-
PDF
- Split View
-
Views
-
Cite
Cite
Karen Tan, James Nguyen, Kevin Nguyen, Holly K Huse, Paul H Nieberg, Annie Wong-Beringer, Prevalence of the carbapenem-heteroresistant phenotype among ESBL-producing Escherichia coli and Klebsiella pneumoniae clinical isolates, Journal of Antimicrobial Chemotherapy, Volume 75, Issue 6, June 2020, Pages 1506–1512, https://doi.org/10.1093/jac/dkaa048
Close - Share Icon Share
Abstract
Carbapenem-heteroresistant (cHR) Enterobacteriaceae strains have been reported worldwide; however, the prevalence among clinical ESBL-producing Enterobacteriaceae isolates obtained from patients with repeated hospital admissions remains largely unknown.
Heteroresistance was screened by disc diffusion and confirmed by a modified population analysis profiling (PAP) method against ertapenem, imipenem, meropenem and ceftolozane/tazobactam. MIC testing was performed by broth microdilution against carbapenems and a panel of agents with potential activity against ESBL-producing strains.
One hundred and seventy-three ESBL-producing meropenem-susceptible Escherichia coli and Klebsiella pneumoniae isolates were selected for testing. A total of 519 bacteria/carbapenem combinations were screened by disc diffusion; 84 combinations were identified as cHR. Modified PAP confirmed 70 bacteria/carbapenem combinations as heteroresistant; most (63%, 44/70) confirmed cHR colonies grew within the ertapenem zone of inhibition, followed by imipenem (30%, 21/70), then meropenem (7%, 5/70). In total, one-third of the unique patient isolates (32%, 55/173) were identified as being heteroresistant to at least one carbapenem; of those patients, 16% (9/55) had a carbapenem-non-susceptible isolate on subsequent visits. Only two cHR isolates screened positive for ceftolozane/tazobactam heteroresistance (1%, 2/173), of which one was confirmed heteroresistant by modified PAP. cHR isolates were more likely to be collected from a non-urinary source (e.g. respiratory) compared with non-cHR isolates (31% versus 19%, P = 0.02). MIC distributions of all tested antibiotic agents did not differ between non-cHR and cHR isolates.
Our findings raise concerns for the continued use of carbapenems as first-line therapy for ESBL infections and for the potential selection for strains with full carbapenem resistance.
Introduction
ESBL-producing Enterobacteriaceae (ESBL-E) infections have been reported worldwide and are estimated to cause 140 000 healthcare-associated infections annually in the USA, with ESBL-producing Escherichia coli (ESBL-EC) and ESBL-producing Klebsiella pneumoniae (ESBL-KP) as the two predominant pathogens.1–3 A carbapenem agent is recommended as first-line therapy for serious, invasive ESBL-EC and ESBL-KP infections.4–7 Despite receipt of carbapenem therapy with apparent in vitro susceptibility, a subset of patients infected with ESBL-E may still develop clinical and/or microbiological failure.8–10
Expression of an antibiotic-heteroresistant phenotype among infecting strains is one potential factor that contributes to treatment failure but is not routinely identified in the clinical setting.11 Heteroresistance is defined as a population-wide variation of antibiotic resistance, where different bacterial subpopulations from the same isolate exhibit varying susceptibilities to the same antimicrobial agent.12 Specifically for Enterobacteriaceae, the carbapenem-heteroresistant (cHR) phenotype has been described in several reports worldwide.13–17 Nicoloff et al.13 screened for heteroresistance in 41 Gram-negative isolates from Sweden against 28 different antibiotics (n = 766 bacteria/antibiotic combinations). Overall, 27% of the tested bacteria/antibiotic combinations were found to be heteroresistant. Combinations included E. coli and K. pneumoniae strains tested against four carbapenem agents, where ertapenem showed the highest frequency of confirmed heteroresistance, followed by imipenem and doripenem, then meropenem. Similarly, another group from China screened 332 clinical E. coli isolates for cHR by disc diffusion or Etest and reported high rates of heteroresistance towards ertapenem and imipenem (17% and 25%, respectively). ESBL production was significantly associated with the cHR phenotype in E. coli isolates (adjusted OR 2.891, 95% CI 1.686–4.959, P < 0.01).14 Notably, one study exposed imipenem-heteroresistant K. pneumoniae isolates (n = 8) to imipenem in vitro at 4- to 16-fold above the MIC over 8 h and observed the emergence of subpopulations with high-level imipenem resistance (MIC >64 mg/L).18 These in vitro findings raise significant concerns for the progression from cHR to high-level resistance in ESBL-E strains following repeated carbapenem exposure, especially in patients with recurrent ESBL-E infections. Thus, there is a clear need to determine the overall prevalence of cHR among ESBL-E clinical isolates from patients with recurrent infections particularly when a carbapenem agent would be likely to be prescribed on repeated occasions.
Methods to screen for heteroresistance entail inoculating a bacterial suspension on medium and then exposing the isolate to different concentrations of the antibiotic of interest.12 Published studies have employed methods differing by inoculum, incubation time and medium for antibiotic exposure.12 Disc diffusion, Etest and population analysis profiling (PAP) are the most commonly used approaches to identify heteroresistant subpopulations. For PAP, no standardized antibiotic concentrations have been established to screen for heteroresistance. Published studies have used antibiotic concentrations ranging from 0.1 to 1 mg/L in increments. While most groups use 2-fold antibiotic increments, some have used >2-fold antibiotic increments. Without a standardized approach to antibiotic concentration increments, results are difficult to compare across studies. A review published in 2015 suggested that the heteroresistant phenotype be defined by growth of subpopulations at ≥8× MIC of the antibiotic of interest.12 Unlike PAP, drug concentrations for both disc diffusion and Etest are standardized by the manufacturer. Additionally, disc diffusion and Etest methods are less labour-intensive and may be incorporated into clinical microbiology workflow for initial screening for the heteroresistant phenotype compared with PAP.
While the clinical relevance of cHR in ESBL-E remains unclear, the potential for subsequent emergence of high-level carbapenem resistance with repeated carbapenem exposure is of grave concern in clinical practice. Agents with activity against ESBL-E, such as ceftolozane/tazobactam, a cephalosporin with confirmed activity against most Ambler class A-type ESBLs except for K. pneumoniae carbapenemase (KPC), may serve as non-carbapenem alternative treatment options but their activity against cHR Enterobacteriaceae remains unknown. To the best of our knowledge, the MIC distributions of standard antibiotic panels for heteroresistant ESBL-E have not been characterized previously. Therefore, we sought to: (i) screen for carbapenem and ceftolozane/tazobactam heteroresistance in ESBL-EC and ESBL-KP clinical isolates from patients with recurrent infections using the disc diffusion method; (ii) confirm carbapenem and ceftolozane/tazobactam heteroresistance by PAP; and (iii) compare the antimicrobial susceptibility profile between cHR and non-cHR clinical isolates, with a focus on carbapenems and ceftolozane/tazobactam.
Materials and methods
Isolates and setting
Clinical isolates were collected from a 619 bed university-affiliated community hospital. We examined our repository of clinical ESBL-EC and ESBL-KP isolates saved from 2012 to 2017. Isolates were selected from patients with more than one hospital encounter and a positive ESBL-E culture during each hospitalization. All isolates were tested for ESBL production using the NMIC panel on the BD Phoenix™ automated system. Routine susceptibility testing was performed using the BD Phoenix™ NMIC susceptibility panel, which included the following antibiotics consistent with the hospital’s antibiotic formulary: amikacin, cefepime, ceftazidime, ceftriaxone, ciprofloxacin, ertapenem, gentamicin, meropenem, piperacillin/tazobactam, trimethoprim/sulfamethoxazole and tobramycin. ESBL confirmatory testing was performed following the CLSI disc diffusion method on isolates that were either (i) ESBL-negative but resistant to any tested third-generation cephalosporin or (ii) ESBL-positive but susceptible to all tested third-generation cephalosporins.
Laboratory study
All bacterial isolates were stored at −80°C until further testing. Frozen isolates were thawed and subcultured at least twice prior to susceptibility testing. E. coli ATCC® 25922™ and K. pneumoniae ATCC® BAA-1705™ were included as quality control strains. Carbapenem MIC testing by broth microdilution (BMD) was performed in triplicate over a broad range of concentrations in doubling dilutions (from 0.0625 to 64 mg/L) according to CLSI methods. The colony suspension method of overnight bacterial cultures was performed. Plates were incubated in 35–37°C ambient air for 16 to 20 h and growth was determined spectrophotometrically at an OD of 600 nm. Similar BMD methods were used for ceftolozane/tazobactam susceptibility testing. MIC results were interpreted using CLSI MIC breakpoints for susceptibility: ertapenem, ≤0.5 mg/L; imipenem, ≤1 mg/L; meropenem, ≤1 mg/L; and ceftolozane/tazobactam, ≤2/4 mg/L.19 Isolates with an MIC above the susceptibility breakpoint were considered either intermediate or resistant. Microbiology records were reviewed for source of bacterial isolation and susceptibility results for all tested antibiotics in the panel by the BD Phoenix™ system. All isolates were screened for cHR using the disc diffusion method as outlined by the CLSI.20 Three carbapenems were used for screening: ertapenem, imipenem and meropenem. Briefly, bacterial suspensions were adjusted to a turbidity equivalent to that of a 0.5 McFarland standard at an OD of 600 nm using a spectrophotometer and a McFarland equivalence turbidity standard (Remel™ lot #421226) and plated on Mueller–Hinton agar plates (BBL MH lot #8299728 or Remel MH lot #453876), followed by placement of BD Sensi-Disc™ discs (meropenem 10 μg lot #8057655, imipenem 10 μg lot #8093934 and ertapenem 10 μg lot #8220830). Additionally, isolates were screened for ceftolozane/tazobactam heteroresistance (30/10 μg, Hardy lot #408864). Plates were incubated for 16 to 18 h at 35–37°C in ambient air. We defined a positive cHR screen as having a susceptible disc diffusion zone diameter by CLSI breakpoints (M100 Twenty-Ninth Edition),19 with growth of colonies within the zone of inhibition. Only isolates that screened positive for cHR by the disc diffusion method were further confirmed by a modified PAP method. Colonies grown within the disc diffusion zone of inhibition were resuspended in CAMHB (Sigma–Aldrich). Overnight bacterial cultures were then adjusted to a turbidity equivalent to that of a 2 McFarland standard (Remel™ lot #406387), as described above, and 30 μL was plated on antibiotic-containing plates and incubated for 48 h. The heteroresistant phenotype was confirmed based on growth on carbapenem-containing Mueller–Hinton agar plates at 8-fold MIC, as determined by BMD.
Data analysis
Based on results from modified PAP, isolates were grouped by the cHR phenotype, as either cHR or non-cHR, and compared for organism type, source of isolation, antimicrobial susceptibility profile from the BD Phoenix™ susceptibility panel, and carbapenem and ceftolozane/tazobactam MIC by BMD. In addition, the proportion of cHR isolates displaying a heteroresistant phenotype to ceftolozane/tazobactam was also determined. For patients from whom cHR strains were isolated, medical records were reviewed to determine if a carbapenem-non-susceptible isolate was identified during subsequent hospital encounters. Categorical variables are presented as a percentage (frequency) and compared using χ2 or Fisher’s exact test. A two-sided P value of <0.05 was considered statistically significant. Statistical analyses were performed using IBM SPSS® Version 26.
Results
A total of 1458 clinical ESBL-EC and ESBL-KP isolates were identified from microbiology records. Of those, 408 of 1458 isolates were from patients with more than one hospital encounter; 173 of 408 isolates were saved, reported as meropenem susceptible and thus included in this study. A total of 519 bacteria–carbapenem combinations were tested for the heteroresistant phenotype (Figure 1). Overall, study isolates were comprised primarily of ESBL-EC (87%, 151/173), with the remaining isolates being ESBL-KP (13%, 22/173). Most isolates were collected from a urinary source (77%, 133/173). Among those isolates from a non-urinary source (n = 40), 40% were from blood samples (16/40), 33% were from respiratory samples (13/40) and 28% were from wound samples (11/40). Microbiological characteristics of all study isolates are summarized in Table 1.
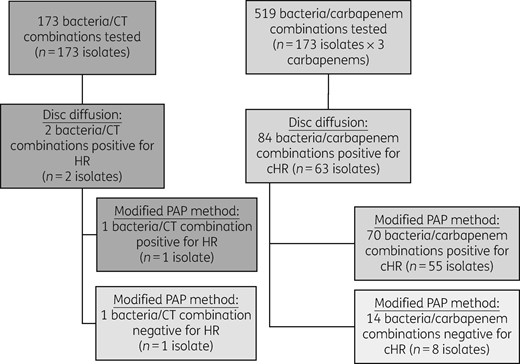
Screening and confirmation for carbapenem and ceftolozane/tazobactam heteroresistance. Ceftolozane/tazobactam and three carbapenem agents (imipenem, meropenem and ertapenem) were tested against a total of 173 isolates. Heteroresistance screening was performed by disc diffusion; strains positive for the heteroresistant phenotype were further tested by PAP for confirmation based on growth of colonies on antibiotic-containing plates at 8× MIC. CT, ceftolozane/tazobactam; HR, heteroresistance.
Summary of microbiological characteristics of PAP-confirmed cHR and non-cHR isolates
| . | All (N = 173), n (%) or n/n (%) . | Non-cHR (N = 118), n (%) or n/n (%) . | cHR (N = 55), n (%) or n/n (%) . | P . |
|---|---|---|---|---|
| Organism | 0.626 | |||
| E. coli | 151 (87) | 102 (86) | 49 (89) | |
| K. pneumoniae | 22 (13) | 16 (14) | 6 (11) | |
| Source | ||||
| urine | 133 (77) | 95 (81) | 38 (69) | 0.018 |
| non-urine source | 40 (23) | 23 (19) | 17 (31) | 0.018 |
| respiratory | 13/40 (33) | 5/23 (22) | 8/17 (47) | 0.008 |
| blood | 16/40 (40) | 10/23 (43) | 6/17 (35) | 0.075 |
| wound | 11/40 (28) | 8/23 (35) | 3/17 (18) | 0.019 |
| Positive heteroresistance testing results | ||||
| disc diffusion | 63 (36) | 8 (7) | 55 (100) | – |
| PAP | 55 (32) | – | 55 (100) | – |
| . | All (N = 173), n (%) or n/n (%) . | Non-cHR (N = 118), n (%) or n/n (%) . | cHR (N = 55), n (%) or n/n (%) . | P . |
|---|---|---|---|---|
| Organism | 0.626 | |||
| E. coli | 151 (87) | 102 (86) | 49 (89) | |
| K. pneumoniae | 22 (13) | 16 (14) | 6 (11) | |
| Source | ||||
| urine | 133 (77) | 95 (81) | 38 (69) | 0.018 |
| non-urine source | 40 (23) | 23 (19) | 17 (31) | 0.018 |
| respiratory | 13/40 (33) | 5/23 (22) | 8/17 (47) | 0.008 |
| blood | 16/40 (40) | 10/23 (43) | 6/17 (35) | 0.075 |
| wound | 11/40 (28) | 8/23 (35) | 3/17 (18) | 0.019 |
| Positive heteroresistance testing results | ||||
| disc diffusion | 63 (36) | 8 (7) | 55 (100) | – |
| PAP | 55 (32) | – | 55 (100) | – |
Summary of microbiological characteristics of PAP-confirmed cHR and non-cHR isolates
| . | All (N = 173), n (%) or n/n (%) . | Non-cHR (N = 118), n (%) or n/n (%) . | cHR (N = 55), n (%) or n/n (%) . | P . |
|---|---|---|---|---|
| Organism | 0.626 | |||
| E. coli | 151 (87) | 102 (86) | 49 (89) | |
| K. pneumoniae | 22 (13) | 16 (14) | 6 (11) | |
| Source | ||||
| urine | 133 (77) | 95 (81) | 38 (69) | 0.018 |
| non-urine source | 40 (23) | 23 (19) | 17 (31) | 0.018 |
| respiratory | 13/40 (33) | 5/23 (22) | 8/17 (47) | 0.008 |
| blood | 16/40 (40) | 10/23 (43) | 6/17 (35) | 0.075 |
| wound | 11/40 (28) | 8/23 (35) | 3/17 (18) | 0.019 |
| Positive heteroresistance testing results | ||||
| disc diffusion | 63 (36) | 8 (7) | 55 (100) | – |
| PAP | 55 (32) | – | 55 (100) | – |
| . | All (N = 173), n (%) or n/n (%) . | Non-cHR (N = 118), n (%) or n/n (%) . | cHR (N = 55), n (%) or n/n (%) . | P . |
|---|---|---|---|---|
| Organism | 0.626 | |||
| E. coli | 151 (87) | 102 (86) | 49 (89) | |
| K. pneumoniae | 22 (13) | 16 (14) | 6 (11) | |
| Source | ||||
| urine | 133 (77) | 95 (81) | 38 (69) | 0.018 |
| non-urine source | 40 (23) | 23 (19) | 17 (31) | 0.018 |
| respiratory | 13/40 (33) | 5/23 (22) | 8/17 (47) | 0.008 |
| blood | 16/40 (40) | 10/23 (43) | 6/17 (35) | 0.075 |
| wound | 11/40 (28) | 8/23 (35) | 3/17 (18) | 0.019 |
| Positive heteroresistance testing results | ||||
| disc diffusion | 63 (36) | 8 (7) | 55 (100) | – |
| PAP | 55 (32) | – | 55 (100) | – |
Out of the 173 isolates screened by the disc diffusion method, 36% (63/173) were heteroresistant to at least one carbapenem. Over 70% (45/63) of cHR isolates were heteroresistant to one of three carbapenem agents tested, while 24% (15/63) were heteroresistant to two carbapenems and 5% (3/63) were heteroresistant to all three carbapenems. In total, 84 (16%) of 519 bacteria/carbapenem combinations screened positive for the cHR phenotype by disc diffusion (Figure 1). Modified PAP confirmed 83% (70/84) of the bacteria/carbapenem combinations as heteroresistant; of those confirmed, most (63%, 44/70) of the cHR colonies grew within the ertapenem zone of inhibition, followed by imipenem (30%, 21/70), then meropenem (7%, 5/70). In total, one-third of the unique patient isolates (32%, 55/173) were identified as being heteroresistant to at least one carbapenem; of those patients, 16% (9/55) had a carbapenem-non-susceptible isolate on subsequent hospital encounter, with a median interval time to isolation of 94 days (IQR = 52–232 days).
In addition to cHR screening, isolates were also screened for ceftolozane/tazobactam heteroresistance, which yielded two positive isolates (1%, 2/173). The modified PAP method also confirmed one of the two aforementioned isolates as ceftolozane/tazobactam heteroresistant; this isolate was also confirmed to be heteroresistant to ertapenem. Heteroresistance results are summarized in Table 2.
Detection of the heteroresistant phenotype by disc diffusion and confirmation by the PAP method for each bacteria/drug combination tested
| Antibiotic tested . | Disc diffusion . | Modified PAP . |
|---|---|---|
| Ertapenem | 11% (55/519) | 63% (44/70) |
| Imipenem | 4% (23/519) | 30% (21/70) |
| Meropenem | 1% (6/519) | 7% (5/70) |
| Ceftolozane/tazobactam | 1% (2/173) | 50% (1/2) |
| Antibiotic tested . | Disc diffusion . | Modified PAP . |
|---|---|---|
| Ertapenem | 11% (55/519) | 63% (44/70) |
| Imipenem | 4% (23/519) | 30% (21/70) |
| Meropenem | 1% (6/519) | 7% (5/70) |
| Ceftolozane/tazobactam | 1% (2/173) | 50% (1/2) |
Eighty-four out of a total of 519 bacteria/carbapenem combinations tested positive for the cHR phenotype by disc diffusion; 70 of the 84 combinations were confirmed by PAP based on growth of colonies on antibiotic-containing plates at 8× MIC by BMD. Two out of a total of 173 isolates tested positive for the heteroresistant phenotype to ceftolozane/tazobactam by disc diffusion; one of them was confirmed by PAP.
Detection of the heteroresistant phenotype by disc diffusion and confirmation by the PAP method for each bacteria/drug combination tested
| Antibiotic tested . | Disc diffusion . | Modified PAP . |
|---|---|---|
| Ertapenem | 11% (55/519) | 63% (44/70) |
| Imipenem | 4% (23/519) | 30% (21/70) |
| Meropenem | 1% (6/519) | 7% (5/70) |
| Ceftolozane/tazobactam | 1% (2/173) | 50% (1/2) |
| Antibiotic tested . | Disc diffusion . | Modified PAP . |
|---|---|---|
| Ertapenem | 11% (55/519) | 63% (44/70) |
| Imipenem | 4% (23/519) | 30% (21/70) |
| Meropenem | 1% (6/519) | 7% (5/70) |
| Ceftolozane/tazobactam | 1% (2/173) | 50% (1/2) |
Eighty-four out of a total of 519 bacteria/carbapenem combinations tested positive for the cHR phenotype by disc diffusion; 70 of the 84 combinations were confirmed by PAP based on growth of colonies on antibiotic-containing plates at 8× MIC by BMD. Two out of a total of 173 isolates tested positive for the heteroresistant phenotype to ceftolozane/tazobactam by disc diffusion; one of them was confirmed by PAP.
Among the 55 of 173 total study isolates confirmed for the cHR phenotype by both disc diffusion and PAP, the proportion of confirmed cHR isolates did not differ between ESBL-EC and ESBL-KP (32%, 49/151 versus 27%, 6/22, respectively). More cHR isolates originated from a non-urinary source compared with non-cHR isolates (31%, 17/55 versus 19% 23/118, P = 0.018). Nearly half (47%, 8/17) of the cHR non-urinary isolates were collected from a respiratory site.
Antimicrobial susceptibilities generated by the BD Phoenix™ system are summarized in Table 3. All tested isolates were identified as meropenem susceptible but three isolates were ertapenem intermediate (n = 2) or resistant (n = 1; 2%, 3/140). One of the two ertapenem-intermediate isolates screened positive and was confirmed for imipenem heteroresistance. For the other tested agents, the susceptibility patterns were similar overall except for gentamicin, where a trend towards more cHR isolates being resistant was observed (59%, 32/54 versus 42%, 45/106, P = 0.05). In agreement with the BD Phoenix™ results, BMD yielded concordant results on carbapenem susceptibility where all 173 isolates were susceptible to imipenem and meropenem (Table 4). However, compared with results from the automated system, BMD identified an additional 9 isolates, for a total of 12 isolates, as either intermediate (4%, 7/173) or resistant (3%, 5/173) to ertapenem; 8 of 12 isolates (4 intermediate, 4 resistant) screened positive for cHR with disc diffusion and 6 of 8 isolates were confirmed heteroresistant with the modified PAP method to be heteroresistant to ertapenem (5/6) and/or imipenem (2/6). Overall MIC distributions of imipenem and meropenem were similar across study strains, regardless of the heteroresistant phenotype. Ertapenem susceptibility was slightly lower for non-cHR isolates compared with cHR isolates (95%, 112/118 versus 89%, 49/55). No significant differences in MIC values by BMD testing of ceftolozane/tazobactam were observed between non-heteroresistant and cHR isolates. Ceftolozane/tazobactam showed excellent susceptibility for ESBL-E regardless of the cHR phenotype (non-cHR ESBL-E, 100%, 118/118 versus cHR ESBL-E, 96%, 53/55).
Antimicrobial susceptibility results from the BD Phoenix™ automated testing system
| . | Total number of isolates tested . | All . | Non-cHR . | cHR . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S (%) . | I (%) . | R (%) . | number of isolates tested . | S (%) . | I (%) . | R (%) . | number of isolates tested . | S (%) . | I (%) . | R (%) . | ||
| Ertapenem | 140 | 98 | 1 | 1 | 91 | 99 | 1 | 0 | 49 | 96 | 2 | 2 |
| Imipenema | 20 | 100 | 0 | 0 | 15 | 100 | 0 | 0 | 5 | 100 | 0 | 0 |
| Meropenem | 100 | 100 | 0 | 0 | 70 | 100 | 0 | 0 | 30 | 100 | 0 | 0 |
| Amikacin | 103 | 95 | 3 | 2 | 60 | 98 | 1 | 1 | 43 | 96 | 2 | 2 |
| Gentamicin | 160 | 51 | 1 | 48 | 106 | 57 | 1 | 42 | 54 | 41 | 0 | 59 |
| Tobramycin | 102 | 2 | 24 | 74 | 60 | 0 | 20 | 80 | 42 | 5 | 29 | 66 |
| Ciprofloxacin | 160 | 6 | 2 | 92 | 106 | 5 | 4 | 91 | 54 | 9 | 0 | 91 |
| Piperacillin/tazobactam | 158 | 81 | 8 | 11 | 106 | 81 | 8 | 11 | 52 | 83 | 8 | 9 |
| Ceftriaxone | 161 | 2 | 0 | 98 | 107 | 1 | 0 | 99 | 54 | 4 | 0 | 96 |
| Ceftazidime | 106 | 2 | 1 | 97 | 73 | 3 | 1 | 96 | 33 | 0 | 0 | 100 |
| Cefepime | 160 | 1 | 0 | 99 | 106 | 1 | 0 | 99 | 54 | 0 | 0 | 100 |
| Trimethoprim/sulfamethoxazole | 159 | 26 | 0 | 74 | 106 | 26 | 0 | 74 | 53 | 26 | 0 | 74 |
| . | Total number of isolates tested . | All . | Non-cHR . | cHR . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S (%) . | I (%) . | R (%) . | number of isolates tested . | S (%) . | I (%) . | R (%) . | number of isolates tested . | S (%) . | I (%) . | R (%) . | ||
| Ertapenem | 140 | 98 | 1 | 1 | 91 | 99 | 1 | 0 | 49 | 96 | 2 | 2 |
| Imipenema | 20 | 100 | 0 | 0 | 15 | 100 | 0 | 0 | 5 | 100 | 0 | 0 |
| Meropenem | 100 | 100 | 0 | 0 | 70 | 100 | 0 | 0 | 30 | 100 | 0 | 0 |
| Amikacin | 103 | 95 | 3 | 2 | 60 | 98 | 1 | 1 | 43 | 96 | 2 | 2 |
| Gentamicin | 160 | 51 | 1 | 48 | 106 | 57 | 1 | 42 | 54 | 41 | 0 | 59 |
| Tobramycin | 102 | 2 | 24 | 74 | 60 | 0 | 20 | 80 | 42 | 5 | 29 | 66 |
| Ciprofloxacin | 160 | 6 | 2 | 92 | 106 | 5 | 4 | 91 | 54 | 9 | 0 | 91 |
| Piperacillin/tazobactam | 158 | 81 | 8 | 11 | 106 | 81 | 8 | 11 | 52 | 83 | 8 | 9 |
| Ceftriaxone | 161 | 2 | 0 | 98 | 107 | 1 | 0 | 99 | 54 | 4 | 0 | 96 |
| Ceftazidime | 106 | 2 | 1 | 97 | 73 | 3 | 1 | 96 | 33 | 0 | 0 | 100 |
| Cefepime | 160 | 1 | 0 | 99 | 106 | 1 | 0 | 99 | 54 | 0 | 0 | 100 |
| Trimethoprim/sulfamethoxazole | 159 | 26 | 0 | 74 | 106 | 26 | 0 | 74 | 53 | 26 | 0 | 74 |
S, susceptible; I, intermediate; R, resistant.
Imipenem is not on the hospital formulary and therefore not included in our standard susceptibility panel for automated testing; only a small subset of isolates was tested.
Antimicrobial susceptibility results from the BD Phoenix™ automated testing system
| . | Total number of isolates tested . | All . | Non-cHR . | cHR . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S (%) . | I (%) . | R (%) . | number of isolates tested . | S (%) . | I (%) . | R (%) . | number of isolates tested . | S (%) . | I (%) . | R (%) . | ||
| Ertapenem | 140 | 98 | 1 | 1 | 91 | 99 | 1 | 0 | 49 | 96 | 2 | 2 |
| Imipenema | 20 | 100 | 0 | 0 | 15 | 100 | 0 | 0 | 5 | 100 | 0 | 0 |
| Meropenem | 100 | 100 | 0 | 0 | 70 | 100 | 0 | 0 | 30 | 100 | 0 | 0 |
| Amikacin | 103 | 95 | 3 | 2 | 60 | 98 | 1 | 1 | 43 | 96 | 2 | 2 |
| Gentamicin | 160 | 51 | 1 | 48 | 106 | 57 | 1 | 42 | 54 | 41 | 0 | 59 |
| Tobramycin | 102 | 2 | 24 | 74 | 60 | 0 | 20 | 80 | 42 | 5 | 29 | 66 |
| Ciprofloxacin | 160 | 6 | 2 | 92 | 106 | 5 | 4 | 91 | 54 | 9 | 0 | 91 |
| Piperacillin/tazobactam | 158 | 81 | 8 | 11 | 106 | 81 | 8 | 11 | 52 | 83 | 8 | 9 |
| Ceftriaxone | 161 | 2 | 0 | 98 | 107 | 1 | 0 | 99 | 54 | 4 | 0 | 96 |
| Ceftazidime | 106 | 2 | 1 | 97 | 73 | 3 | 1 | 96 | 33 | 0 | 0 | 100 |
| Cefepime | 160 | 1 | 0 | 99 | 106 | 1 | 0 | 99 | 54 | 0 | 0 | 100 |
| Trimethoprim/sulfamethoxazole | 159 | 26 | 0 | 74 | 106 | 26 | 0 | 74 | 53 | 26 | 0 | 74 |
| . | Total number of isolates tested . | All . | Non-cHR . | cHR . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S (%) . | I (%) . | R (%) . | number of isolates tested . | S (%) . | I (%) . | R (%) . | number of isolates tested . | S (%) . | I (%) . | R (%) . | ||
| Ertapenem | 140 | 98 | 1 | 1 | 91 | 99 | 1 | 0 | 49 | 96 | 2 | 2 |
| Imipenema | 20 | 100 | 0 | 0 | 15 | 100 | 0 | 0 | 5 | 100 | 0 | 0 |
| Meropenem | 100 | 100 | 0 | 0 | 70 | 100 | 0 | 0 | 30 | 100 | 0 | 0 |
| Amikacin | 103 | 95 | 3 | 2 | 60 | 98 | 1 | 1 | 43 | 96 | 2 | 2 |
| Gentamicin | 160 | 51 | 1 | 48 | 106 | 57 | 1 | 42 | 54 | 41 | 0 | 59 |
| Tobramycin | 102 | 2 | 24 | 74 | 60 | 0 | 20 | 80 | 42 | 5 | 29 | 66 |
| Ciprofloxacin | 160 | 6 | 2 | 92 | 106 | 5 | 4 | 91 | 54 | 9 | 0 | 91 |
| Piperacillin/tazobactam | 158 | 81 | 8 | 11 | 106 | 81 | 8 | 11 | 52 | 83 | 8 | 9 |
| Ceftriaxone | 161 | 2 | 0 | 98 | 107 | 1 | 0 | 99 | 54 | 4 | 0 | 96 |
| Ceftazidime | 106 | 2 | 1 | 97 | 73 | 3 | 1 | 96 | 33 | 0 | 0 | 100 |
| Cefepime | 160 | 1 | 0 | 99 | 106 | 1 | 0 | 99 | 54 | 0 | 0 | 100 |
| Trimethoprim/sulfamethoxazole | 159 | 26 | 0 | 74 | 106 | 26 | 0 | 74 | 53 | 26 | 0 | 74 |
S, susceptible; I, intermediate; R, resistant.
Imipenem is not on the hospital formulary and therefore not included in our standard susceptibility panel for automated testing; only a small subset of isolates was tested.
Comparison of susceptibility to ertapenem, imipenem, meropenem and ceftolozane/tazobactam between non-cHR and cHR isolates based on manual testing by BMD
| . | All (N = 173) . | Non-cHR (N = 118) . | cHR (N = 55) . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC50 (mg/L)/ MIC90 (mg/L) . | S (%) . | I (%) . | R (%) . | MIC50 (mg/L)/ MIC90 (mg/L) . | S (%) . | I (%) . | R (%) . | MIC50 (mg/L)/ MIC90 (mg/L) . | S (%) . | I (%) . | R (%) . | |
| Ertapenem | 0.25/0.50 | 93 | 4 | 3 | 0.125/0.50 | 95 | 4 | 1 | 0.25/1 | 89 | 5 | 5 |
| Imipenem | 0.25/0.50 | 100 | 0 | 0 | 0.25/0.50 | 100 | 0 | 0 | 0.25/0.50 | 100 | 0 | 0 |
| Meropenem | 0.0625/0.125 | 100 | 0 | 0 | 0.0625/0.125 | 100 | 0 | 0 | 0.0625/0.0625 | 100 | 0 | 0 |
| Ceftolozane/ tazobactam | 0.25/0.50 | 99 | 0 | 1 | 0.25–4/0.5–4 | 100 | 0 | 0 | 0.25–4/0.5–4 | 96 | 0 | 4 |
| . | All (N = 173) . | Non-cHR (N = 118) . | cHR (N = 55) . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC50 (mg/L)/ MIC90 (mg/L) . | S (%) . | I (%) . | R (%) . | MIC50 (mg/L)/ MIC90 (mg/L) . | S (%) . | I (%) . | R (%) . | MIC50 (mg/L)/ MIC90 (mg/L) . | S (%) . | I (%) . | R (%) . | |
| Ertapenem | 0.25/0.50 | 93 | 4 | 3 | 0.125/0.50 | 95 | 4 | 1 | 0.25/1 | 89 | 5 | 5 |
| Imipenem | 0.25/0.50 | 100 | 0 | 0 | 0.25/0.50 | 100 | 0 | 0 | 0.25/0.50 | 100 | 0 | 0 |
| Meropenem | 0.0625/0.125 | 100 | 0 | 0 | 0.0625/0.125 | 100 | 0 | 0 | 0.0625/0.0625 | 100 | 0 | 0 |
| Ceftolozane/ tazobactam | 0.25/0.50 | 99 | 0 | 1 | 0.25–4/0.5–4 | 100 | 0 | 0 | 0.25–4/0.5–4 | 96 | 0 | 4 |
S, susceptible; I, intermediate; R, resistant.
Comparison of susceptibility to ertapenem, imipenem, meropenem and ceftolozane/tazobactam between non-cHR and cHR isolates based on manual testing by BMD
| . | All (N = 173) . | Non-cHR (N = 118) . | cHR (N = 55) . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC50 (mg/L)/ MIC90 (mg/L) . | S (%) . | I (%) . | R (%) . | MIC50 (mg/L)/ MIC90 (mg/L) . | S (%) . | I (%) . | R (%) . | MIC50 (mg/L)/ MIC90 (mg/L) . | S (%) . | I (%) . | R (%) . | |
| Ertapenem | 0.25/0.50 | 93 | 4 | 3 | 0.125/0.50 | 95 | 4 | 1 | 0.25/1 | 89 | 5 | 5 |
| Imipenem | 0.25/0.50 | 100 | 0 | 0 | 0.25/0.50 | 100 | 0 | 0 | 0.25/0.50 | 100 | 0 | 0 |
| Meropenem | 0.0625/0.125 | 100 | 0 | 0 | 0.0625/0.125 | 100 | 0 | 0 | 0.0625/0.0625 | 100 | 0 | 0 |
| Ceftolozane/ tazobactam | 0.25/0.50 | 99 | 0 | 1 | 0.25–4/0.5–4 | 100 | 0 | 0 | 0.25–4/0.5–4 | 96 | 0 | 4 |
| . | All (N = 173) . | Non-cHR (N = 118) . | cHR (N = 55) . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC50 (mg/L)/ MIC90 (mg/L) . | S (%) . | I (%) . | R (%) . | MIC50 (mg/L)/ MIC90 (mg/L) . | S (%) . | I (%) . | R (%) . | MIC50 (mg/L)/ MIC90 (mg/L) . | S (%) . | I (%) . | R (%) . | |
| Ertapenem | 0.25/0.50 | 93 | 4 | 3 | 0.125/0.50 | 95 | 4 | 1 | 0.25/1 | 89 | 5 | 5 |
| Imipenem | 0.25/0.50 | 100 | 0 | 0 | 0.25/0.50 | 100 | 0 | 0 | 0.25/0.50 | 100 | 0 | 0 |
| Meropenem | 0.0625/0.125 | 100 | 0 | 0 | 0.0625/0.125 | 100 | 0 | 0 | 0.0625/0.0625 | 100 | 0 | 0 |
| Ceftolozane/ tazobactam | 0.25/0.50 | 99 | 0 | 1 | 0.25–4/0.5–4 | 100 | 0 | 0 | 0.25–4/0.5–4 | 96 | 0 | 4 |
S, susceptible; I, intermediate; R, resistant.
Discussion
Global reports of cHR among ESBL-producing Enterobacteriaceae raise concerns about the impact of continued use of carbapenems as first-line therapy for ESBL-E infections on the emergence of Enterobacteriaceae with high-level carbapenem resistance. However, it is not known what proportion of clinical isolates harbouring the cHR phenotype are reported to be susceptible to carbapenems when applying current CLSI interpretive criteria for BMD testing that is routinely performed in the clinical microbiology laboratory. Thus, the goal of our study was to characterize the prevalence of the cHR phenotype in meropenem-susceptible clinical ESBL-EC and ESBL-KP isolates collected from patients who are likely to have had carbapenem exposure due to repeated hospital admission with growth of ESBL-E. In addition, our aim was to identify whether differences in antimicrobial susceptibility profiles exist between cHR and non-cHR isolates. Our use of a modified PAP method identified almost one-third of our ESBL-E isolates as harbouring the cHR phenotype to at least one of the tested carbapenems. Our cHR rate of 32% is higher than the previously published cHR rate of 25% from China by Sun et al.14 This difference can probably be attributed to differences in study design as they included both ESBL-EC and non-ESBL-EC, while our study only included ESBL-positive EC and KP. They found that ESBL production was associated with an almost three times greater risk for carrying the cHR phenotype and thus may explain the higher cHR rate observed in our study. Among the three carbapenem agents tested, differences in cHR rates were observed. We found that the majority of ESBL-E colonies grew around the ertapenem disc. This finding is consistent with that observed in the study by Nicoloff et al.,13 while Sun et al.14 observed the greatest growth around the imipenem disc. It is likely that the cHR phenotype is biased towards a specific carbapenem agent that corresponds to its frequency of use. As is in the case at our institution, ertapenem is the carbapenem agent of choice for treating serious ESBL-E infections, consistent with the observed cHR phenotype.
Carbapenem MIC results for ESBL-EC and ESBL-KP isolates obtained from both automated and manual BMD testing were similar between those with cHR and non-cHR phenotypes. Similarly, Nicoloff et al.13 reported that all cHR E. coli and K. pneumoniae had MICs indicating susceptibility to ertapenem, imipenem and meropenem. While carbapenem susceptibility results generated by BMD were more sensitive with manual testing than with automated systems in identifying the heteroresistant phenotype, it is worth noting that carbapenem susceptibility as determined by BMD testing missed up to 89% (49/55) of ESBL-E isolates that carried the heteroresistant phenotype, depending on the specific carbapenem agent tested. Taken together, our results suggest that MIC values obtained from either an automated system or manual BMD do not differentiate ESBL-EC or ESBL-KP strains with the cHR phenotype.
Our data suggest that isolates with the cHR phenotype may be enriched in particular body niches. Sun et al.14 only tested isolates from sterile body fluid, but did not report a comparison of non-cHR and cHR isolates for differences in body site of collection. We found that a higher proportion of non-urinary isolates, particularly those from a respiratory source, had the cHR phenotype compared with urine isolates. It is possible that higher-inoculum infections coupled with a relatively lower antibiotic exposure at the respiratory site compared with urine may have contributed to the selection of heteroresistant subpopulations. Our findings deserve confirmation with further studies involving larger numbers of respiratory samples.
This study has several limitations. First, methods to study heteroresistance have yet to be standardized. Test conditions have varied by bacterial inoculum (turbidity equivalent to that of a 0.5–2.0 McFarland standard) and incubation time (of up to 24 to 48 h).14–18,21 It is notable that an inoculum effect in which diminished antibacterial activity is observed at high bacterial density has been observed in vitro with β-lactam agents against some pathogens.22 Therefore, to minimize the potential for false-positive detection of the heteroresistance phenotype when testing at the higher bacterial inoculum, we chose to perform disc diffusion testing at the standard inoculum (turbidity equivalent to that of a 0.5 McFarland standard) and incubation time of 16 to 18 h commonly employed in a clinical microbiology laboratory setting to screen for the heteroresistant phenotype. Not using a higher than standard inoculum or extending the incubation time may have limited the growth of heteroresistant subpopulations. As there is no standardized method for heteroresistance testing and considering that the heteroresistance phenotype is characterized by instability, low frequency and a transient nature, testing at higher inoculum for a prolonged incubation period has been recommended.23,24 Thus, to confirm the colonies grown within the zone of inhibition on disc diffusion testing as heteroresistant, we used an inoculum with a turbidity equivalent to that of a 2.0 McFarland standard by PAP. Second, the majority of our isolates tested were ESBL-EC, thus results may not apply to other Enterobacteriaceae. Nonetheless, our results are highly clinically relevant as E. coli is the most commonly encountered ESBL-E from community-acquired infections. Third, clinical factors, such as cumulative antibiotic exposure, were not recorded though our study isolates were obtained from patients with repeated admission with growth of ESBL-E who would probably have received prior carbapenem therapy. Finally, our study did not evaluate the activity of other FDA-approved antibiotics active against both ESBL- and KPC-producing Enterobacteriaceae such as ceftazidime/avibactam, imipenem/relebactam, meropenem/vaborbactam or plazomicin against ESBL-producing strains with the cHR phenotype. Since heteroresistance probably precedes emergence of full resistance, we chose to focus on ceftolozane/tazobactam, rather than the aforementioned antimicrobial agents, to reserve KPC-active agents for the treatment of infections involving strains expressing full carbapenem resistance.
Conclusions
The cHR phenotype was observed in almost one-third of clinical isolates from patients who had repeated hospital admissions with a positive culture for ESBL-E at a community hospital. The heteroresistant phenotype was most frequently observed against ertapenem despite testing ‘susceptible’ to ertapenem, imipenem and meropenem by BMD when applying CLSI interpretive criteria. Isolates from respiratory sources were more likely to harbour the cHR phenotype compared with urinary isolates, probably related to differential drug exposure in the respective body compartments where the infection took place and cumulative drug exposure with multiple infections. Our findings raise significant concerns for the continued use of carbapenems as first-line therapy and underscore the need for future studies to examine the contribution of antibiotic exposure to the development of cHR phenotypes and the clinical outcomes of cHR ESBL-E infections treated with a carbapenem agent versus non-carbapenem alternatives.
Acknowledgements
We thank Chao Niu for technical assistance with susceptibility testing.
Funding
This is an investigator-initiated study supported in part by Merck, Inc. The sponsor had no role in data collection and interpretation. K.T. was funded by the USC School of Pharmacy Clinical Translational Research Fellowship Award.
Transparency declarations
A.W.-B. has received grants from Merck and Allergan, and consulting fees from Nabriva Therapeutics, Insmed, Rempex Pharmaceuticals, Paratek Pharmaceuticals, Achaogen, Inc., Bayer Healthcare, SIGA Technologies and GlaxoSmithKline. All other authors: none to declare.
References
CDC.
CLSI.
CLSI.



