-
PDF
- Split View
-
Views
-
Cite
Cite
Massimiliano Fabbiani, Dario Cattaneo, Andrea Lombardi, Marta Colaneri, Margherita Sambo, Stefano Novati, Marta Fusi, Raffaele Bruno, Pharmacokinetic profile of dolutegravir after transjugular intrahepatic portosystemic shunt placement, Journal of Antimicrobial Chemotherapy, Volume 75, Issue 5, May 2020, Pages 1354–1356, https://doi.org/10.1093/jac/dkz572
Close - Share Icon Share
Sir,
Chronic liver diseases are commonly observed in HIV-infected patients and the progression of liver fibrosis can occur faster in such a population. Placement of a transjugular intrahepatic portosystemic shunt (TIPS) is a procedure used in advanced chronic liver diseases to reduce portal hypertension and its related complications; it consists of the creation of an artificial shunt between the portal tree and the systemic circulation, bypassing the obstruction represented by the liver and thus lowering the portosystemic pressure gradient.1 Despite TIPS being currently indicated for several conditions related to portal hypertension, with positive results for survival, very few data are available on its efficacy and tolerability in HIV-infected patients.2 Indeed, no data are currently available about the impact of TIPS on the pharmacokinetics of antiretroviral drugs. Here, we describe the modification of the pharmacokinetic profile of dolutegravir after TIPS placement.
An HIV-infected man in his fifties with liver cirrhosis (Child–Pugh score A5) related to HCV coinfection (eradicated 4 years before with direct-acting antivirals), treated with 50 mg of dolutegravir + 300 mg of lamivudine once daily with adequate viroimmunological control (HIV-RNA stably undetectable, last CD4 count 355 cells/mm3), underwent endoscopic variceal ligation for upper gastrointestinal bleeding from oesophageal varices. Subsequently, TIPS placement was planned to reduce portal hypertension. Pre-procedure portosystemic pressure gradient was 21 mmHg, which decreased to 5 mmHg after TIPS [expanded polytetrafluorethylene-covered nitinol stent (Viatorr®10/20/60; WL Gore & Associates, Flagstaff, AZ, USA) dilated with a 7 mm angioplasty balloon (DORADO®; Bard Peripheral Vascular Inc., Tempe, AZ, USA)]. The procedure was well tolerated and the patient was discharged after a few days. Of note, 2 weeks after TIPS placement the patient was newly admitted for asthenia and confusion. Ammonia plasma levels were initially within the normal range and a head magnetic resonance scan did not demonstrate ischaemic alterations or recent bleeding. Such symptoms resolved with fluid infusion. However, in the following months the patient showed some episodes of transient hepatic encephalopathy with elevated ammonia levels. Six months after TIPS, HIV-RNA was still undetectable and CD4 count remained stable.
We obtained a complete pharmacokinetic profile [plasma samples drawn before antiretroviral drug administration (Ctrough) and 1, 2, 3, 4, 6 and 12 h after drug intake] before and 4 weeks after TIPS placement. Dolutegravir plasma levels were measured by HPLC coupled with UV detection. The lower limit of detection was set at 50 ng/mL and the method was linear in the range of 20–8000 ng/mL.3
The pharmacokinetic curves and main pharmacokinetic parameters of dolutegravir (administered after a low-fat meal; no drug with known interactions was coadministered) before and after TIPS are shown in Figure 1. After TIPS, Tmax was reduced to 60 min (versus 240 min before TIPS). We observed a 56.1% and 49.1% increase in Cmax (from 3434 to 5361 ng/mL) and AUC0–24 (from 64 949 to 96 827 ng·h/mL), respectively; Ctrough did not show significant modifications (+7%, from 2912 to 3117 ng/mL).
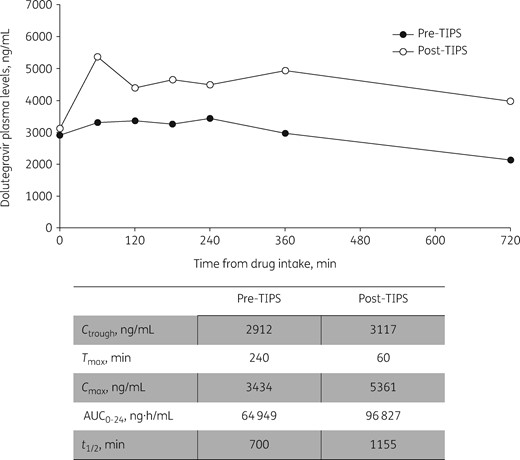
Main pharmacokinetic parameters of dolutegravir before and after TIPS placement.
To the best of our knowledge, this is the first report to describe the changes of dolutegravir pharmacokinetics after TIPS. Our data show that in our patient, pharmacokinetic parameters were higher before TIPS (AUC0–24 +33%, Cmax +6%, Ctrough +170%), when compared with median values reported in a real-life setting.4 Several factors could contribute to pharmacokinetic variability of antiretroviral drugs.5 Dolutegravir is mainly metabolized in the liver by UGT1A1 and to a lesser extent by CYP3A4. Advanced liver diseases could affect dolutegravir pharmacokinetics due to changes in metabolism via CYP enzymes or glucuronidation and also due to modifications in liver blood flow or altered synthesis of plasma proteins.6 However, further studies are needed to determine the impact of liver diseases on dolutegravir pharmacokinetics since very few data are available in patients with hepatic impairment.7
After TIPS placement, even if Ctrough did not significantly change, dolutegravir exposure was markedly increased due to elevations of Cmax and AUC0–24. This could be ascribed to an increased bioavailability as a consequence of reduced first-pass metabolism and possibly to an effect on metabolization as a consequence of the artificial shunt bypassing the liver.
Despite no other data being available for antiretrovirals, our results are in accordance with the few data showing an increased exposure to non-antiretroviral drugs after TIPS. Indeed, Chalasani et al.8 demonstrated how TIPS placement modified midazolam pharmacokinetic properties, with increases in bioavailability and Cmax. Similarly, Spriet et al.9 observed an increase in half-life and AUC for caspofungin after TIPS placement. These data are particularly interesting considering that midazolam is primarily metabolized by CYP3A4, whereas caspofungin is not, suggesting that an interplay between several factors should be taken into account to justify pharmacokinetic changes after TIPS placement.
Interestingly, some reports on non-antiretroviral drugs have shown development of drug-related toxicity after TIPS that has been related to an increased drug bioavailability as a consequence of the shunt bypassing the liver.10 Recently, dolutegravir use has been associated with the occurrence of neuropsychiatric adverse events11,12 and a possible association between these adverse events and dolutegravir concentration has been suggested.13 The increased exposure to dolutegravir might explain the transient neurological symptoms initially reported by our patient.
In conclusion, we described (for the first time, to the best of our knowledge) the pharmacokinetic profile of dolutegravir after TIPS placement, demonstrating an increase in drug exposure. Whether a sudden increase in dolutegravir concentration related to TIPS could be associated with the occurrence of neuropsychiatric symptoms remains to be determined. However, such an observation should be taken into account at the time of planning TIPS in HIV-infected patients treated with ART.
Funding
This study was carried out as part of our routine work.
Transparency declarations
M.F. has received speakers’ honoraria and support for travel to meetings from Bristol-Myers Squibb (BMS), Gilead, Janssen-Cilag, Merck Sharp & Dohme (MSD) and ViiV Healthcare, and fees for attending advisory boards from BMS and Gilead. D.C. has received speakers’ honoraria and support for travel to meetings from MSD, ViiV Healthcare, Janssen-Cilag, Angelini and Pfizer. All other authors: none to declare.



