-
PDF
- Split View
-
Views
-
Cite
Cite
Maurizio Sanguinetti, Brunella Posteraro, Catherine Beigelman-Aubry, Frederic Lamoth, Vincent Dunet, Monica Slavin, Malcolm D Richardson, Diagnosis and treatment of invasive fungal infections: looking ahead, Journal of Antimicrobial Chemotherapy, Volume 74, Issue Supplement_2, March 2019, Pages ii27–ii37, https://doi.org/10.1093/jac/dkz041
Close - Share Icon Share
Abstract
Improved standards of care depend on the development of new laboratory diagnostic and imaging procedures and the development of new antifungal compounds. Immunochromatography technologies have led to the development of lateral flow devices for the diagnosis of cryptococcal meningitis and invasive aspergillosis (IA). Similar devices are being developed for the detection of histoplasmosis that meet the requirements for speed (∼15 min assay time) and ease of use for point-of-care diagnostics. The evolution of molecular tools for the detection of fungal pathogens has been slow but the introduction of new nucleic acid amplification techniques appears to be helpful, for example T2Candida. An Aspergillus proximity ligation assay has been developed for a rapid near-patient bedside diagnosis of IA. CT remains the cornerstone for radiological diagnosis of invasive pulmonary fungal infections. MRI of the lungs may be performed to avoid radiation exposure. MRI with T2-weighted turbo-spin-echo sequences exhibits sensitivity and specificity approaching that of CT for the diagnosis of invasive pulmonary aspergillosis. The final part of this review looks at new approaches to drug discovery that have yielded new classes with novel mechanisms of action. There are currently two new classes of antifungal drugs in Phase 2 study for systemic invasive fungal disease and one in Phase 1. These new antifungal drugs show promise in meeting unmet needs with oral and intravenous formulations available and some with decreased potential for drug–drug interactions. Novel mechanisms of action mean these agents are not susceptible to the common resistance mechanisms seen in Candida or Aspergillus. Modification of existing antifungal susceptibility testing techniques may be required to incorporate these new compounds.
Introduction
Invasive fungal and fungus-like infections contribute to substantial morbidity and mortality in immunocompromised individuals. The incidence of these infections is increasing, largely because of rising numbers of immunocompromised patients, including those with neutropenia, HIV, chronic immunosuppression, indwelling prostheses, burns and diabetes mellitus, and those taking broad-spectrum antibiotics. Fungal and fungus-like infections can affect a variety of organ systems and include conditions such as meningitis, sinusitis, osteomyelitis and enteritis. As awareness of these infections increases, timely diagnosis and treatment will become even more important. Imaging has a critical role in the evaluation of disease activity, therapy response and related complications. Using an organ-based approach with CT, MRI and ultrasonography to gain familiarity with the appearances of these infections enables timely and accurate diagnoses. Future success in diagnosing proven invasive fungal infection earlier will drive and support the development of new therapeutic drugs. We have reviewed the current state of the art in diagnostics and new antifungal compounds and have provided an insight into how all this activity might impact on improved patient care.
Developments in laboratory-based and point-of-care tests
Taking advantage of emerging technologies, molecular methods have supplanted culture for the clinical laboratory diagnosis of both viral and intracellular bacterial infections.1 Notably, some of these technologies led to the development of lateral flow immunoassay (LFIA) formats, which meet the requirements for speed (∼15 min assay time) and ease of use for point-of-care (POC) diagnostics.2 Conversely, laboratory adoption of molecular methods, in particular PCR-based assays,3,4 for detection of fungal pathogens direct from clinical samples is still limited.5–7 Thus, it is not surprising that FDA-cleared fungal diagnostics in routine clinical use are scarce. Consequently, clinicians are forced to continue or change antifungal therapy in the face of negative cultures.8 As for invasive Candida infections,9 this usually occurs for deep-seated candidiasis, which is most associated with early or transient candidaemia.10 While the performance of blood cultures may also vary depending on the type of causative Candida species,11 the overall rate of positive blood cultures is 38% (range 21%–71%) based on autopsy studies.12 However, at the time of first positive blood culture, the median concentration of Candida organisms was 1 cfu/mL (mode 0.1, range 0.1 to >1000), and >50% of cultures had ≤1 cfu/mL when candidaemia was detected using the lysis–centrifugation blood culture system.13 These findings, published in 2011, suggested that blood cultures are sufficiently sensitive to detect viable Candida cells, and supported the rationale for the emergence of molecular (DNA-based) diagnostic systems with greater ability to detect organisms in blood. In 2013, a T2Candida nanodiagnostic panel that uses T2 magnetic resonance and a dedicated instrument platform (T2Dx) to amplify and detect (with target probe-bearing nanoparticles) Candida DNA directly in whole blood (i.e. not subjected to any previous incubation or nucleic acid extraction) was developed.14 The amplification signal originates from not just the amplified target but, remarkably, the nanoparticle clustering that yields changes in the T2 relaxation time, making it detectable by magnetic resonance.14 The T2Candida system is the only FDA-cleared PCR assay for Candida to date.15,16 It detects the five most common species of Candida (C. albicans, C. glabrata, C. parapsilosis, C. tropicalis and C. krusei), has a detection limit of 1 cfu/mL and results in a >10-fold decrease in time to result (compared with blood cultures), and is devised to not amplify freely circulating, non-cell-associated DNA.9,14 In the recently completed multicentre DIRECT trial, with 1801 samples tested, overall sensitivity and specificity (excluding invalid results) were 91.1% and 99.4%, respectively, with detection sensitivities of 1–3 cfu/mL (depending on the Candida species), a negative predictive value of 99.5% in a 5% prevalence population, and a mean time to negative result of 4.2 ± 0.9 h.17 In the follow-up, multicentre DIRECT2 trial, the clinical sensitivity for the five Candida species identified by T2Candida was found to be 89% (excluding invalid results) in 36 of 152 patients, who were identified as having a positive diagnostic blood culture for Candida species and then resampled (within 1–6 days) with both blood cultures and T2Candida testing.18 Among the study patients, T2Candida tests were more likely to remain positive than concomitantly collected blood cultures [45% (69/152) and 24% (36/152); P < 0.0001]. Interestingly, among the patients who were receiving antifungal therapy by the time repeat blood samples were collected, a higher positivity for T2Candida was found, indicating that the system is able to reveal the presence of non-viable or drug-inhibited Candida organisms if the patient is on antifungal therapy.18 While the ability of T2Candida to detect Candida species in culture-negative invasive candidiasis, including deep-seated candidiasis,15 remains unproven on a larger scale,10 it will be necessary to define the effects of antifungal treatment on the T2Candida performance as well as the role of T2Candida in monitoring candidaemia during antifungal therapy19 in the near future.
As for Aspergillus infections, cultures of respiratory tract specimens, obtained by sputum induction or bronchoalveolar lavage (BAL), have a low positive predictive value (around 72%), which can be even lower in non-haematology patients or those who are already receiving antifungal therapy.20 Although detection of galactomannan (Bio-Rad Aspergillus Platelia assay) in serum or BAL fluid represents a great advance in the diagnosis of invasive aspergillosis,21,22 the test performance for routine patient blood screening is compromised by antifungal prophylaxis and therapy, which reduce its specificity and sensitivity, respectively.23,24 Among LFIA-based fungal antigen detection methods, the Aspergillus-specific lateral-flow device (LFD) is an immunochromatographic test that uses the JF5 monoclonal antibody to detect an extracellular glycoprotein (mannoprotein) antigen secreted by actively growing Aspergillus species.25 The LFD format is a ‘pregnancy test-like’ near-POC test for the diagnosis of invasive aspergillosis, and it has demonstrated convincing clinical performance, in particular with BAL fluid samples.26,27 Although the LFD may also be used with serum samples, BAL fluid samples have the advantage of being tested without any pre-treatment28 and are less influenced by systemic antifungal prophylaxis/therapy compared with serum samples.29,30 A recent meta-analysis of seven studies (mainly with solid organ transplant recipients) reported a pooled sensitivity of 86% (95% CI 76%–93%), specificity of 93% (95% CI 89%–96%) and diagnostic odds ratio of 65.94 (95% CI 27.21–159.81) for proven/probable versus no invasive aspergillosis cases.31 Analysis of summarized data on the performance of the BAL fluid LFD, with 792 samples from different patient populations, resulted in an overall sensitivity of 73% and specificity of 90% for probable/proven invasive aspergillosis versus no evidence for invasive aspergillosis.27 Interestingly, LFD sensitivity was lower in patients with underlying haematological malignancies (67% sensitivity) compared with other patient groups, perhaps due to the frequent use of antifungal prophylaxis/empirical antifungal therapy in this patient population.27 While a commercial assay version is now available (http://olmdiagnostics.com/products/asplfd/), more data on the impact of mould-active antifungal agents on the performance of the LFD are necessary.30 The Aspergillus proximity ligation assay (PLA) was developed for a POC diagnosis of invasive aspergillosis and is now commercialized (http://www.olminnovations.com/_assets/media/editor/file/maa_olm_inn_pla_a4leaflet_2015_vs03.pdf). This immuno-PCR assay32 uses a single monoclonal antibody, JF5, to generate two pools of proximity probes (A and B), targeting the above-mentioned Aspergillus-specific extracellular mannoprotein, that are linked to two different, non-complementary DNA oligonucleotides. In the presence of antigen, binding of probes to adjacent epitopes brings the oligonucleotides together and allows them to be joined to form a single, amplifiable DNA sequence.32 One study shows that the Aspergillus PLA provided positive results in all BAL fluid samples from three cases with probable invasive aspergillosis, which were also positive in the Aspergillus-specific LFD and the Aspergillus Platelia galactomannan assays.26 These findings are preliminary and warrant further evaluation with a larger number of clinical samples.
Unlike for Candida and Aspergillus diseases, detection of cryptococcal antigen (CrAg), a component of the cryptococcal polysaccharide capsule glucuronoxylomannan, is invaluable for establishing Cryptococcus disease.33 Thus, it is not surprising that the latex agglutination assay for the detection of CrAg, one of the first immunoassays for the diagnosis of infectious diseases,2 is still used in routine clinical practice. However, a recent improvement of CrAg testing is the LFIA format, which was developed and FDA-cleared as a prescription-use laboratory assay for the detection of CrAg in body fluids.34 The CrAg lateral flow assay (LFA) has been validated in serum and CSF, with 99.3% sensitivity and >99.1% specificity in CSF.35 Before widespread ART was available, estimated cases of cryptococcal disease per year reached ∼1 million,36 with the mortality rate being substantial, particularly in sub-Saharan and South Africa. Since then, extensive ART expansion has occurred, but cryptococcal meningitis remains globally responsible for 15% of AIDS-related deaths (95% CI 10%–19%) according to recent estimates.37 As the CrAg LFA satisfies most of the WHO affordable, sensitive, specific, user-friendly, rapid/robust, equipment-free and deliverable to end users (ASSURED) criteria for POC tests, this assay has been proposed as a serum or plasma screening test in ‘at-risk’ patients (<100 CD4 cells/mm3 and HIV infection) prior to ART initiation.38 Furthermore, CrAg LFA testing with fingerstick whole blood in resource-limited settings can simplify screening of large numbers of patients and facilitate the prioritization of patients on whom to perform a diagnostic lumbar puncture with the measurement of CSF opening pressure.39 Importantly, bedside testing can be performed rapidly, with informed consent, during the lumbar puncture, because it does not require a centrifuge for serum or plasma separation. As the CrAg test is able to detect subclinical cryptococcal disease in patients entering ART programmes,34 a positive CrAg allows the treating physician to initiate fluconazole preemptive treatment, thus preventing many of these patients from developing severe cryptococcosis.38
Finally, rapid detection of Histoplasma capsulatum in clinical samples remains challenging, particularly in resource-limited regions in which the organism is endemic.40 While delayed diagnosis of progressive disseminated histoplasmosis (PDH) results in high mortality rates,41 it is undisputed that traditional PCR assays, requiring expensive reagents and equipment, may be unsustainable in clinical laboratories with limited funding. As an alternative, loop-mediated isothermal amplification (LAMP) is a cheaper and simpler assay that can provide results to the laboratory in less than 60 min.42 Briefly, LAMP utilizes a Bacillus stearothermophilus polymerase with helicase activity and four primers forming stem–loop DNA structures, which become the template DNA for further amplification. Reactions are performed in single tubes at a single temperature, and results are readable under UV light. Findings from one study that tested urine samples from healthy persons (n = 10) and HIV patients with PDH (n = 6) showed that the LAMP assay was more sensitive than traditional PCR assays in detecting the H. capsulatum Hcp100 DNA in clinical samples.43 Within an incubation time of 1.5 h, the LAMP assay did not cross-react with DNA isolated from urine specimens from healthy persons, showing proof of the concept that this assay may be a valuable tool for detection of disseminated histoplasmosis. As only 67% of samples (4/6 samples) were positive in the Hcp100 LAMP assay in this pilot study,43 it is expected that a large number of urine samples will be tested to determine the clinical sensitivity of this method.
In conclusion, the present landscape of the most promising new molecular platforms and POC tests for detection of fungal DNA or antigens shows that, at this time, important diagnostic tools are available for the most common fungal diseases. These tools may have the potential to help improve the prognosis of patients, allowing earlier diagnosis and treatment. However, the implementation of new rapid diagnostics or POC tests and the actionable information that would be triggered by the test results (i.e. fluconazole treatment for a CrAg-positive AIDS patient) may be hindered by costs and infrastructure requirements. Although these limitations concern both resource-rich and resource-limited settings, rapid fungal diagnostics-based preemptive screening and/or treatment reduce reliance on presumptive antifungal use and thereby facilitate antimicrobial stewardship and effective patient care. Informative diagnostic markers can assist in antifungal stewardship programmes by reducing inappropriate use of antifungals and improving patient outcomes.
For a summary of laboratory diagnostic tests discussed in this review see Table 1.
Summary of new non-culture laboratory diagnostic tests for systemic fungal diseases described in this review
| Disease . | Test . | Comments . |
|---|---|---|
| Candidaemia/deep-seated candidaemia | T2 Candida panel. | Detects Candida DNA directly in whole blood. Detection limit of 1 cfu/mL. Greater than 10-fold decrease in reporting time. |
| Pulmonary aspergillosis | Immunochromatographic technology – lateral flow devices/dip sticks. | POC or near-POC test for detecting cell wall components in serum, BAL and urine. Impact of antifungal prophylaxis on sensitivity not known. |
| Aspergillus proximity ligation assay. | Detects cell wall mannoprotein. | |
| Cryptococcosis | Various lateral flow formats. | Designed to detect cryptococcal polysaccharide antigen in CSF, whole blood and saliva. Adopted by WHO on their Essential Diagnostics List. |
| Histoplasmosis | Various ELISA platforms, lateral flow devices, and a loop-mediated isothermal amplification assay. | Rapid, lateral flow devices are suitable for POC testing, urine is the preferred clinical sample. |
| Disease . | Test . | Comments . |
|---|---|---|
| Candidaemia/deep-seated candidaemia | T2 Candida panel. | Detects Candida DNA directly in whole blood. Detection limit of 1 cfu/mL. Greater than 10-fold decrease in reporting time. |
| Pulmonary aspergillosis | Immunochromatographic technology – lateral flow devices/dip sticks. | POC or near-POC test for detecting cell wall components in serum, BAL and urine. Impact of antifungal prophylaxis on sensitivity not known. |
| Aspergillus proximity ligation assay. | Detects cell wall mannoprotein. | |
| Cryptococcosis | Various lateral flow formats. | Designed to detect cryptococcal polysaccharide antigen in CSF, whole blood and saliva. Adopted by WHO on their Essential Diagnostics List. |
| Histoplasmosis | Various ELISA platforms, lateral flow devices, and a loop-mediated isothermal amplification assay. | Rapid, lateral flow devices are suitable for POC testing, urine is the preferred clinical sample. |
Summary of new non-culture laboratory diagnostic tests for systemic fungal diseases described in this review
| Disease . | Test . | Comments . |
|---|---|---|
| Candidaemia/deep-seated candidaemia | T2 Candida panel. | Detects Candida DNA directly in whole blood. Detection limit of 1 cfu/mL. Greater than 10-fold decrease in reporting time. |
| Pulmonary aspergillosis | Immunochromatographic technology – lateral flow devices/dip sticks. | POC or near-POC test for detecting cell wall components in serum, BAL and urine. Impact of antifungal prophylaxis on sensitivity not known. |
| Aspergillus proximity ligation assay. | Detects cell wall mannoprotein. | |
| Cryptococcosis | Various lateral flow formats. | Designed to detect cryptococcal polysaccharide antigen in CSF, whole blood and saliva. Adopted by WHO on their Essential Diagnostics List. |
| Histoplasmosis | Various ELISA platforms, lateral flow devices, and a loop-mediated isothermal amplification assay. | Rapid, lateral flow devices are suitable for POC testing, urine is the preferred clinical sample. |
| Disease . | Test . | Comments . |
|---|---|---|
| Candidaemia/deep-seated candidaemia | T2 Candida panel. | Detects Candida DNA directly in whole blood. Detection limit of 1 cfu/mL. Greater than 10-fold decrease in reporting time. |
| Pulmonary aspergillosis | Immunochromatographic technology – lateral flow devices/dip sticks. | POC or near-POC test for detecting cell wall components in serum, BAL and urine. Impact of antifungal prophylaxis on sensitivity not known. |
| Aspergillus proximity ligation assay. | Detects cell wall mannoprotein. | |
| Cryptococcosis | Various lateral flow formats. | Designed to detect cryptococcal polysaccharide antigen in CSF, whole blood and saliva. Adopted by WHO on their Essential Diagnostics List. |
| Histoplasmosis | Various ELISA platforms, lateral flow devices, and a loop-mediated isothermal amplification assay. | Rapid, lateral flow devices are suitable for POC testing, urine is the preferred clinical sample. |
Imaging: best practice
Imaging of invasive pulmonary fungal infections
CT remains the cornerstone for radiological diagnosis of invasive pulmonary fungal infections (IPFI) (Figure 1). Thin-section CT evaluation has to be performed within 12–24 h of symptom onset in case of suspicion of IPFI at optimized dose, according to the ALARA (as low as reasonably achievable) principle.44 Although chest CT can be done without contrast media in most cases, CT with intravenous (iv) contrast administration (CT-angiography) may identify direct peripheral vascular occlusion in lesions with a large diameter and not localized in peripheral lung parenchyma with high sensitivity and negative predictive value for invasive pulmonary aspergillosis (IPA).45,46 CT-angiography is also required in case of haemoptysis.
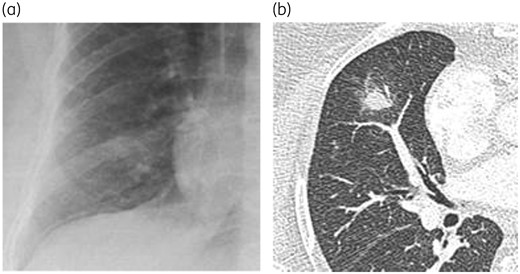
Chest X-ray (a) and axial CT slice (b) in a patient with invasive aspergillosis. Note the much higher value of the axial CT scan compared with the X-ray, perfectly demonstrating a macronodule with a halo sign at the level of the middle lobe. Such an aspect is very suggestive of the diagnosis in a specific context.
As an alternative to thin-section CT, MRI of the lungs may be performed to avoid radiation exposure.47 MRI with T2-weighted turbo-spin-echo sequences exhibits sensitivity and specificity approaching that of CT for the diagnosis of IPA.47–49 New sequences are also promising, even without gadolinium injection, to potentially assess parenchymal lesions as well as vascular involvement.
The role of positron-emission tomography (PET) for the diagnosis of invasive fungal infections is not well defined. Some studies suggest a role for initial assessment of the extent of the disease.50,51
During the patient’s follow-up, reduced-dose CT is preferred to chest X-ray.52 PET could also have a role in the assessment of disease activity in the case of a persistent lesion in order to guide therapeutic decisions.50
No CT scan pattern is either sensitive or specific for IPA or IPFI in general,53 and in all cases differential diagnoses must be kept in mind. The classical well-known CT findings in the angio-invasive form of IPA consist of dense and well-circumscribed lesions >1 cm, with the presence of a halo of ground-glass attenuation (halo sign) at early stages.54,55 While these anomalies are observed in about 90% of patients with haematological cancer, additional findings may be observed, some of them being described in non-haematological patients, particularly in solid organ transplant recipients as well as in other non-neutropenic patients.56,57 The latter include alveolar consolidations or masses, internal low-attenuation or hypodense signs, tree-in-bud pattern and pleural effusion. A distinct entity, referred to as airway-invasive aspergillosis, characterized by destruction of the bronchiolar wall by Aspergillus, may present with tracheal or bronchial wall thickening, centrilobular nodules with tree-in-bud appearance in a patchy distribution, or predominant peribronchial areas of consolidation (Figure 2).58 Alveolar consolidations that may occur in Aspergillus bronchopneumonia (in airway-invasive disease) may not be differentiated from aspergillosis-induced haemorrhagic infarctions encountered in the angio-invasive form.59
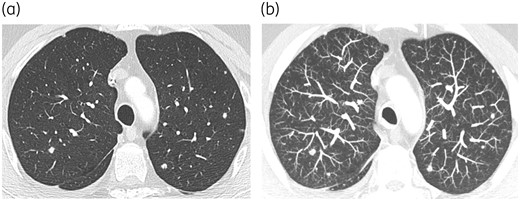
Chronic disseminated candidosis in a patient suffering from AML under salvage chemotherapy (a and b). Lung involvement appearing as multiple nodules is much better assessed by using the maximum intensity projection (suppress CR) post-processing tool (b). This tool, which consists of projecting the voxel with the highest attenuation value on every view throughout the volume onto a 2D image, improves the detection of pulmonary nodules in this setting. There was an associated hepato-splenic involvement (not shown).
Overall, various and non-specific presentations may be observed, partly due to the increased diversity of immunosuppression types and increased incidence of IPA in non-neutropenic patients. In particular, IPA is increasingly reported among ICU patients, cirrhotic patients or patients with severe influenza infection,60,61 but no typical radiological pattern has been described up to now.
Delayed findings, including the air-crescent sign (crescent-shaped collection of air surrounding an infarcted sequester) correlating with bone marrow recovery62 and cavitation, are less observed nowadays owing to the improved diagnostic procedures leading to early IPA detection and treatment.
Mucormycosis, the second most frequent IPFI after IPA, should be suspected in the presence of a reversed halo sign63,64 or a bird’s nest sign, multiple (>10) nodular lesions or pleural effusion.65 The same angio-invasive profile as angio-invasive aspergillosis may be observed.
Lung nodules may also be encountered in the context of chronic disseminated candidosis (Figure 2), in addition to hepato-splenic involvement.
![Invasive sinonasal aspergillosis. A 76-year-old immunocompromised woman presented with right eye proptosis. MRI and PET showed a mass in the right nasal fossa and sphenoid sinus appearing hypointense on T2-weighted images (a), with poor to intermediate enhancement on gadolinium T1-weighted images (b) and high [18F]FDG uptake (c). Intraorbital extension with pathological infiltration of the extraconal fat, of the lower, medial and lateral extrinsic eye muscles and infiltration of the intraconal fat was noted on fluid-attenuated inversion recovery (FLAIR) ‘fat sat’ coronal images (d).](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jac/74/Supplement_2/10.1093_jac_dkz041/1/m_dkz041f3.jpeg?Expires=1747910131&Signature=qNWofOUNAf7xqGMdO3OCfWZlKa4RzU~syJuPO21xDyDmsPQPuftru32ollESiqzmc7uNocnOIlYXInW-oDWN7QZu56DZANP2jVfLgB0qLuLsW7NC6mAhotsn4IeyVFI0SRQfenkcTE8q80QYdvIIqSqP8cNOGmHhi2C3r9IRyQCSqwOnHUBpruE8WqBGTvX8kSh0Nh~mkTrLgfo-PTumI2Jf6NbD4xsyRWjKtN7chASIx0H2ngh6rKKwSa~nar4ycsW--mjg7APTitVPg0y2-izA1TVkZf1CFjVHmA35WtTcXFrPjsSdm-TmTmVlukokg1ocdhqpSVWneCSDMmjnFQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Invasive sinonasal aspergillosis. A 76-year-old immunocompromised woman presented with right eye proptosis. MRI and PET showed a mass in the right nasal fossa and sphenoid sinus appearing hypointense on T2-weighted images (a), with poor to intermediate enhancement on gadolinium T1-weighted images (b) and high [18F]FDG uptake (c). Intraorbital extension with pathological infiltration of the extraconal fat, of the lower, medial and lateral extrinsic eye muscles and infiltration of the intraconal fat was noted on fluid-attenuated inversion recovery (FLAIR) ‘fat sat’ coronal images (d).
Rhinosinusal fungal infections
CT of the nasal fossae and paranasal sinuses may allow an early diagnosis of rhinosinusal fungal infections, such as invasive aspergillosis or mucormycosis, with an optimal assessment of osseous erosion. Nevertheless, MRI has higher sensitivity and specificity and may more clearly visualize the extent of the lesions, particularly in the brain.66 Common CT findings of rhinosinusal fungal infections include unilateral involvement of the maxillary and/or ethmoid sinuses with or without facial soft tissue thickening, bone erosion, mucosal thickening or soft tissue masses, and fluid level. On MRI, rhinosinusal disease (Figure 3) demonstrates variable T1 intensity, T2 intermediate to low intensity, and typical loss of contrast enhancement (also known as the ‘black turbinate sign’) due to devitalized mucosa that have to be surgically removed.67 Soft-tissue infiltration, as well as invasion of the optic nerve, may be subtle and are better demonstrated by using MRI with fat saturation and gadolinium injection.68
Cerebral fungal infections
Diagnosis of cerebral aspergillosis or other invasive fungal infections affecting the brain is based on CT and MRI.69,70 CT is the ideal tool for assessment of bone involvement, and has to include a non-enhanced series to exclude bleeding. A normal CT requires an additional MRI, which has better sensitivity for small lesions that may be undetected by CT.70 Multiple, poorly defined hypoattenuated lesions with little or no mass effect and slight or no contrast enhancement can be observed on CT scans. MRI allows the detection of additional >1 cm diameter lesions. Focal hypo/hyperintense T1 and hypo/isointense T2/proton density (PD) lesions related to septic infarctions with haemorrhage may be seen in ∼25% of cases.69 While weak or no enhancement is most common after gadolinium injection in immunocompromised patients, annular enhancement consistent with capsule formation has been reported in immunocompetent patients, overall suggesting that enhancement also depends on the degree of patient immune system integrity. Annular restricted diffusion, decreased cerebral blood volume on perfusion-weighted imaging, as well as potential interest of MR spectroscopy have also been reported.71
Novel antifungal agents
With globalization, antifungal drug resistance is an emerging public health problem. Options for treatment of multidrug-resistant fungi and oral antifungal treatment are limited and new antifungals are urgently needed.72,73 There are several additional unmet needs in the treatment of invasive fungal disease (IFD) that may be addressed by new antifungals in development.72 Key issues are the lack of agents that are active against emerging antifungal resistance mechanisms, such as echinocandin and azole resistance mediated by FKS and CYP mutations, respectively, and that target difficult-to-treat yeasts or moulds harbouring intrinsic antifungal resistance. Other desirable features include agents that are fungicidal, and provide options for combination antifungal therapy, that have a favourable pharmacokinetic (PK)/pharmacodynamic (PD) profile and few drug–drug interactions and can be administered via both the oral and the iv route.72 Until recently there has been no new class of antifungal drugs discovered in over 40 years and no new class brought into practice since the echinocandins in 2006.72,73
Recent discoveries of novel antifungal agents address some of these unmet needs in antifungal treatment. New antifungal drugs have been identified through various approaches, including drug screening libraries, repurposing of old drugs74 and advances in understanding cell metabolic pathways.73 Fast tracking and awarding orphan drug status by the FDA have assisted in bringing much-needed new drugs into clinical practice.72 There are now several new antifungal drug classes undergoing clinical trials.
New antifungals in clinical trials (Table 2) include those with activity against the fungal cell wall, cell membrane metabolic pathways, novel CYP inhibitors and drugs targeting cell signalling pathways. New formulations of existing agents such as nanoparticles or encochleated amphotericin B and new approaches to treating IFD such as immunotherapy,75 manipulation of the microbiome76 and modifying gene expression are under also investigation but will not be discussed further here.
| Agent name . | Target . | Activity . | Gap . | Phase of trial . |
|---|---|---|---|---|
| F901318 |
|
|
|
|
|
|
| Some moulds | Phase 2: VVC. |
| VL2397 |
|
| Unknown | Phase 2: first-line IA in adults. |
| SCY-078 |
|
| Unknown |
|
|
|
| Some moulds |
|
| APX-001 | GPI synthesis inhibitor. |
|
|
|
| Agent name . | Target . | Activity . | Gap . | Phase of trial . |
|---|---|---|---|---|
| F901318 |
|
|
|
|
|
|
| Some moulds | Phase 2: VVC. |
| VL2397 |
|
| Unknown | Phase 2: first-line IA in adults. |
| SCY-078 |
|
| Unknown |
|
|
|
| Some moulds |
|
| APX-001 | GPI synthesis inhibitor. |
|
|
|
BDG, (1–3) β-d-glucan; GPI, glycosylphosphatidyl inositol; IA, invasive aspergillosis; IC, invasive candidiasis; PJP, Pneumocystis jiroveci pneumonia; VVC, vulvovaginal candidiasis.
| Agent name . | Target . | Activity . | Gap . | Phase of trial . |
|---|---|---|---|---|
| F901318 |
|
|
|
|
|
|
| Some moulds | Phase 2: VVC. |
| VL2397 |
|
| Unknown | Phase 2: first-line IA in adults. |
| SCY-078 |
|
| Unknown |
|
|
|
| Some moulds |
|
| APX-001 | GPI synthesis inhibitor. |
|
|
|
| Agent name . | Target . | Activity . | Gap . | Phase of trial . |
|---|---|---|---|---|
| F901318 |
|
|
|
|
|
|
| Some moulds | Phase 2: VVC. |
| VL2397 |
|
| Unknown | Phase 2: first-line IA in adults. |
| SCY-078 |
|
| Unknown |
|
|
|
| Some moulds |
|
| APX-001 | GPI synthesis inhibitor. |
|
|
|
BDG, (1–3) β-d-glucan; GPI, glycosylphosphatidyl inositol; IA, invasive aspergillosis; IC, invasive candidiasis; PJP, Pneumocystis jiroveci pneumonia; VVC, vulvovaginal candidiasis.
Agents targeting fungal metabolic pathways
The pyrimidine salvage pathway is targeted by olorofim (formerly F901318; F2G Inc., Manchester, UK), a dihydro-orotate dehydrogenase inhibitor of the orotomide class with activity against Aspergillus species, including isolates with azole resistance mediated by CYP51A mutations.77 Further, it has activity against Scedosporium and Lomentospora spp. and some other moulds and endemic fungi such as certain species of Fusarium and Penicillium spp., as well as Taloromyces marneffei.77 It has no activity against the Mucorales or yeasts.77 The enzyme is critical to fungal pyrimidine synthesis and this orotomide class drug has ∼2000 times the affinity for the fungal than the human enzyme.77 It is available in oral and iv formulations. It was more effective than posaconazole in treating IPA in neutropenic mice and rabbit studies as determined by galactomannan response.78 It is currently under Phase 2 study as antifungal prophylaxis in patients with AML (Clinicaltrials.gov NCT02856178) and a salvage study for treatment of resistant Aspergillus or other moulds.79
VL2397 (Vical Pharmaceuticals San Diego, CA, USA) utilizes the siderophore transport pathway to enter the fungus and complexes aluminium; however, its precise mechanism of antifungal action is unknown.80 It has activity against Aspergillus, including CYP51A mutants with azole resistance, drug-resistant Candida, Cryptococcus and Trichosporon spp.80 It is undergoing Phase 2 study for first-line treatment of invasive aspergillosis (Clinicaltrials.gov NCT03327727).81
VT-1161, VT-1129 and VT-1598 (Viamet Pharmaceuticals Inc., Durham, NC, USA) are oral agents that inhibit fungal CYP51 and have greater specificity for fungal CYP51 than mammalian CYP450 enzymes, which may result in fewer significant drug–drug interactions.80 The structural modification resulting in this feature is the replacement of the triazole group with a tetrazole group.80 Activity against dimorphics, Cryptococcus and Candida, has been demonstrated by all three agents whilst a broader spectrum including Aspergillus, other moulds and endemic fungi was seen with VT-1598.80 In murine models VT-1161 was protective against Rhizopus arrhizus infection82 and VT-1598 was efficacious in experimental coccidioidomycosis.83 VT-1161 was efficacious and well tolerated in recurrent vulvovaginal candidiasis in a human Phase 2 study.84 These agents are available in oral or topical formulation and VT-1161 is undergoing Phase 2 study in vulvovaginal candidiasis (Clinicaltrials.gov NCT01891331).85
Agents targeting fungal cell wall integrity
APX-001 (Amplyx Pharmaceuticals, San Diego, CA, USA) is a glycosylphosphatidyl inositol inhibitor that blocks maturation of anchoring cell proteins that act as adhesins, allowing microorganisms to adhere to host mucosal and epithelial surfaces prior to colonization and replication. It has broad activity including Candida spp. such as Candida auris, FKS mutants exhibiting echinocandin resistance, Aspergillus spp. including azole-resistant isolates, Fusarium spp., Scedosporium and some Mucorales.72,80 It is available in oral or iv formulations and is undergoing Phase 1 study (Clinicaltrials.gov NCT02956499).86
SCY-078 (Synexis Inc., Jersey City, NJ, USA), an enfumafungin derivative, inhibits glucan synthase and although structurally not an echinocandin, belonging to the triterpene drug class, it inhibits (1–3) β-d-glucan.87 It is active against Candida spp. including C. auris and FKS mutants, and has good activity against Aspergillus, including azole-resistant isolates.88,89 Variable activity is seen against other moulds.80 It was effective in a neutropenic murine model of invasive candidiasis caused by wild-type and echinocandin-resistant Candida glabrata.90 It is available in oral and iv formulations and is undergoing Phase 2 human studies in invasive and mucocutaneous candidiasis (Clinicaltrials.gov NCT02244606)91 and an open-label Phase 3 study comparing it with standard of care for refractoriness or antifungal intolerance in invasive and mucocutaneous candidiasis (Clinicaltrials.gov NCT02679456).92
Rezafungin (CD101) (Cidara Therapeutics, San Diego, CA, USA) acts by 1,3-β-d-glucan synthase inhibition. There is a structural modification of the ether ring that increases potency and half-life compared with other echinocandins, meaning that the drug can be administered weekly.93,94 It has iv or topical formulations and activity against Candida spp. including C. auris and FKS mutants exhibiting echinocandin resistance.95,96 It has activity against Aspergillus spp. including azole-resistant isolates.93 It is in Phase 2 studies in invasive candidiasis, vulvovaginal candidiasis and for prophylaxis of fungal infection in patients undergoing stem cell transplantation (NCT02734862, NCT02733432).97,98
Cell signal transduction pathways and epigenetic/gene regulation are also targets for novel antifungals. Signal transduction pathways targeted include mitogen-activated protein (MAP) kinase, phosphoinositide-dependent kinase 1 (PDK-1) and calcium signalling. Gene expression transcription factors and epigenetically acting agents such as histone deacetylase (HDAC) inhibitors, which regulate apoptosis, transcription and chromatin structure, are being explored.72,73 These approaches are all in Phase 1 study but show promise.
Repurposing old drugs
Through screening of drug libraries,74 drugs such as Cox2 inhibitors,99 tamoxifen,100 sertraline101 and aurofin102 have been identified as having antifungal activity (Table 3) through various mechanisms including acetyl CoA, protein synthesis and calmodulin inhibition. Some, such as aurofin, which acts through oxidative cell death, have broad-spectrum antifungal activity including Lomentospora and Fusarium spp.102 All are in Phase 1/2 study.
Repurposed drugs as antifungals, mechanism of action, spectrum and clinical studies
| Agent . | Mechanism . | Spectrum . | Trial Phase . | Derivative . | Use . |
|---|---|---|---|---|---|
| AR-12 | Acetyl-CoA inhibition. | Broad activity: yeasts/moulds including Fusarium and Scedosporium spp. and dimorphics. | Animal studies. |
| Anti-cancer |
| Tamoxifen | Calmodulin inhibition. | Cryptococcus. | Phase 2: cryptococcal meningitis. | Oestrogen receptor blocker. | Anti-cancer |
| Sertraline | Inhibition of protein synthesis via translation initiation factor. |
| Phase 2: cryptococcal meningitis. | Selective serotonin reuptake inhibitor. | Anti-anxiety |
| Aurofin | Oxidative cell death. |
| None. | Gold thiol. | Rheumatoid arthritis |
| Agent . | Mechanism . | Spectrum . | Trial Phase . | Derivative . | Use . |
|---|---|---|---|---|---|
| AR-12 | Acetyl-CoA inhibition. | Broad activity: yeasts/moulds including Fusarium and Scedosporium spp. and dimorphics. | Animal studies. |
| Anti-cancer |
| Tamoxifen | Calmodulin inhibition. | Cryptococcus. | Phase 2: cryptococcal meningitis. | Oestrogen receptor blocker. | Anti-cancer |
| Sertraline | Inhibition of protein synthesis via translation initiation factor. |
| Phase 2: cryptococcal meningitis. | Selective serotonin reuptake inhibitor. | Anti-anxiety |
| Aurofin | Oxidative cell death. |
| None. | Gold thiol. | Rheumatoid arthritis |
Repurposed drugs as antifungals, mechanism of action, spectrum and clinical studies
| Agent . | Mechanism . | Spectrum . | Trial Phase . | Derivative . | Use . |
|---|---|---|---|---|---|
| AR-12 | Acetyl-CoA inhibition. | Broad activity: yeasts/moulds including Fusarium and Scedosporium spp. and dimorphics. | Animal studies. |
| Anti-cancer |
| Tamoxifen | Calmodulin inhibition. | Cryptococcus. | Phase 2: cryptococcal meningitis. | Oestrogen receptor blocker. | Anti-cancer |
| Sertraline | Inhibition of protein synthesis via translation initiation factor. |
| Phase 2: cryptococcal meningitis. | Selective serotonin reuptake inhibitor. | Anti-anxiety |
| Aurofin | Oxidative cell death. |
| None. | Gold thiol. | Rheumatoid arthritis |
| Agent . | Mechanism . | Spectrum . | Trial Phase . | Derivative . | Use . |
|---|---|---|---|---|---|
| AR-12 | Acetyl-CoA inhibition. | Broad activity: yeasts/moulds including Fusarium and Scedosporium spp. and dimorphics. | Animal studies. |
| Anti-cancer |
| Tamoxifen | Calmodulin inhibition. | Cryptococcus. | Phase 2: cryptococcal meningitis. | Oestrogen receptor blocker. | Anti-cancer |
| Sertraline | Inhibition of protein synthesis via translation initiation factor. |
| Phase 2: cryptococcal meningitis. | Selective serotonin reuptake inhibitor. | Anti-anxiety |
| Aurofin | Oxidative cell death. |
| None. | Gold thiol. | Rheumatoid arthritis |
Conclusions
This review has summarized the application of currently available diagnostic strategies and ongoing developments to take advantage of our ever-increasing understanding of the pathogenesis of fungal disease. The development of POC tests for fungal infections has gained momentum over the past 5 years. LFD tests for cryptococcal antigen are now the standard of care for diagnosing cryptococcal meningitis. Similar immune-chromatography technology is being developed for Histoplasma antigen, and immunoglobulin G for the diagnosis of chronic aspergillosis and coccidioidomycosis. Many nucleic acid amplification techniques (PCR) have been developed for the diagnosis of fungal disease since the early 1990s but still need standardization and clinical validation in different disease settings. European initiatives to address these issues are ongoing. Exciting new developments in the radiology of fungal lesions include the application of antibody-based PET/MRI imaging. This type of approach is being developed for therapeutic purposes as well. Although until recently there have been no new drug classes under study, several promising new agents are now undergoing clinical trials. New approaches to drug discovery have yielded multiple new classes with novel mechanisms of action. There are currently two new classes of antifungal drugs in Phase 2 study for systemic IFD and one in Phase 1 study. These new antifungal drugs show promise in meeting unmet needs with oral and iv formulations available and some with decreased potential for drug–drug interactions. Novel mechanisms of action mean these agents are not susceptible to the common resistance mechanisms seen in Candida or Aspergillus. They also provide new treatment options for intrinsically resistant organisms such as C. auris, Lichtheimia prolificans and Fusarium spp. Favourable PK/PD and options for combination therapy are provided by some of these agents.
In conclusion, partnerships between industry, regulators and clinicians are critical to fast-track new diagnostics and new antifungal agents and maintain the momentum to bring these urgently needed new diagnostics and treatment options into clinical practice to enhance patient care.
Transparency declarations
M. Sanguinetti has participated in meeting and advisory boards for Gilead, Merck and ThermoFisher Scientific, and has received research grants and/or speaker honoraria from Merck, Pfizer and Gilead. C. B.-A. has received speaker honoraria from Gilead. F. L. has been a member of advisory boards for MSD and Basilea. M. Slavin has received research grants, consultancy fees and speaker honoraria from Gilead Sciences, Merck, and Pfizer. M. D. R. has been an advisor and lecturer for Gilead Sciences, MSD, Basilea, and Pfizer. B. P. and V. D. have nothing to declare.
This article forms part of a Supplement sponsored and funded by Gilead Sciences. Editorial assistance was provided by Tribal Healthcare. The content of this Supplement is based on the sessions presented at the CARE X meeting, held in Barcelona in November 2017.
References
World Health Organization.
Clinicaltrials.gov. Study on Safety and Pharmacokinetics of Intravenous F901318 for Fungal Prophylaxis in AML Patients (SAFEGUARD). https://clinicaltrials.gov/ct2/show/NCT02856178.
Clinicaltrials.gov. VL-2397 Compared to Standard First-Line Treatment for Invasive Aspergillosis (IA) in Adults. https://clinicaltrials.gov/ct2/show/NCT03327727.
Clinicaltrials.gov. A Study to Evaluate Oral VT-1161 in the Treatment of Patients With Acute Vaginal Candidiasis (Yeast Infection). https://clinicaltrials.gov/ct2/show/NCT02267382.
Clinicaltrials.gov. Safety, Tolerability and Pharmacokinetics of APX001 Administered Intravenously. https://clinicaltrials.gov/ct2/show/NCT02956499.
Clinicaltrials.gov. Oral SCY-078 vs Standard-of-Care Following IV Echinocandin in the Treatment of Invasive Candidiasis. https://clinicaltrials.gov/ct2/show/NCT02244606.
Clinicaltrials.gov. Safety and Efficacy of Oral SCY-078 vs. Oral Fluconazole in Subjects With Vulvovaginal Candidiasis. https://clinicaltrials.gov/ct2/show/NCT02679456.
Clinicaltrials.gov. CD101 Compared to Caspofungin Followed by Oral Step Down in Subjects with Candidemia and/or Invasive Candidiasis (STRIVE). https://clinicaltrials.gov/ct2/show/NCT02734862.
Clinicaltrials.gov. RADIANT: CD101 vs Standard of Care in Subjects with Acute Vaginal Yeast Infections. https://clinicaltrials.gov/ct2/show/NCT02733432.
- magnetic resonance imaging
- echocardiography
- drug interactions
- lung
- antifungal agents
- cryptococcal meningitis
- radiation exposure
- mycoses
- systemic mycosis
- histoplasmosis
- aspergillus
- ligation
- nucleic acid amplification techniques
- technology
- diagnosis
- diagnostic imaging
- pharmacokinetics
- pathogenic organism
- aspergillosis, invasive
- aspergillosis, invasive pulmonary
- drug discovery
- candida
- standard of care
- immunochromatographic test
- mri of lungs
- transverse spin relaxation time
- candida test
- fluid flow



