-
PDF
- Split View
-
Views
-
Cite
Cite
Brendan J McMullan, Lisa Hall, Rodney James, Mona Mostaghim, Cheryl A Jones, Pamela Konecny, Christopher C Blyth, Karin A Thursky, on behalf of the National Antimicrobial Prescribing Survey (NAPS), Antibiotic appropriateness and guideline adherence in hospitalized children: results of a nationwide study, Journal of Antimicrobial Chemotherapy, Volume 75, Issue 3, March 2020, Pages 738–746, https://doi.org/10.1093/jac/dkz474
Close - Share Icon Share
Abstract
Information on the nature and appropriateness of antibiotic prescribing for children in hospitals is important, but scarce.
To analyse antimicrobial prescribing and appropriateness, and guideline adherence, in hospitalized children across Australia.
We analysed data from the National Antimicrobial Prescribing Survey (NAPS) from 2014 to 2017. Surveys were performed in hospital facilities of all types (public and private; major city, regional and remote). Participants were admitted children <18 years old. Risk factors associated with inappropriate prescribing were explored using logistic regression models.
Among 6219 prescriptions for 3715 children in 253 facilities, 19.6% of prescriptions were deemed inappropriate. Risk factors for inappropriate prescribing included non-tertiary paediatric hospital admission [OR 1.37 (95% CI 1.20–1.55)] and non-major city hospital location [OR 1.52 (95% CI 1.30–1.77)]. Prescriptions for neonates, immunocompromised children and those admitted to an ICU were less frequently inappropriate. If a restricted antimicrobial was prescribed and not approved, the prescription was more likely to be inappropriate [OR 12.9 (95% CI 8.4–19.8)]. Surgical prophylaxis was inappropriate in 59% of prescriptions.
Inappropriate antimicrobial prescribing in children was linked to specific risk factors identified here, presenting opportunities for targeted interventions to improve prescribing. This information, using a NAPS dataset, allows for analysis of antimicrobial prescribing among different groups of hospitalized children. Further exploration of barriers to appropriate prescribing and facilitators of best practice in this population is recommended.
Introduction
Antimicrobial resistance is a grave threat to human health and economic development.1 Antimicrobial stewardship (AMS) is recognized as a key intervention to improve prescribing and limit spread of resistance.2 Prescribing of antibiotics to children is common, and inappropriate or unnecessary prescription is frequently reported.3 Assessment of prescribing appropriateness has been described as a gold-standard metric of effectiveness of AMS programmes,4 but no nationwide standardized assessment of antibiotic prescribing in hospitalized children has been reported.
Resources to support AMS in Australia exist: all hospitals were mandated in 2012 to have an AMS programme for accreditation and specific recommendations were outlined by the Australian Commission on Safety and Quality in Health Care.5 Hospitals are required to provide prescribers access to appropriate guidelines for antimicrobial prescribing, specifically including access to the national Australian antibiotic guidelines, Therapeutic Guidelines.6 These guidelines are published independently from government, with regular updates carried out by an expert reviewer group. They include information for most infectious syndromes encountered in Australia. During the period covered by this study, however, these guidelines lacked recommendations for some infections in infants and children and included no antimicrobial prescribing recommendations for infections in neonates. For this reason, guidelines from tertiary children’s hospitals are often accessed by non-tertiary facilities and facilities may develop their own local guidelines for antimicrobial prescribing. Hospitals are also required to have ‘an antimicrobial formulary that includes restriction rules and approval processes’.7,8
Hospitals and other healthcare facilities in Australia have access to the National Antimicrobial Prescribing Survey (NAPS), a standardized online auditing programme developed in 2013 to assist in monitoring antimicrobial prescribing practices, facilitate quality improvement activities and meet national accreditation requirements.9,10 Aggregated (adult and paediatric) data have been reported in annual reports and publications.9–12 Areas for improvement identified in the most recent report included documentation of indication for antimicrobials and adherence to guidelines; particular note was made of inappropriate prescribing for surgical prophylaxis and pneumonia, inappropriate broad-spectrum antimicrobial use and prolonged duration of therapy.12 The majority of prescriptions included in these reports were in adults; no paediatric-specific analyses have been conducted to date. In this study we report on appropriateness of antimicrobial prescribing in hospitalized children in a range of healthcare settings, using a large national NAPS dataset.
Patients and methods
De-identified data were obtained from the NAPS database of point-prevalence prescribing surveys for Australian hospitals from all six Australian states and two territories (1 January 2014 to 31 December 2017).11 Participation in surveys is voluntary and data are submitted through a web-based interface to a central database.11 Contributing facilities encompass a spectrum from major city to regional and remote care centres. Facilities can choose to perform the survey at any time, but over two-thirds conduct a survey between October and November each year, to coincide with World Antibiotic Awareness Week. For this reason, we did not assess for seasonality of prescribing. Participation increased from 32 facilities (30 public and 2 private) in a paper-based pilot survey conducted in 2011 to 314 facilities (228 public and 86 private) in the 2017 web-based survey.12
Data are included in the NAPS for all inpatients with an antimicrobial prescription at 08:00 on the audit day or those who had received a single dose in the previous 24 h, including for surgical prophylaxis. As previously described, outpatients, day-stay and non-admitted emergency department patients were excluded.12 For this study, we included data from patients aged 0 days to less than 18 years at the time of survey. Children may have been prescribed more than one antimicrobial and the principal unit of analysis here is prescription. Aggregate data on appropriateness and guideline adherence in all patients, including adults and children from NAPS surveys conducted during the same time period, were also obtained for comparison with paediatric-specific data in this study.
The dataset includes the following: antimicrobial usage (agent, dose, frequency, route); baseline demographics [age, gender, documented antimicrobial allergies, admitting specialty, hospital location, funding type (public/private), hospital size]; infection history (infection site, infection type, documentation); whether surgical prophylaxis had been given for more than 24 h; adherence to local or national Australian antibiotic guidelines; and antimicrobial appropriateness.6,10,11 Local guidelines include any locally endorsed hospital, network or regional guidelines, other than the nationally used Therapeutic Guidelines,6 which inform antibiotic prescribing. Assessments are conducted by trained local surveyors (infectious diseases specialists and trainees, clinical microbiologists, pharmacists, nurses or infection control practitioners) according to a structured algorithm and appropriateness is classified as follows: 1 (optimal); 2 (adequate); 3 (suboptimal); 4 (inadequate); or 5 (not assessable), as shown in Figure 1.9,13 A score of 1 or 2 is considered to be ‘appropriate’ and 3 or 4 as ‘inappropriate’. Assessors can choose to select from various potential reasons for inappropriate prescribing, including ‘spectrum too broad’, ‘spectrum too narrow’, ‘incorrect route’, ‘incorrect dose or frequency’, ‘incorrect duration’, ‘allergy mismatch’ or ‘microbiology mismatch’. These are not mutually exclusive. In facilities without on-site expertise, appropriateness may be assessed remotely by a NAPS support team by prior arrangement. Local and remote assessors have been compared and agreement between antimicrobial assessment validated as sufficiently accurate.14 Dedicated children’s hospitals or combined maternity/children’s hospitals were classed as tertiary paediatric facilities. Hospital size and type were classified according to categories from the Australian Institute of Health and Welfare (AIHW).15 Year of survey was analysed by calendar year (2014–17). Antimicrobials were categorized in classes by type.
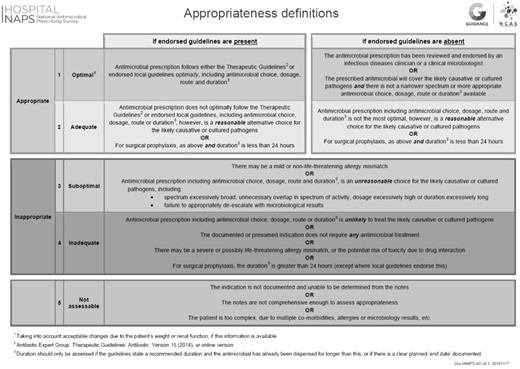
Ethics approval as a quality assurance project was obtained from the Melbourne Health Human Research Ethics Committee to coordinate the NAPS (no. QA2013066).
Statistical analysis
Categorical variables and proportions were summarized and compared between groups using a χ2 test. Test for trend over time was also calculated using a χ2 test. A P value of 0.05 (two-tailed) was deemed statistically significant. Explanatory variables for overall appropriateness were analysed using multivariable logistic regression with bootstrapping of regression coefficients. Factors included in the initial multivariable regression model were age category, sex, year of survey, tertiary paediatric site, major city site, ICU admission, immunocompromised status, restricted antimicrobial approval and indication documented. Statistical analyses were performed using Stata 12.1 (StataCorp, College Station, TX, USA).
Results
All Australian states and territories were represented (Figure 2). There were 96 901 antimicrobial prescriptions entered in hospital surveys from 2014 to 2017 and entered into the entire NAPS database for persons of all ages. Of these, 6219 (6.4%) were prescriptions for 3715 children under 18 years of age. These paediatric data were obtained from 253 facilities, ranging from tertiary paediatric hospitals (including 75% of dedicated paediatric facilities nationally) to large general principal referral hospitals caring for adults and children, and small remote facilities. Demographic factors are summarized in Table 1 and hospital peer-group classification in Table S1 (available as Supplementary data at JAC Online). The median number of antibiotic prescriptions per child was two and this did not vary by tertiary or metropolitan location.
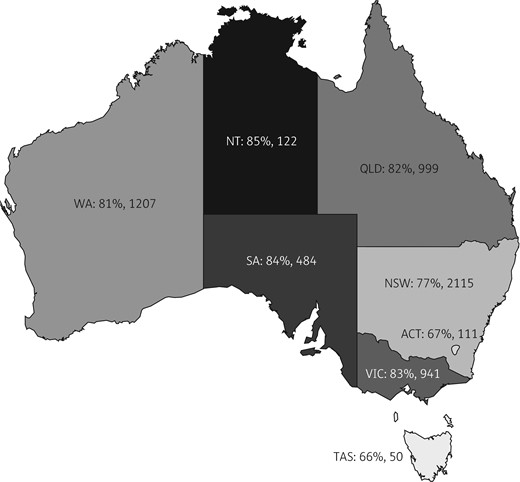
Percentage of prescriptions for children (<18 years old) in NAPS surveys 2014–17 assessed as appropriate by Australian state or territory. Figures are expressed by state/territory (ACT, Australian Capital Territory; NSW, New South Wales; NT, Northern Territory; QLD, Queensland; SA, South Australia; TAS, Tasmania; VIC, Victoria; WA, Western Australia): percentage appropriate, total number of prescriptions. Total (n=6029) excludes those for whom appropriateness was not assessable. If NSW appropriateness (77%) is taken as the reference, NT, SA, VIC and WA had significantly higher appropriateness and ACT had significantly lower appropriateness overall.
| Demographic . | Number of prescriptions (%), total=6219 . |
|---|---|
| Sex: male | 3416 (54.9) |
| Age | |
| neonates (<28 days) | 760 (12.2) |
| infants (28 days to <1 year) | 1108 (17.8) |
| toddlers (1 year to <3 years) | 854 (13.7) |
| children (3 years to <12 years) | 1901 (30.6) |
| adolescents (12 years to <18 years) | 1596 (25.7) |
| Hospital type | |
| specialist children’s | 2706 (43.5) |
| specialist women’s and children’s | 401 (6.5) |
| other public facilities | 2779 (44.7) |
| private hospital | 333 (5.4) |
| Hospital location | |
| major city | 5001 (80.4) |
| inner regional | 583 (9.4) |
| outer regional | 338 (5.4) |
| remote/very remote | 295 (4.7) |
| unknown | 2 (0.03) |
| Other factors | |
| immunocompromised | 699 (11.2) |
| allergic to any antimicrobial | 599 (9.6) |
| admitted to an ICU/neonatal ICU | 810 (13) |
| Demographic . | Number of prescriptions (%), total=6219 . |
|---|---|
| Sex: male | 3416 (54.9) |
| Age | |
| neonates (<28 days) | 760 (12.2) |
| infants (28 days to <1 year) | 1108 (17.8) |
| toddlers (1 year to <3 years) | 854 (13.7) |
| children (3 years to <12 years) | 1901 (30.6) |
| adolescents (12 years to <18 years) | 1596 (25.7) |
| Hospital type | |
| specialist children’s | 2706 (43.5) |
| specialist women’s and children’s | 401 (6.5) |
| other public facilities | 2779 (44.7) |
| private hospital | 333 (5.4) |
| Hospital location | |
| major city | 5001 (80.4) |
| inner regional | 583 (9.4) |
| outer regional | 338 (5.4) |
| remote/very remote | 295 (4.7) |
| unknown | 2 (0.03) |
| Other factors | |
| immunocompromised | 699 (11.2) |
| allergic to any antimicrobial | 599 (9.6) |
| admitted to an ICU/neonatal ICU | 810 (13) |
| Demographic . | Number of prescriptions (%), total=6219 . |
|---|---|
| Sex: male | 3416 (54.9) |
| Age | |
| neonates (<28 days) | 760 (12.2) |
| infants (28 days to <1 year) | 1108 (17.8) |
| toddlers (1 year to <3 years) | 854 (13.7) |
| children (3 years to <12 years) | 1901 (30.6) |
| adolescents (12 years to <18 years) | 1596 (25.7) |
| Hospital type | |
| specialist children’s | 2706 (43.5) |
| specialist women’s and children’s | 401 (6.5) |
| other public facilities | 2779 (44.7) |
| private hospital | 333 (5.4) |
| Hospital location | |
| major city | 5001 (80.4) |
| inner regional | 583 (9.4) |
| outer regional | 338 (5.4) |
| remote/very remote | 295 (4.7) |
| unknown | 2 (0.03) |
| Other factors | |
| immunocompromised | 699 (11.2) |
| allergic to any antimicrobial | 599 (9.6) |
| admitted to an ICU/neonatal ICU | 810 (13) |
| Demographic . | Number of prescriptions (%), total=6219 . |
|---|---|
| Sex: male | 3416 (54.9) |
| Age | |
| neonates (<28 days) | 760 (12.2) |
| infants (28 days to <1 year) | 1108 (17.8) |
| toddlers (1 year to <3 years) | 854 (13.7) |
| children (3 years to <12 years) | 1901 (30.6) |
| adolescents (12 years to <18 years) | 1596 (25.7) |
| Hospital type | |
| specialist children’s | 2706 (43.5) |
| specialist women’s and children’s | 401 (6.5) |
| other public facilities | 2779 (44.7) |
| private hospital | 333 (5.4) |
| Hospital location | |
| major city | 5001 (80.4) |
| inner regional | 583 (9.4) |
| outer regional | 338 (5.4) |
| remote/very remote | 295 (4.7) |
| unknown | 2 (0.03) |
| Other factors | |
| immunocompromised | 699 (11.2) |
| allergic to any antimicrobial | 599 (9.6) |
| admitted to an ICU/neonatal ICU | 810 (13) |
Appropriateness and guideline adherence are compared with figures from aggregate NAPS data from the same time period (>92% of which is from adults over 18 years old) in Table 2. Of the paediatric prescriptions surveyed, 19.6% were assessed as inappropriate (compared with 22.6% overall, P<0.0001) and 3.1% of prescriptions were recorded as not assessable. Guideline adherence was variable: 28.8% of prescriptions were adherent to national Therapeutic Guidelines6 and 32.7% adherent to local guidelines. For 5.3% of assessments, no guidelines were available. Directed therapy (therapy based on microbiology results) was reported for 10% of prescriptions. Prescribing was more likely to be deemed appropriate for tertiary paediatric facilities compared with other facilities [OR 1.37 (95% CI 1.20–1.55)] and for major city facilities compared with regional or remote facilities [OR 1.52 (95% CI 1.30–1.77)]. Appropriateness also varied by state/territory (P<0.0001; Figure 2). For 35 facilities that entered data for all four survey years, appropriateness increased from 82.2% in 2014 to 85.3% in 2017, but without a statistically significant trend (P=0.13; Table S2). The top 10 antimicrobials prescribed varied between tertiary and non-tertiary facilities (Table S3).
| . | Frequency (total=6219) . | Percentage . | Overall NAPS percentagea . |
|---|---|---|---|
| Appropriateness | |||
| appropriate | 4810 | 77.3 | 72.3 |
| inappropriate | 1219 | 19.6 | 22.6 |
| not assessable | 190 | 3.1 | 5.1 |
| Guideline adherenceb | |||
| adherent to Therapeutic Guidelines | 1793 | 28.8 | 43.7 |
| adherent to local guidelines | 2035 | 32.7 | 10.4 |
| directed therapy | 622 | 10 | 11.9 |
| no guidelines available | 329 | 5.3 | 4.1 |
| non-adherent | 1267 | 20.4 | 25.3 |
| not assessable | 173 | 2.8 | 4.5 |
| . | Frequency (total=6219) . | Percentage . | Overall NAPS percentagea . |
|---|---|---|---|
| Appropriateness | |||
| appropriate | 4810 | 77.3 | 72.3 |
| inappropriate | 1219 | 19.6 | 22.6 |
| not assessable | 190 | 3.1 | 5.1 |
| Guideline adherenceb | |||
| adherent to Therapeutic Guidelines | 1793 | 28.8 | 43.7 |
| adherent to local guidelines | 2035 | 32.7 | 10.4 |
| directed therapy | 622 | 10 | 11.9 |
| no guidelines available | 329 | 5.3 | 4.1 |
| non-adherent | 1267 | 20.4 | 25.3 |
| not assessable | 173 | 2.8 | 4.5 |
Comparison with overall NAPS data (aggregated adult and paediatric data) from hospital surveys collected during 2014–17.
These categories are mutually exclusive. Assessors were instructed to choose ‘compliant with Therapeutic Guidelines’, which are the national guidelines, if prescribing was adherent to these and if it was not, but was adherent to local guidelines, then to choose ‘compliant with local guidelines’.
| . | Frequency (total=6219) . | Percentage . | Overall NAPS percentagea . |
|---|---|---|---|
| Appropriateness | |||
| appropriate | 4810 | 77.3 | 72.3 |
| inappropriate | 1219 | 19.6 | 22.6 |
| not assessable | 190 | 3.1 | 5.1 |
| Guideline adherenceb | |||
| adherent to Therapeutic Guidelines | 1793 | 28.8 | 43.7 |
| adherent to local guidelines | 2035 | 32.7 | 10.4 |
| directed therapy | 622 | 10 | 11.9 |
| no guidelines available | 329 | 5.3 | 4.1 |
| non-adherent | 1267 | 20.4 | 25.3 |
| not assessable | 173 | 2.8 | 4.5 |
| . | Frequency (total=6219) . | Percentage . | Overall NAPS percentagea . |
|---|---|---|---|
| Appropriateness | |||
| appropriate | 4810 | 77.3 | 72.3 |
| inappropriate | 1219 | 19.6 | 22.6 |
| not assessable | 190 | 3.1 | 5.1 |
| Guideline adherenceb | |||
| adherent to Therapeutic Guidelines | 1793 | 28.8 | 43.7 |
| adherent to local guidelines | 2035 | 32.7 | 10.4 |
| directed therapy | 622 | 10 | 11.9 |
| no guidelines available | 329 | 5.3 | 4.1 |
| non-adherent | 1267 | 20.4 | 25.3 |
| not assessable | 173 | 2.8 | 4.5 |
Comparison with overall NAPS data (aggregated adult and paediatric data) from hospital surveys collected during 2014–17.
These categories are mutually exclusive. Assessors were instructed to choose ‘compliant with Therapeutic Guidelines’, which are the national guidelines, if prescribing was adherent to these and if it was not, but was adherent to local guidelines, then to choose ‘compliant with local guidelines’.
An indication for antimicrobials was documented in 79% (4903/6219) of prescriptions and these were diverse: the most frequent indications included empirical therapy for sepsis (586, 9.4% of prescriptions) and prophylaxis of fungal infection (571, 9.2% of prescriptions, mainly in immunocompromised children and neonates), as shown in Table 3. Agents used most commonly for fungal prophylaxis were oral nystatin, followed by trimethoprim/sulfamethoxazole and then azole antifungal agents. These were generally assessed as appropriate. In contrast, prescriptions for surgical prophylaxis, also among the top five indications, were assessed as appropriate in 41% of prescriptions. The most frequent reasons noted for inappropriate prescribing with regard to surgical prophylaxis included ‘incorrect duration’ (generally indicating prolonged use) in 21% (103/498) of assessments and ‘spectrum too broad’ in 8% (38/459). For another common indication, community-acquired pneumonia (CAP), with overall appropriateness in 75%, ‘spectrum too broad’ was noted in 19% (73/378). Appropriateness also varied substantially by antimicrobial class, from >95% appropriate for antivirals to 60% appropriate for carbapenems (Table 3). Carbapenems were prescribed in 172 instances: 170/172 (99%) were for meropenem, representing 2.7% of all prescriptions in the survey. In 65/170 (38%) meropenem prescriptions, prescribing was assessed as inappropriate due to ‘spectrum too broad’.
| . | Appropriate . | Inappropriate . | Total . | Percentage appropriate . |
|---|---|---|---|---|
| Antibiotic class | ||||
| first-generation cephalosporin | 342 | 244 | 586 | 58 |
| carbapenem | 103 | 69 | 172 | 60 |
| macrolide | 161 | 72 | 233 | 69 |
| nitroimidazole | 171 | 69 | 240 | 71 |
| fluoroquinolone | 80 | 27 | 107 | 75 |
| third-generation cephalosporin | 494 | 137 | 631 | 78 |
| penicillin | 1368 | 375 | 1743 | 78 |
| lincosamide | 90 | 21 | 111 | 81 |
| other agentsa | 167 | 37 | 204 | 82 |
| glycopeptide | 164 | 26 | 190 | 86 |
| aminoglycoside | 470 | 65 | 535 | 88 |
| trimethoprim/cotrimoxazole | 315 | 24 | 339 | 93 |
| antifungal | 695 | 45 | 740 | 94 |
| antiviral | 134 | 6 | 140 | 96 |
| fourth-generation cephalosporin | 28 | 1 | 29 | 97 |
| tetracycline | 28 | 1 | 29 | 97 |
| total | 4810 | 1219 | 6029 | 80 |
| Top five indications | ||||
| surgical prophylaxis | 261 | 368 | 629 | 41 |
| CAP | 362 | 119 | 481 | 75 |
| cystic fibrosis: infective exacerbation | 235 | 25 | 260 | 90 |
| sepsis: empirical therapy | 586 | 48 | 634 | 92 |
| medical prophylaxis: fungal | 571 | 18 | 589 | 97 |
| total | 2015 | 578 | 2593 | 78 |
| . | Appropriate . | Inappropriate . | Total . | Percentage appropriate . |
|---|---|---|---|---|
| Antibiotic class | ||||
| first-generation cephalosporin | 342 | 244 | 586 | 58 |
| carbapenem | 103 | 69 | 172 | 60 |
| macrolide | 161 | 72 | 233 | 69 |
| nitroimidazole | 171 | 69 | 240 | 71 |
| fluoroquinolone | 80 | 27 | 107 | 75 |
| third-generation cephalosporin | 494 | 137 | 631 | 78 |
| penicillin | 1368 | 375 | 1743 | 78 |
| lincosamide | 90 | 21 | 111 | 81 |
| other agentsa | 167 | 37 | 204 | 82 |
| glycopeptide | 164 | 26 | 190 | 86 |
| aminoglycoside | 470 | 65 | 535 | 88 |
| trimethoprim/cotrimoxazole | 315 | 24 | 339 | 93 |
| antifungal | 695 | 45 | 740 | 94 |
| antiviral | 134 | 6 | 140 | 96 |
| fourth-generation cephalosporin | 28 | 1 | 29 | 97 |
| tetracycline | 28 | 1 | 29 | 97 |
| total | 4810 | 1219 | 6029 | 80 |
| Top five indications | ||||
| surgical prophylaxis | 261 | 368 | 629 | 41 |
| CAP | 362 | 119 | 481 | 75 |
| cystic fibrosis: infective exacerbation | 235 | 25 | 260 | 90 |
| sepsis: empirical therapy | 586 | 48 | 634 | 92 |
| medical prophylaxis: fungal | 571 | 18 | 589 | 97 |
| total | 2015 | 578 | 2593 | 78 |
Results from classes with <20 prescriptions not listed individually.
| . | Appropriate . | Inappropriate . | Total . | Percentage appropriate . |
|---|---|---|---|---|
| Antibiotic class | ||||
| first-generation cephalosporin | 342 | 244 | 586 | 58 |
| carbapenem | 103 | 69 | 172 | 60 |
| macrolide | 161 | 72 | 233 | 69 |
| nitroimidazole | 171 | 69 | 240 | 71 |
| fluoroquinolone | 80 | 27 | 107 | 75 |
| third-generation cephalosporin | 494 | 137 | 631 | 78 |
| penicillin | 1368 | 375 | 1743 | 78 |
| lincosamide | 90 | 21 | 111 | 81 |
| other agentsa | 167 | 37 | 204 | 82 |
| glycopeptide | 164 | 26 | 190 | 86 |
| aminoglycoside | 470 | 65 | 535 | 88 |
| trimethoprim/cotrimoxazole | 315 | 24 | 339 | 93 |
| antifungal | 695 | 45 | 740 | 94 |
| antiviral | 134 | 6 | 140 | 96 |
| fourth-generation cephalosporin | 28 | 1 | 29 | 97 |
| tetracycline | 28 | 1 | 29 | 97 |
| total | 4810 | 1219 | 6029 | 80 |
| Top five indications | ||||
| surgical prophylaxis | 261 | 368 | 629 | 41 |
| CAP | 362 | 119 | 481 | 75 |
| cystic fibrosis: infective exacerbation | 235 | 25 | 260 | 90 |
| sepsis: empirical therapy | 586 | 48 | 634 | 92 |
| medical prophylaxis: fungal | 571 | 18 | 589 | 97 |
| total | 2015 | 578 | 2593 | 78 |
| . | Appropriate . | Inappropriate . | Total . | Percentage appropriate . |
|---|---|---|---|---|
| Antibiotic class | ||||
| first-generation cephalosporin | 342 | 244 | 586 | 58 |
| carbapenem | 103 | 69 | 172 | 60 |
| macrolide | 161 | 72 | 233 | 69 |
| nitroimidazole | 171 | 69 | 240 | 71 |
| fluoroquinolone | 80 | 27 | 107 | 75 |
| third-generation cephalosporin | 494 | 137 | 631 | 78 |
| penicillin | 1368 | 375 | 1743 | 78 |
| lincosamide | 90 | 21 | 111 | 81 |
| other agentsa | 167 | 37 | 204 | 82 |
| glycopeptide | 164 | 26 | 190 | 86 |
| aminoglycoside | 470 | 65 | 535 | 88 |
| trimethoprim/cotrimoxazole | 315 | 24 | 339 | 93 |
| antifungal | 695 | 45 | 740 | 94 |
| antiviral | 134 | 6 | 140 | 96 |
| fourth-generation cephalosporin | 28 | 1 | 29 | 97 |
| tetracycline | 28 | 1 | 29 | 97 |
| total | 4810 | 1219 | 6029 | 80 |
| Top five indications | ||||
| surgical prophylaxis | 261 | 368 | 629 | 41 |
| CAP | 362 | 119 | 481 | 75 |
| cystic fibrosis: infective exacerbation | 235 | 25 | 260 | 90 |
| sepsis: empirical therapy | 586 | 48 | 634 | 92 |
| medical prophylaxis: fungal | 571 | 18 | 589 | 97 |
| total | 2015 | 578 | 2593 | 78 |
Results from classes with <20 prescriptions not listed individually.
The most frequently inappropriately prescribed antimicrobials were cefazolin (159 cases, 13%), amoxicillin (107 cases, 9%) and ceftriaxone (83 cases, 7%). Other frequently inappropriately prescribed antimicrobials are shown in Table S4. A prescription was given where an antimicrobial was not indicated in 298 cases (6%). Reasons for inappropriate prescribing varied, with the most frequent reasons being ‘incorrect dose or frequency’ or ‘spectrum too broad’ (these are not mutually exclusive and are shown in Table 4). Appropriateness also varied by admitting specialty (Table S5) with surgical specialties less likely to have appropriate prescriptions, e.g. only 36% (92/255) of Ear, Nose and Throat (ENT) surgery prescriptions were assessed as appropriate. Appropriateness also varied by age and was highest in neonates at 95% and lowest, at 74.6%, in children aged 3 to <12 years (Table S6).
| Category . | Number . | Evaluable populationa . | Percentage . |
|---|---|---|---|
| Incorrect dose or frequency | 458 | 5172 | 8.9 |
| Spectrum too broad | 423 | 5066 | 8.4 |
| Surgical prophylaxis given for >24 h | 309 | 6219 | 5 |
| Incorrect duration | 243 | 5123 | 4.7 |
| Spectrum too narrow | 106 | 5010 | 2.1 |
| Microbiology mismatch | 94 | 6219 | 1.5 |
| Incorrect route | 69 | 5111 | 1.4 |
| Allergy mismatch | 12 | 6219 | 0.2 |
| Category . | Number . | Evaluable populationa . | Percentage . |
|---|---|---|---|
| Incorrect dose or frequency | 458 | 5172 | 8.9 |
| Spectrum too broad | 423 | 5066 | 8.4 |
| Surgical prophylaxis given for >24 h | 309 | 6219 | 5 |
| Incorrect duration | 243 | 5123 | 4.7 |
| Spectrum too narrow | 106 | 5010 | 2.1 |
| Microbiology mismatch | 94 | 6219 | 1.5 |
| Incorrect route | 69 | 5111 | 1.4 |
| Allergy mismatch | 12 | 6219 | 0.2 |
This includes those for whom a result was entered: ‘incorrect route’, ‘incorrect dose or frequency’, ‘incorrect duration’, ‘spectrum too broad’ and ‘spectrum too narrow’. These are non-compulsory fields.
| Category . | Number . | Evaluable populationa . | Percentage . |
|---|---|---|---|
| Incorrect dose or frequency | 458 | 5172 | 8.9 |
| Spectrum too broad | 423 | 5066 | 8.4 |
| Surgical prophylaxis given for >24 h | 309 | 6219 | 5 |
| Incorrect duration | 243 | 5123 | 4.7 |
| Spectrum too narrow | 106 | 5010 | 2.1 |
| Microbiology mismatch | 94 | 6219 | 1.5 |
| Incorrect route | 69 | 5111 | 1.4 |
| Allergy mismatch | 12 | 6219 | 0.2 |
| Category . | Number . | Evaluable populationa . | Percentage . |
|---|---|---|---|
| Incorrect dose or frequency | 458 | 5172 | 8.9 |
| Spectrum too broad | 423 | 5066 | 8.4 |
| Surgical prophylaxis given for >24 h | 309 | 6219 | 5 |
| Incorrect duration | 243 | 5123 | 4.7 |
| Spectrum too narrow | 106 | 5010 | 2.1 |
| Microbiology mismatch | 94 | 6219 | 1.5 |
| Incorrect route | 69 | 5111 | 1.4 |
| Allergy mismatch | 12 | 6219 | 0.2 |
This includes those for whom a result was entered: ‘incorrect route’, ‘incorrect dose or frequency’, ‘incorrect duration’, ‘spectrum too broad’ and ‘spectrum too narrow’. These are non-compulsory fields.
If an antimicrobial was restricted at the site assessed, assessors were asked whether approval was recorded as given. Approval was recorded as given in 625 cases (12%), not given in 640 cases (13%) and ‘not applicable’ (that is, not required) in 3829 cases (75%), with a further 1125 missing values for this field. Antimicrobial classes listed by sites audited as ‘not applicable’ for restriction at their site (and thus not subject to approval at that site) are shown in Table S7.
Multivariable regression was performed for risk factors for inappropriate therapy. Sex and year of survey were not significantly associated with appropriateness and were removed from the final analysis. Major city site was collinear with tertiary paediatric hospital and was also removed. On final multivariable logistic regression, older age category was significantly associated with inappropriate therapy [all age groups compared with neonates, but highest for those 3 to <12 years: OR 6.2 (95% CI 4.3–8.8)]. Conversely, prescriptions for children with indication documented, ICU admission, immunocompromised status or admission at a tertiary paediatric hospital were more likely to be appropriate (Table 5). If approval was given for a restricted antimicrobial, the prescription was significantly more likely to be appropriate [OR 12.9 (95% CI 8.4–19.8)]. This parameter was assessed by univariate regression for prescriptions where antimicrobials were recorded as restricted (n=1265).
| Category . | ORa . | 95% CI . |
|---|---|---|
| Age | ||
| neonates (<28 days) | 1.0 | reference |
| infants (28 days to <1 year) | 3.6 | 2.5–5.1 |
| toddlers (1 year to <3 years) | 5.6 | 3.8–8.1 |
| children (3 years to <12 years) | 6.2 | 4.3–8.8 |
| adolescents (12 years to <18 years) | 5.2 | 3.6–7.5 |
| Indication documented | 0.4 | 0.4–0.5 |
| Tertiary site | 0.7 | 0.6–0.9 |
| Admitted to an ICU/neonatal ICU | 0.7 | 0.5–0.9 |
| Immunocompromised | 0.5 | 0.4–0.6 |
| Category . | ORa . | 95% CI . |
|---|---|---|
| Age | ||
| neonates (<28 days) | 1.0 | reference |
| infants (28 days to <1 year) | 3.6 | 2.5–5.1 |
| toddlers (1 year to <3 years) | 5.6 | 3.8–8.1 |
| children (3 years to <12 years) | 6.2 | 4.3–8.8 |
| adolescents (12 years to <18 years) | 5.2 | 3.6–7.5 |
| Indication documented | 0.4 | 0.4–0.5 |
| Tertiary site | 0.7 | 0.6–0.9 |
| Admitted to an ICU/neonatal ICU | 0.7 | 0.5–0.9 |
| Immunocompromised | 0.5 | 0.4–0.6 |
Multivariable logistic regression with bootstrapping.
| Category . | ORa . | 95% CI . |
|---|---|---|
| Age | ||
| neonates (<28 days) | 1.0 | reference |
| infants (28 days to <1 year) | 3.6 | 2.5–5.1 |
| toddlers (1 year to <3 years) | 5.6 | 3.8–8.1 |
| children (3 years to <12 years) | 6.2 | 4.3–8.8 |
| adolescents (12 years to <18 years) | 5.2 | 3.6–7.5 |
| Indication documented | 0.4 | 0.4–0.5 |
| Tertiary site | 0.7 | 0.6–0.9 |
| Admitted to an ICU/neonatal ICU | 0.7 | 0.5–0.9 |
| Immunocompromised | 0.5 | 0.4–0.6 |
| Category . | ORa . | 95% CI . |
|---|---|---|
| Age | ||
| neonates (<28 days) | 1.0 | reference |
| infants (28 days to <1 year) | 3.6 | 2.5–5.1 |
| toddlers (1 year to <3 years) | 5.6 | 3.8–8.1 |
| children (3 years to <12 years) | 6.2 | 4.3–8.8 |
| adolescents (12 years to <18 years) | 5.2 | 3.6–7.5 |
| Indication documented | 0.4 | 0.4–0.5 |
| Tertiary site | 0.7 | 0.6–0.9 |
| Admitted to an ICU/neonatal ICU | 0.7 | 0.5–0.9 |
| Immunocompromised | 0.5 | 0.4–0.6 |
Multivariable logistic regression with bootstrapping.
Discussion
This nationwide study of over 6000 prescriptions for almost 4000 children from 253 facilities is unique, to our knowledge, in reporting an assessment of appropriateness and guideline adherence of antimicrobial prescribing in a wide range of hospitalized paediatric patients using this standardized methodology. It provides a rich dataset for further research and quality improvement. The assessment was undertaken across a range of healthcare settings in all national jurisdictions. Overall inappropriate prescribing accounted for almost 20% of prescriptions and risk factors for inappropriate prescribing included older age, non-tertiary and non-metropolitan location. We also found a high proportion of inappropriate prescribing for surgical patients, especially with regard to surgical antimicrobial prophylaxis.
Standardized assessment of paediatric antimicrobial prescribing has been limited to date, with few multicentre studies using standardized methodology. Recently, the European ARPEC (Antimicrobial Resistance, Prescribing and Efficacy in Neonates and Children) and GARPEC (Global ARPEC) projects have promoted collaboration to analyse data on antimicrobial prescriptions among neonates and children worldwide. This has led to several publications, though most have focused on drug usage, rather than assessments of individual appropriateness by prescription.16–24 A multicentre paediatric AMS ‘SHARPS’ collaboration has been reported in the USA, with sharing of resources and quality improvement methodology, but this group has not reported assessment of prescribing appropriateness across facilities at the time of publication.4 Appropriateness of paediatric prescribing was assessed in a survey of eight Australian specialist paediatric facilities in 2012 by Osowicki et al.,25 who found a similar level of inappropriate prescribing to that in our study (18.5% versus our 19.6%). As in our study, inappropriate prescribing for surgical prophylaxis was identified as an area for improvement. In contrast, however, our study includes prescriptions from diverse facility types, including not only specialist tertiary facilities, but non-tertiary, regional, remote and very remote facilities, over several years, thus our results may be more generalizable across settings.
Inappropriate prescribing in children was somewhat less common compared with aggregate hospital NAPS data (19.6% versus 22.6%), which are dominated by prescriptions for adult patients, and guideline-non-adherent prescribing was also less common in our dataset (20.4% versus 25.3%). Prescribing was also more likely to be appropriate in major cities and in tertiary paediatric facilities. These suggest that paediatric prescribing may differ systematically from adult prescribing and that a lack of resources or targeted education may contribute to inappropriate paediatric prescribing in smaller or non-paediatric centres.
Prescribing for surgical prophylaxis was inappropriate in 59% of prescriptions, worse than the 42.6% reported in the aggregate NAPS data from 2016,11 and again emphasizing an area of unmet need in practice. International guidelines recommend most surgical procedures require only a single dose of prophylaxis and no post-operative doses,26,27 though surgical prophylaxis is commonly continued post-operatively, as was found in our study. Prescribing was frequently inappropriate for patients admitted by several surgical specialties, especially by ENT surgeons. This was also observed in an Australian 2012 study by Osowicki et al.25 and suggests a need for further research and quality-improvement work directed to engagement with representatives from these specialties to support optimal prescribing. The Royal Australasian College of Surgeons (RACS) has formally recognized a problem with antimicrobial misuse in surgical prophylaxis and established a committee to assist in efforts to improve this: the Prevention of Healthcare-Associated Infection Control Committee. In a letter sent in 2017, the RACS advocated for specific training for surgeons in antibiotic prescribing.28 This study provides further evidence that a systematic approach with all stakeholders is required to achieve meaningful improvements in surgical antimicrobial prescribing.
Prescribing was inappropriate in 25% of prescriptions for CAP in our study. Although guidelines exist to support prescribing for this condition,29 actual practice varies substantially, suggesting a need for further education and review of barriers and facilitators to support best-practice prescribing for this common childhood infection.
In our regression analysis, appropriateness was higher in more specialized populations: immunocompromised children, those admitted to an ICU and neonates, despite these groups potentially requiring more complex care. This may reflect the fact that resources (guidelines, protocols, human resources and training) may be more abundant in these settings or potentially that assessors were less willing to label prescribing ‘inappropriate’ in these populations, perhaps reflecting uncertainty in auditing paediatric or specialized patients. While remote and varied local assessors using NAPS tools have been shown to agree moderately well on guideline-adherence assessment, there was lower agreement on assessment of appropriateness, at least with the training available to assessors in 2013.14 Since this time, assessors must undergo an online training process to standardize assessment of appropriateness and guideline adherence. This assessment is unlikely to ever achieve a ‘perfect’ standard of agreement for assessment of appropriate prescribing and we acknowledge this as a potential limitation of the assessment methodology. Appropriateness, however, was assessed in this study as higher in tertiary settings, where expert assessors are more readily available, supporting a hypothesis that more resources may genuinely result in more appropriate prescribing. In contrast, older children and adolescents, as well as non-tertiary and non-major city facilities had less appropriate prescribing. We also noted antibiotics listed by some assessors as ‘not applicable’ for restriction (Table S7) that we believe generally ought to be restricted, such as carbapenems and fluoroquinolones. These, taken together, suggest areas for improvement for these populations, requiring collaboration between health services, tertiary and non-tertiary facilities and government organizations.
Compared with overall NAPS data (Table 2), there was less use of the national resource Therapeutic Guidelines6 and more use of local guidelines for children. This may reflect fewer paediatric-specific recommendations in the national guidelines, as discussed above. While local guidelines may be appropriate and evidence-based, a greater variation in prescribing generates greater potential for local, historical-based rather than evidence-based practice and potential for harm when prescribers or patients move from one facility to another with different recommendations. In addition, frequent use of local guidelines has implications for governance of AMS programmes, especially outside tertiary centres, and may create additional demands on resources to support paediatric AMS, where these resources are already constrained.
Formulary restriction and approval processes for antimicrobials are requirements for hospital accreditation in Australia.8 Facilities have diverse restriction models, with variation in antimicrobials restricted, prescriber and patient groups affected and structural processes. In some Australian systems, restricted antimicrobials may be prescribed for a limited time without approval and are then ‘flagged’ for review by AMS teams. This is in contrast to ‘zero tolerance’ systems where it is impossible to dispense unapproved medications. More permissive systems may tolerate short-term access to unapproved antimicrobials where potential delays are anticipated or there is lack of access to timely expert advice, e.g. after hours. These systems might facilitate some inappropriate prescribing due to inadvertent or deliberate failure by prescribers to follow system processes and guidelines. Detailed analysis of subverted antibiotic restriction processes and behaviours is warranted, but is beyond the scope of this study. Here we have demonstrated that restricted antimicrobials that have been approved within AMS systems are significantly more likely to be appropriately prescribed. This supports the value of restriction and approval processes and demonstrates a potential limitation of permissive systems. Further work to adapt and improve adherence to approval processes is needed, including provision of adequate resources.
This study has several limitations: the point-prevalence survey methodology generates cross-sectional rather than longitudinal data, though we reviewed results of repeated surveys in multiple facilities over time and demonstrated improvement in this regard. Secondly, participation in surveys is voluntary and thus does not represent a comprehensive survey of prescribing in all Australian children in hospital. Tertiary facilities were somewhat over-represented in proportion to population, though our results suggest these surveys capture a large majority of facilities that admit children. The study is limited to admitted patients and does not capture data on emergency or day-stay patients, community or primary care, the last of which constitutes the bulk of antibiotic prescribing. According to the Australian Institute of Health and Welfare, children and young people (aged less than 20 years) contributed 7.4% of hospital patient days during 2017–18.30 Children, however, have a high rate of antibiotic prescriptions when admitted to hospital, but account for only 6.4% of prescriptions in NAPS surveys. Some children admitted to general hospitals may have been excluded from surveys if auditors felt ill-equipped to assess prescribing for them. This would be supported by the fact that tertiary facilities, which contributed over 50% of prescription data to this dataset, admit a minority of child and neonatal hospital admissions in Australia overall, though of higher complexity. Generally, these facilities have specific AMS resources for children that may be lacking in other facilities. Metropolitan hospitals are likely to have been relatively oversampled due to availability of assessors. In addition, children with more complex care needs (many of whom require antibiotics) are frequently transferred from regional or remote to metropolitan centres. It may be that children are not being included in NAPS assessments in some facilities, due to lack of auditors assessing paediatric prescribing. These children are potentially being exempted from AMS surveillance entirely.
This survey has implications for policy and practice change: almost 20% inappropriate prescribing for hospitalized children demonstrates room for improvement and this figure is higher in non-tertiary and non-major city settings, where fewer resources to support prescribing are available. Healthcare facilities need to work in partnership with governments and other organizations to support appropriate prescribing for children in all environments. Resources should be made available at multiple levels: at a national level, serious consideration should be given to how to support paediatric AMS governance and programmes, especially outside tertiary facilities, e.g. provision of enhanced paediatric content in guidelines, surveillance and audit tools, with consultation and training to support implementation in non-tertiary facilities. Strategies to improve antimicrobial prescribing for children should be reflected in the National Antimicrobial Resistance Strategy31 and Implementation Plan.32 At a local level, hospitals need to be resourced to provide AMS for children and should be required to do so by hospital accreditors. At an individual level, prescribers, pharmacists, nurses and other healthcare professionals require education and training to support improvement. Prescriber culture and etiquette also needs to be addressed in quality-improvement efforts.33 Consumers should also be made aware of the gaps in prescribing for children and be engaged in process improvements in the future.
Conclusions
In this study we have identified gaps in appropriateness of prescribing for children and areas for improvement with implications for national policy change. The NAPS programme provides a valuable dataset for analysing antimicrobial prescribing in hospitalized children, allowing for further exploration of barriers to and facilitators of best-practice prescribing in more specific patient groups in the future.
Acknowledgements
We acknowledge the support of the Australian Commission for Safety and Quality in Health Care, the NAPS staff and programme and NAPS assessors and participating facilities.
Funding
This study was carried out as part of B.J.M.’s PhD. B.J.M. is supported by a PhD scholarship from the University of Melbourne. Since 2013 the Australian Commission on Safety and Quality in Health Care has supported the National Antimicrobial Prescribing Survey (NAPS) program for the Antimicrobial Use and Resistance in Australia (AURA) Surveillance System.
Transparency declarations
None to declare.
References
Australian Commission for Safety and Quality in Healthcare.
Australian Commission for Safety and Quality in Healthcare.
National Centre for Antimicrobial Stewardship and Australian Commission on Safety and Quality in Health Care.
National Centre for Antimicrobial Stewardship and Australian Commission on Safety and Quality in Health Care.
National Centre for Antimicrobial Stewardship and Australian Commission on Safety and Quality in Health Care.
Australian Institute of Health and Welfare.
Royal Australasian College of Surgeons.
Australian Institute of Health and Welfare.
Australian Government Department of Health.
Australian Government Department of Health.



