-
PDF
- Split View
-
Views
-
Cite
Cite
David W Wareham, M H F Abdul Momin, Lynette M Phee, Michael Hornsey, Joseph F Standing, Cefepime/sulbactam as an enhanced antimicrobial combination therapy for the treatment of MDR Gram-negative infections, Journal of Antimicrobial Chemotherapy, Volume 75, Issue 1, January 2020, Pages 135–139, https://doi.org/10.1093/jac/dkz420
Close - Share Icon Share
Abstract
β-Lactam (BL)/β-lactamase inhibitor (BLI) combinations are widely used for the treatment of Gram-negative infections. Cefepime has not been widely studied in combination with BLIs. Sulbactam, with dual BL/BLI activity, has been partnered with very few BLs. We investigated the potential of cefepime/sulbactam as an unorthodox BL/BLI combination against MDR Gram-negative bacteria.
In vitro activity of cefepime/sulbactam (1:1, 1:2 and 2:1) was assessed against 157 strains. Monte Carlo simulation was used to predict the PTA with a number of simulated cefepime combination regimens, modelled across putative cefepime/sulbactam breakpoints (≤16/≤0.25 mg/L).
Cefepime/sulbactam was more active (MIC50/MIC90 8/8–64/128 mg/L) compared with either drug alone (MIC50/MIC90 128 to >256 mg/L). Activity was enhanced when sulbactam was added at 1:1 or 1:2 (P<0.05). Reduction in MIC was most notable against Acinetobacter baumannii and Enterobacterales (MIC 8/8–32/64 mg/L). Pharmacokinetic/pharmacodynamic modelling highlighted that up to 48% of all isolates and 73% of carbapenem-resistant A. baumannii with a cefepime/sulbactam MIC of ≤16/≤8 mg/L may be treatable with a high-dose, fixed-ratio (1:1 or 1:2) combination of cefepime/sulbactam.
Cefepime/sulbactam (1:1 or 1:2) displays enhanced in vitro activity versus MDR Gram-negative pathogens. It could be a potential alternative to existing BL/BLI combinations for isolates with a cefepime/sulbactam MIC of 16/8 mg/L either as a definitive treatment or as a carbapenem-sparing option.
Introduction
β-Lactams (BLs) are the most widely used antibiotics in the empirical and targeted treatment of bacterial infections. Efficacy against many Gram-negative pathogens (Enterobacterales, Pseudomonas and Acinetobacter) is increasingly compromised by the emergence and spread of MDR strains that produce β-lactamases. These can confer resistance to one or more penicillin, cephalosporin, monobactam or carbapenem drugs routinely used in clinical practice.1 A potential solution is to combine BLs with β-lactamase inhibitors (BLIs). These include BLs such as clavulanic acid, tazobactam, sulbactam and the diazabicyclooctane BLIs avibactam, zidebactam and nacubactam; able to act either as direct or competitive suicide inhibitors of β-lactamase enzymes. Those licensed, and most widely used in the UK, are fixed-ratio combinations of amoxicillin/clavulanate (2:1), ticarcillin/clavulanate (15:1), piperacillin/tazobactam (8:1), ceftolozane/tazobactam (2:1), ampicillin/sulbactam (2:1) and ceftazidime/avibactam (4:1). Other BL/BLI combinations such as cefoperazone/sulbactam are available in some regions of the world (South and South-East Asia). There are also a number of novel combinations in the later stages of clinical development (aztreonam/avibactam, imipenem/relebactam, meropenem/vaborbactam, aztreonam/nacubactam and meropenem/nacubactam).2,3
None of the existing BL/BLI combinations has been shown to have reliable activity against all important BL-resistant species or provide functional inhibition of all clinically relevant β-lactamases (Table S1, available as Supplementary data at JAC Online). Resistance to BL/BLI combinations is further influenced by the permeability (porin), active efflux and target site modifications (PBP) typically found in MDR strains,4 along with the capacity of the BL component to induce or enhance the production of β-lactamases. With treatment options limited, clinicians are increasingly using unorthodox BL/BLI combination therapies, often as salvage treatments for MDR infections, especially those with resistance to carbapenems.5,6
Here, we undertook in vitro studies using a collection of contemporary MDR Gram-negative isolates to determine whether cefepime/sulbactam might be a useful combination therapy for development as a treatment for MDR Gram-negative infections.
Materials and methods
Isolates (n=157) were from the collection held at Queen Mary University of London, recovered from routine specimens submitted to Barts Health NHS Trust and associated London hospitals. Species identification was performed by MALDI-TOF MS (Bruker, Coventry, UK) with resistance to cephalosporins, carbapenems and monobactams determined by a combination of disc diffusion (ertapenem, imipenem, meropenem), the Microscan WalkAway System (Beckman Coulter, High Wycombe, UK) and Etest (bioMérieux, Basingstoke UK) interpreted according to current EUCAST/CLSI breakpoints. Genes encoding common class A (KPC, IMI), B (NDM, IMP, VIM) and D (OXA CHDL) β-lactamases were identified using a range of multiplex PCRs and WGS methods.7
Initial screens for cefepime/sulbactam synergy were performed by double-disc diffusion tests using cefepime (30 μg) and ampicillin/sulbactam (10 μg/10 μg) discs (Oxoid, Basingstoke, UK) placed 10–15 mm apart with >3 mm zones of expansion or ‘keyhole’ effects used to identify synergistic activity. See Figure S1.
Antibiotics (cefepime hydrochloride Lot no. LRA9570, sulbactam Lot no. 3100156) purchased from Sigma–Aldrich (Poole, UK) and Cambridge Bioscience Ltd (Cambridge, UK) were made as stock solutions of 10000 mg/L in PBS. MICs of cefepime, sulbactam and cefepime/sulbactam at 2:1, 1:1 and 1:2 ratios were determined by the agar dilution method using Mueller–Hinton agar, supplemented with doubling dilutions of cefepime/sulbactam from 0.125/0.0625 to 128/256 mg/L according to Andrews.8 Control organisms used in MIC determinations were ATCC 25922 (Escherichia coli), ATCC 27853 (Pseudomonas aeruginosa), ATCC 9633 (Klebsiella pneumoniae) and ATCC 19606 (Acinetobacter baumannii). Assays were only considered valid if the MIC of controls fell within ±1 dilution of the reference MIC.
The MIC distribution of cefepime combined with sulbactam (2:1, 1:1 and 1:2) was used to predict the likelihood of therapeutic success with a number of simulated cefepime dosing regimens. Monte Carlo simulation was performed in R using the linpk package. The cefepime pharmacokinetic model was taken from Jonckheere et al.,9 whereas the sulbactam model was taken from Soto et al.10 In both cases a two-compartment model was used and fraction unbound assumed to be 81% for cefepime and 62% for sulbactam. A population of 10000 adult ICU patients was sampled from a real adult demographic dataset, with a plot of the age, weight and creatinine clearance given in Figure S2.
The PTA was set at 60% fT>MIC at steady state for cefepime and sulbactam, with PTA for 1:1, 2:1 and 1:2 ratios compared across 36 possible cefepime/sulbactam dosing regimens (3–8 g/day) administered by bolus, extended infusion or continuous infusion. The proportion of isolates for which the PTA was achieved for both cefepime and sulbactam11 was compared by species and by the dosing regimen.
Results and discussion
A total of 157 cephalosporin/carbapenem-resistant E. coli (n=36), Klebsiella spp. (n=49), A. baumannii (n=66) and P. aeruginosa (n=6) were tested (Table 1). Synergy was observed in cefepime/sulbactam double disc diffusion assays with 73% of the E. coli and 78% of the A. baumannii isolates. Most isolates exhibited high-level resistance to both cefepime and sulbactam (MIC90 >256 mg/L) alone. The exception was for ESBL-producing E. coli and K. pneumoniae, which retained some susceptibility to cefepime (MIC50 ≤0.25–1 mg/L), and for A. baumannii, where an enhanced activity of sulbactam was observed (MIC50/90 16 to ≥256 mg/L). At a ratio of 2:1 the activity of cefepime/sulbactam was improved against ESBL producers (MIC50/90 2/1–64/32 mg/L), but had little effect on carbapenem-resistant Enterobacterales (MIC50/90 64/32 to ≥256/128 mg/L). A stepwise increase in the ratio of sulbactam to cefepime (1:1 to 1:2) resulted in a decrease in the cefepime MIC (Figure 1). This was most marked with respect to A. baumannii (MIC50/90 8/8–32/64 mg/L) and for all isolates with carbapenem resistance (MIC50/90 4/8–32/64 mg/L). Activity was most enhanced when sulbactam was added to cefepime at a concentration of 1:1 or 1:2 (P<0.05). Reduction in MIC was then most notable against carbapenem-resistant A. baumannii and Enterobacterales isolates harbouring OXA-like carbapenemases (MIC 8/8–32/64).
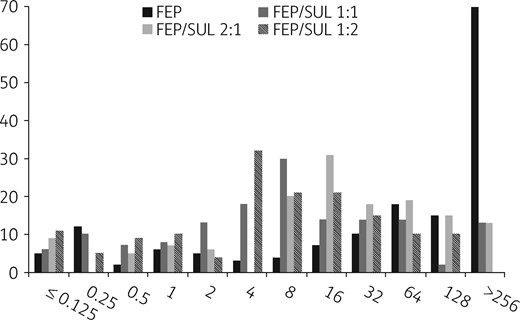
Distribution of cefepime MIC versus 157 MDR Gram-negative pathogens (either alone or combined with sulbactam at fixed ratios). FEP, cefepime; SUL, sulbactam.
In vitro activity of cefepime, sulbactam and cefepime/sulbactam fixed-ratio combinations (1:1, 2:1 and 1:2) versus MDR pathogens
| Pathogen . | Cefepime . | Sulbactam . | Cefepime/sulbactam 1:1 . | Cefepime/sulbactam 2:1 . | Cefepime/sulbactam 1:2 . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| MIC50 (mg/L) . | MIC90 (mg/L) . | MIC50 (mg/L) . | MIC90 (mg/L) . | MIC50 (mg/L) . | MIC90 (mg/L) . | MIC50 (mg/L) . | MIC90 (mg/L) . | MIC50 (mg/L) . | MIC90 (mg/L) . | |
| E. coli (n=36) | 4 | >256 | 32 | >256 | 1/1 | >256/256 | 8/4 | 128/64 | 0.5/1 | 16/32 |
| ESBL positive (28) | ≤0.25 | ≥256 | 32 | ≥256 | 1/1 | 64/64 | 8/4 | 64/32 | 0.5/1 | 32/64 |
| blaCTX-14/15, OXA-1 | ||||||||||
| carbapenem resistant (8) | 128 | ≥256 | 32 | ≥256 | 32/32 | ≥256/256 | 64/32 | ≥256/128 | 4/8 | 32/64 |
| blaOXA-48, NDM, IMI | ||||||||||
| Klebsiella spp. (n=49) | ≥256 | ≥256 | ≥256 | ≥256 | 32/32 | ≥256/256 | 64/32 | ≥256/128 | 16/32 | ≥128/256 |
| ESBL positive (7) | 1 | 32 | ≥256 | ≥256 | 1/1 | 4/4 | 2/2 | 16/8 | 1/2 | 4/8 |
| blaSHV, CTX-14/15, OXA-1 | ||||||||||
| carbapenem resistant (42) | ≥256 | ≥256 | ≥256 | ≥256 | 32/32 | ≥256/256 | 64/32 | ≥256/128 | 32/84 | ≥128/256 |
| blaNDM, KPC, VIM | ||||||||||
| A. baumannii (n=66) | 128 | >256 | 16 | >256 | 8/8 | 32/32 | 16/8 | 64/32 | 8/16 | 32/64 |
| carbapenem resistant (59) | ≥256 | ≥256 | 16 | ≥256 | 8/8 | 64/64 | 16/8 | 64/32 | 8/16 | 32/64 |
| blaOXA-23 | ||||||||||
| P. aeruginosa (n=6) | 4 | 16 | ≥256 | ≥256 | 4/4 | 8/8 | 2/1 | 16/8 | 4/8 | 16/32 |
| carbapenem resistant (2) | 2 | 2 | ≥256 | ≥256 | 2/2 | 2/2 | 1/0.5 | 1/0.5 | 2/4 | 16/32 |
| blaVIM-2 | ||||||||||
| Total (n=157) | 128 | >256 | 128 | >256 | 8/8 | 128/128 | 16/8 | 128/64 | 8/16 | 64/128 |
| Pathogen . | Cefepime . | Sulbactam . | Cefepime/sulbactam 1:1 . | Cefepime/sulbactam 2:1 . | Cefepime/sulbactam 1:2 . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| MIC50 (mg/L) . | MIC90 (mg/L) . | MIC50 (mg/L) . | MIC90 (mg/L) . | MIC50 (mg/L) . | MIC90 (mg/L) . | MIC50 (mg/L) . | MIC90 (mg/L) . | MIC50 (mg/L) . | MIC90 (mg/L) . | |
| E. coli (n=36) | 4 | >256 | 32 | >256 | 1/1 | >256/256 | 8/4 | 128/64 | 0.5/1 | 16/32 |
| ESBL positive (28) | ≤0.25 | ≥256 | 32 | ≥256 | 1/1 | 64/64 | 8/4 | 64/32 | 0.5/1 | 32/64 |
| blaCTX-14/15, OXA-1 | ||||||||||
| carbapenem resistant (8) | 128 | ≥256 | 32 | ≥256 | 32/32 | ≥256/256 | 64/32 | ≥256/128 | 4/8 | 32/64 |
| blaOXA-48, NDM, IMI | ||||||||||
| Klebsiella spp. (n=49) | ≥256 | ≥256 | ≥256 | ≥256 | 32/32 | ≥256/256 | 64/32 | ≥256/128 | 16/32 | ≥128/256 |
| ESBL positive (7) | 1 | 32 | ≥256 | ≥256 | 1/1 | 4/4 | 2/2 | 16/8 | 1/2 | 4/8 |
| blaSHV, CTX-14/15, OXA-1 | ||||||||||
| carbapenem resistant (42) | ≥256 | ≥256 | ≥256 | ≥256 | 32/32 | ≥256/256 | 64/32 | ≥256/128 | 32/84 | ≥128/256 |
| blaNDM, KPC, VIM | ||||||||||
| A. baumannii (n=66) | 128 | >256 | 16 | >256 | 8/8 | 32/32 | 16/8 | 64/32 | 8/16 | 32/64 |
| carbapenem resistant (59) | ≥256 | ≥256 | 16 | ≥256 | 8/8 | 64/64 | 16/8 | 64/32 | 8/16 | 32/64 |
| blaOXA-23 | ||||||||||
| P. aeruginosa (n=6) | 4 | 16 | ≥256 | ≥256 | 4/4 | 8/8 | 2/1 | 16/8 | 4/8 | 16/32 |
| carbapenem resistant (2) | 2 | 2 | ≥256 | ≥256 | 2/2 | 2/2 | 1/0.5 | 1/0.5 | 2/4 | 16/32 |
| blaVIM-2 | ||||||||||
| Total (n=157) | 128 | >256 | 128 | >256 | 8/8 | 128/128 | 16/8 | 128/64 | 8/16 | 64/128 |
In vitro activity of cefepime, sulbactam and cefepime/sulbactam fixed-ratio combinations (1:1, 2:1 and 1:2) versus MDR pathogens
| Pathogen . | Cefepime . | Sulbactam . | Cefepime/sulbactam 1:1 . | Cefepime/sulbactam 2:1 . | Cefepime/sulbactam 1:2 . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| MIC50 (mg/L) . | MIC90 (mg/L) . | MIC50 (mg/L) . | MIC90 (mg/L) . | MIC50 (mg/L) . | MIC90 (mg/L) . | MIC50 (mg/L) . | MIC90 (mg/L) . | MIC50 (mg/L) . | MIC90 (mg/L) . | |
| E. coli (n=36) | 4 | >256 | 32 | >256 | 1/1 | >256/256 | 8/4 | 128/64 | 0.5/1 | 16/32 |
| ESBL positive (28) | ≤0.25 | ≥256 | 32 | ≥256 | 1/1 | 64/64 | 8/4 | 64/32 | 0.5/1 | 32/64 |
| blaCTX-14/15, OXA-1 | ||||||||||
| carbapenem resistant (8) | 128 | ≥256 | 32 | ≥256 | 32/32 | ≥256/256 | 64/32 | ≥256/128 | 4/8 | 32/64 |
| blaOXA-48, NDM, IMI | ||||||||||
| Klebsiella spp. (n=49) | ≥256 | ≥256 | ≥256 | ≥256 | 32/32 | ≥256/256 | 64/32 | ≥256/128 | 16/32 | ≥128/256 |
| ESBL positive (7) | 1 | 32 | ≥256 | ≥256 | 1/1 | 4/4 | 2/2 | 16/8 | 1/2 | 4/8 |
| blaSHV, CTX-14/15, OXA-1 | ||||||||||
| carbapenem resistant (42) | ≥256 | ≥256 | ≥256 | ≥256 | 32/32 | ≥256/256 | 64/32 | ≥256/128 | 32/84 | ≥128/256 |
| blaNDM, KPC, VIM | ||||||||||
| A. baumannii (n=66) | 128 | >256 | 16 | >256 | 8/8 | 32/32 | 16/8 | 64/32 | 8/16 | 32/64 |
| carbapenem resistant (59) | ≥256 | ≥256 | 16 | ≥256 | 8/8 | 64/64 | 16/8 | 64/32 | 8/16 | 32/64 |
| blaOXA-23 | ||||||||||
| P. aeruginosa (n=6) | 4 | 16 | ≥256 | ≥256 | 4/4 | 8/8 | 2/1 | 16/8 | 4/8 | 16/32 |
| carbapenem resistant (2) | 2 | 2 | ≥256 | ≥256 | 2/2 | 2/2 | 1/0.5 | 1/0.5 | 2/4 | 16/32 |
| blaVIM-2 | ||||||||||
| Total (n=157) | 128 | >256 | 128 | >256 | 8/8 | 128/128 | 16/8 | 128/64 | 8/16 | 64/128 |
| Pathogen . | Cefepime . | Sulbactam . | Cefepime/sulbactam 1:1 . | Cefepime/sulbactam 2:1 . | Cefepime/sulbactam 1:2 . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| MIC50 (mg/L) . | MIC90 (mg/L) . | MIC50 (mg/L) . | MIC90 (mg/L) . | MIC50 (mg/L) . | MIC90 (mg/L) . | MIC50 (mg/L) . | MIC90 (mg/L) . | MIC50 (mg/L) . | MIC90 (mg/L) . | |
| E. coli (n=36) | 4 | >256 | 32 | >256 | 1/1 | >256/256 | 8/4 | 128/64 | 0.5/1 | 16/32 |
| ESBL positive (28) | ≤0.25 | ≥256 | 32 | ≥256 | 1/1 | 64/64 | 8/4 | 64/32 | 0.5/1 | 32/64 |
| blaCTX-14/15, OXA-1 | ||||||||||
| carbapenem resistant (8) | 128 | ≥256 | 32 | ≥256 | 32/32 | ≥256/256 | 64/32 | ≥256/128 | 4/8 | 32/64 |
| blaOXA-48, NDM, IMI | ||||||||||
| Klebsiella spp. (n=49) | ≥256 | ≥256 | ≥256 | ≥256 | 32/32 | ≥256/256 | 64/32 | ≥256/128 | 16/32 | ≥128/256 |
| ESBL positive (7) | 1 | 32 | ≥256 | ≥256 | 1/1 | 4/4 | 2/2 | 16/8 | 1/2 | 4/8 |
| blaSHV, CTX-14/15, OXA-1 | ||||||||||
| carbapenem resistant (42) | ≥256 | ≥256 | ≥256 | ≥256 | 32/32 | ≥256/256 | 64/32 | ≥256/128 | 32/84 | ≥128/256 |
| blaNDM, KPC, VIM | ||||||||||
| A. baumannii (n=66) | 128 | >256 | 16 | >256 | 8/8 | 32/32 | 16/8 | 64/32 | 8/16 | 32/64 |
| carbapenem resistant (59) | ≥256 | ≥256 | 16 | ≥256 | 8/8 | 64/64 | 16/8 | 64/32 | 8/16 | 32/64 |
| blaOXA-23 | ||||||||||
| P. aeruginosa (n=6) | 4 | 16 | ≥256 | ≥256 | 4/4 | 8/8 | 2/1 | 16/8 | 4/8 | 16/32 |
| carbapenem resistant (2) | 2 | 2 | ≥256 | ≥256 | 2/2 | 2/2 | 1/0.5 | 1/0.5 | 2/4 | 16/32 |
| blaVIM-2 | ||||||||||
| Total (n=157) | 128 | >256 | 128 | >256 | 8/8 | 128/128 | 16/8 | 128/64 | 8/16 | 64/128 |
The probability of individualized cefepime/sulbactam dosing regimens achieving the cefepime/sulbactam pharmacokinetic/pharmacodynamic target of 60% fT>MIC at each ratio is shown in Table 2. Up to 48% of all isolates and 73% of carbapenem-resistant A. baumannii with a cefepime/sulbactam MIC of ≤16/≤8 mg/L were predicted to be treatable with a high-dose (6–8 g/day) cefepime/sulbactam (1:1 or 1:2) combination. Furthermore, if a cefepime/sulbactam (>1:1) regimen of 8 g/day was administered by continuous or extended infusion, efficacy against 62% of the carbapenem-resistant organisms tested is predicted for those with a cefepime/sulbactam MIC of up to 16/16 mg/L (Figure S3).
Susceptibility of carbapenem-resistant strains to simulated cefepime (3–8 g/day)/sulbactam (1:1, 1:2 and 2:1) dosing regimens; PTA (>0.9) for isolates with MIC ≤2 to ≤16 mg/L
| Dosing regimen . | Cefepime/sulbactam target MIC (mg/L), 60% fT>MIC . | Percentage susceptible . | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E. coli . | Klebsiella spp. . | A. baumannii . | P. aeruginosa . | all isolates . | |||||||||||||||
| cefepime/ sulbactam ratio . | cefepime/ sulbactam ratio . | cefepime/ sulbactam ratio . | cefepime/ sulbactam ratio . | cefepime/ sulbactam ratio . | |||||||||||||||
| 1:1 . | 2:1 . | 1:2 . | 1:1 . | 2:1 . | 1:2 . | 1:1 . | 2:1 . | 1:2 . | 1:1 . | 2:1 . | 1:2 . | 1:1 . | 2:1 . | 1:2 . | |||||
| sulbactam 3 g | sulbactam 1.5 g | sulbactam 6 g | |||||||||||||||||
| Cefepime 3 g | bolus | ≤2/≤0.25 | ≤2/≤0.25 | ≤2/≤0.5 | 33 | 40 | 53 | 6 | 10 | 10 | 5 | 3 | 5 | 0 | 14 | 0 | 11 | 10 | 11 |
| extended infusion | ≤4/≤1 | ≤4/≤0.5 | ≤4/≤2 | 53 | 43 | 73 | 17 | 12 | 17 | 9 | 6 | 8 | 14 | 43 | 0 | 21 | 15 | 24 | |
| continuous infusion | ≤4/≤2 | ≤4/≤1 | ≤4/≤4 | 60 | 46 | 73 | 23 | 19 | 17 | 19 | 9 | 9 | 43 | 57 | 29 | 30 | 19 | 26 | |
| sulbactam 4 g | sulbactam 2 g | sulbactam 8 g | |||||||||||||||||
| Cefepime 4 g | bolus | ≤4/≤0.5 | ≤4/≤0.25 | ≤4/≤1 | 47 | 40 | 53 | 12 | 10 | 10 | 5 | 6 | 6 | 0 | 14 | 0 | 15 | 10 | 17 |
| extended infusion | ≤8/≤4 | ≤8/≤2 | ≤8/≤8 | 63 | 50 | 83 | 31 | 19 | 25 | 24 | 9 | 47 | 57 | 57 | 43 | 42 | 19 | 48 | |
| continuous infusion | ≤8/≤4 | ≤8/≤2 | ≤8/≤8 | 63 | 50 | 83 | 31 | 19 | 25 | 24 | 9 | 47 | 57 | 57 | 43 | 42 | 19 | 48 | |
| sulbactam 6 g | sulbactam 3 g | sulbactam 12 g | |||||||||||||||||
| Cefepime 6 g | bolus | ≤4/≤0.5 | ≤4/≤0.5 | ≤4/≤1 | 47 | 43 | 53 | 12 | 12 | 10 | 5 | 6 | 6 | 0 | 43 | 0 | 15 | 15 | 17 |
| extended infusion | ≤8/≤2 | ≤8/≤1 | ≤8/≤4 | 60 | 46 | 73 | 23 | 19 | 25 | 19 | 6 | 47 | 43 | 57 | 29 | 30 | 19 | 26 | |
| continuous infusion | ≤8/≤4 | ≤8/≤8 | ≤8/≤8 | 63 | 63 | 83 | 31 | 23 | 25 | 38 | 28 | 47 | 57 | 86 | 43 | 42 | 33 | 48 | |
| sulbactam 8 g | sulbactam 4 g | sulbactam 16 g | |||||||||||||||||
| Cefepime 8 g | bolus | ≤8/≤1 | ≤8/≤0.5 | ≤8/≤2 | 53 | 43 | 73 | 16 | 15 | 17 | 9 | 6 | 47 | 14 | 43 | 29 | 21 | 15 | 27 |
| extended infusion | ≤16/≤8 | ≤16/≤4 | ≤16/≤8 | 67 | 63 | 83 | 37 | 23 | 33 | 73 | 28 | 73 | 100 | 86 | 43 | 62 | 47 | 48 | |
| continuous infusion | ≤16/≤8 | ≤16/≤4 | ≤16/≤16 | 67 | 63 | 83 | 37 | 23 | 33 | 73 | 28 | 73 | 100 | 86 | 57 | 62 | 47 | 62 | |
| Dosing regimen . | Cefepime/sulbactam target MIC (mg/L), 60% fT>MIC . | Percentage susceptible . | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E. coli . | Klebsiella spp. . | A. baumannii . | P. aeruginosa . | all isolates . | |||||||||||||||
| cefepime/ sulbactam ratio . | cefepime/ sulbactam ratio . | cefepime/ sulbactam ratio . | cefepime/ sulbactam ratio . | cefepime/ sulbactam ratio . | |||||||||||||||
| 1:1 . | 2:1 . | 1:2 . | 1:1 . | 2:1 . | 1:2 . | 1:1 . | 2:1 . | 1:2 . | 1:1 . | 2:1 . | 1:2 . | 1:1 . | 2:1 . | 1:2 . | |||||
| sulbactam 3 g | sulbactam 1.5 g | sulbactam 6 g | |||||||||||||||||
| Cefepime 3 g | bolus | ≤2/≤0.25 | ≤2/≤0.25 | ≤2/≤0.5 | 33 | 40 | 53 | 6 | 10 | 10 | 5 | 3 | 5 | 0 | 14 | 0 | 11 | 10 | 11 |
| extended infusion | ≤4/≤1 | ≤4/≤0.5 | ≤4/≤2 | 53 | 43 | 73 | 17 | 12 | 17 | 9 | 6 | 8 | 14 | 43 | 0 | 21 | 15 | 24 | |
| continuous infusion | ≤4/≤2 | ≤4/≤1 | ≤4/≤4 | 60 | 46 | 73 | 23 | 19 | 17 | 19 | 9 | 9 | 43 | 57 | 29 | 30 | 19 | 26 | |
| sulbactam 4 g | sulbactam 2 g | sulbactam 8 g | |||||||||||||||||
| Cefepime 4 g | bolus | ≤4/≤0.5 | ≤4/≤0.25 | ≤4/≤1 | 47 | 40 | 53 | 12 | 10 | 10 | 5 | 6 | 6 | 0 | 14 | 0 | 15 | 10 | 17 |
| extended infusion | ≤8/≤4 | ≤8/≤2 | ≤8/≤8 | 63 | 50 | 83 | 31 | 19 | 25 | 24 | 9 | 47 | 57 | 57 | 43 | 42 | 19 | 48 | |
| continuous infusion | ≤8/≤4 | ≤8/≤2 | ≤8/≤8 | 63 | 50 | 83 | 31 | 19 | 25 | 24 | 9 | 47 | 57 | 57 | 43 | 42 | 19 | 48 | |
| sulbactam 6 g | sulbactam 3 g | sulbactam 12 g | |||||||||||||||||
| Cefepime 6 g | bolus | ≤4/≤0.5 | ≤4/≤0.5 | ≤4/≤1 | 47 | 43 | 53 | 12 | 12 | 10 | 5 | 6 | 6 | 0 | 43 | 0 | 15 | 15 | 17 |
| extended infusion | ≤8/≤2 | ≤8/≤1 | ≤8/≤4 | 60 | 46 | 73 | 23 | 19 | 25 | 19 | 6 | 47 | 43 | 57 | 29 | 30 | 19 | 26 | |
| continuous infusion | ≤8/≤4 | ≤8/≤8 | ≤8/≤8 | 63 | 63 | 83 | 31 | 23 | 25 | 38 | 28 | 47 | 57 | 86 | 43 | 42 | 33 | 48 | |
| sulbactam 8 g | sulbactam 4 g | sulbactam 16 g | |||||||||||||||||
| Cefepime 8 g | bolus | ≤8/≤1 | ≤8/≤0.5 | ≤8/≤2 | 53 | 43 | 73 | 16 | 15 | 17 | 9 | 6 | 47 | 14 | 43 | 29 | 21 | 15 | 27 |
| extended infusion | ≤16/≤8 | ≤16/≤4 | ≤16/≤8 | 67 | 63 | 83 | 37 | 23 | 33 | 73 | 28 | 73 | 100 | 86 | 43 | 62 | 47 | 48 | |
| continuous infusion | ≤16/≤8 | ≤16/≤4 | ≤16/≤16 | 67 | 63 | 83 | 37 | 23 | 33 | 73 | 28 | 73 | 100 | 86 | 57 | 62 | 47 | 62 | |
Susceptibility of carbapenem-resistant strains to simulated cefepime (3–8 g/day)/sulbactam (1:1, 1:2 and 2:1) dosing regimens; PTA (>0.9) for isolates with MIC ≤2 to ≤16 mg/L
| Dosing regimen . | Cefepime/sulbactam target MIC (mg/L), 60% fT>MIC . | Percentage susceptible . | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E. coli . | Klebsiella spp. . | A. baumannii . | P. aeruginosa . | all isolates . | |||||||||||||||
| cefepime/ sulbactam ratio . | cefepime/ sulbactam ratio . | cefepime/ sulbactam ratio . | cefepime/ sulbactam ratio . | cefepime/ sulbactam ratio . | |||||||||||||||
| 1:1 . | 2:1 . | 1:2 . | 1:1 . | 2:1 . | 1:2 . | 1:1 . | 2:1 . | 1:2 . | 1:1 . | 2:1 . | 1:2 . | 1:1 . | 2:1 . | 1:2 . | |||||
| sulbactam 3 g | sulbactam 1.5 g | sulbactam 6 g | |||||||||||||||||
| Cefepime 3 g | bolus | ≤2/≤0.25 | ≤2/≤0.25 | ≤2/≤0.5 | 33 | 40 | 53 | 6 | 10 | 10 | 5 | 3 | 5 | 0 | 14 | 0 | 11 | 10 | 11 |
| extended infusion | ≤4/≤1 | ≤4/≤0.5 | ≤4/≤2 | 53 | 43 | 73 | 17 | 12 | 17 | 9 | 6 | 8 | 14 | 43 | 0 | 21 | 15 | 24 | |
| continuous infusion | ≤4/≤2 | ≤4/≤1 | ≤4/≤4 | 60 | 46 | 73 | 23 | 19 | 17 | 19 | 9 | 9 | 43 | 57 | 29 | 30 | 19 | 26 | |
| sulbactam 4 g | sulbactam 2 g | sulbactam 8 g | |||||||||||||||||
| Cefepime 4 g | bolus | ≤4/≤0.5 | ≤4/≤0.25 | ≤4/≤1 | 47 | 40 | 53 | 12 | 10 | 10 | 5 | 6 | 6 | 0 | 14 | 0 | 15 | 10 | 17 |
| extended infusion | ≤8/≤4 | ≤8/≤2 | ≤8/≤8 | 63 | 50 | 83 | 31 | 19 | 25 | 24 | 9 | 47 | 57 | 57 | 43 | 42 | 19 | 48 | |
| continuous infusion | ≤8/≤4 | ≤8/≤2 | ≤8/≤8 | 63 | 50 | 83 | 31 | 19 | 25 | 24 | 9 | 47 | 57 | 57 | 43 | 42 | 19 | 48 | |
| sulbactam 6 g | sulbactam 3 g | sulbactam 12 g | |||||||||||||||||
| Cefepime 6 g | bolus | ≤4/≤0.5 | ≤4/≤0.5 | ≤4/≤1 | 47 | 43 | 53 | 12 | 12 | 10 | 5 | 6 | 6 | 0 | 43 | 0 | 15 | 15 | 17 |
| extended infusion | ≤8/≤2 | ≤8/≤1 | ≤8/≤4 | 60 | 46 | 73 | 23 | 19 | 25 | 19 | 6 | 47 | 43 | 57 | 29 | 30 | 19 | 26 | |
| continuous infusion | ≤8/≤4 | ≤8/≤8 | ≤8/≤8 | 63 | 63 | 83 | 31 | 23 | 25 | 38 | 28 | 47 | 57 | 86 | 43 | 42 | 33 | 48 | |
| sulbactam 8 g | sulbactam 4 g | sulbactam 16 g | |||||||||||||||||
| Cefepime 8 g | bolus | ≤8/≤1 | ≤8/≤0.5 | ≤8/≤2 | 53 | 43 | 73 | 16 | 15 | 17 | 9 | 6 | 47 | 14 | 43 | 29 | 21 | 15 | 27 |
| extended infusion | ≤16/≤8 | ≤16/≤4 | ≤16/≤8 | 67 | 63 | 83 | 37 | 23 | 33 | 73 | 28 | 73 | 100 | 86 | 43 | 62 | 47 | 48 | |
| continuous infusion | ≤16/≤8 | ≤16/≤4 | ≤16/≤16 | 67 | 63 | 83 | 37 | 23 | 33 | 73 | 28 | 73 | 100 | 86 | 57 | 62 | 47 | 62 | |
| Dosing regimen . | Cefepime/sulbactam target MIC (mg/L), 60% fT>MIC . | Percentage susceptible . | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E. coli . | Klebsiella spp. . | A. baumannii . | P. aeruginosa . | all isolates . | |||||||||||||||
| cefepime/ sulbactam ratio . | cefepime/ sulbactam ratio . | cefepime/ sulbactam ratio . | cefepime/ sulbactam ratio . | cefepime/ sulbactam ratio . | |||||||||||||||
| 1:1 . | 2:1 . | 1:2 . | 1:1 . | 2:1 . | 1:2 . | 1:1 . | 2:1 . | 1:2 . | 1:1 . | 2:1 . | 1:2 . | 1:1 . | 2:1 . | 1:2 . | |||||
| sulbactam 3 g | sulbactam 1.5 g | sulbactam 6 g | |||||||||||||||||
| Cefepime 3 g | bolus | ≤2/≤0.25 | ≤2/≤0.25 | ≤2/≤0.5 | 33 | 40 | 53 | 6 | 10 | 10 | 5 | 3 | 5 | 0 | 14 | 0 | 11 | 10 | 11 |
| extended infusion | ≤4/≤1 | ≤4/≤0.5 | ≤4/≤2 | 53 | 43 | 73 | 17 | 12 | 17 | 9 | 6 | 8 | 14 | 43 | 0 | 21 | 15 | 24 | |
| continuous infusion | ≤4/≤2 | ≤4/≤1 | ≤4/≤4 | 60 | 46 | 73 | 23 | 19 | 17 | 19 | 9 | 9 | 43 | 57 | 29 | 30 | 19 | 26 | |
| sulbactam 4 g | sulbactam 2 g | sulbactam 8 g | |||||||||||||||||
| Cefepime 4 g | bolus | ≤4/≤0.5 | ≤4/≤0.25 | ≤4/≤1 | 47 | 40 | 53 | 12 | 10 | 10 | 5 | 6 | 6 | 0 | 14 | 0 | 15 | 10 | 17 |
| extended infusion | ≤8/≤4 | ≤8/≤2 | ≤8/≤8 | 63 | 50 | 83 | 31 | 19 | 25 | 24 | 9 | 47 | 57 | 57 | 43 | 42 | 19 | 48 | |
| continuous infusion | ≤8/≤4 | ≤8/≤2 | ≤8/≤8 | 63 | 50 | 83 | 31 | 19 | 25 | 24 | 9 | 47 | 57 | 57 | 43 | 42 | 19 | 48 | |
| sulbactam 6 g | sulbactam 3 g | sulbactam 12 g | |||||||||||||||||
| Cefepime 6 g | bolus | ≤4/≤0.5 | ≤4/≤0.5 | ≤4/≤1 | 47 | 43 | 53 | 12 | 12 | 10 | 5 | 6 | 6 | 0 | 43 | 0 | 15 | 15 | 17 |
| extended infusion | ≤8/≤2 | ≤8/≤1 | ≤8/≤4 | 60 | 46 | 73 | 23 | 19 | 25 | 19 | 6 | 47 | 43 | 57 | 29 | 30 | 19 | 26 | |
| continuous infusion | ≤8/≤4 | ≤8/≤8 | ≤8/≤8 | 63 | 63 | 83 | 31 | 23 | 25 | 38 | 28 | 47 | 57 | 86 | 43 | 42 | 33 | 48 | |
| sulbactam 8 g | sulbactam 4 g | sulbactam 16 g | |||||||||||||||||
| Cefepime 8 g | bolus | ≤8/≤1 | ≤8/≤0.5 | ≤8/≤2 | 53 | 43 | 73 | 16 | 15 | 17 | 9 | 6 | 47 | 14 | 43 | 29 | 21 | 15 | 27 |
| extended infusion | ≤16/≤8 | ≤16/≤4 | ≤16/≤8 | 67 | 63 | 83 | 37 | 23 | 33 | 73 | 28 | 73 | 100 | 86 | 43 | 62 | 47 | 48 | |
| continuous infusion | ≤16/≤8 | ≤16/≤4 | ≤16/≤16 | 67 | 63 | 83 | 37 | 23 | 33 | 73 | 28 | 73 | 100 | 86 | 57 | 62 | 47 | 62 | |
These in vitro activity data suggest that cefepime/sulbactam could be developed as a BL/BLI-based treatment for some MDR Gram-negative infections. There are a number of reasons to progress it as a preferred combination, but also some challenges.
Cefepime monotherapy has been licensed and used for decades in the treatment of bacterial infections. There is a wealth of data on its efficacy, safety and tolerability, including at high doses for the treatment of susceptible Gram-negative infections. As the primary component of a BL/BLI therapy, cefepime also offers some advantages over other cephalosporins (cefoperazone, ceftazidime). Of note, it is stable to hydrolysis by many class C (AmpC) β-lactamases and, carrying a neutral (zwitterionic) charge, is less affected by permeability (porin)- and efflux-mediated resistance mechanisms.12 The potential of cefepime is evident from recent studies assessing its activity in combination with tazobactam,13 enmetazobactam (AAI101),14 zidebactam,15 avibactam16 and nacubactam3 as BLIs. These all demonstrate in vitro activity against MDR Gram-negatives that produce ESBLs and/or carbapenemases comparable to that which we have observed with cefepime/sulbactam.
Sulbactam, a BL, is licensed and used as a competitive BLI usually in combination with ampicillin or cefoperazone. It also has intrinsic antimicrobial activity, through inhibition of PBPs, with most affinity for PBP1a and 2. The ability to inhibit PBP2 makes it particularly active against A. baumannii, including those with carbapenem resistance.17
Sulbactam is susceptible to hydrolysis by most class A (TEM, SHV, CTX-M, KPC), B (IMP, VIM, NDM) and D (OXA-10, 23, 24, 48) β-lactamases, but appears relatively stable to many class C (AmpC-like) enzymes.17,18 Although the majority of the carbapenem-resistant A. baumannii we assessed here were positive for blaOXA-23, we still observed a significant increase in the activity of a cefepime/sulbactam combination. This could in part be due to preferential hydrolysis of sulbactam and preservation of enough cefepime activity to withstand degradation by Acinetobacter ADC cephalosporinases. This contrasts with the activity of cefepime/sulbactam we saw against carbapenem-resistant E. coli and K. pneumoniae, whereby the activity of both cefepime and sulbactam is likely compromised by the co-production of class A (CTX-M, KPC) and B (NDM, VIM) ESBLs and carbapenemases. Sulbactam has little intrinsic activity against P. aeruginosa and did not seem to enhance the activity of high-dose cefepime in vitro (Table 2).
The importance of the ratio of BL to sulbactam is evident from studies of cefoperazone/sulbactam, most widely available as a 2:1 formulation. Adjusting the cefoperazone/sulbactam ratio to 1:1 or 1:2 increases the in vitro susceptibility of ESBL-producing E. coli and carbapenem-resistant A. baumannii by up to 90%.19,20 Furthermore, meta-analysis of clinical studies identifies the importance of higher doses of sulbactam when combined with ampicillin or cefoperazone.21 This is entirely in keeping with our findings for cefepime/sulbactam, in which a 1:1 or 1:2 ratio is optimal. Whether higher ratios (1:3) are likely to be more effective would require synthesis of enzyme kinetic and MIC data on a strain-by-strain basis.
From the pharmacokinetic/pharmacodynamic modelling analysis, both a 1:1 and a 1:2 cefepime/sulbactam therapy would require dosing at the upper range of both drugs to provide useful activity against carbapenem-resistant strains. Cefepime has been safely used at 8 g/day and sulbactam at 9 g/8 h in the treatment of bloodstream infections and pneumonia.18 A combined cefepime/sulbactam dosing regimen of 8 g/8 g should enable treatment of ESBL-producing and carbapenem-resistant isolates with cefepime/sulbactam MIC up to 16 mg/L.
Effective targeted antimicrobial therapy is fundamental in the treatment of Gram-negative sepsis. The increasing prevalence of ESBLs in Enterobacterales has led to increased empirical use of carbapenems, a strategy that further drives carbapenem resistance. Existing BL/BLI therapies, in the formulations and doses currently used, are increasingly shown to be suboptimal in severe infections as alternatives to carbapenems.22
Given the current challenges in antimicrobial drug development, it is unlikely that all of the cefepime/BLI therapies currently under investigation will enter widespread clinical use. The data for cefepime/sulbactam suggest it could be most useful to progress as a 1:1 formulation targeting ESBLs and in particular carbapenem-resistant Acinetobacter infections. It could also be employed as a BL/BLI carbapenem-sparing agent that still retains some useful activity against emerging carbapenem-resistant strains.
Acknowledgements
We would like to thank members of ANTRUK’s Science Committee for their valuable input into both the design and interpretation of the studies reported here.
Funding
This study was funded by an award from Antibiotic Research UK (ANTRUK) a UK registered charity (no. 1157884). J.F.S. was supported by a United Kingdom Medical Research Council Fellowship (MRC grant MR/M008665/1).
Transparency declarations
D.W.W. is a member of the Scientific Committee of ANTRUK and has served on advisory boards for Shionogi and Pfizer. All other authors: none to declare.



