | 5120:640 | 4612 | NR | NR | |
| NB29084 | 16/4 | 5120:640 | 2663 | 4301 | NR | |
| NB29316 | 16/4 | 5120:640 | 2297 | 4664 | NR | |
| NB29339 | 16/4 | 5120:640 | 4249 | 5529 | NR | |
| S. aureus | NB01001 | 0.5/2 | 640:80 | 195 | 332 | NR |
| NB01421 | 1/2 | 5120:640 | 222 | 267 | 342 |
| Species . | Novartis strain . | MIC (mg/L) . | Highest dose piperacillin:IID572 (mg/kg/day) . | Stasisa (mg/kg/day) . | 1 Log10 killa (mg/kg/day) . | 2 Log10 killa (mg/kg/day) . |
|---|---|---|---|---|---|---|
| E. coli | NB27001 | 4/4 | 5120:640 | 1870 | 3187 | 3721 |
| NB27235 | 2/4 | 5120:640 | 1344 | 2521 | 4393 | |
| NB27169 | 4/4 | 5120:640 | 1948 | 3511 | NR | |
| K. pneumoniae | NB29002 | 2/4 | 2560:320 | 2196 | NR | NR |
| NB29257 | 4/4 | 5120:640 | 495 | NR | NR | |
| NB29001 | 8/4 | 5120:640 | 1271 | 4530 | NR | |
| NB29293 | 8/4 | 5120:640 | 3379 | NR | NR | |
| NB29355 | 8/4 | 5120:640 | 4612 | NR | NR | |
| NB29084 | 16/4 | 5120:640 | 2663 | 4301 | NR | |
| NB29316 | 16/4 | 5120:640 | 2297 | 4664 | NR | |
| NB29339 | 16/4 | 5120:640 | 4249 | 5529 | NR | |
| S. aureus | NB01001 | 0.5/2 | 640:80 | 195 | 332 | NR |
| NB01421 | 1/2 | 5120:640 | 222 | 267 | 342 |
NR, not reached.
As piperacillin is the active component of the combination, only the dose of piperacillin is recorded; piperacillin was dosed in a ratio of 8:1 with IID572.
Finally, piperacillin/IID572 was tested against S. aureus. The combination was efficacious against reference strain S. aureus ATCC 29213 (Figure 2c), requiring 195 and 332 mg/kg/day to achieve bacterial stasis and a 1 log10 reduction, respectively (Table 2). The highest dose of piperacillin/IID572 tested (640/80 mg/kg/day) was similar in efficacy to a single dose of 5120/640 mg/kg/day piperacillin/tazobactam (Figure 2c). IID572 was able to restore piperacillin activity against a β-lactamase-producing MSSA strain with a piperacillin MIC value 16 times greater than the MIC value determined for ATCC 29213 (Table 2), requiring 222 and 267 mg/kg/day to achieve bacterial stasis and a 1 log10 reduction, respectively. Furthermore, 342 mg/kg/day piperacillin/IID572 reduced bacterial load 2 log10 below stasis. The mean static dose for piperacillin/IID572 against these two S. aureus strains was 209 mg/kg/day.
When considering all the strains examined, piperacillin/IID572 was able to induce bacterial stasis in all strains and the calculated static doses ranged from 195 to 4612 mg/kg/day. The combination was found to be efficacious against all the β-lactamase-expressing strains, which included those expressing class A, C and D β-lactamases (Table 2), demonstrating the ability of IID572 to rescue piperacillin. Of the 13 strains tested, a 1 log10 bacterial reduction was achieved in 9 isolates, while a 2 log10 reduction was achieved in 3 of the strains (Table 2). To further examine bacterial kill, the reduction below stasis (Δ log10 cfu/thigh) was calculated for the top dose of piperacillin/IID572 tested against each of the isolates (Table 3). Bacterial reduction was observed in all strains tested, with the average Δ log10 cfu/thigh from stasis ranging from −2.69 ± 0.68 to −0.23 ± 0.35. For comparison, the effect of a high dose of piperacillin/tazobactam treatment was also calculated and piperacillin/tazobactam reduced burden in seven of the strains tested. In the remaining six strains, piperacillin/tazobactam was not efficacious and bacterial growth was observed (Table 3). Of these six strains, five were resistant to piperacillin/tazobactam according to CSLI (≥128/4 mg/L) and EUCAST (>16/4 mg/L) Enterobacteriaceae guidelines.19,20
Bacterial kill from stasis for the piperacillin/IID572 combination and the piperacillin/tazobactam combination in vivo
| Species . | Novartis strain . | Piperacillin/IID572a . | Piperacillin/tazobactama . | ||
|---|---|---|---|---|---|
| MIC (mg/L) . | Δ log10 cfu/thigh . | MIC (mg/L) . | Δ log10 cfu/thigh . | ||
| E. coli | NB27001 | 4/4 | −1.71 ± 0.29 | 4/4 | −1.3 ± 0.41 |
| NB27235 | 2/4 | −2.20 ± 0.22 | 4/4 | −1.61 ± 0.44 | |
| NB27169 | 4/4 | −1.56 ± 0.48 | >64/4 | 1.43 ± 0.50 | |
| K. pneumoniae | NB29002 | 2/4 | −0.33 ± 0.55 | 4/4 | 0.26 ± 1.01 |
| NB29257 | 4/4 | −0.77 ± 0.34 | 16/4 | −0.81 ± 0.15 | |
| NB29001 | 8/4 | −1.09 ± 0.16 | 8/4 | −0.87 ± 0.16 | |
| NB29293 | 8/4 | −0.54 ± 0.42 | >64/4 | 2.42 ± 0.10 | |
| NB29355 | 8/4 | −0.23 ± 0.35 | >64/4 | 2.90 ± 0.46 | |
| NB29084 | 16/4 | −1.64 ± 0.38 | 16/4 | −0.63 ± 0.33 | |
| NB29316 | 16/4 | −1.07 ± 0.47 | >64/4 | 0.68 ± 0.60b | |
| NB29339 | 16/4 | −0.99 ± 1.70 | >64/4 | 3.19 ± 0.19 | |
| S. aureus | NB01001 | 0.5/2 | −1.65 ± 0.19 | 1/4 | −2.01 ± 0.55 |
| NB01421 | 1/2 | −2.69 ± 0.68 | 1/4 | −2.78 ± 0.35 | |
| Species . | Novartis strain . | Piperacillin/IID572a . | Piperacillin/tazobactama . | ||
|---|---|---|---|---|---|
| MIC (mg/L) . | Δ log10 cfu/thigh . | MIC (mg/L) . | Δ log10 cfu/thigh . | ||
| E. coli | NB27001 | 4/4 | −1.71 ± 0.29 | 4/4 | −1.3 ± 0.41 |
| NB27235 | 2/4 | −2.20 ± 0.22 | 4/4 | −1.61 ± 0.44 | |
| NB27169 | 4/4 | −1.56 ± 0.48 | >64/4 | 1.43 ± 0.50 | |
| K. pneumoniae | NB29002 | 2/4 | −0.33 ± 0.55 | 4/4 | 0.26 ± 1.01 |
| NB29257 | 4/4 | −0.77 ± 0.34 | 16/4 | −0.81 ± 0.15 | |
| NB29001 | 8/4 | −1.09 ± 0.16 | 8/4 | −0.87 ± 0.16 | |
| NB29293 | 8/4 | −0.54 ± 0.42 | >64/4 | 2.42 ± 0.10 | |
| NB29355 | 8/4 | −0.23 ± 0.35 | >64/4 | 2.90 ± 0.46 | |
| NB29084 | 16/4 | −1.64 ± 0.38 | 16/4 | −0.63 ± 0.33 | |
| NB29316 | 16/4 | −1.07 ± 0.47 | >64/4 | 0.68 ± 0.60b | |
| NB29339 | 16/4 | −0.99 ± 1.70 | >64/4 | 3.19 ± 0.19 | |
| S. aureus | NB01001 | 0.5/2 | −1.65 ± 0.19 | 1/4 | −2.01 ± 0.55 |
| NB01421 | 1/2 | −2.69 ± 0.68 | 1/4 | −2.78 ± 0.35 | |
Experiments were performed using a ratio of 8:1 piperacillin:BLI.
Separate experiment to that where piperacillin/IID572 was tested.
Bacterial kill from stasis for the piperacillin/IID572 combination and the piperacillin/tazobactam combination in vivo
| Species . | Novartis strain . | Piperacillin/IID572a . | Piperacillin/tazobactama . | ||
|---|---|---|---|---|---|
| MIC (mg/L) . | Δ log10 cfu/thigh . | MIC (mg/L) . | Δ log10 cfu/thigh . | ||
| E. coli | NB27001 | 4/4 | −1.71 ± 0.29 | 4/4 | −1.3 ± 0.41 |
| NB27235 | 2/4 | −2.20 ± 0.22 | 4/4 | −1.61 ± 0.44 | |
| NB27169 | 4/4 | −1.56 ± 0.48 | >64/4 | 1.43 ± 0.50 | |
| K. pneumoniae | NB29002 | 2/4 | −0.33 ± 0.55 | 4/4 | 0.26 ± 1.01 |
| NB29257 | 4/4 | −0.77 ± 0.34 | 16/4 | −0.81 ± 0.15 | |
| NB29001 | 8/4 | −1.09 ± 0.16 | 8/4 | −0.87 ± 0.16 | |
| NB29293 | 8/4 | −0.54 ± 0.42 | >64/4 | 2.42 ± 0.10 | |
| NB29355 | 8/4 | −0.23 ± 0.35 | >64/4 | 2.90 ± 0.46 | |
| NB29084 | 16/4 | −1.64 ± 0.38 | 16/4 | −0.63 ± 0.33 | |
| NB29316 | 16/4 | −1.07 ± 0.47 | >64/4 | 0.68 ± 0.60b | |
| NB29339 | 16/4 | −0.99 ± 1.70 | >64/4 | 3.19 ± 0.19 | |
| S. aureus | NB01001 | 0.5/2 | −1.65 ± 0.19 | 1/4 | −2.01 ± 0.55 |
| NB01421 | 1/2 | −2.69 ± 0.68 | 1/4 | −2.78 ± 0.35 | |
| Species . | Novartis strain . | Piperacillin/IID572a . | Piperacillin/tazobactama . | ||
|---|---|---|---|---|---|
| MIC (mg/L) . | Δ log10 cfu/thigh . | MIC (mg/L) . | Δ log10 cfu/thigh . | ||
| E. coli | NB27001 | 4/4 | −1.71 ± 0.29 | 4/4 | −1.3 ± 0.41 |
| NB27235 | 2/4 | −2.20 ± 0.22 | 4/4 | −1.61 ± 0.44 | |
| NB27169 | 4/4 | −1.56 ± 0.48 | >64/4 | 1.43 ± 0.50 | |
| K. pneumoniae | NB29002 | 2/4 | −0.33 ± 0.55 | 4/4 | 0.26 ± 1.01 |
| NB29257 | 4/4 | −0.77 ± 0.34 | 16/4 | −0.81 ± 0.15 | |
| NB29001 | 8/4 | −1.09 ± 0.16 | 8/4 | −0.87 ± 0.16 | |
| NB29293 | 8/4 | −0.54 ± 0.42 | >64/4 | 2.42 ± 0.10 | |
| NB29355 | 8/4 | −0.23 ± 0.35 | >64/4 | 2.90 ± 0.46 | |
| NB29084 | 16/4 | −1.64 ± 0.38 | 16/4 | −0.63 ± 0.33 | |
| NB29316 | 16/4 | −1.07 ± 0.47 | >64/4 | 0.68 ± 0.60b | |
| NB29339 | 16/4 | −0.99 ± 1.70 | >64/4 | 3.19 ± 0.19 | |
| S. aureus | NB01001 | 0.5/2 | −1.65 ± 0.19 | 1/4 | −2.01 ± 0.55 |
| NB01421 | 1/2 | −2.69 ± 0.68 | 1/4 | −2.78 ± 0.35 | |
Experiments were performed using a ratio of 8:1 piperacillin:BLI.
Separate experiment to that where piperacillin/IID572 was tested.
Discussion
Here, the in vivo efficacy piperacillin in combination with the novel BLI IID572 was evaluated utilizing murine neutropenic thigh infection models.
Clinically, piperacillin is combined with the BLI tazobactam and used to treat a plethora of conditions, including intra-abdominal infections and pneumonia. However, tazobactam is not an effective inhibitor of some β-lactamase enzymes such as ESBLs or KPCs. IID572 is a novel BLI that effectively protects piperacillin against serine β-lactamase enzymes and thus has a broader coverage when compared with piperacillin/tazobactam, restoring activity against many piperacillin/tazobactam-resistant strains.12
This potential for broader coverage was initially demonstrated by the fact that IID572 was able to rescue the antibacterial activity of piperacillin against all the strains expressing β-lactamase enzymes in vitro, reducing the MIC of piperacillin alone by a minimum of 4- to 32-fold. In vivo, piperacillin/IID572 was able to treat murine thigh infections established with Enterobacteriaceae, including strains expressing class A, C and D β-lactamases, as defined by Ambler.21 IID572 is not active against MBLs (class B)12 and therefore no strains expressing class B enzymes were tested. The tested strains included those harbouring ESBLs (e.g. CTX-M-14, CTX-M-15) and serine carbapenemases (e.g. KPC-11) as well as TEM-1, SHV-1, SHV-12, SHV-18, CYM-2 and OXA-48 enzymes. The strains expressing KPC-11 and OXA-48 (NB29316 and NB29339) were carbapenem resistant.16,22 Interestingly, piperacillin/IID572 was able to treat a K. pneumoniae strain expressing SHV-12, CTX-M-15 and DHA-1, which the novel monobactam LYS228 had limited activity against in a murine thigh infection model.16 This suggests that the use of a BLI such as IID572 could be effective in rescuing LYS228 against this K. pneumoniae.23 KPCs, CTX-Ms, SHVs, TEMs and CMY-2 are frequently present in drug-resistant Enterobacteriaceae found in the clinic24,25 and, globally, KPC epidemics are an issue.4 There have been outbreaks of carbapenemase-producing Enterobacteriaceae due to a KPC-bearing plasmid in the UK,26 while, in the USA, isolates producing KPCs are now endemic and large-scale outbreaks have been reported.27 In addition to covering Enterobacteriaceae expressing β-lactamases, piperacillin/IID572 was also efficacious against a β-lactamase-producing MSSA strain.
A high dose of piperacillin/tazobactam lacked efficacy against six of the tested strains as evidenced by bacterial growth from stasis. Against one of these isolates (NB29002) piperacillin/tazobactam did not reduce bacterial burden below stasis despite the MIC indicating it was susceptible (MIC = 8 mg/L). This strain, K. pneumoniae ATCC 43816, is particularly virulent in vivo as evidenced by the fact it has previously been shown to be one of the few strains able to establish an infection in the presence of neutrophils.16 This may be a factor as to why efficacy was not observed with piperacillin/tazobactam. The remaining five strains where a high dose of piperacillin/tazobactam was not efficacious were resistant to piperacillin/tazobactam and the lack of in vivo efficacy in these strains was unsurprising given the elevated MIC, and expression of β-lactamases not inhibited by tazobactam. The ability of IID572 in rescuing piperacillin efficacy for treatment of in vivo infections established with β-lactamase-producing bacteria, where piperacillin/tazobactam is not efficacious, suggests an improvement in coverage of piperacillin/IID572 compared with piperacillin/tazobactam, while maintaining similar activity against other species. This supports IID572 as a potential replacement for tazobactam in combination with piperacillin, restoring broad-spectrum activity of this antibiotic against drug-resistant Enterobacteriaceae.12
Following the investigation of dosing different ratios of piperacillin to IID572, a ratio of 8:1 was selected and this ratio is the same as that which is used clinically for piperacillin/tazobactam. It is worth noting, as suggested by Reck et al.,12 that human dose predictions have yet to be performed and these predictions may indicate the need for a different ratio for piperacillin/IID572 for optimal clinically efficacy. Going forward, the use of the murine thigh model to analyse pharmacokinetic/pharmacodynamic relationships may also help to inform on the optimal dosing for clinical efficacy.
In conclusion the combination of piperacillin with IID572 was found to be efficacious in the neutropenic mouse thigh infection model against β-lactamase-producing Enterobacteriaceae and S. aureus strains. These data support the further development of IID572 as a novel BLI.
Acknowledgements
We wish to thank JMI Labs for strains NB27235 and NB29084, IHMA for strains NB29293, NB29355, NB29316 and NB29339, and William Weiss for strain NB27169. We thank Johanne Blais and Jennifer Leeds for editorial support.
Funding
This study was carried out as part of routine work as part of a Novartis drug discovery programme.
Transparency declarations
All authors were employed by Novartis at the time the work was performed. E.J.G., S.L. and C.S.O. are also shareholders of Novartis.
References
ECDC. Summary of the Latest Data on Antibiotic Consumption in the European Union, ESAC-Net Surveillance Data.
CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically—Eleventh Edition: M07.
CLSI. Performance Standards for Antimicrobial Susceptibility Testing—Twenty-Ninth Edition: M100.
EUCAST. Breakpoint Tables for Interpretation of MICs and Zone Diameters, Version 8.1.
Author notes
Present address: Casebia Therapeutics, San Francisco, CA, USA.
Present address: Cortexyme Inc., South San Francisco, CA, USA.
§Present address: Durect, Cupertino, CA, USA.



