-
PDF
- Split View
-
Views
-
Cite
Cite
Lisa Leon, Philippe Guerci, Elise Pape, Nathalie Thilly, Amandine Luc, Adeline Germain, Anne-Lise Butin-Druoton, Marie-Reine Losser, Julien Birckener, Julien Scala-Bertola, Emmanuel Novy, Serum and peritoneal exudate concentrations after high doses of β-lactams in critically ill patients with severe intra-abdominal infections: an observational prospective study, Journal of Antimicrobial Chemotherapy, Volume 75, Issue 1, January 2020, Pages 156–161, https://doi.org/10.1093/jac/dkz407
Close - Share Icon Share
Abstract
Critically ill patients with severe intra-abdominal infections (IAIs) requiring surgery may undergo several pharmacokinetic (PK) alterations that can lead to β-lactam underdosage.
To measure serum and peritoneal exudate concentrations of β-lactams after high doses and optimal administration schemes.
This observational prospective study included critically ill patients with suspicion of IAI who required surgery and a β-lactam antibiotic as empirical therapy. Serum and peritoneal exudate concentrations were measured during surgery and after a 24 h steady-state period. The PK/pharmacodynamic (PD) target was to obtain serum β-lactam concentrations of 100% fT>4×MIC based on a worst-case scenario (based on the EUCAST highest epidemiological cut-off values) before bacterial documentation (a priori) and redefined following determination of the MIC for the isolated bacteria (a posteriori). Registered with ClinicalTrials.gov (NCT03310606).
Forty-eight patients were included with a median (IQR) age of 64 (53–74) years and a SAPS II of 40 (32–65). The main diagnosis was secondary nosocomial peritonitis. Piperacillin/tazobactam was the most administered β-lactam antibiotic (75%). The serum/peritoneal piperacillin/tazobactam ratio was 0.88 (0.64–0.97) after a 24 h steady-state period. Prior to bacterial documentation, 16 patients (33.3%) achieved the a priori PK/PD target. The identification of microorganisms was available for 34 patients (71%). Based on the MIC for isolated bacteria, 78% of the patients achieved the serum PK/PD target.
In severe IAIs, high doses of β-lactams ensured 100% fT>4×MIC in the serum for 78% of critically ill patients with severe IAIs within the first 24 h. In order to define optimal β-lactam dosing, the PK/PD target should take into account the tissue penetration and local ecology.
Introduction
Early surgery and appropriate antibiotic therapy are cornerstones of management of intra-abdominal infections (IAIs). Among the pivotal measures of optimal antibiotic therapy, adequate dosing and administration schemes are recommended to achieve pharmacokinetic (PK)/pharmacodynamic (PD) targets.1 Insufficient serum antibiotic concentrations secondary to PK alterations are well described in critically ill patients with sepsis.2,3 In cases of IAIs, impaired tissue penetration and presence of indwelling surgical drains may increase PK alterations.4–6
β-Lactams are the first-line antimicrobial agents for IAIs.7 Current guidelines regarding the use of β-lactams for critically ill patients recommend higher doses at the onset of treatment and continuous infusion.8 There are few PK/PD studies focused on critically ill patients requiring emergent surgery, and data regarding peritoneal diffusion are scarce.9,10
In the present study, we hypothesized that underdosage of β-lactams was likely to occur despite a high dosing regimen. The concentrations of β-lactams in the serum and in the peritoneal exudate of critically ill patients with IAI requiring surgical management were measured. The rates of PK/PD target attainment at the empirical phase and after bacterial documentation were assessed.
Methods
Study design and population
A single-centre observational prospective study was conducted in a surgical ICU at the University Hospital of Nancy, France. The study protocol was reviewed and approved by the Patient Protection Committee Sud Méditerranée (IDRCB: 2017-A00710-53) and was registered with ClinicalTrials.gov (NCT03310606). Written informed consent was obtained from the patients or their substitute decision-makers.
All consecutive patients aged ≥18 years admitted to the ICU with a suspected IAI were eligible if their empirical antibiotic treatment included a β-lactam (see the Supplementary data available at JAC Online) and if they underwent abdominal surgery.8 Patients (i) with inappropriate β-lactam administration, (ii) with a history of allergy to β-lactams or (iii) who were pregnant were excluded.
Clinical data collected from the electronic medical record included demographics, ICU hospitalization details, IAI characteristics and β-lactam exposure. Septic shock was defined according to Sepsis-3.11
β-Lactam analysis in serum and peritoneal fluid
Serum and peritoneal samples were simultaneously collected at three different timepoints: peritoneal incision (T0); end of surgery (T1); and 24 h after the first sampling (T2). Peritoneal samples were sterilely collected after peritoneal incision with aspiration of 5 mL of peritoneal exudate (T0) and then drawn from intra-abdominal drains in the ICU (T1 and T2).
The total concentrations of β-lactams in the serum and peritoneal exudate were assessed using HPLC (see the Supplementary data available at JAC Online). The linearity ranged from 1 to 500 mg/L. The coefficients of variation for inter- and intra-assay precision were <10% and the accuracy was within 10% for all antibiotics. The free concentration was approximated using the literature estimates of the free unbound percentage of each β-lactam: 80% for cefotaxime, imipenem and piperacillin, and 10% and 100% for ceftriaxone and meropenem, respectively.8
PK/PD targets
In cases of undocumented IAI or empirical antibiotic therapy, the a priori PK/PD target was to achieve a serum concentration above 4×MIC [based on the EUCAST highest epidemiological cut-off (ECOFF) values].
In the case of documented IAI, the a posteriori PK/PD target was defined as a serum free concentration >4×MIC for the isolated bacteria. The MIC of the β-lactam infused was determined using an automated system, i.e. the VITEK® 2 system (bioMérieux, Marcy-l’Étoile, France).
Overall, the PK/PD target was to obtain an adequate serum concentration at the three timepoints, corresponding to 100% of T>4×MIC in susceptible pathogens (see the Supplementary data available at JAC Online).
Outcomes
The primary outcome was to determine the percentage of patients who met the a priori and the a posteriori PK/PD targets.
Secondary outcomes included the impact of the factors associated with changes in PK/PD and the mortality at 28 days.
A subgroup analysis for piperacillin/tazobactam was performed to evaluate the correlation between serum and peritoneal concentrations and the concentration ratio.
Statistical analysis
The characteristics of the sample were described as percentages for categorical variables and as median (IQR) values for continuous variables. Parametric and non-parametric statistics were used to analyse risk factors of underdosage. Analysis of the explanatory factors of the T2 underdosing was performed using logistic regression models. Comparison tests between concentrations at T2 and T1 (on paired samples) were performed using the Wilcoxon signed rank test. Correlations were evaluated using the Spearman coefficient. The alpha risk was 5% for all analyses. These statistical analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Patients
From October 2017 to January 2019, 48 adult patients were prospectively included [Table 1 and Figure S1 (available as Supplementary data at JAC Online)]. All IAIs were secondary peritonitis. Thirty-nine (81%) were healthcare-associated IAIs. Piperacillin/tazobactam was the most administered β-lactam antibiotic (75%). The identification of microorganisms was available for 34 patients (71%). Among these, an MIC was available for 23 patients. The observed total serum and peritoneal piperacillin/tazobactam concentrations are presented in Figure 1 (those of other β-lactams are shown in Figure S2). Gram-negative species were the most identified bacteria. The MICs of piperacillin/tazobactam ranged from a minimum of 0.3 mg/L to a maximum of 8 mg/L. Overall, only 11% of bacteria isolated were MDR bacteria. Documented microorganisms are presented in Table S1.
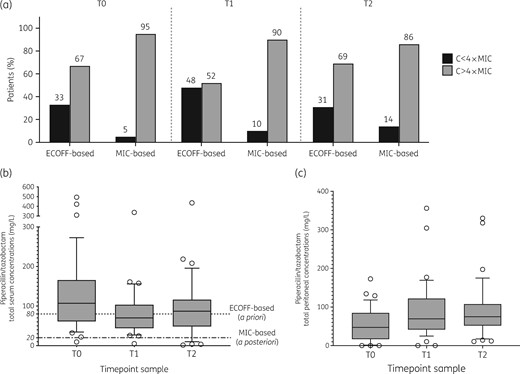
(a) Serum β-lactam PK/PD target attainment and total serum (b) and peritoneal (c) concentrations of piperacillin/tazobactam at each timepoint. (a) Difference in target attainment between a priori target (worst-case scenario) and a posteriori target (documented MIC, based on local ecology). ECOFF-based target: a priori PK/PD target attainment defined as a total concentration of β-lactams above 4× the MIC (based on ECOFF values and a worst-case scenario). MIC-based target: a posteriori PK/PD objective attainment defined as a free concentration above 4× the MIC for isolated bacteria. (b and c) The dotted line represents the ECOFF-based target (worst-case scenario) according to guidelines: the threshold was calculated at 80 mg/L corresponding to 4× the MIC for Pseudomonas aeruginosa (16 mg/L) and considering a free fraction of 80%. The dotted-dashed line represents the MIC-based target: the threshold was calculated at 20 mg/L corresponding to 4× the MIC of 4 mg/L and considering a free fraction of 80%. The boxplots represent median and IQR. C, serum concentration of β-lactam.
Table 1. Demographics and clinical data for included patients; N=48
| Demographic data and medical history | |
| age (years), median (IQR) | 64 (54–74) |
| female, n (%) | 12 (25) |
| BMI (kg/m2), median (IQR) | 25.3 (20.5–30.3) |
| arterial hypertension, n (%) | 23 (48) |
| chronic renal failurea, n (%) | 14 (29) |
| ischaemic heart disease, n (%) | 10 (21) |
| IAI: type and localization, n (%) | |
| healthcare associated | 39 (81) |
| localization | |
| colon | 17 (35) |
| small intestine | 14 (29) |
| gastric | 6 (13) |
| hepato-biliary | 6 (13) |
| multiple localizations | 4 (8) |
| pancreatic | 1 (2) |
| β-Lactams administered, n (%) | |
| piperacillin/tazobactam | 36 (75) |
| carbapenem | 3 (6) |
| imipenem | 1 (2) |
| meropenem | 2 (4) |
| third-generation cephalosporin | 9 (19) |
| ceftriaxone | 6 (13) |
| cefotaxime | 3 (6) |
| β-Lactam total concentrations (mg/L), serum/peritoneal exudate, median (IQR) | |
| piperacillin concentrations | |
| T0 | 107.9 (62.2–179.9)/47.3 (19.1–84.5) |
| T1 | 70 (43.8–103.5)/69.3 (41.4–112.7) |
| T2 | 88 (51.5–116.3)/77.4 (56.8–108.7) |
| ceftriaxone concentrations | |
| T0 | 99.6 (78.3–135.7)/71.5 (33.9–112.8) |
| T1 | 58.8 (38.1–75.6)/60.1 (31.2–77.3) |
| T2 | 32.2 (19.2–70.6)/55.9 (27.8–91.4) |
| cefotaxime concentrations | |
| T0 | –/– |
| T1 | 114.5 (55.4–135.9)/71.1 (36.8–124.5) |
| T2 | 49 (45.9–156.6)/74.7 (56–93.5) |
| imipenem concentrations | |
| T0 | 212 |
| T1 | 160 |
| T2 | 58 |
| meropenem concentrations | |
| T0 | 26.8 (10.5–43.2)/23.6 (10.1–37.2) |
| T1 | 17.4 (5.7–29.2)/21.1 (8.8–33.5) |
| T2 | 10 (3.8–32)/11.9 (10.7–54.6) |
| Clinical and biological data prior to surgery | |
| leucocytes (×109/L), median (IQR) | 13.8 (9.4–19) |
| protein at T2 (g/L), median (IQR) | 50 (46–55) |
| C-reactive protein (mg/L), median (IQR) | 193 (123–312) |
| norepinephrine infusion, n (%) | 13 (27) |
| temperature (°C), median (IQR) | 37.5 (37–38) |
| Intraoperative data | |
| open surgery, n (%) | 44 (92) |
| vascular filling (mL), median (IQR) | 2000 (1375–2250) |
| norepinephrine maximal dose (μg/kg/min), median (IQR) | 0.3 (0.1–0.8) |
| septic shock, n (%) | 11 (23) |
| ICU characteristics | |
| mechanical ventilation at ICU admission, n (%) | 24 (50) |
| SOFA score at ICU admission, median (IQR) | 6 (2–10) |
| SAPS II, median (IQR) | 40 (32–65) |
| measured creatinine clearance (mL/min) at 24 hb, median (IQR) | 46.5 (20–87) |
| drain fluid output at T2 (mL), median (IQR) | 200 (100–250) |
| mortality at day 28, n (%) | 7 (15) |
| Microbiological data | |
| MIC of piperacillin/tazobactam, median (IQR) | 4 (4–4) |
| microbiological identification in peritoneal exudate, n (%) | |
| monobacterial | 7 (15) |
| polymicrobial | 23 (48) |
| fungal | 4 (8) |
| no identification | 14 (29) |
| Demographic data and medical history | |
| age (years), median (IQR) | 64 (54–74) |
| female, n (%) | 12 (25) |
| BMI (kg/m2), median (IQR) | 25.3 (20.5–30.3) |
| arterial hypertension, n (%) | 23 (48) |
| chronic renal failurea, n (%) | 14 (29) |
| ischaemic heart disease, n (%) | 10 (21) |
| IAI: type and localization, n (%) | |
| healthcare associated | 39 (81) |
| localization | |
| colon | 17 (35) |
| small intestine | 14 (29) |
| gastric | 6 (13) |
| hepato-biliary | 6 (13) |
| multiple localizations | 4 (8) |
| pancreatic | 1 (2) |
| β-Lactams administered, n (%) | |
| piperacillin/tazobactam | 36 (75) |
| carbapenem | 3 (6) |
| imipenem | 1 (2) |
| meropenem | 2 (4) |
| third-generation cephalosporin | 9 (19) |
| ceftriaxone | 6 (13) |
| cefotaxime | 3 (6) |
| β-Lactam total concentrations (mg/L), serum/peritoneal exudate, median (IQR) | |
| piperacillin concentrations | |
| T0 | 107.9 (62.2–179.9)/47.3 (19.1–84.5) |
| T1 | 70 (43.8–103.5)/69.3 (41.4–112.7) |
| T2 | 88 (51.5–116.3)/77.4 (56.8–108.7) |
| ceftriaxone concentrations | |
| T0 | 99.6 (78.3–135.7)/71.5 (33.9–112.8) |
| T1 | 58.8 (38.1–75.6)/60.1 (31.2–77.3) |
| T2 | 32.2 (19.2–70.6)/55.9 (27.8–91.4) |
| cefotaxime concentrations | |
| T0 | –/– |
| T1 | 114.5 (55.4–135.9)/71.1 (36.8–124.5) |
| T2 | 49 (45.9–156.6)/74.7 (56–93.5) |
| imipenem concentrations | |
| T0 | 212 |
| T1 | 160 |
| T2 | 58 |
| meropenem concentrations | |
| T0 | 26.8 (10.5–43.2)/23.6 (10.1–37.2) |
| T1 | 17.4 (5.7–29.2)/21.1 (8.8–33.5) |
| T2 | 10 (3.8–32)/11.9 (10.7–54.6) |
| Clinical and biological data prior to surgery | |
| leucocytes (×109/L), median (IQR) | 13.8 (9.4–19) |
| protein at T2 (g/L), median (IQR) | 50 (46–55) |
| C-reactive protein (mg/L), median (IQR) | 193 (123–312) |
| norepinephrine infusion, n (%) | 13 (27) |
| temperature (°C), median (IQR) | 37.5 (37–38) |
| Intraoperative data | |
| open surgery, n (%) | 44 (92) |
| vascular filling (mL), median (IQR) | 2000 (1375–2250) |
| norepinephrine maximal dose (μg/kg/min), median (IQR) | 0.3 (0.1–0.8) |
| septic shock, n (%) | 11 (23) |
| ICU characteristics | |
| mechanical ventilation at ICU admission, n (%) | 24 (50) |
| SOFA score at ICU admission, median (IQR) | 6 (2–10) |
| SAPS II, median (IQR) | 40 (32–65) |
| measured creatinine clearance (mL/min) at 24 hb, median (IQR) | 46.5 (20–87) |
| drain fluid output at T2 (mL), median (IQR) | 200 (100–250) |
| mortality at day 28, n (%) | 7 (15) |
| Microbiological data | |
| MIC of piperacillin/tazobactam, median (IQR) | 4 (4–4) |
| microbiological identification in peritoneal exudate, n (%) | |
| monobacterial | 7 (15) |
| polymicrobial | 23 (48) |
| fungal | 4 (8) |
| no identification | 14 (29) |
Chronic renal failure was defined as glomerular filtration rate <60 mL/min/1.73 m2 according to KDIGO recommendations (where KDIGO stands for Kidney Disease Improving Global Outcomes).
Creatinine clearance was measured using the formula (urinary creatinine×urinary volume)/plasma creatinine.
Table 1. Demographics and clinical data for included patients; N=48
| Demographic data and medical history | |
| age (years), median (IQR) | 64 (54–74) |
| female, n (%) | 12 (25) |
| BMI (kg/m2), median (IQR) | 25.3 (20.5–30.3) |
| arterial hypertension, n (%) | 23 (48) |
| chronic renal failurea, n (%) | 14 (29) |
| ischaemic heart disease, n (%) | 10 (21) |
| IAI: type and localization, n (%) | |
| healthcare associated | 39 (81) |
| localization | |
| colon | 17 (35) |
| small intestine | 14 (29) |
| gastric | 6 (13) |
| hepato-biliary | 6 (13) |
| multiple localizations | 4 (8) |
| pancreatic | 1 (2) |
| β-Lactams administered, n (%) | |
| piperacillin/tazobactam | 36 (75) |
| carbapenem | 3 (6) |
| imipenem | 1 (2) |
| meropenem | 2 (4) |
| third-generation cephalosporin | 9 (19) |
| ceftriaxone | 6 (13) |
| cefotaxime | 3 (6) |
| β-Lactam total concentrations (mg/L), serum/peritoneal exudate, median (IQR) | |
| piperacillin concentrations | |
| T0 | 107.9 (62.2–179.9)/47.3 (19.1–84.5) |
| T1 | 70 (43.8–103.5)/69.3 (41.4–112.7) |
| T2 | 88 (51.5–116.3)/77.4 (56.8–108.7) |
| ceftriaxone concentrations | |
| T0 | 99.6 (78.3–135.7)/71.5 (33.9–112.8) |
| T1 | 58.8 (38.1–75.6)/60.1 (31.2–77.3) |
| T2 | 32.2 (19.2–70.6)/55.9 (27.8–91.4) |
| cefotaxime concentrations | |
| T0 | –/– |
| T1 | 114.5 (55.4–135.9)/71.1 (36.8–124.5) |
| T2 | 49 (45.9–156.6)/74.7 (56–93.5) |
| imipenem concentrations | |
| T0 | 212 |
| T1 | 160 |
| T2 | 58 |
| meropenem concentrations | |
| T0 | 26.8 (10.5–43.2)/23.6 (10.1–37.2) |
| T1 | 17.4 (5.7–29.2)/21.1 (8.8–33.5) |
| T2 | 10 (3.8–32)/11.9 (10.7–54.6) |
| Clinical and biological data prior to surgery | |
| leucocytes (×109/L), median (IQR) | 13.8 (9.4–19) |
| protein at T2 (g/L), median (IQR) | 50 (46–55) |
| C-reactive protein (mg/L), median (IQR) | 193 (123–312) |
| norepinephrine infusion, n (%) | 13 (27) |
| temperature (°C), median (IQR) | 37.5 (37–38) |
| Intraoperative data | |
| open surgery, n (%) | 44 (92) |
| vascular filling (mL), median (IQR) | 2000 (1375–2250) |
| norepinephrine maximal dose (μg/kg/min), median (IQR) | 0.3 (0.1–0.8) |
| septic shock, n (%) | 11 (23) |
| ICU characteristics | |
| mechanical ventilation at ICU admission, n (%) | 24 (50) |
| SOFA score at ICU admission, median (IQR) | 6 (2–10) |
| SAPS II, median (IQR) | 40 (32–65) |
| measured creatinine clearance (mL/min) at 24 hb, median (IQR) | 46.5 (20–87) |
| drain fluid output at T2 (mL), median (IQR) | 200 (100–250) |
| mortality at day 28, n (%) | 7 (15) |
| Microbiological data | |
| MIC of piperacillin/tazobactam, median (IQR) | 4 (4–4) |
| microbiological identification in peritoneal exudate, n (%) | |
| monobacterial | 7 (15) |
| polymicrobial | 23 (48) |
| fungal | 4 (8) |
| no identification | 14 (29) |
| Demographic data and medical history | |
| age (years), median (IQR) | 64 (54–74) |
| female, n (%) | 12 (25) |
| BMI (kg/m2), median (IQR) | 25.3 (20.5–30.3) |
| arterial hypertension, n (%) | 23 (48) |
| chronic renal failurea, n (%) | 14 (29) |
| ischaemic heart disease, n (%) | 10 (21) |
| IAI: type and localization, n (%) | |
| healthcare associated | 39 (81) |
| localization | |
| colon | 17 (35) |
| small intestine | 14 (29) |
| gastric | 6 (13) |
| hepato-biliary | 6 (13) |
| multiple localizations | 4 (8) |
| pancreatic | 1 (2) |
| β-Lactams administered, n (%) | |
| piperacillin/tazobactam | 36 (75) |
| carbapenem | 3 (6) |
| imipenem | 1 (2) |
| meropenem | 2 (4) |
| third-generation cephalosporin | 9 (19) |
| ceftriaxone | 6 (13) |
| cefotaxime | 3 (6) |
| β-Lactam total concentrations (mg/L), serum/peritoneal exudate, median (IQR) | |
| piperacillin concentrations | |
| T0 | 107.9 (62.2–179.9)/47.3 (19.1–84.5) |
| T1 | 70 (43.8–103.5)/69.3 (41.4–112.7) |
| T2 | 88 (51.5–116.3)/77.4 (56.8–108.7) |
| ceftriaxone concentrations | |
| T0 | 99.6 (78.3–135.7)/71.5 (33.9–112.8) |
| T1 | 58.8 (38.1–75.6)/60.1 (31.2–77.3) |
| T2 | 32.2 (19.2–70.6)/55.9 (27.8–91.4) |
| cefotaxime concentrations | |
| T0 | –/– |
| T1 | 114.5 (55.4–135.9)/71.1 (36.8–124.5) |
| T2 | 49 (45.9–156.6)/74.7 (56–93.5) |
| imipenem concentrations | |
| T0 | 212 |
| T1 | 160 |
| T2 | 58 |
| meropenem concentrations | |
| T0 | 26.8 (10.5–43.2)/23.6 (10.1–37.2) |
| T1 | 17.4 (5.7–29.2)/21.1 (8.8–33.5) |
| T2 | 10 (3.8–32)/11.9 (10.7–54.6) |
| Clinical and biological data prior to surgery | |
| leucocytes (×109/L), median (IQR) | 13.8 (9.4–19) |
| protein at T2 (g/L), median (IQR) | 50 (46–55) |
| C-reactive protein (mg/L), median (IQR) | 193 (123–312) |
| norepinephrine infusion, n (%) | 13 (27) |
| temperature (°C), median (IQR) | 37.5 (37–38) |
| Intraoperative data | |
| open surgery, n (%) | 44 (92) |
| vascular filling (mL), median (IQR) | 2000 (1375–2250) |
| norepinephrine maximal dose (μg/kg/min), median (IQR) | 0.3 (0.1–0.8) |
| septic shock, n (%) | 11 (23) |
| ICU characteristics | |
| mechanical ventilation at ICU admission, n (%) | 24 (50) |
| SOFA score at ICU admission, median (IQR) | 6 (2–10) |
| SAPS II, median (IQR) | 40 (32–65) |
| measured creatinine clearance (mL/min) at 24 hb, median (IQR) | 46.5 (20–87) |
| drain fluid output at T2 (mL), median (IQR) | 200 (100–250) |
| mortality at day 28, n (%) | 7 (15) |
| Microbiological data | |
| MIC of piperacillin/tazobactam, median (IQR) | 4 (4–4) |
| microbiological identification in peritoneal exudate, n (%) | |
| monobacterial | 7 (15) |
| polymicrobial | 23 (48) |
| fungal | 4 (8) |
| no identification | 14 (29) |
Chronic renal failure was defined as glomerular filtration rate <60 mL/min/1.73 m2 according to KDIGO recommendations (where KDIGO stands for Kidney Disease Improving Global Outcomes).
Creatinine clearance was measured using the formula (urinary creatinine×urinary volume)/plasma creatinine.
Primary outcome
Overall, 16 patients (33%) achieved the a priori PK/PD target (Figure 1). In the 23 patients with documented infection and MIC determination, the a posteriori PK/PD target was achieved in 18 patients (78%). Among these 18 patients, 6 (33%) achieved both a priori and a posteriori PK/PD.
Secondary outcomes
In the bivariate analysis (Table S2), high creatinine clearance was associated with an underdosage (P=0.01). The presence of septic shock (P=0.30), the presence of acute renal failure (P=0.40), vascular filling (P=0.61) and BMI (P=0.29) were not associated with underdosage. For patients who were still treated with norepinephrine after the end of surgery, less underdosage at T2 was observed (P=0.04).
In the multivariate analysis, age ≤64 years increased the risk of underdosage at T2 by 9.82-fold (OR=9.82; 95% CI=1.84–52.39; P=0.008) (Table S2).
Correlation between piperacillin/tazobactam serum and peritoneal concentrations
At T1, a trend of correlation was observed, with the Spearman coefficient calculated at 0.73 (P<0.0001) (Figure S3). The median ratio of serum to peritoneal exudate was 0.48 (0.24–0.82), 0.96 (0.88–1.4) and 0.88 (0.64–0.97) at T0, T1 and T2, respectively.
Discussion
Optimal β-lactam administration schemes ensured 100% fT>4×MIC in 78% of critically ill patients with severe IAIs within the first 24 h. Considering solely a PK/PD target based on ECOFF values, this rate would have decreased to 33%. This therapeutic scheme resulted in a favourable concentration in the peritoneal exudate. Age ≤64 years and high creatinine clearance were identified as risk factors for target non-attainment.
Dhaese et al.12 showed that in 253 critically ill patients, <40% of patients receiving continuous infusion of 16 g/day of piperacillin/tazobactam achieved the chosen PK/PD target. In their study, the PK/PD target was based on a worst-case scenario. One of the major findings of our study was the difference in target attainment between the a priori target (worst-case scenario) and the a posteriori target (documented MIC). The identification of microorganisms was available for 34 patients (71%) and the MIC for 23 patients. Consequently, the rate of target attainment in undocumented cases may be underestimated because most of the cases reached a concentration of unbound β-lactam >16 mg/L (corresponding to 4×MIC of 4 mg/L). As one of the pivotal measures of antimicrobial stewardship, the consideration of local ecology and resistance patterns is crucial.13 In a recent survey, having access to local bacterial resistance data was reported in >80% of cases.14 Herein, the median MIC of piperacillin/tazobactam was calculated at 4 (4–4) mg/L and very few MDR bacteria were identified.
Another finding was the favourable diffusion of piperacillin/tazobactam in the peritoneal exudate, contrary to the findings of a previous study.6 Indeed, while there was a large difference between serum and peritoneal concentrations (ratio of 0.48) at the onset, this difference was offset at T1 (ratio of 0.96) and T2 (ratio of 0.88) probably due to the use of continuous infusion. Further studies are needed to confirm the benefit from continuous infusion on peritoneal concentration. The other factors that could explain the favourable peritoneal diffusion were: (i) the type of IAI; (ii) a relatively low vascular filling; and (iii) a low drain fluid output.5
Limitations
First, because this study was a pragmatic and non-interventional study, we did not compare different dosing and/or administration schemes for the same antibiotic. Consequently, the rate of PK/PD target attainment observed here reflected real-life practices with an optimal dosing regimen. Second, free concentration was not measured but estimated. We acknowledge that the measurement of total antibiotic concentrations could lead to misinterpretation in the presence of hypoalbuminaemia, which is frequent in critically ill patients.15,16 Nevertheless, the most prescribed β-lactams were piperacillin/tazobactam and ceftriaxone. For piperacillin, the difference between estimated and measured free concentration was described in the case of low concentration (i.e. <50 mg/L), which was not the case here. For ceftriaxone, which is highly protein bound, the estimated free concentration was thought to be underestimated.16 Consequently, the resulting bias related to the estimation of free concentrations in terms of PK/PD objectives can be considered low.
Conclusions
In severe IAIs, high doses of β-lactams ensured 100% fT>4×MIC in the serum for 78% of critically ill patients within the first 24 h. In order to define optimal β-lactam dosing, the PK/PD target should take into account the tissue penetration and local ecology.
Acknowledgements
We thank all operating room staff of the University Hospital of Nancy for their invaluable help in blood and fluid sampling.
Funding
This work was funded by the Department of Anaesthesiology and Intensive Care Medicine of the University Hospital of Nancy.
Transparency declarations
None to declare.



