-
PDF
- Split View
-
Views
-
Cite
Cite
Jason Kim, Firdose Lambey Nakwa, Fábio Araujo Motta, Hong Liu, Mary Beth Dorr, Leah J Anderson, Nicholas Kartsonis, A randomized, double-blind trial investigating the efficacy of caspofungin versus amphotericin B deoxycholate in the treatment of invasive candidiasis in neonates and infants younger than 3 months of age, Journal of Antimicrobial Chemotherapy, Volume 75, Issue 1, January 2020, Pages 215–220, https://doi.org/10.1093/jac/dkz398
Close - Share Icon Share
Abstract
Investigate the efficacy of caspofungin in participants <3 months of age with invasive Candida infection (ICI).
This multicentre, randomized, double-blind, comparator-controlled, Phase 2 study (protocol MK0991-064; NCT01945281) enrolled participants <3 months of age with culture-confirmed ICI within 96 h of study entry. Participants were randomly assigned 2:1 to once-daily intravenous 2 mg/kg caspofungin or intravenous 1 mg/kg amphotericin B deoxycholate (dAMB). The primary endpoint was fungal-free survival (FFS) 2 weeks after treatment in the full-analysis-set (FAS) population, defined as participants with culture-confirmed ICI who received ≥1 dose of therapy. Planned enrolment was 90 participants.
Fifty-one participants were enrolled; 49 received treatment (caspofungin, n=33; dAMB, n=16); 2 additional participants did not have confirmed infections at study entry. The study was terminated after ∼ 3.5 years because of low enrolment. Forty-seven participants were included in the FAS population (caspofungin, n=31; dAMB, n=16). FFS rate at 2 weeks after treatment was 71.0% (22/31) in the caspofungin arm and 68.8% (11/16) in the dAMB arm [difference, stratified by weight, − 0.9% (95% CI, − 24.3%–27.7%)]. Adverse events developed in 84.8% (28/33) of participants in the caspofungin arm and 100% (16/16) in the dAMB arm.
Among neonates and infants with confirmed ICI, FFS at 2 weeks was similar in the caspofungin and dAMB treatment arms. A smaller proportion of participants who received caspofungin experienced adverse events.
Introduction
Candida species are among the most common fungal pathogens in premature neonates and in children with compromised immune systems.1 Premature neonates have several risk factors for invasive Candida infection (ICI): compromised immunity based on immature neutrophil function; high reliance on medical devices (e.g. mechanical ventilation and central venous catheters); parenteral nutrition; postnatal systemic corticosteroid use; frequent exposure to broad-spectrum antibiotics; and high incidence of intestinal pathology, often necessitating abdominal surgery.1
The 2016 update of the clinical practice guideline for the management of candidiasis by IDSA, which has been endorsed by the American Academy of Pediatrics, and the 2012 ESCMID guidelines recommend the use of amphotericin B deoxycholate (dAMB) for the treatment of ICIs in neonates.2,3 The echinocandin caspofungin has less toxicity than dAMB4 and is approved for the treatment of candidaemia and other ICIs in paediatric populations 3 months to 17 years of age.5 We investigated the efficacy of caspofungin compared with dAMB for the treatment of ICIs in neonates and infants <3 months of age.
Methods
Ethics
Parents or guardians of all participants signed informed consent and the trial was conducted in conformance with the Declaration of Helsinki, Good Clinical Practice requirements and applicable country and/or local statutes and regulations regarding independent ethics committee review (Table S1, available as Supplementary data at JAC Online).
Study design and participants
This was a multicentre, double-blind, randomized, comparative-controlled, Phase 2 trial to evaluate the safety, tolerability and efficacy of caspofungin versus dAMB in the treatment of ICI in neonates and infants (protocol MK0991-064; ClinicalTrials.gov, NCT01945281) at 12 study sites in Brazil, Colombia, Mexico, South Africa and Turkey.
Neonates and infants <3 months of age with a diagnosis of ICI documented within 96 h before study entry were eligible for enrolment. Documented (culture-confirmed) ICI included any of the following: positive culture for Candida spp. collected from a normally sterile body fluid within 96 h of study entry; positive culture for Candida spp. collected from a newly placed drain inserted into a normally sterile body site; and urine cultures positive for Candida spp. that surpassed a pre-specified threshold (>10 000 cfu/mL).
Participants were excluded for any of the following reasons: Candida infection limited to oropharynx, oesophagus, other mucosal or superficial skin surfaces; Candida isolated from sputum, bronchoalveolar lavage fluid, catheter tip or previously placed indwelling non-vascular catheter/drain; suspected Candida infection of prosthetic device; or active coinfection with a non-Candida fungal organism. Participants were also excluded if they previously received ≥48 h of systemic antifungal treatment as therapy for the qualifying ICI episode and if previous systemic antifungal therapy for the qualifying ICI episode failed. Exclusionary laboratory values ≤48 h before therapy initiation included AST level >3× upper limit of normal (ULN) for age and ALT level >3× ULN for age. Also excluded were: participants who had a life expectancy of <5 days; those with acute hepatitis or cirrhosis; those with renal insufficiency who were scheduled to receive rifampicin; and those who, in the opinion of the principal investigator, had a history or evidence that would limit their participation in the study.
Randomization, blinding and treatment duration
Eligible participants were randomly assigned in a 2:1 ratio by an interactive voice response system to either intravenous 2 mg/kg caspofungin once daily infused over 2 h (with no loading dose) or intravenous 1 mg/kg dAMB once daily infused over 2 h. Randomization was stratified into three weight categories (based on weight at study entry): <1000 g, 1000 to 1500 g and >1500 g. Treatments were prepared by a pharmacist not blinded to study treatment. Because the two drugs were different colours, the prepared study medications and infusion materials were covered with opaque masking to maintain blinding during administration. In addition, although the caspofungin label recommends administration over 1 h,5 a 2 h caspofungin infusion was implemented to maintain study blinding with respect to dAMB.
Participants in the current study received caspofungin at a daily dose of 2 mg/kg. The daily dose of 2 mg/kg caspofungin was chosen based on a previous Phase 2 study in neonates and infants <3 months of age with documented or highly suspected Candida infections who received either single- or multiple-dose 25 mg/m2 caspofungin as a 1 h infusion.6 On day 4, the geometric mean for neonates and infants at risk for ICI was 10.9 μg/mL at 1 h and 2.3 μg/mL at 24 h after dose.6 After calculating the actual dose received (in milligrams) based on each participant’s body surface area and adjusting by weight, the 25 mg/m2 dose equated to a mean of ∼ 2.1 mg/kg/day and a median of ∼ 2.0 mg/kg/day.
The duration of study therapy with either caspofungin or dAMB was variable and dependent on a variety of factors. In general, participants were to be treated for ≥14 days after documented negative cultures from the site of ICI and improvement of any clinical signs and symptoms. The maximum duration of permitted study therapy was 90 days. If warranted, the study investigator could ask the sponsor (Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA) to extend the duration of therapy.
Endpoints and procedures
The primary efficacy endpoint was fungal-free survival (FFS) 2 weeks after the completion of treatment and the secondary endpoint was FFS at the end of treatment. FFS at the 2 week post-treatment period was defined as survival through the 2 week post-treatment period and documented microbiological eradication of Candida spp. from follow-up cultures collected after the initiation of study treatment. If the participant received rescue antifungal treatment, FFS was imputed as not met. FFS was evaluated in the full-analysis-set (FAS) population, which included those participants who received ≥1 full dose of study treatment and had a documented (culture-confirmed) diagnosis of ICI. The PP population excluded participants with important deviations from the protocol, which might have confounded efficacy results. Participants included in the PP population met the following criteria: had documented (culture-confirmed) ICI; did not receive concomitant systemic antifungal therapy for >1 day while receiving study therapy if the total duration of study therapy was ≤3 weeks; received concomitant systemic antifungal therapy along with study medication for ≤5% of the total duration of study therapy if the total duration of study therapy was >3 weeks; had a 2 week post-treatment efficacy evaluation; and received ≥5 days of study therapy. The study design is summarized in Figure S1.
Detailed evaluation of the ICI was performed at screening, daily during study treatment, on the last day of study treatment and at the 2 and 8 week post-treatment follow-up visits. Resolution or progression of the ICI was documented by assessment of the participant’s signs and symptoms, radiographic studies (when clinically indicated) and follow-up blood cultures or, as appropriate, other follow-up culture (including urine or CSF). Safety and tolerability data were collected throughout the study period.
Pharmacokinetic analyses
To minimize the blood volume collected from each participant, three blood samples for pharmacokinetics were collected from half of the participants on day 4 and three blood samples were collected from the other half on day 7. Samples were collected immediately after the end of study therapy infusion (approximate time of peak concentration) and immediately before the next dose (∼ 24 h after study therapy infusion, at the time of trough concentration). A third sample was collected at an unspecified time between peak and trough times (intermediate concentration). CSF samples for caspofungin assay were to be collected whenever possible. Only one CSF sample was obtained, however, and it was not assayed because it would not have provided meaningful information.
Plasma samples were analysed for caspofungin concentration by inVentiv Health Clinique (now Syneos Health) using an LC–MS/MS method, as described previously in detail.7 The lower limit of quantitation was 25 ng/mL.
Caspofungin plasma concentrations at the actual elapsed plasma sampling times relative to the corresponding time of caspofungin dosing were used to determine mean peak (C2) and trough (C24) concentrations.
Statistical analyses
A sample size of ∼ 90 participants randomly assigned 2:1 to caspofungin/dAMB was chosen to ensure 80% power to demonstrate the superiority of caspofungin over dAMB at an overall α=0.05 (two-sided) level if the underlying treatment difference in FFS at 2 weeks after treatment was ∼ 30% or larger. The minimum criterion for success of the primary endpoint was that the lower bound of 95% CI be >0 for the difference between the two treatment groups (caspofungin minus dAMB). The 95% CI for the difference in response rates was calculated based on the Miettinen and Nurminen8 method stratified by participant weight at study entry with Cochran–Mantel–Haenszel weights. Unless otherwise stated, all statistical tests were conducted at α=0.05 (two-sided) level.
Descriptive statistics for pharmacokinetic parameters were calculated for peak, trough and intermediate caspofungin plasma concentrations on days 4 and 7.
Safety was monitored during treatment and the 2 week follow-up period. Selected laboratory safety tests were performed using blood collected at screening, on day 4 (±1 day) and day 7 of study treatment, twice weekly thereafter during study treatment, on the last day of study treatment and at the 2 week post-treatment follow-up visit. The safety population included all participants who received ≥1 dose of study medication.
Results
Study population
The study was terminated early, ∼ 3.5 years after initiation, because of low enrolment. Between 27 March 2014 and 28 February 2018, 61 participants were screened and 51 were randomly assigned to treatment with caspofungin (34 participants) or dAMB (17 participants) (Figure 1). Forty-nine participants received treatment; one participant assigned to the caspofungin arm died before dosing and one assigned to the dAMB arm was withdrawn from the study at the treating physician’s discretion. Two participants in the caspofungin arm did not have culture-confirmed ICI at study entry; therefore, the FAS population comprised 47 participants (31 receiving caspofungin and 16 receiving dAMB). All 49 participants were included in the analysis of safety (33 receiving caspofungin and 16 receiving dAMB).
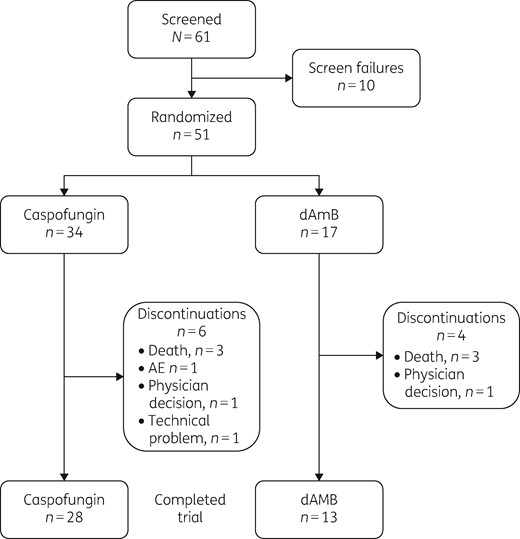
Median age (minimum–maximum) at enrolment was 22 days (7–88 days) and 53.2% of the participants were male. Median birth weight (minimum–maximum) was 1445 g (510–4175 g), median baseline weight (minimum–maximum) was 1860 g (425–6540 g) and median gestational age (minimum–maximum) was 30.4 weeks (26–41 weeks). Baseline characteristics are summarized in Table 1. Candida albicans was the most common species isolated at baseline [39/47 participants (83.0%)] (Table 1). Candidaemia was the most common manifestation of invasive candidiasis in the study [32/47 participants (68.1%)] (Table 1). Most participants across both treatment arms (51%) received study treatment for 15 to 21 days (Table S2).
| Characteristic . | Caspofungin (2 mg/kg), n=31 . | dAMB (1 mg/kg), n=16 . | Total, N=47 . |
|---|---|---|---|
| Age (days), median (minimum–maximum) | 22.0 (11–88) | 25.0 (7–74) | 22 (7–88) |
| Sex, n (%) | |||
| male | 18 (58.1) | 7 (43.8) | 25 (53.2) |
| female | 13 (41.9) | 9 (56.3) | 22 (46.8) |
| Race, n (%) | |||
| white | 12 (38.7) | 7 (43.8) | 19 (40.4) |
| black or African American | 11 (35.5) | 6 (37.5) | 17 (36.2) |
| multiracial | 5 (16.1) | 2 (12.5) | 7 (14.9) |
| American Indian/Alaskan Native | 3 (9.7) | 1 (6.3) | 4 (8.5) |
| Ethnicity, n (%) | |||
| Hispanic or Latino | 11 (35.5) | 7 (43.8) | 18 (38.3) |
| not Hispanic or Latino | 19 (61.3) | 8 (50.0) | 27 (57.4) |
| unknown/not reported | 1 (3.2) | 1 (6.3) | 2 (4.3) |
| Baseline weight (g) | |||
| median (range) | 1910 (810–4410) | 1684 (425–6540) | 1860 (425–6540) |
| <1000, n (%) | 4 (12.9) | 3 (18.8) | 7 (14.9) |
| 1000 to 1500, n (%) | 9 (29.0) | 4 (25.0) | 13 (27.7) |
| >1500, n (%) | 18 (58.1) | 9 (56.3) | 27 (57.4) |
| Gestational age (weeks), median (minimum–maximum) | 32.0 (26.0–41.0) | 30.0 (26.7–40.0) | 30.4 (26.0–41.0) |
| Birth weight (g) | |||
| median (minimum–maximum) | 1335.0 (850–3985) | 1462.5 (510–4175) | 1445.0 (510–4175) |
| <1000, n (%) | 7 (22.6) | 3 (18.8) | 10 (21.3) |
| 1000 to 1500, n (%) | 11 (35.5) | 6 (37.5) | 17 (36.2) |
| >1500, n (%) | 13 (41.9) | 7 (43.8) | 20 (42.6) |
| Baseline fungal isolates, n (%) | |||
| C. albicans | 27 (87.1) | 12 (75.0) | 39 (83.0) |
| Candida glabrata | 0 | 1 (6.3) | 1 (2.1) |
| Candida intermedia | 1 (3.2) | 0 | 1 (2.1) |
| Candida parapsilosis | 1 (3.2) | 1 (6.3) | 2 (4.3) |
| Candida tropicalis | 1 (3.2) | 0 | 1 (2.1) |
| C. albicans/C. glabrata | 1 (3.2) | 0 | 1 (2.1) |
| Pichia kudriavzevii | 0 | 1 (6.3) | 1 (2.1) |
| unspecified | 0 | 1 (6.3) | 1 (2.1) |
| Associated fungal diagnosis, n/N (%) | |||
| candidaemia | 24/31 (77.4) | 8/16 (50.0) | 32/47 (68.1) |
| urinary tract infection | 3/31 (9.7) | 2/16 (12.5) | 5/47 (10.6) |
| endocarditis | 1/31 (3.2) | 1/16 (6.3) | 2/47 (4.3) |
| lung infection | 1/31 (3.2) | 1/16 (6.3) | 2/47 (4.3) |
| meningoencephalitisa | 1/31 (3.2) | 1/16 (6.3) | 2/47 (4.3) |
| endophthalmitis | 0 | 1/16 (6.3) | 1/47 (2.1) |
| peritonitis | 0 | 1/16 (6.3) | 1/47 (2.1) |
| Characteristic . | Caspofungin (2 mg/kg), n=31 . | dAMB (1 mg/kg), n=16 . | Total, N=47 . |
|---|---|---|---|
| Age (days), median (minimum–maximum) | 22.0 (11–88) | 25.0 (7–74) | 22 (7–88) |
| Sex, n (%) | |||
| male | 18 (58.1) | 7 (43.8) | 25 (53.2) |
| female | 13 (41.9) | 9 (56.3) | 22 (46.8) |
| Race, n (%) | |||
| white | 12 (38.7) | 7 (43.8) | 19 (40.4) |
| black or African American | 11 (35.5) | 6 (37.5) | 17 (36.2) |
| multiracial | 5 (16.1) | 2 (12.5) | 7 (14.9) |
| American Indian/Alaskan Native | 3 (9.7) | 1 (6.3) | 4 (8.5) |
| Ethnicity, n (%) | |||
| Hispanic or Latino | 11 (35.5) | 7 (43.8) | 18 (38.3) |
| not Hispanic or Latino | 19 (61.3) | 8 (50.0) | 27 (57.4) |
| unknown/not reported | 1 (3.2) | 1 (6.3) | 2 (4.3) |
| Baseline weight (g) | |||
| median (range) | 1910 (810–4410) | 1684 (425–6540) | 1860 (425–6540) |
| <1000, n (%) | 4 (12.9) | 3 (18.8) | 7 (14.9) |
| 1000 to 1500, n (%) | 9 (29.0) | 4 (25.0) | 13 (27.7) |
| >1500, n (%) | 18 (58.1) | 9 (56.3) | 27 (57.4) |
| Gestational age (weeks), median (minimum–maximum) | 32.0 (26.0–41.0) | 30.0 (26.7–40.0) | 30.4 (26.0–41.0) |
| Birth weight (g) | |||
| median (minimum–maximum) | 1335.0 (850–3985) | 1462.5 (510–4175) | 1445.0 (510–4175) |
| <1000, n (%) | 7 (22.6) | 3 (18.8) | 10 (21.3) |
| 1000 to 1500, n (%) | 11 (35.5) | 6 (37.5) | 17 (36.2) |
| >1500, n (%) | 13 (41.9) | 7 (43.8) | 20 (42.6) |
| Baseline fungal isolates, n (%) | |||
| C. albicans | 27 (87.1) | 12 (75.0) | 39 (83.0) |
| Candida glabrata | 0 | 1 (6.3) | 1 (2.1) |
| Candida intermedia | 1 (3.2) | 0 | 1 (2.1) |
| Candida parapsilosis | 1 (3.2) | 1 (6.3) | 2 (4.3) |
| Candida tropicalis | 1 (3.2) | 0 | 1 (2.1) |
| C. albicans/C. glabrata | 1 (3.2) | 0 | 1 (2.1) |
| Pichia kudriavzevii | 0 | 1 (6.3) | 1 (2.1) |
| unspecified | 0 | 1 (6.3) | 1 (2.1) |
| Associated fungal diagnosis, n/N (%) | |||
| candidaemia | 24/31 (77.4) | 8/16 (50.0) | 32/47 (68.1) |
| urinary tract infection | 3/31 (9.7) | 2/16 (12.5) | 5/47 (10.6) |
| endocarditis | 1/31 (3.2) | 1/16 (6.3) | 2/47 (4.3) |
| lung infection | 1/31 (3.2) | 1/16 (6.3) | 2/47 (4.3) |
| meningoencephalitisa | 1/31 (3.2) | 1/16 (6.3) | 2/47 (4.3) |
| endophthalmitis | 0 | 1/16 (6.3) | 1/47 (2.1) |
| peritonitis | 0 | 1/16 (6.3) | 1/47 (2.1) |
Meningoencephalitis was confirmed by lumbar puncture.
| Characteristic . | Caspofungin (2 mg/kg), n=31 . | dAMB (1 mg/kg), n=16 . | Total, N=47 . |
|---|---|---|---|
| Age (days), median (minimum–maximum) | 22.0 (11–88) | 25.0 (7–74) | 22 (7–88) |
| Sex, n (%) | |||
| male | 18 (58.1) | 7 (43.8) | 25 (53.2) |
| female | 13 (41.9) | 9 (56.3) | 22 (46.8) |
| Race, n (%) | |||
| white | 12 (38.7) | 7 (43.8) | 19 (40.4) |
| black or African American | 11 (35.5) | 6 (37.5) | 17 (36.2) |
| multiracial | 5 (16.1) | 2 (12.5) | 7 (14.9) |
| American Indian/Alaskan Native | 3 (9.7) | 1 (6.3) | 4 (8.5) |
| Ethnicity, n (%) | |||
| Hispanic or Latino | 11 (35.5) | 7 (43.8) | 18 (38.3) |
| not Hispanic or Latino | 19 (61.3) | 8 (50.0) | 27 (57.4) |
| unknown/not reported | 1 (3.2) | 1 (6.3) | 2 (4.3) |
| Baseline weight (g) | |||
| median (range) | 1910 (810–4410) | 1684 (425–6540) | 1860 (425–6540) |
| <1000, n (%) | 4 (12.9) | 3 (18.8) | 7 (14.9) |
| 1000 to 1500, n (%) | 9 (29.0) | 4 (25.0) | 13 (27.7) |
| >1500, n (%) | 18 (58.1) | 9 (56.3) | 27 (57.4) |
| Gestational age (weeks), median (minimum–maximum) | 32.0 (26.0–41.0) | 30.0 (26.7–40.0) | 30.4 (26.0–41.0) |
| Birth weight (g) | |||
| median (minimum–maximum) | 1335.0 (850–3985) | 1462.5 (510–4175) | 1445.0 (510–4175) |
| <1000, n (%) | 7 (22.6) | 3 (18.8) | 10 (21.3) |
| 1000 to 1500, n (%) | 11 (35.5) | 6 (37.5) | 17 (36.2) |
| >1500, n (%) | 13 (41.9) | 7 (43.8) | 20 (42.6) |
| Baseline fungal isolates, n (%) | |||
| C. albicans | 27 (87.1) | 12 (75.0) | 39 (83.0) |
| Candida glabrata | 0 | 1 (6.3) | 1 (2.1) |
| Candida intermedia | 1 (3.2) | 0 | 1 (2.1) |
| Candida parapsilosis | 1 (3.2) | 1 (6.3) | 2 (4.3) |
| Candida tropicalis | 1 (3.2) | 0 | 1 (2.1) |
| C. albicans/C. glabrata | 1 (3.2) | 0 | 1 (2.1) |
| Pichia kudriavzevii | 0 | 1 (6.3) | 1 (2.1) |
| unspecified | 0 | 1 (6.3) | 1 (2.1) |
| Associated fungal diagnosis, n/N (%) | |||
| candidaemia | 24/31 (77.4) | 8/16 (50.0) | 32/47 (68.1) |
| urinary tract infection | 3/31 (9.7) | 2/16 (12.5) | 5/47 (10.6) |
| endocarditis | 1/31 (3.2) | 1/16 (6.3) | 2/47 (4.3) |
| lung infection | 1/31 (3.2) | 1/16 (6.3) | 2/47 (4.3) |
| meningoencephalitisa | 1/31 (3.2) | 1/16 (6.3) | 2/47 (4.3) |
| endophthalmitis | 0 | 1/16 (6.3) | 1/47 (2.1) |
| peritonitis | 0 | 1/16 (6.3) | 1/47 (2.1) |
| Characteristic . | Caspofungin (2 mg/kg), n=31 . | dAMB (1 mg/kg), n=16 . | Total, N=47 . |
|---|---|---|---|
| Age (days), median (minimum–maximum) | 22.0 (11–88) | 25.0 (7–74) | 22 (7–88) |
| Sex, n (%) | |||
| male | 18 (58.1) | 7 (43.8) | 25 (53.2) |
| female | 13 (41.9) | 9 (56.3) | 22 (46.8) |
| Race, n (%) | |||
| white | 12 (38.7) | 7 (43.8) | 19 (40.4) |
| black or African American | 11 (35.5) | 6 (37.5) | 17 (36.2) |
| multiracial | 5 (16.1) | 2 (12.5) | 7 (14.9) |
| American Indian/Alaskan Native | 3 (9.7) | 1 (6.3) | 4 (8.5) |
| Ethnicity, n (%) | |||
| Hispanic or Latino | 11 (35.5) | 7 (43.8) | 18 (38.3) |
| not Hispanic or Latino | 19 (61.3) | 8 (50.0) | 27 (57.4) |
| unknown/not reported | 1 (3.2) | 1 (6.3) | 2 (4.3) |
| Baseline weight (g) | |||
| median (range) | 1910 (810–4410) | 1684 (425–6540) | 1860 (425–6540) |
| <1000, n (%) | 4 (12.9) | 3 (18.8) | 7 (14.9) |
| 1000 to 1500, n (%) | 9 (29.0) | 4 (25.0) | 13 (27.7) |
| >1500, n (%) | 18 (58.1) | 9 (56.3) | 27 (57.4) |
| Gestational age (weeks), median (minimum–maximum) | 32.0 (26.0–41.0) | 30.0 (26.7–40.0) | 30.4 (26.0–41.0) |
| Birth weight (g) | |||
| median (minimum–maximum) | 1335.0 (850–3985) | 1462.5 (510–4175) | 1445.0 (510–4175) |
| <1000, n (%) | 7 (22.6) | 3 (18.8) | 10 (21.3) |
| 1000 to 1500, n (%) | 11 (35.5) | 6 (37.5) | 17 (36.2) |
| >1500, n (%) | 13 (41.9) | 7 (43.8) | 20 (42.6) |
| Baseline fungal isolates, n (%) | |||
| C. albicans | 27 (87.1) | 12 (75.0) | 39 (83.0) |
| Candida glabrata | 0 | 1 (6.3) | 1 (2.1) |
| Candida intermedia | 1 (3.2) | 0 | 1 (2.1) |
| Candida parapsilosis | 1 (3.2) | 1 (6.3) | 2 (4.3) |
| Candida tropicalis | 1 (3.2) | 0 | 1 (2.1) |
| C. albicans/C. glabrata | 1 (3.2) | 0 | 1 (2.1) |
| Pichia kudriavzevii | 0 | 1 (6.3) | 1 (2.1) |
| unspecified | 0 | 1 (6.3) | 1 (2.1) |
| Associated fungal diagnosis, n/N (%) | |||
| candidaemia | 24/31 (77.4) | 8/16 (50.0) | 32/47 (68.1) |
| urinary tract infection | 3/31 (9.7) | 2/16 (12.5) | 5/47 (10.6) |
| endocarditis | 1/31 (3.2) | 1/16 (6.3) | 2/47 (4.3) |
| lung infection | 1/31 (3.2) | 1/16 (6.3) | 2/47 (4.3) |
| meningoencephalitisa | 1/31 (3.2) | 1/16 (6.3) | 2/47 (4.3) |
| endophthalmitis | 0 | 1/16 (6.3) | 1/47 (2.1) |
| peritonitis | 0 | 1/16 (6.3) | 1/47 (2.1) |
Meningoencephalitis was confirmed by lumbar puncture.
Efficacy
In the FAS population, FFS at 2 weeks after treatment was 71.0% (22/31) in the caspofungin arm and 68.8% (11/16) in the dAMB arm [difference, − 0.9% (95% CI, − 24.3%–27.7%)] (Figure S2A). In the PP population, FFS at 2 weeks after treatment was 82.6% (19/23) in the caspofungin arm and 83.3% (10/12) in the dAMB arm [difference, − 1.9% (95% CI, − 28.2%–28.4%)] (Figure S2B). Of participants whose baseline pathogen was verified by the central laboratory, FFS at 2 weeks after treatment was higher in the subgroup infected with C. albicans at baseline who received caspofungin (72.7%; 16/22) than in the subgroup who received dAMB (55.6%; 5/9) (Figure S3).
FFS at the end of treatment was similar to what it was 2 weeks after treatment in the FAS population: 71.0% (22/31) in the caspofungin arm and 75.0% (12/16) in the dAMB arm [difference, − 6.3% (95% CI, − 30.2%–22.6%)] (Figure S2C). In the PP population, it was 82.6% (19/23) in the caspofungin arm and 91.7% (11/12) in the dAMB arm [difference, − 9.5% (95% CI, − 34.0%–20.4%)] (Figure S2D).
Pharmacokinetics
Caspofungin peak and trough plasma concentrations were comparable across days 4 and 7, supporting that steady-state was achieved by day 4 (Table 2).
Summary statistics for caspofungin peak (end of 2 h infusion; C2) and trough plasma concentration (∼24 h after end of infusion; C24) in neonates and infants (<3 months of age) with ICI receiving 2 mg/kg/day caspofungin
| Statistics . | C2, μg/mL . | C24, μg/mL . | ||
|---|---|---|---|---|
| day 4a, n=12 . | day 7a, n=11 . | day 4a, n=13 . | day 7a, n=12 . | |
| Mean (SD) | 9.31 (3.46) | 7.22 (4.52) | 3.23 (1.40) | 3.85 (2.51) |
| CV% | 37.1 | 62.6 | 43.3 | 65.2 |
| Median (minimum–maximum) | 9.25 (2.93–15.6) | 6.33 (2.43–16.1) | 2.97 (1.07–5.89) | 3.04 (0.862–7.84) |
| Geometric mean (CV% geometric mean) | 8.58 (48.4) | 5.99 (72.4) | 2.93 (51.3) | 3.12 (80.0) |
| Statistics . | C2, μg/mL . | C24, μg/mL . | ||
|---|---|---|---|---|
| day 4a, n=12 . | day 7a, n=11 . | day 4a, n=13 . | day 7a, n=12 . | |
| Mean (SD) | 9.31 (3.46) | 7.22 (4.52) | 3.23 (1.40) | 3.85 (2.51) |
| CV% | 37.1 | 62.6 | 43.3 | 65.2 |
| Median (minimum–maximum) | 9.25 (2.93–15.6) | 6.33 (2.43–16.1) | 2.97 (1.07–5.89) | 3.04 (0.862–7.84) |
| Geometric mean (CV% geometric mean) | 8.58 (48.4) | 5.99 (72.4) | 2.93 (51.3) | 3.12 (80.0) |
CV, coefficient of variance.
Data on day 4 and day 7 were obtained from different participants so that blood volumes drawn from each participant would be minimized.
Summary statistics for caspofungin peak (end of 2 h infusion; C2) and trough plasma concentration (∼24 h after end of infusion; C24) in neonates and infants (<3 months of age) with ICI receiving 2 mg/kg/day caspofungin
| Statistics . | C2, μg/mL . | C24, μg/mL . | ||
|---|---|---|---|---|
| day 4a, n=12 . | day 7a, n=11 . | day 4a, n=13 . | day 7a, n=12 . | |
| Mean (SD) | 9.31 (3.46) | 7.22 (4.52) | 3.23 (1.40) | 3.85 (2.51) |
| CV% | 37.1 | 62.6 | 43.3 | 65.2 |
| Median (minimum–maximum) | 9.25 (2.93–15.6) | 6.33 (2.43–16.1) | 2.97 (1.07–5.89) | 3.04 (0.862–7.84) |
| Geometric mean (CV% geometric mean) | 8.58 (48.4) | 5.99 (72.4) | 2.93 (51.3) | 3.12 (80.0) |
| Statistics . | C2, μg/mL . | C24, μg/mL . | ||
|---|---|---|---|---|
| day 4a, n=12 . | day 7a, n=11 . | day 4a, n=13 . | day 7a, n=12 . | |
| Mean (SD) | 9.31 (3.46) | 7.22 (4.52) | 3.23 (1.40) | 3.85 (2.51) |
| CV% | 37.1 | 62.6 | 43.3 | 65.2 |
| Median (minimum–maximum) | 9.25 (2.93–15.6) | 6.33 (2.43–16.1) | 2.97 (1.07–5.89) | 3.04 (0.862–7.84) |
| Geometric mean (CV% geometric mean) | 8.58 (48.4) | 5.99 (72.4) | 2.93 (51.3) | 3.12 (80.0) |
CV, coefficient of variance.
Data on day 4 and day 7 were obtained from different participants so that blood volumes drawn from each participant would be minimized.
Safety
At least one adverse event (AE) was reported by 84.8% (28/33) of participants in the caspofungin arm and 100% (16/16) in the dAMB arm (Table 3). Two participants in each arm (6.1% and 12.5%, respectively) had investigator-assessed, drug-related AEs [caspofungin: infusion-site oedema and cholestatic jaundice, n=1 each; dAMB: anaemia (n=1) and increased blood lactate dehydrogenase level and metabolic alkalosis (n=1 for both)]. Five participants discontinued due to six AEs: four participants in the caspofungin arm experienced one each of cholestasis, endocarditis, accidental overdose and superior vena cava syndrome and one participant in the dAMB arm experienced cardiac arrest and procedural pneumothorax. No participant discontinued study medication because of a drug-related AE. Although the groups were small, the proportion of participants who experienced serious AEs (SAEs) was larger in the dAMB group (43.8%) than in the caspofungin group (18.2%) (Table 3). Six participants died: three in the caspofungin arm (one after randomization but before initiation of caspofungin, one of septic shock on day 1 and one of neonatal necrotizing enterocolitis on day 28) and three in the dAMB arm (one of suture rupture on day 16, one of cardiac arrest/procedural pneumothorax on day 4 and one of pulmonary haemorrhage on day 56). All SAEs and deaths were considered unrelated to the study drug.
Summary of AEs during treatment and first 2 weeks after treatment in the all-subjects-as-treated population
| Event, n (%) . | Caspofungin (2 mg/kg), n=33 . | dAMB (1 mg/kg), n=16 . |
|---|---|---|
| Any AE | 28 (84.8) | 16 (100.0) |
| Most common (≥10% of participants) AEs | ||
| anaemia | 10 (30.3) | 8 (50.0) |
| pyrexia | 6 (18.2) | 3 (18.8) |
| sepsis | 3 (9.1) | 4 (25.0) |
| hypoglycaemia | 2 (6.1) | 2 (12.5) |
| tachycardia | 2 (6.1) | 2 (12.5) |
| abdominal distension | 1 (3.0) | 2 (12.5) |
| ALT level increased | 0 | 2 (12.5) |
| AST level increased | 0 | 3 (18.8) |
| blood bilirubin level increased | 0 | 2 (12.5) |
| Drug-related AE | 2 (6.1) | 2 (12.5) |
| SAE | 6 (18.2) | 7 (43.8) |
| cardiac arresta,b | 0 | 1 (6.3) |
| necrotizing colitis | 1 (3.0) | 0 |
| neonatal necrotizing enterocolitisa | 1 (3.0) | 0 |
| bacterial sepsis | 0 | 1 (6.3) |
| device-related sepsis | 0 | 1 (6.3) |
| endocarditis | 1 (3.0) | 0 |
| Escherichia sepsis | 1 (3.0) | 0 |
| fungal infection | 0 | 1 (6.3) |
| bacterial meningitis | 0 | 1 (6.3) |
| Escherichia coli pneumonia | 0 | 1 (6.3) |
| septic shocka | 1 (3.0) | 0 |
| anastomotic complication | 0 | 1 (6.3) |
| procedural pneumothoraxa,b | 0 | 1 (6.3) |
| suture rupturea | 0 | 1 (6.3) |
| apnoea | 1 (3.0) | 0 |
| dyspnoea | 0 | 1 (6.3) |
| superior vena cava syndrome | 1 (3.0) | 0 |
| Discontinued study drug because of AE | 4 (12.1) | 1 (6.3) |
| Discontinued study drug because of SAE | 2 (6.1) | 1 (6.3) |
| Event, n (%) . | Caspofungin (2 mg/kg), n=33 . | dAMB (1 mg/kg), n=16 . |
|---|---|---|
| Any AE | 28 (84.8) | 16 (100.0) |
| Most common (≥10% of participants) AEs | ||
| anaemia | 10 (30.3) | 8 (50.0) |
| pyrexia | 6 (18.2) | 3 (18.8) |
| sepsis | 3 (9.1) | 4 (25.0) |
| hypoglycaemia | 2 (6.1) | 2 (12.5) |
| tachycardia | 2 (6.1) | 2 (12.5) |
| abdominal distension | 1 (3.0) | 2 (12.5) |
| ALT level increased | 0 | 2 (12.5) |
| AST level increased | 0 | 3 (18.8) |
| blood bilirubin level increased | 0 | 2 (12.5) |
| Drug-related AE | 2 (6.1) | 2 (12.5) |
| SAE | 6 (18.2) | 7 (43.8) |
| cardiac arresta,b | 0 | 1 (6.3) |
| necrotizing colitis | 1 (3.0) | 0 |
| neonatal necrotizing enterocolitisa | 1 (3.0) | 0 |
| bacterial sepsis | 0 | 1 (6.3) |
| device-related sepsis | 0 | 1 (6.3) |
| endocarditis | 1 (3.0) | 0 |
| Escherichia sepsis | 1 (3.0) | 0 |
| fungal infection | 0 | 1 (6.3) |
| bacterial meningitis | 0 | 1 (6.3) |
| Escherichia coli pneumonia | 0 | 1 (6.3) |
| septic shocka | 1 (3.0) | 0 |
| anastomotic complication | 0 | 1 (6.3) |
| procedural pneumothoraxa,b | 0 | 1 (6.3) |
| suture rupturea | 0 | 1 (6.3) |
| apnoea | 1 (3.0) | 0 |
| dyspnoea | 0 | 1 (6.3) |
| superior vena cava syndrome | 1 (3.0) | 0 |
| Discontinued study drug because of AE | 4 (12.1) | 1 (6.3) |
| Discontinued study drug because of SAE | 2 (6.1) | 1 (6.3) |
Resulted in death.
Same participant.
Summary of AEs during treatment and first 2 weeks after treatment in the all-subjects-as-treated population
| Event, n (%) . | Caspofungin (2 mg/kg), n=33 . | dAMB (1 mg/kg), n=16 . |
|---|---|---|
| Any AE | 28 (84.8) | 16 (100.0) |
| Most common (≥10% of participants) AEs | ||
| anaemia | 10 (30.3) | 8 (50.0) |
| pyrexia | 6 (18.2) | 3 (18.8) |
| sepsis | 3 (9.1) | 4 (25.0) |
| hypoglycaemia | 2 (6.1) | 2 (12.5) |
| tachycardia | 2 (6.1) | 2 (12.5) |
| abdominal distension | 1 (3.0) | 2 (12.5) |
| ALT level increased | 0 | 2 (12.5) |
| AST level increased | 0 | 3 (18.8) |
| blood bilirubin level increased | 0 | 2 (12.5) |
| Drug-related AE | 2 (6.1) | 2 (12.5) |
| SAE | 6 (18.2) | 7 (43.8) |
| cardiac arresta,b | 0 | 1 (6.3) |
| necrotizing colitis | 1 (3.0) | 0 |
| neonatal necrotizing enterocolitisa | 1 (3.0) | 0 |
| bacterial sepsis | 0 | 1 (6.3) |
| device-related sepsis | 0 | 1 (6.3) |
| endocarditis | 1 (3.0) | 0 |
| Escherichia sepsis | 1 (3.0) | 0 |
| fungal infection | 0 | 1 (6.3) |
| bacterial meningitis | 0 | 1 (6.3) |
| Escherichia coli pneumonia | 0 | 1 (6.3) |
| septic shocka | 1 (3.0) | 0 |
| anastomotic complication | 0 | 1 (6.3) |
| procedural pneumothoraxa,b | 0 | 1 (6.3) |
| suture rupturea | 0 | 1 (6.3) |
| apnoea | 1 (3.0) | 0 |
| dyspnoea | 0 | 1 (6.3) |
| superior vena cava syndrome | 1 (3.0) | 0 |
| Discontinued study drug because of AE | 4 (12.1) | 1 (6.3) |
| Discontinued study drug because of SAE | 2 (6.1) | 1 (6.3) |
| Event, n (%) . | Caspofungin (2 mg/kg), n=33 . | dAMB (1 mg/kg), n=16 . |
|---|---|---|
| Any AE | 28 (84.8) | 16 (100.0) |
| Most common (≥10% of participants) AEs | ||
| anaemia | 10 (30.3) | 8 (50.0) |
| pyrexia | 6 (18.2) | 3 (18.8) |
| sepsis | 3 (9.1) | 4 (25.0) |
| hypoglycaemia | 2 (6.1) | 2 (12.5) |
| tachycardia | 2 (6.1) | 2 (12.5) |
| abdominal distension | 1 (3.0) | 2 (12.5) |
| ALT level increased | 0 | 2 (12.5) |
| AST level increased | 0 | 3 (18.8) |
| blood bilirubin level increased | 0 | 2 (12.5) |
| Drug-related AE | 2 (6.1) | 2 (12.5) |
| SAE | 6 (18.2) | 7 (43.8) |
| cardiac arresta,b | 0 | 1 (6.3) |
| necrotizing colitis | 1 (3.0) | 0 |
| neonatal necrotizing enterocolitisa | 1 (3.0) | 0 |
| bacterial sepsis | 0 | 1 (6.3) |
| device-related sepsis | 0 | 1 (6.3) |
| endocarditis | 1 (3.0) | 0 |
| Escherichia sepsis | 1 (3.0) | 0 |
| fungal infection | 0 | 1 (6.3) |
| bacterial meningitis | 0 | 1 (6.3) |
| Escherichia coli pneumonia | 0 | 1 (6.3) |
| septic shocka | 1 (3.0) | 0 |
| anastomotic complication | 0 | 1 (6.3) |
| procedural pneumothoraxa,b | 0 | 1 (6.3) |
| suture rupturea | 0 | 1 (6.3) |
| apnoea | 1 (3.0) | 0 |
| dyspnoea | 0 | 1 (6.3) |
| superior vena cava syndrome | 1 (3.0) | 0 |
| Discontinued study drug because of AE | 4 (12.1) | 1 (6.3) |
| Discontinued study drug because of SAE | 2 (6.1) | 1 (6.3) |
Resulted in death.
Same participant.
Discussion
The current study was among the largest double-blind, randomized, controlled trials in neonatal ICI to date and provided many important insights into the efficacy, pharmacokinetics and safety profile of caspofungin compared with dAMB. Our study found that, among neonates and infants with confirmed ICI, FFS at 2 weeks after completion of treatment was similar between the caspofungin treatment arm and the dAMB treatment arm. One participant receiving dAMB achieved FFS at the end of treatment, but experienced re-emergence of fungaemia during the 2 week follow-up period, whereas all participants who received caspofungin and achieved FFS at the end of treatment maintained FFS at 2 weeks after the end of treatment.
Anecdotal data have suggested the benefit of caspofungin in preterm neonates with ICI.9–14 In multiple reports, caspofungin therapy was started as salvage therapy in very low birth weight preterm neonates only after initial therapy with conventional antifungals (dAMB or fluconazole, with or without flucytosine) had failed, either because of pathogen resistance or intolerance to the initial therapy.12,13 Other anecdotes reported clearance of shunt-associated C. albicans meningitis.10
Prior to the current study, the largest prospective, randomized, double-blind study in neonates with ICI was performed at a single centre in Saudi Arabia.9 The Saudi study showed significantly higher rates of favourable responses among 32 neonates with ICI treated with 2 mg/kg/day caspofungin (86.7%; n=15) than with 1 mg/kg/day dAMB (41.7%; n=12) (P=0.04) and showed efficacy in all sites of ICI, including three cases of meningitis and four of five cases of endocarditis.9 The current trial was unable to demonstrate the superiority of caspofungin over dAMB at a difference of 25% in FFS, which is smaller than the difference seen in the Saudi study.
Caspofungin (2 mg/kg) was generally well tolerated in neonates and infants <3 months of age with ICIs. Participants who received caspofungin experienced proportionally fewer AEs and SAEs and fewer treatment-emergent AEs and SAEs compared with dAMB recipients. This aligns with other published studies of caspofungin use in neonatal ICI.10–14 A recent meta-analysis shows that study treatment is discontinued less often in neonates with ICI who receive an echinocandin (caspofungin or micafungin) than in neonates who receive liposomal amphotericin B.15
The major limitation of the current study was the small sample size and the study was prematurely halted because of slow enrolment. This small sample size precluded detection of any difference in efficacy between caspofungin and dAMB. Many factors contributed to the lower-than-anticipated enrolment. First and foremost, the standardized use of fluconazole prophylaxis in neonatal ICUs worldwide drastically altered the epidemiology of ICI, diminishing the neonatal population who had ICI.2,16 Another was that the study population (neonates <3 months of age with ICI) included very ill participants with a disease that carries high morbidity and mortality.17 Culture-confirmed ICI is often difficult to diagnose given its protean manifestations and culture confirmation is sometimes delayed. Thus, by the time diagnosis is made, participants are critically ill (and have a mortality rate of up to 30%).18,19 Parental consent may be difficult to acquire in such stressful conditions. Additional study-related procedures dissuaded parents from giving consent to participate in the study. Thus, conclusions from this trial cannot be made with regard to the safety and efficacy of caspofungin compared with those of dAMB in the treatment of neonates and infants <3 months of age with invasive candidiasis.
Acknowledgements
Previous presentation: IDWeek 2018, San Francisco, CA, USA (Abstract 1955).
We would like to thank the participants and their families and the clinicians involved in this trial (Table S1).
Funding
Funding for this research was provided by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. The medical writing assistance (see below) was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
Transparency declarations
H.L. and L.J.A. are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA (MSD). F.L.N. and F.A.M. have received grants from MSD. J.K., M.B.D. and N.K. are employees of MSD and shareholders in Merck & Co., Inc., Kenilworth, NJ, USA.
Medical writing assistance was provided by Jennifer M. Kulak, PhD, of ApotheCom (Yardley, PA, USA).
Author contributions
J.K.: analysis and acquisition of the data, interpretation of the results, drafting of the manuscript, reviewing and revising the manuscript, and approval of the manuscript for submission. F.L.N.: acquisition of the data, interpretation of the results, reviewing and revising the manuscript, and approval of the manuscript for submission. F.A.M.: acquisition of the data, drafting of the manuscript, and approval of the manuscript for submission. H.L.: analysis of the data, interpretation of the results, reviewing and revising the manuscript, and approval of the manuscript for submission. M.B.D.: study design, reviewing and revising the manuscript, and approval of the manuscript for submission. L.J.A.: interpretation of the results, reviewing and revising the manuscript, and approval of the manuscript for submission. N.K.: study design, interpretation of results, reviewing and revising the manuscript, and approval of the manuscript for submission.
References
Merck & Co., Inc.



