-
PDF
- Split View
-
Views
-
Cite
Cite
Melissa G McCracken, Heather J Adam, Joseph M Blondeau, Andrew J Walkty, James A Karlowsky, Daryl J Hoban, George G Zhanel, Michael R Mulvey, Canadian Antimicrobial Resistance Alliance (CARA) and CANWARD , Characterization of carbapenem-resistant and XDR Pseudomonas aeruginosa in Canada: results of the CANWARD 2007–16 study, Journal of Antimicrobial Chemotherapy, Volume 74, Issue Supplement_4, August 2019, Pages iv32–iv38, https://doi.org/10.1093/jac/dkz285
Close - Share Icon Share
Abstract
Carbapenem-resistant Pseudomonas aeruginosa are emerging worldwide with increasing reports of carbapenemase-producing isolates. Carbapenem-resistant isolates may also be XDR. This study characterized carbapenem-resistant and XDR P. aeruginosa isolated from patients receiving care at Canadian hospitals from 2007 to 2016.
Antimicrobial susceptibility testing was performed using CLSI broth microdilution methods. PCR was used to detect carbapenemases (GES, KPC, NDM, IMP, VIM, OXA-48) and other resistance markers; specific carbapenemase gene variants were identified by DNA sequencing. Genetic relatedness was assessed by MLST and PFGE.
From 2007 to 2016, 3864 isolates of P. aeruginosa were collected; 466 (12.1%) isolates were carbapenem resistant. The prevalence of carbapenem-resistant P. aeruginosa reached a peak of 17.3% in 2014. Colistin (94% susceptible) and ceftolozane/tazobactam (92.5%) were the most active agents against carbapenem-resistant P. aeruginosa. XDR P. aeruginosa comprised 4.5% of isolates; they were found to be genetically diverse and remained susceptible to colistin and ceftolozane/tazobactam. Only 4.3% (n = 20) of carbapenem-resistant P. aeruginosa harboured a carbapenemase; most were blaGES-5 (35%, n = 7). Wide genetic diversity was observed among carbapenem-resistant P. aeruginosa with >200 different sequence types identified.
Although the prevalence of carbapenem-resistant P. aeruginosa in Canada spiked in 2014 and 2015, carbapenemase-producing P. aeruginosa remain rare with only 20 (4.3%) isolates identified over a 10 year period. Broad genetic diversity was observed among both carbapenem-resistant and XDR phenotypes of P. aeruginosa. Pan-drug-resistant P. aeruginosa have not yet been identified in Canada.
Introduction
Pseudomonas aeruginosa is an aerobic, Gram-negative bacillus often implicated in nosocomial infections.1,2P. aeruginosa may demonstrate both intrinsic and acquired resistance mechanisms and some clinical isolates may be pan-resistant to all currently marketed antimicrobial classes.
Carbapenems are commonly used to treat P. aeruginosa infections.3 However, carbapenem resistance in P. aeruginosa is not unusual.4,5 Carbapenem resistance can be mediated by overproduction of multidrug efflux pumps, alteration of outer-membrane porins or elevated AmpC production, or a combination of these mechanisms.3,5,6 To add to the complexity of carbapenem resistance in this organism, there are increasingly frequent reports of clinical isolates of P. aeruginosa acquiring carbapenemase genes.3,5–7 These carbapenemases include IMP, VIM, KPC, GES, NDM, GIM and SPM.8,9
Carbapenem-resistant P. aeruginosa can also be XDR. Although the definitions of XDR may vary slightly worldwide, there is little doubt XDR P. aeruginosa are emerging globally.10 Internationally, XDR P. aeruginosa infections have been associated with a number of high-risk clones, including ST111, ST175 and ST235, which have caused outbreaks worldwide.2,5,9 The successful spread of these clones in conjunction with acquired resistance in the form of carbapenemases has highlighted the growing concern in monitoring and treating these organisms.
In this study, patient isolates of carbapenem-resistant P. aeruginosa and XDR P. aeruginosa were examined over a 10 year period in Canada as a part of the CANWARD surveillance study.
Materials and methods
Bacterial isolates
From January 2007 to December 2016, 10–15 tertiary-care medical centre sites per year (12 centres in 2007, 10 in 2008, 15 in 2009, 14 in 2010, 15 in 2011, 12 in 2012, 15 in 2013, 13 in 2014, 13 in 2015 and 13 in 2016) in 8 of the 10 Canadian provinces submitted bacterial pathogens from patients attending hospital clinics, emergency rooms, medical and surgical wards and ICUs to a central clinical microbiology laboratory (Health Sciences Centre, Winnipeg, Canada).11 Each site was asked to submit clinical isolates of any type of bacteria (consecutive, one per patient, per infection site) from inpatients and outpatients with respiratory, urinary, wound and bloodstream infections. By year, each site was asked to provide the following: in 2007, 200 respiratory, 50 wound, 100 urinary, 30/month blood isolates; in 2008, 150 respiratory, 50 wound, 100 urinary, 20/month blood isolates; in 2009–10, 100 respiratory, 50 wound, 50 urinary, 15/month (January to November) blood isolates; and in 2011–16, 100 respiratory, 25 wound, 25 urinary, 10/month (January to October) blood isolates. Carbapenem-resistant P. aeruginosa were identified using CLSI guidelines, where meropenem resistance was ≥8 mg/L. All further testing was completed on carbapenem-resistant P. aeruginosa.
Ethics
The CANWARD study receives annual approval by the University of Manitoba Ethics Board (H2009:059).
Antimicrobial susceptibility testing
Antimicrobial susceptibility testing was performed in the Department of Clinical Microbiology at the Winnipeg Health Sciences Centre using the standard CLSI broth microdilution method.12 All isolates were tested against amikacin, ceftazidime, ceftolozane/tazobactam, ciprofloxacin, colistin, meropenem, piperacillin/tazobactam and tobramycin. MIC interpretive criteria were defined according to CLSI breakpoints.12 MDR P. aeruginosa were not defined in this study as there are currently no final MDR recommendations for this pathogen.13 XDR P. aeruginosa was defined as resistant to all of the following five antimicrobial agents: ceftazidime, ciprofloxacin, meropenem, piperacillin/tazobactam and tobramycin.13
Molecular testing
Genetic relatedness was determined using PFGE.14 PFGE fingerprints generated with SpeI were analysed using BioNumerics version 6.1 software and compared with previously typed strains in the Canadian national database. A 1.5% band tolerance was used for comparisons, cluster analysis was performed using the unweighted pair-group method, and DNA relatedness was calculated based on the Dice coefficient.
Genetic relatedness was also determined by MLST.15 Genes for the following metabolic enzymes were sequenced: AcsA, AroE, GuaA, MutL, NuoD, PpsA and TrpE, and, as a whole, STs were generated and compared with the Canadian national P. aeruginosa database (www.pubmlst.org/paeruginosa/). Genetic relatedness was assessed using goeBURST.16
To gain perspective on genetic relatedness, MLST and PFGE were only used to investigate isolates from 2012 to 2016. Isolates positive for blaGES-5 were also investigated by PFGE and MLST to evaluate suspicions of clonal spread.
PCR was used to detect carbapenemases GES, KPC, NDM, IMP, VIM and OXA-48;17 ESBLs SHV, TEM, CTX-M, OXA-1 and CMY-2; and the mcr-1 and mcr-2 genes for colistin resistance.18 Identified carbapenemase genes were DNA sequenced to identify the specific carbapenemase gene variant. In order to ensure no carbapenemases were missed with PCR, the phenotypic Carba NP test was performed on all carbapenem-resistant isolates.19
Results
A total of 3864 P. aeruginosa isolates were collected across Canada from 2007 to 2016; 466 (12.1%) of the isolates were carbapenem resistant. The annual distribution of carbapenem-resistant isolates was as follows: 2007, 12.0% (n = 76); 2008, 9.4% (n = 35); 2009, 11.5% (n = 54); 2010, 11.7% (n = 44); 2011, 9.7% (n = 32); 2012, 9.5% (n = 25); 2013, 12.2% (n = 47); 2014, 17.3% (n = 59); 2015, 15.8% (n = 58); and 2016, 11.1% (n = 36) (Figure 1). Over the course of the surveillance period, carbapenem-resistant P. aeruginosa reached a peak of 17.3% in 2014. Among the 466 isolates of carbapenem-resistant P. aeruginosa, we identified 21 (4.5%) XDR isolates.13 The annual distribution of the XDR isolates was less than five isolates per year with no discernible trend from 2007 (0.8%) to 2016 (1.5%) (P = 0.3226) (Figure 1).

Incidence of carbapenem-resistant and XDR phenotypes among P. aeruginosa in Canada, 2007–16 (n = 3684).
Patient demographic information associated with the 466 carbapenem-resistant isolates is presented in Table 1. Patient age ranged from 1 to 93 years with a median age of 58 years. Males contributed 64.4% (n = 300) of the isolates. The majority of isolates were obtained from respiratory specimens (74.7%; n = 348), followed by blood (13.5%; n = 63), wound (8.4%; n = 39) and urine (3.4%; n = 16) specimens. The majority of isolates were submitted by sites in central Canada (68.5%, n = 319), followed by western Canada (26.6%, n = 124) and eastern Canada (4.9%, n = 23).
Patient demographic information for carbapenem-resistant P. aeruginosa, 2007–16 (n = 466)
| . | Number (%) of isolates per year . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Demographic parameter . | 2007 . | 2008 . | 2009 . | 2010 . | 2011 . | 2012 . | 2013 . | 2014 . | 2015 . | 2016 . |
| Gender | ||||||||||
| male | 42 (55.3) | 24 (68.6) | 34 (63.0) | 29 (65.9) | 18 (56.2) | 18 (72.0) | 33 (70.2) | 42 (71.2) | 34 (58.6) | 26 (72.2) |
| female | 34 (44.7) | 11 (31.4) | 20 (37.0) | 15 (34.1) | 14 (43.8) | 7 (28.0) | 14 (29.8) | 17 ( 28.8) | 24 (41.4) | 10 (27.8) |
| Hospital ward type | ||||||||||
| clinic | 19 (25.0) | 6 (17.1) | 14 (26.0) | 7 (15.9) | 4 (12.5) | 5 (20.0) | 7 (14.9) | 5 (8.5) | 2 (3.4) | 5 (13.9) |
| emergency room | 5 (6.6) | 3 (8.6) | 0 (0.0) | 4 (9.1) | 2 (6.3) | 0 (0.0) | 4 (8.5) | 3 (5.1) | 1 (1.7) | 1 (2.8) |
| ICU | 26 (34.2) | 10 (28.6) | 20 (37.0) | 13 (29.5) | 13 (40.6) | 10 (40.0) | 18 (38.3) | 17 (28.8) | 19 (32.8) | 13 (36.1) |
| medical | 21 (27.6) | 9 (25.7) | 16 (29.6) | 15 (34.1) | 7 (21.9) | 8 (32.0) | 17 (36.2) | 33 (55.9) | 31 (53.4) | 13 (36.1) |
| surgical | 5 (6.6) | 7 (20.0) | 4 (7.4) | 5 (11.4) | 6 (18.7) | 2 (8.0) | 1 (2.1) | 1 (1.7) | 5 (8.6) | 4 (11.1) |
| Specimen type | ||||||||||
| urine | 6 (7.9) | 2 (5.7) | 0 (0.0) | 3 (6.8) | 0 (0.0) | 0 (0.0) | 1 (2.1) | 1 (1.7) | 2 (3.4) | 1 (2.8) |
| blood | 16 (21.1) | 8 (22.9) | 8 (14.8) | 7 (15.9) | 4 (12.5) | 4 (16.0) | 4 (8.5) | 6 (10.1) | 5 (8.6) | 1 (2.8) |
| wound | 8 (10.5) | 3 (8.6) | 8 (14.8) | 4 (9.1) | 2 (6.3) | 0 (0.0) | 2 (4.3) | 5 (8.5) | 5 (8.6) | 2 (5.6) |
| respiratory | 46 (60.5) | 22 (62.8) | 38 (70.4) | 30 (68.2) | 26 (81.2) | 21 (84.0) | 40 (85.1) | 47 (79.7) | 46 (79.3) | 32 (88.9) |
| Geographic region | ||||||||||
| western Canada | 23 (30.3) | 13 (37.1) | 16 (29.6) | 15 (34.1) | 5 (15.6) | 8 (32.0) | 8 (17.0) | 8 (13.6) | 18 (31.0) | 10 (27.8) |
| central Canada | 50 (65.8) | 21 (60.0) | 36 (66.7) | 25 (56.8) | 24 (75.0) | 15 (60.0) | 39 (83.0) | 49 (83.1) | 37 (63.8) | 23 (63.9) |
| eastern Canada | 3 (3.9) | 1 (2.9) | 2 (3.7) | 4 (9.1) | 3 (9.4) | 2 (8.0) | 0 (0.0) | 2 (3.4) | 3 (5.2) | 3 (8.3) |
| Patient age | ||||||||||
| ≤17 years | 6 (7.9) | 4 (11.4) | 3 (5.6) | 2 (4.6) | 0 (0.0) | 0 (0.0) | 2 (4.3) | 4 (6.8) | 1 (1.7) | 4 (11.1) |
| 18–65 years | 49 (64.5) | 17 (48.6) | 29 (53.7) | 29 (65.9) | 18 (56.2) | 16 (64.0) | 27 (57.4) | 31 (52.5) | 28 (48.3) | 23 (63.9) |
| ≥66 years | 21 (27.6) | 14 (40.0) | 22 (40.7) | 13 (29.5) | 14 (43.8) | 9 (36.0) | 18 (38.3) | 24 (40.7) | 29 (50.0) | 9 (25.0) |
| Total number of isolates | 76 | 35 | 54 | 44 | 32 | 25 | 47 | 59 | 58 | 36 |
| . | Number (%) of isolates per year . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Demographic parameter . | 2007 . | 2008 . | 2009 . | 2010 . | 2011 . | 2012 . | 2013 . | 2014 . | 2015 . | 2016 . |
| Gender | ||||||||||
| male | 42 (55.3) | 24 (68.6) | 34 (63.0) | 29 (65.9) | 18 (56.2) | 18 (72.0) | 33 (70.2) | 42 (71.2) | 34 (58.6) | 26 (72.2) |
| female | 34 (44.7) | 11 (31.4) | 20 (37.0) | 15 (34.1) | 14 (43.8) | 7 (28.0) | 14 (29.8) | 17 ( 28.8) | 24 (41.4) | 10 (27.8) |
| Hospital ward type | ||||||||||
| clinic | 19 (25.0) | 6 (17.1) | 14 (26.0) | 7 (15.9) | 4 (12.5) | 5 (20.0) | 7 (14.9) | 5 (8.5) | 2 (3.4) | 5 (13.9) |
| emergency room | 5 (6.6) | 3 (8.6) | 0 (0.0) | 4 (9.1) | 2 (6.3) | 0 (0.0) | 4 (8.5) | 3 (5.1) | 1 (1.7) | 1 (2.8) |
| ICU | 26 (34.2) | 10 (28.6) | 20 (37.0) | 13 (29.5) | 13 (40.6) | 10 (40.0) | 18 (38.3) | 17 (28.8) | 19 (32.8) | 13 (36.1) |
| medical | 21 (27.6) | 9 (25.7) | 16 (29.6) | 15 (34.1) | 7 (21.9) | 8 (32.0) | 17 (36.2) | 33 (55.9) | 31 (53.4) | 13 (36.1) |
| surgical | 5 (6.6) | 7 (20.0) | 4 (7.4) | 5 (11.4) | 6 (18.7) | 2 (8.0) | 1 (2.1) | 1 (1.7) | 5 (8.6) | 4 (11.1) |
| Specimen type | ||||||||||
| urine | 6 (7.9) | 2 (5.7) | 0 (0.0) | 3 (6.8) | 0 (0.0) | 0 (0.0) | 1 (2.1) | 1 (1.7) | 2 (3.4) | 1 (2.8) |
| blood | 16 (21.1) | 8 (22.9) | 8 (14.8) | 7 (15.9) | 4 (12.5) | 4 (16.0) | 4 (8.5) | 6 (10.1) | 5 (8.6) | 1 (2.8) |
| wound | 8 (10.5) | 3 (8.6) | 8 (14.8) | 4 (9.1) | 2 (6.3) | 0 (0.0) | 2 (4.3) | 5 (8.5) | 5 (8.6) | 2 (5.6) |
| respiratory | 46 (60.5) | 22 (62.8) | 38 (70.4) | 30 (68.2) | 26 (81.2) | 21 (84.0) | 40 (85.1) | 47 (79.7) | 46 (79.3) | 32 (88.9) |
| Geographic region | ||||||||||
| western Canada | 23 (30.3) | 13 (37.1) | 16 (29.6) | 15 (34.1) | 5 (15.6) | 8 (32.0) | 8 (17.0) | 8 (13.6) | 18 (31.0) | 10 (27.8) |
| central Canada | 50 (65.8) | 21 (60.0) | 36 (66.7) | 25 (56.8) | 24 (75.0) | 15 (60.0) | 39 (83.0) | 49 (83.1) | 37 (63.8) | 23 (63.9) |
| eastern Canada | 3 (3.9) | 1 (2.9) | 2 (3.7) | 4 (9.1) | 3 (9.4) | 2 (8.0) | 0 (0.0) | 2 (3.4) | 3 (5.2) | 3 (8.3) |
| Patient age | ||||||||||
| ≤17 years | 6 (7.9) | 4 (11.4) | 3 (5.6) | 2 (4.6) | 0 (0.0) | 0 (0.0) | 2 (4.3) | 4 (6.8) | 1 (1.7) | 4 (11.1) |
| 18–65 years | 49 (64.5) | 17 (48.6) | 29 (53.7) | 29 (65.9) | 18 (56.2) | 16 (64.0) | 27 (57.4) | 31 (52.5) | 28 (48.3) | 23 (63.9) |
| ≥66 years | 21 (27.6) | 14 (40.0) | 22 (40.7) | 13 (29.5) | 14 (43.8) | 9 (36.0) | 18 (38.3) | 24 (40.7) | 29 (50.0) | 9 (25.0) |
| Total number of isolates | 76 | 35 | 54 | 44 | 32 | 25 | 47 | 59 | 58 | 36 |
Patient demographic information for carbapenem-resistant P. aeruginosa, 2007–16 (n = 466)
| . | Number (%) of isolates per year . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Demographic parameter . | 2007 . | 2008 . | 2009 . | 2010 . | 2011 . | 2012 . | 2013 . | 2014 . | 2015 . | 2016 . |
| Gender | ||||||||||
| male | 42 (55.3) | 24 (68.6) | 34 (63.0) | 29 (65.9) | 18 (56.2) | 18 (72.0) | 33 (70.2) | 42 (71.2) | 34 (58.6) | 26 (72.2) |
| female | 34 (44.7) | 11 (31.4) | 20 (37.0) | 15 (34.1) | 14 (43.8) | 7 (28.0) | 14 (29.8) | 17 ( 28.8) | 24 (41.4) | 10 (27.8) |
| Hospital ward type | ||||||||||
| clinic | 19 (25.0) | 6 (17.1) | 14 (26.0) | 7 (15.9) | 4 (12.5) | 5 (20.0) | 7 (14.9) | 5 (8.5) | 2 (3.4) | 5 (13.9) |
| emergency room | 5 (6.6) | 3 (8.6) | 0 (0.0) | 4 (9.1) | 2 (6.3) | 0 (0.0) | 4 (8.5) | 3 (5.1) | 1 (1.7) | 1 (2.8) |
| ICU | 26 (34.2) | 10 (28.6) | 20 (37.0) | 13 (29.5) | 13 (40.6) | 10 (40.0) | 18 (38.3) | 17 (28.8) | 19 (32.8) | 13 (36.1) |
| medical | 21 (27.6) | 9 (25.7) | 16 (29.6) | 15 (34.1) | 7 (21.9) | 8 (32.0) | 17 (36.2) | 33 (55.9) | 31 (53.4) | 13 (36.1) |
| surgical | 5 (6.6) | 7 (20.0) | 4 (7.4) | 5 (11.4) | 6 (18.7) | 2 (8.0) | 1 (2.1) | 1 (1.7) | 5 (8.6) | 4 (11.1) |
| Specimen type | ||||||||||
| urine | 6 (7.9) | 2 (5.7) | 0 (0.0) | 3 (6.8) | 0 (0.0) | 0 (0.0) | 1 (2.1) | 1 (1.7) | 2 (3.4) | 1 (2.8) |
| blood | 16 (21.1) | 8 (22.9) | 8 (14.8) | 7 (15.9) | 4 (12.5) | 4 (16.0) | 4 (8.5) | 6 (10.1) | 5 (8.6) | 1 (2.8) |
| wound | 8 (10.5) | 3 (8.6) | 8 (14.8) | 4 (9.1) | 2 (6.3) | 0 (0.0) | 2 (4.3) | 5 (8.5) | 5 (8.6) | 2 (5.6) |
| respiratory | 46 (60.5) | 22 (62.8) | 38 (70.4) | 30 (68.2) | 26 (81.2) | 21 (84.0) | 40 (85.1) | 47 (79.7) | 46 (79.3) | 32 (88.9) |
| Geographic region | ||||||||||
| western Canada | 23 (30.3) | 13 (37.1) | 16 (29.6) | 15 (34.1) | 5 (15.6) | 8 (32.0) | 8 (17.0) | 8 (13.6) | 18 (31.0) | 10 (27.8) |
| central Canada | 50 (65.8) | 21 (60.0) | 36 (66.7) | 25 (56.8) | 24 (75.0) | 15 (60.0) | 39 (83.0) | 49 (83.1) | 37 (63.8) | 23 (63.9) |
| eastern Canada | 3 (3.9) | 1 (2.9) | 2 (3.7) | 4 (9.1) | 3 (9.4) | 2 (8.0) | 0 (0.0) | 2 (3.4) | 3 (5.2) | 3 (8.3) |
| Patient age | ||||||||||
| ≤17 years | 6 (7.9) | 4 (11.4) | 3 (5.6) | 2 (4.6) | 0 (0.0) | 0 (0.0) | 2 (4.3) | 4 (6.8) | 1 (1.7) | 4 (11.1) |
| 18–65 years | 49 (64.5) | 17 (48.6) | 29 (53.7) | 29 (65.9) | 18 (56.2) | 16 (64.0) | 27 (57.4) | 31 (52.5) | 28 (48.3) | 23 (63.9) |
| ≥66 years | 21 (27.6) | 14 (40.0) | 22 (40.7) | 13 (29.5) | 14 (43.8) | 9 (36.0) | 18 (38.3) | 24 (40.7) | 29 (50.0) | 9 (25.0) |
| Total number of isolates | 76 | 35 | 54 | 44 | 32 | 25 | 47 | 59 | 58 | 36 |
| . | Number (%) of isolates per year . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Demographic parameter . | 2007 . | 2008 . | 2009 . | 2010 . | 2011 . | 2012 . | 2013 . | 2014 . | 2015 . | 2016 . |
| Gender | ||||||||||
| male | 42 (55.3) | 24 (68.6) | 34 (63.0) | 29 (65.9) | 18 (56.2) | 18 (72.0) | 33 (70.2) | 42 (71.2) | 34 (58.6) | 26 (72.2) |
| female | 34 (44.7) | 11 (31.4) | 20 (37.0) | 15 (34.1) | 14 (43.8) | 7 (28.0) | 14 (29.8) | 17 ( 28.8) | 24 (41.4) | 10 (27.8) |
| Hospital ward type | ||||||||||
| clinic | 19 (25.0) | 6 (17.1) | 14 (26.0) | 7 (15.9) | 4 (12.5) | 5 (20.0) | 7 (14.9) | 5 (8.5) | 2 (3.4) | 5 (13.9) |
| emergency room | 5 (6.6) | 3 (8.6) | 0 (0.0) | 4 (9.1) | 2 (6.3) | 0 (0.0) | 4 (8.5) | 3 (5.1) | 1 (1.7) | 1 (2.8) |
| ICU | 26 (34.2) | 10 (28.6) | 20 (37.0) | 13 (29.5) | 13 (40.6) | 10 (40.0) | 18 (38.3) | 17 (28.8) | 19 (32.8) | 13 (36.1) |
| medical | 21 (27.6) | 9 (25.7) | 16 (29.6) | 15 (34.1) | 7 (21.9) | 8 (32.0) | 17 (36.2) | 33 (55.9) | 31 (53.4) | 13 (36.1) |
| surgical | 5 (6.6) | 7 (20.0) | 4 (7.4) | 5 (11.4) | 6 (18.7) | 2 (8.0) | 1 (2.1) | 1 (1.7) | 5 (8.6) | 4 (11.1) |
| Specimen type | ||||||||||
| urine | 6 (7.9) | 2 (5.7) | 0 (0.0) | 3 (6.8) | 0 (0.0) | 0 (0.0) | 1 (2.1) | 1 (1.7) | 2 (3.4) | 1 (2.8) |
| blood | 16 (21.1) | 8 (22.9) | 8 (14.8) | 7 (15.9) | 4 (12.5) | 4 (16.0) | 4 (8.5) | 6 (10.1) | 5 (8.6) | 1 (2.8) |
| wound | 8 (10.5) | 3 (8.6) | 8 (14.8) | 4 (9.1) | 2 (6.3) | 0 (0.0) | 2 (4.3) | 5 (8.5) | 5 (8.6) | 2 (5.6) |
| respiratory | 46 (60.5) | 22 (62.8) | 38 (70.4) | 30 (68.2) | 26 (81.2) | 21 (84.0) | 40 (85.1) | 47 (79.7) | 46 (79.3) | 32 (88.9) |
| Geographic region | ||||||||||
| western Canada | 23 (30.3) | 13 (37.1) | 16 (29.6) | 15 (34.1) | 5 (15.6) | 8 (32.0) | 8 (17.0) | 8 (13.6) | 18 (31.0) | 10 (27.8) |
| central Canada | 50 (65.8) | 21 (60.0) | 36 (66.7) | 25 (56.8) | 24 (75.0) | 15 (60.0) | 39 (83.0) | 49 (83.1) | 37 (63.8) | 23 (63.9) |
| eastern Canada | 3 (3.9) | 1 (2.9) | 2 (3.7) | 4 (9.1) | 3 (9.4) | 2 (8.0) | 0 (0.0) | 2 (3.4) | 3 (5.2) | 3 (8.3) |
| Patient age | ||||||||||
| ≤17 years | 6 (7.9) | 4 (11.4) | 3 (5.6) | 2 (4.6) | 0 (0.0) | 0 (0.0) | 2 (4.3) | 4 (6.8) | 1 (1.7) | 4 (11.1) |
| 18–65 years | 49 (64.5) | 17 (48.6) | 29 (53.7) | 29 (65.9) | 18 (56.2) | 16 (64.0) | 27 (57.4) | 31 (52.5) | 28 (48.3) | 23 (63.9) |
| ≥66 years | 21 (27.6) | 14 (40.0) | 22 (40.7) | 13 (29.5) | 14 (43.8) | 9 (36.0) | 18 (38.3) | 24 (40.7) | 29 (50.0) | 9 (25.0) |
| Total number of isolates | 76 | 35 | 54 | 44 | 32 | 25 | 47 | 59 | 58 | 36 |
Patient demographic information associated with XDR isolates was as follows: patient age ranged from 1 to 84 years with a median age of 54 years; males contributed 61.9% (n = 13) of the isolates; and the majority of isolates were obtained from respiratory specimens (61.9%; n = 13), followed by blood (23.8%; n = 5), urine (9.5%; n = 2) and wound (4.8%; n = 1) specimens. The majority of isolates were from central Canada (85.7%, n = 18), followed by western Canada (9.5%, n = 2) and eastern Canada (4.8%, n = 1). Although numbers are much smaller, these trends in patient demographic information are similar to those seen among all carbapenem-resistant P. aeruginosa in the study.
Antimicrobial susceptibility testing results are presented in Table 2. Colistin and ceftolozane/tazobactam were the most active agents, with susceptibilities of 94.0% and 92.5%, respectively. Lower susceptibility rates were observed for amikacin (80.7%), tobramycin (72.7%), piperacillin/tazobactam (43.1%), ceftazidime (41.2%) and ciprofloxacin (37.6%).
Antimicrobial susceptibility profiles of carbapenem-resistant P. aeruginosa, 2007–16 (n = 466)
| Antimicrobial agent . | MIC (mg/L) . | MIC breakpoint interpretationsa . | ||||
|---|---|---|---|---|---|---|
| MIC50 . | MIC90 . | MIC range . | %S . | %I . | %R . | |
| Amikacin | 8 | >64 | ≤1 to ≥64 | 80.7 | 6.9 | 12.4 |
| Ceftazidime | 16 | >32 | 1 to ≥32 | 41.2 | 17.2 | 41.6 |
| Ceftolozane/tazobactam | 1 | 4 | 0.25 to ≥64 | 92.5 | 3 | 4.5 |
| Ciprofloxacin | 2 | >16 | ≤0.06 to ≥16 | 37.6 | 15 | 47.4 |
| Colistin | 1 | 2 | 0.12 to ≥16 | 94 | 0 | 6 |
| Meropenem | 16 | 32 | 8 to ≥64 | 0 | 0 | 100 |
| Piperacillin/tazobactam | 32 | 256 | ≤1 to ≥512 | 43.1 | 26 | 30.9 |
| Tobramycin | 1 | 64 | ≤0.5 to ≥64 | 72.7 | 3.7 | 23.6 |
| Antimicrobial agent . | MIC (mg/L) . | MIC breakpoint interpretationsa . | ||||
|---|---|---|---|---|---|---|
| MIC50 . | MIC90 . | MIC range . | %S . | %I . | %R . | |
| Amikacin | 8 | >64 | ≤1 to ≥64 | 80.7 | 6.9 | 12.4 |
| Ceftazidime | 16 | >32 | 1 to ≥32 | 41.2 | 17.2 | 41.6 |
| Ceftolozane/tazobactam | 1 | 4 | 0.25 to ≥64 | 92.5 | 3 | 4.5 |
| Ciprofloxacin | 2 | >16 | ≤0.06 to ≥16 | 37.6 | 15 | 47.4 |
| Colistin | 1 | 2 | 0.12 to ≥16 | 94 | 0 | 6 |
| Meropenem | 16 | 32 | 8 to ≥64 | 0 | 0 | 100 |
| Piperacillin/tazobactam | 32 | 256 | ≤1 to ≥512 | 43.1 | 26 | 30.9 |
| Tobramycin | 1 | 64 | ≤0.5 to ≥64 | 72.7 | 3.7 | 23.6 |
Using CLSI M100 27th edition (2017) MIC interpretative breakpoints.
Antimicrobial susceptibility profiles of carbapenem-resistant P. aeruginosa, 2007–16 (n = 466)
| Antimicrobial agent . | MIC (mg/L) . | MIC breakpoint interpretationsa . | ||||
|---|---|---|---|---|---|---|
| MIC50 . | MIC90 . | MIC range . | %S . | %I . | %R . | |
| Amikacin | 8 | >64 | ≤1 to ≥64 | 80.7 | 6.9 | 12.4 |
| Ceftazidime | 16 | >32 | 1 to ≥32 | 41.2 | 17.2 | 41.6 |
| Ceftolozane/tazobactam | 1 | 4 | 0.25 to ≥64 | 92.5 | 3 | 4.5 |
| Ciprofloxacin | 2 | >16 | ≤0.06 to ≥16 | 37.6 | 15 | 47.4 |
| Colistin | 1 | 2 | 0.12 to ≥16 | 94 | 0 | 6 |
| Meropenem | 16 | 32 | 8 to ≥64 | 0 | 0 | 100 |
| Piperacillin/tazobactam | 32 | 256 | ≤1 to ≥512 | 43.1 | 26 | 30.9 |
| Tobramycin | 1 | 64 | ≤0.5 to ≥64 | 72.7 | 3.7 | 23.6 |
| Antimicrobial agent . | MIC (mg/L) . | MIC breakpoint interpretationsa . | ||||
|---|---|---|---|---|---|---|
| MIC50 . | MIC90 . | MIC range . | %S . | %I . | %R . | |
| Amikacin | 8 | >64 | ≤1 to ≥64 | 80.7 | 6.9 | 12.4 |
| Ceftazidime | 16 | >32 | 1 to ≥32 | 41.2 | 17.2 | 41.6 |
| Ceftolozane/tazobactam | 1 | 4 | 0.25 to ≥64 | 92.5 | 3 | 4.5 |
| Ciprofloxacin | 2 | >16 | ≤0.06 to ≥16 | 37.6 | 15 | 47.4 |
| Colistin | 1 | 2 | 0.12 to ≥16 | 94 | 0 | 6 |
| Meropenem | 16 | 32 | 8 to ≥64 | 0 | 0 | 100 |
| Piperacillin/tazobactam | 32 | 256 | ≤1 to ≥512 | 43.1 | 26 | 30.9 |
| Tobramycin | 1 | 64 | ≤0.5 to ≥64 | 72.7 | 3.7 | 23.6 |
Using CLSI M100 27th edition (2017) MIC interpretative breakpoints.
None of the XDR isolates was resistant to colistin and only 19.0% (n = 4) were resistant to ceftolozane/tazobactam. Reduced susceptibility to amikacin was observed for 57.1% of XDR isolates.
PCR for six prominent carbapenemases (GES, KPC, NDM, IMP, VIM and OXA-48) was completed on all carbapenem-resistant isolates. Only 4.3% (n = 20) of all carbapenem-resistant isolates harboured a carbapenemase. The distribution of carbapenemase genes was as follows: GES-5, 1.5% (n = 7); VIM-2, 0.6% (n = 3); VIM-4, 0.6% (n = 3); IMP-1, 0.4% (n = 2); IMP-18, 0.4% (n = 2); IMP-7, 0.4% (n = 2); and IMP-13, 0.2% (n = 1). Only two (9.5%) XDR P. aeruginosa harboured a carbapenemase: VIM-4 and IMP-7. A positive Carba NP result, indicative of the presence of a carbapenemase, was not found on any carbapenemase-negative isolates (data not shown). The mcr gene was not found in any of the 6.5% (n = 25) carbapenem-resistant P. aeruginosa that were colistin resistant.
Molecular typing by PFGE revealed the majority of carbapenem-resistant P. aeruginosa from 2012 to 2016 (n = 225) were genetically diverse. These findings were further supported by the identification of >150 different STs using MLST (Figure 2). Both PFGE and MLST found four genetically related clusters with more than five isolates per cluster. The STs of these clusters were ST235 (3.6%, n = 8), ST357 (3.1%, n = 7), ST17 (2.7%, n = 6) and ST274 (2.2%, n = 5). From 2012 to 2016 there were only 10 XDR P. aeruginosa. The majority of XDR P. aeruginosa were genetically diverse with nine different STs observed (Figure 2). Although genetically diverse, only 30% (n = 3) of XDR P. aeruginosa were associated with high-risk clones: ST357 (20%, n = 2) and ST298 (10%, n = 1). Further investigation into the seven blaGES-5 isolates determined that all were genetically related by PFGE and MLST (ST17) and were all from two hospitals in western Canada (Figure 3). None of the blaGES-5 isolates was XDR; however, 57.1% (n = 4) of blaGES-5 isolates were resistant to ceftolozane/tazobactam.
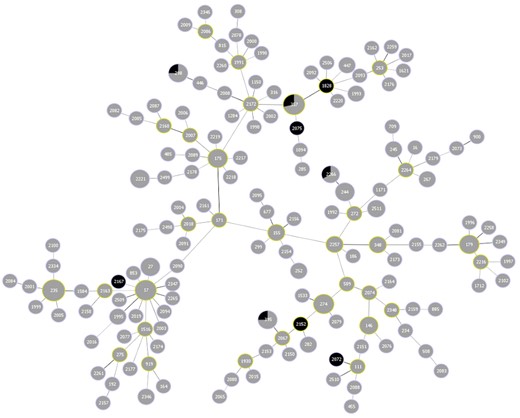
goeBURST diagram of all STs identified among carbapenem-resistant P. aeruginosa (n = 225) from 2012 to 2016. More than 150 unique STs were identified. The most common STs were 17, 235 and 357. XDR isolates are shown in black and non-XDR isolates in grey. The relative sizes of the circles correlate to the number of isolates of a particular ST (i.e. larger circles have greater numbers of isolates with the same ST). This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.

PFGE subtyping with SpeI of all GES-5 isolates (n = 7). Genetic relatedness was observed with MLST (all seven isolates were ST17). PFGE also demonstrated high discriminatory power with certain band changes over time.
Discussion
The annual rates of carbapenem resistance among isolates of P. aeruginosa from Canadian patients varied over the 10 year (2007–16) study period. There was a significant increase (P = 0.0136) in the rate of carbapenem-resistant P. aeruginosa from 2012 (9.5%) to 2014 (17.3%) followed by decreasing rates in 2015 (15.8%) and 2016 (11.1%). The rates of carbapenem-resistant P. aeruginosa identified in the current study are higher than the 3.3% identified over a 1 year period (2009–10) in a previous study conducted by the Canadian Nosocomial Infection Surveillance Program (CNISP).17 The higher rates per year observed in the present study may be due to study design as the CNISP study involved a larger number of hospital sites. Similar to previous studies, the majority of isolates in the current study were from respiratory samples, and from the ICU or medical units of the hospital.17,20 Furthermore, it has been suggested an ICU stay is a risk factor for carbapenemase-producing P. aeruginosa infection.20
XDR P. aeruginosa have remained rare (0.54%, n = 21) in Canada compared with other countries, which have reported up to 22% of P. aeruginosa to be XDR.21 The difference in XDR guidelines may play a role in the discrepancies being seen. For instance, interim definitions proposed by Magiorakos et al.22 defined XDR as non-susceptible (i.e. resistant or intermediate) whereas the Canadian recommendations include only resistant isolates.13 In addition, there are differences in the number of antimicrobial classes involved in the two definitions that may add to the discrepancies. Ideally, the adoption of a single definition of XDR worldwide would allow better comparability of data and promote a better understanding of the extent of the problem with highly resistant P. aeruginosa.22 Interestingly, the increase in carbapenem-resistant P. aeruginosa over time did not correlate with any significant change in XDR P. aeruginosa. This leads us to believe that, in this study, the existence of carbapenem-resistant and that of XDR isolates are independent of each other. The XDR profile also existed independently from the presence of a carbapenemase as only two XDR isolates were found to harbour a carbapenemase.
From 2012 to 2016, most carbapenem-resistant P. aeruginosa belonged to different STs and did not cluster by PFGE, suggesting little clonal dissemination of isolates in or between hospitals in this study. This diversity is not unusual among P. aeruginosa collected from nosocomial infections.9 Nevertheless, many countries, such as Brazil, the Czech Republic and Spain, have reported significant clonal spread.9,20,23 Although we did not observe much clonal dissemination among our carbapenem-resistant P. aeruginosa, there were a few high-risk clones circulating, which included ST235 (3.6%) and ST357 (3.1%). High-risk clones are typically MDR or XDR and have been subject to widespread dissemination.5 Common high-risk clones identified internationally include ST175, ST233, ST244, ST274, ST277, ST298, ST308, ST357 and ST654, with ST235 being the most established globally.9 Past studies have also suggested that clonal diversity is linked to the resistance in the organism. For example, in Spain, clonal diversity of P. aeruginosa was much lower among MDR or XDR isolates.2,24 Among our XDR subset from 2012–16, this was not entirely the case as 9 (90%) different STs were observed among 10 isolates. However, only 20% (n = 2) of the XDR isolates were in fact associated with a high-risk clone, ST357.9
Carbapenem-resistant P. aeruginosa have been associated with a variety of carbapenemases: IMP, VIM, KPC, GES, NDM, GIM and SPM.4,8 From 2007 to 2016, our study showed that only a small portion of carbapenem-resistant P. aeruginosa in Canada was due to the presence of a carbapenemase (4.3%). Similarly, results previously reported by CNISP found 5.3% of carbapenem-resistant P. aeruginosa to harbour a carbapenemase.17 Not only have the prevalences of carbapenemases remained low in Canada, but they have not shown any significant change from 2007 to 2016. In contrast, other studies have shown increases in carbapenemases over time.7,20,25 The majority of carbapenem resistance in P. aeruginosa identified in this study was likely due to a combination of chromosomal changes in porin, efflux and β-lactamase genes.3,5,6
The predominant carbapenemase gene found circulating in this study was blaGES-5 (35%). This integron-encoded gene was found circulating between two hospitals in western Canada and has previously been described worldwide.9,17,26,27 Although a less common carbapenemase associated with P. aeruginosa, blaGES-5 has been implicated in previous outbreaks.26 The blaGES-5-harbouring P. aeruginosa were all genetically related (ST17) and all clustered at >80% similarity with PFGE. Due to its high discriminatory power, minor variation was revealed with PFGE once the isolates spread over time to a secondary hospital; however, they remained genetically similar. Unlike ST111, ST175 and ST235, ST17 is not a high-risk clone.2,5,9
Carbapenem-resistant P. aeruginosa displayed reduced susceptibilities to all tested antimicrobials but remained highly susceptible to colistin (94%) and ceftolozane/tazobactam (92.5%). Previous reports support these observations.28,29 Colistin and ceftolozane/tazobactam also displayed excellent activity against XDR P. aeruginosa (100% and 81.0%, respectively). Despite our small subset of XDR isolates, previous studies have reported comparable results.29
In conclusion, we observed a spike from 2014 to 2015 in the number of carbapenem-resistant P. aeruginosa in Canada and very low numbers of XDR isolates from 2007 to 2016. Genetic diversity was independent of carbapenem-resistant or XDR phenotypes. Our findings suggest that continued surveillance is warranted considering the circulation and success of high-risk clones and the increase in carbapenemase-producing P. aeruginosa globally.
Acknowledgements
The data in this paper were previously presented in part at the ASM MICROBE meeting (2017) in New Orleans, LA. Poster #121. CANWARD data can also be found at www.can-r.ca, the official website of CARA.
We would like to thank the participating centres, investigators and laboratory site staff from the CANWARD sites for their continued support and cooperation.
Members of CARA
The CARA principal members include George G. Zhanel, Daryl J. Hoban, Heather J. Adam, Melanie R. Baxter, Kimberly A. Nichol, Philippe R.S. Lagacé-Wiens, Andrew Walkty and James A. Karlowsky.
Members of CANWARD
The participating CANWARD sites (investigator) are: Royal University Hospital, Saskatoon, SK (Dr J. Blondeau); Children’s Hospital of Eastern Ontario, Ottawa, ON (Dr R. Slinger); Queen Elizabeth II Health Sciences Centre, Halifax, NS (Dr R. Davidson); Health Sciences Centre, Winnipeg, MB (Dr G. Zhanel/Dr D. Hoban); London Health Sciences Centre, London, ON (Dr J. Delport); South East Health Care Corp., Moncton, NB (Dr C. Ellis); Hôpital Maisonneuve-Rosemont, Montreal, QC (Dr M. Laverdière); Montreal General Hospital, Montreal, QC (Dr V. Loo); Royal Victoria Hospital, Montreal, QC (Dr V. Loo); Mount Sinai Hospital/University Health Network, Toronto, ON (Dr S. Poutanen); University of Alberta Hospital, Edmonton, AB (Dr J. Fuller); Vancouver Hospital, Vancouver, BC (Dr D. Roscoe); The Ottawa Hospital, Ottawa, ON (Dr M. Desjardins); St. Michael’s Hospital, Toronto, ON (Dr L. Matukas); CHRTR Pavillon Ste. Marie, Trois-Rivières, QC (Dr M. Goyette); St. Joseph’s Hospital, Hamilton, ON (Dr C. Lee); Centre Hospitalier Universitaire de Sherbrooke, Sherbrooke, QC (Dr A. Carignan); Cité de la Santé, Laval, QC (Dr M. Bergevin); and L’Hôtel-Dieu de Quebec, Quebec City, QC (Dr R. Pelletier).
Funding
The CANWARD study was supported in part by the University of Manitoba, Diagnostic Services Manitoba, the National Microbiology Laboratory, Astellas, Merck, Pfizer, Sunovion, The Medicines Company, Abbott, Achaogen, Cubist, Paladin Labs, Bayer, Janssen Ortho/Ortho McNeil, Affinium, Basilea, AstraZeneca, Paratek, Tetraphase, Theravance, Sanofi-Aventis and Zoetis.
Transparency declarations
G. G. Z. and D. J. H. have received research grants from Astellas, Merck, Pfizer, Sunovion, The Medicines Company, Abbott, Achaogen, Cubist, Paladin Labs, Bayer, Janssen Ortho/Ortho McNeil, Affinium, Basilea, AstraZeneca, Paratek, Tetraphase, Theravance, Sanofi-Aventis and Zoetis. J. M. B. has received research grants from Bayer, Pfizer, Sunovion, and Abbott. All other authors: none to declare.
This article forms part of a Supplement sponsored by the University of Manitoba and Shared Health Manitoba, Winnipeg, Canada.
Disclaimer
The opinions expressed in this paper are those of the authors and do not necessarily represent those of Astellas, Merck, Pfizer, Sunovion, The Medicines Company, Abbott, Achaogen, Cubist, Paladin Labs, Bayer, Janssen Ortho/Ortho McNeil, Affinium, Basilea, AstraZeneca, Paratek, Tetraphase, Theravance, Sanofi-Aventis or Zoetis.
References
Clinical and Laboratory Standards Institute.
Author notes
Members are listed in the Acknowledgements section.



